Abstract
Cellulose is the most abundant renewable material in nature, which is converted to simple sugar, glucose, by cellulase enzymes. Cellulase enzymes are one of the most important industrial enzymes that are widely used in the production of biofuels. This study aimed to use wheat straw as a carbon source for Saccharomyces cerevisiae yeast cells to produce bioethanol. Physical grinding and phosphoric acid plus hydrogen peroxide chemical pretreatment methods were carried out for the removal of lignin and hemicellulose from the structure of wheat straw and obtaining a cellulose-rich material. Thermophilic filamentous fungi were isolated from cow manure, and two isolates were selected based on the levels of endoglucanase, exoglucanase, and total cellulase activities and identified based on DNA sequences of internal transcribed spacer regions. Hydrolysates were obtained from enzymatic hydrolysis of pretreatment wheat straw by thermophilic cellulase enzymes. Fermentation of Saccharomyces cerevisiae yeast cells was performed in hydrolysates for bioethanol production. Aspergillus fumigatus isolate indicated 147.11, 515.9, and 453.78 U/mg wheat straw for exoglucanase, endoglucanase, and total cellulase activities, respectively. These activities were, respectively, measured as 380.35, 730.24, and 372.76 U/mg wheat straw for Rhizomucor pusillus isolate. Pretreatment procedures functioned efficiently and increased the cellulose part of wheat straw up to 88.91%. Hydrolysates of pretreated wheat straw (obtained from Aspergillus fumigatus and Rhizomucor pusillus cellulase activities) resulted in 21.88 and 24.02 g/L (4.05 and 5.31%) bioethanol by Saccharomyces cerevisiae yeast fermentation, respectively. It seems that utilizing thermophilic cellulase enzymes and wheat straw is a promising economic process for bioethanol production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Fossil fuels, as non-renewable sources, are mainly responsible for emitting greenhouse gases such as CO2, CH4, and NO2, which lead to global warming. Some factors such as excessive consumption of fossil fuels, increasing demand for energy, fluctuating fuel prices, and rising greenhouse gas emissions have changed the global trends from fossil fuels to biofuels [1]. Biofuels divide into three categories according to the type of raw materials: first-generation biofuels (using food, mainly corn and sugarcane as raw materials), second-generation biofuels (utilizing non-food materials such as wheat straw, rice straw, and other crop residues for bioethanol production), and third-generation biofuels (using algae, sludge, and municipal wastes). Second-generation biofuels are the most suitable option due to the abundance of raw materials and lack of competition with food [2]. Wheat straw (containing lignocellulosic compounds) is a promising solution for liquid fuel production (such as bioethanol). Lignocellulosic biomass is mainly composed of three parts: cellulose, hemicellulose, and lignin. Several processes should be performed to produce bioethanol from cellulose and hemicellulose [3]. Annually, about 850 million tons of wheat are produced worldwide, and the European Union, China, India, the USA, and Canada are the leading countries in the field of wheat cultivation. It is possible that 93 million liters of gasoline are processed with these amounts of wheat [4]. Wheat is one of the major grain crops in Iran and accounts for about 14.28% of all agricultural products. Due to the high capacities of wheat in Iran, wheat straw can be used as a raw material to produce bioethanol [5]. Pretreatment of lignocellulosic materials using different physicochemical methods is necessary to decompose the strong and rigid structure of lignocellulose into cellulose, hemicellulose, and lignin. Lignin is a substance that inhibits the effect of cellulase enzymes on cellulose, and one of the goals of the pretreatment phase is to separate this inhibitory compound. Pretreatment would also increase the access of cellulase enzymes to cellulose polymers. Cellulases convert these polymers into sugar monomers. Then, using the fermentation process by microorganisms, the created sugar is converted to bioethanol [6]. Cellulase enzymes include three groups of enzymes: (1) endoglucanases (hydrolyze internal binds of cellulose polymers), (2) exoglucanases (attack to ends of cellulose chains), and (3) β-glucosidases (breakdown cellobiose to glucose). Cellobiose is the product of the activity of the two first enzymes. As a result of the synergistic functions of these three enzymes, lignocellulosic materials would be degraded to simple sugars [7]. Filamentous fungi are one of the primary sources of cellulase enzymes in nature; several thermophilic fungi grow at 40°C and produce all three cellulase enzymes significantly. These enzymes show high hydrolysis activities at high temperatures, cause enzymatic degradation of pretreated lignocellulosic materials, and provide the sugar for the yeast cell fermentation and bioethanol production [8]. Currently, the phosphoric acid plus hydrogen peroxide (PHP) method is a new and developed pretreatment procedure. PHP is a mixture of phosphoric acid and hydrogen peroxide, which efficiently separates lignin and hemicellulose from grinned wheat straw, and finally provides cellulose for cellulase enzymes in an amorphous and porous structure. It was obtained a cellulose-rich part (39.7 g) from dry straw (100 g) [9].
In this study, we aimed to utilize wheat straw as a raw material for bioethanol production. Thermophilic cellulase-producing fungi were isolated from cow manure for the enzymatic hydrolysis step, and harvested sugar solutions were converted to ethanol by Saccharomyces cerevisiae fermentation. Different filamentous fungi have various capabilities in cellulase enzyme production, and their cellulase activity levels could be varied at a wide range. Screening new fungus isolates might be resulted in finding strong cellulase enzyme cocktails. We screened and selected two thermophilic fungal isolates among more than 20 isolates. These two showed the best cellulase enzyme activities, and bioethanol production was performed using these new cellulase enzyme cocktails. It seemed that they are good economic candidate for utilization in bioethanol produce processing.
2 Materials and methods
2.1 Wheat straw
Wheat straw samples were washed and cleaned, then dried at 50°C for 72 h, and stored in zippered plastic. The straw was ground into small pieces by a mill and passed through a sieve with a mesh of 30 until the size of the pieces reached about 1 mm [10]. This straw was also very crushed and passed through a sieve with 100 mesh. The straw particle size reached about 0.1 mm. These straw particles were used for chemical pretreatment and fungal isolates cultivations. The crushed straw was dried at 50°C for 24–48 h and stored in zippered plastic. To ensure complete drying of the straw, it was weighed several times during this time, and when the weight loss occurred no longer, the drying process was completed [9].
2.2 Wheat straw components
Wheat straw consisted of three main parts: cellulose, hemicellulose, and lignin. The percentages of dried wheat straw components were determined based on neutral detergent-insoluble fiber method (NDF) and acid detergent-insoluble fiber method. Wheat straw was also placed at 550°C for 4 h for determining the percentage of ash [11].
2.3 Chemical pretreatment of wheat straw by phosphoric acid plus hydrogen peroxide method
Phosphoric acid plus hydrogen peroxide (PHP) pretreatment was performed on wheat straw as the following protocol:
-
1)
Wheat straw was collected, ground, and passed through mesh 100, so that the particles size was 150–250 µm.
-
2)
PHP pretreatment was performed in a 250-ml bottle containing 10 g of dried wheat straw and 100 g of PHP solution. PHP solution was prepared by mixing 82.6 g (85% H3PO4 w/w) with 17.4 g (30% H2O2 w/w) (final concentration of 70.2% phosphoric acid and 5.2% hydrogen peroxide, respectively).
-
3)
The resulting mixture was incubated at 50°C, 180 rpm for 2 h, and at 40°C, 180 rpm for 3 h.
-
4)
The treated mixture was mixed with 250 ml of 95% ethanol and filtered through filter paper. The solid part was washed three to five times using 95% ethanol.
-
5)
The solid part was washed with distilled water until the pH reached 5. This cellulose-rich solid part was stored at −20°C until use.
-
6)
The washing solution of step four was distilled to recover acid and ethanol [9].
2.4 Thermophilic fungal isolates
The aim of this section was to isolate thermophilic filamentous fungi with the ability of producing cellulase enzymes. These fungi survive and grow in the temperature range of 20 to 60°C. These fungi were isolated from animal manure samples. Different dilutions of samples were prepared and transferred onto plates containing carboxymethyl cellulose (CMC) and cellulose culture medium. These plates were incubated at 45°C for 12–14 h. Fungal colonies were isolated and cultivated on PDA culture medium [12]. After primary growth of fungi, single spore colonies were prepared for each isolate [13, 14].
2.4.1 Qualitative endoglucanase test
Thermophilic fungal isolates (a 1-cm circle of young mycelium) were transferred onto CMC media. Clear zones (fungal growth regions) were determined by Congo red dye, and their diameters were measured after 24 and 48 h [15]. Seven isolates with the largest clear zones (the highest levels of CMCase enzymes) and higher growth rates on potato dextrose agar (PDA) medium were selected for the next step.
2.4.2 Optimization of cellulase enzyme reaction conditions
The cellulase enzymes of thermophilic fungal isolates show the highest hydrolysis activities at specialized pHs and temperatures. Optimization of cellulase enzyme (endoglucanase, exoglucanase, and total cellulase) activities was carried out for the temperature (at the range of 35–60°C) and pH (at the range of 3.5–6.5) parameters.
CMC (2% w/v), Avicel PH 101 (2% w/v), and Whatman filter paper No. 1 were utilized as the substrates for quantitative endoglucanase, exoglucanase, and total cellulase (FPase) activity assays, respectively. The reaction mixtures were prepared with substrates and crude enzyme solutions obtained from thermophilic fungi cultivation. They were incubated at the temperature range of 35–60°C for 30 (endoglucanase and exoglucanase assays) and 60 min (FPase assay), respectively. The concentration of enzymatic reaction production (glucose) was determined by dinitrosalicylic acid (DNS) method. Glucose standard curves were prepared for each enzyme assay and utilized for enzyme activity calculations. Each unit of enzyme is equivalent to the amount of enzyme in the reaction mixture that produces 1 μmol of product per 1 min. Cellulase enzyme activities were expressed based on unit enzyme per mg wheat straw [16,17,18]. Cellulase enzyme assays were repeated at optimized temperature and pH for each crude enzyme solution.
2.4.3 Identification of filamentous fungal isolates
DNA extraction was done for two thermophilic fungal isolates that had been indicated the highest cellulase enzyme activities. Polymerase chain reactions (PCR) were performed using general primers ITS5 (5′-TCCTCCGCTTATTGTATGC-3′) and ITS4 (5′-GGAAGTAAAAGTCGTAAGG-3′) (manufactured by Sinuhe Biotechnology Company, Iran) to amplify ITS region of template DNAs according to Table 1 [19]. Red Master mix PCR was prepared from Amplicon company.
The PCR product of each sample was electrophoresed on agarose gel, and DNA bands were observed by gel documentation. Then PCR products were sequenced, and the resulting nucleotide sequences were utilized for nucleotide BLAST in NCBI database for filamentous fungi identification [20]. These sequences were analyzed by BOLD system software version 4. Phylogenetic tree was constructed using MEGA software version X, based on maximum likelihood statistical method, bootstrap method with 5000 replications, and Tamura–Nei model using ITS sequences.
2.5 Filamentous fungi and yeast cultivations
Primary cultivations of two selected fungal isolates were performed by adding fungal spores (107/ml) to the YPD medium culture. These incubated at 45°C and 100 rpm for 24 h. Growing mycelia were transferred to solid-state fermentation flasks including wheat straw powder (10.0 g) and salt solution (30 mL). Salt solution contained (g/L) KH2PO4, 1; (NH4)2SO4, 0.5; MgSO4.7H2O, 0.5; yeast extract, 0.5; and CaCl2, 0.5, and mineral solution (mg/L) contained FeSO4.7H2O, 7.5; MnSO4.H2O, 2.5; ZnSO4.7H2O, 3.6; CoCl2, 3.7; and ampicillin, 100 [12]. These cultures were incubated at 45°C for 5 days. The moisture of cultures was kept at 75%. Then 30-mL citrate buffer (50 mM) was added into every flask and shaken gently. Citrate buffer pH was adjusted at optimal pH of cellulase enzyme activities. Enzyme solutions were obtained by passing culture media through Whatman filter paper No.1. Enzyme solutions and pretreated wheat straw were mixed (5% w/v) and incubated at optimal temperatures of cellulase activities for 48 h. Hydrolysates were separated by centrifugation at 5000 g for 10 min. Hydrolysate concentration (five times) was carried out by boiling method. Glucose concentration of these hydrolysates was measured using DNS method. Finally, they were used for yeast cell cultivation and bioethanol production.
Saccharomyces cerevisiae PTCC 5052 yeast cells were prepared from Persian Type Culture Collection (Iran). These cells were cultivated in YPD culture medium at 25°C and 150 rpm. Yeast cells were harvested from culture medium in exponential phase (OD600 = 0.5–1) and added to hydrolysates (production of fungal crude enzyme solution and treated wheat straw reaction) for fermentation phase and bioethanol production [21].
2.6 Determination of ethanol concentration
Potassium dichromate reagent was utilized for determining ethanol concentration in Saccharomyces cerevisiae culture medium. A simple distillation apparatus was provided, 10 ml of ethanol-containing solution was placed at the beginning of the condenser, and 4 ml of potassium dichromate reagent was placed at the end of the condenser. Distillation was performed for half an hour at 76°C. After this time, depending on the percentage of soluble ethanol, the reagent color changed from orange into bright green to black. Then, the optical absorbencies were read at 600 nm. The ethanol standard curve was prepared for calculating produced ethanol percentage [22].
2.7 Statistical analysis
Data were analyzed using GraphPad Prism version 9.3.0 and reported as mean ± SEM (standard error of the mean). It considered the significant differences among cellulase enzyme activities obtained from various fungal isolates at p < 0.05. Analysis of variance was performed by one-way ANOVA method.
3 Results and discussion
3.1 Qualitative CMCase test
The qualitative CMCase test was performed to assess the ability of different isolates of fungi to consume CMC as their carbon source. According to this test, the higher levels of CMCase activity of thermophilic fungal isolates resulted in faster decomposition of CMC and more growth of isolates onto the CMC culture medium. We selected seven thermophilic fungal isolates for the next steps of the study, based on the results of this test, and also based on the growth rates of fugal isolates onto PDA medium. These isolates, respectively, indicated 8 cm clear zones and growth zones onto CMC and PDA culture media during 48 h (Table 2).
3.2 Quantitative cellulase enzyme assays
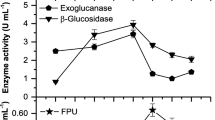
Primarily, the optimization of pH and temperature of enzymatic reaction (endoglucanase, exoglucanase, and total cellulase) were carried out for enzyme solutions obtained from fungal isolates (Figs. 1 and 2), and then their cellulase enzyme activities (U/mg wheat straw) were calculated under optimal conditions (Table 3). Isolates of thermophilic fungi in this study had high enzymatic activity at the pH range of 4.5 to 5.5. The expected optimum pH for the enzymatic activity of fungal cellulases was also at the acidic range. The enzymatic cocktails acquired from co-cultivation of different filamentous fungi indicated the highest activities at pH 5–6 [23]. Some other studies also determined the optimum pH of filamentous fungal cellulase at acidic pH 4–6 [24,25,26]. The temperature range for enzymatic activity of thermophilic fungal isolates has been reported from 40 to 80°C [23, 26,27,28], and the optimum temperature for our thermophilic fungal isolates was in the range of 45–50°C.
Variation of cellulase enzyme activities at different temperatures. Effects of various temperatures (35–60°C) on endoglucanase, exoglucanase, and total cellulase activities of thermophilic filamentous fungi A isolate 3, B isolate 9, and C isolate 10. Quantitative cellulase reactions were performed at pH 5
Variation of cellulase enzyme activities at different pHs. Effects of various pHs (3.5–6.5) on endoglucanase, exoglucanase, and total cellulase activities of thermophilic filamentous fungi A isolate 3, B isolate 9, and C isolate 10. Quantitative cellulase reactions were performed at optimal temperature of each cellulase enzymes
Each unit of enzyme is equivalent to the amount of enzyme in the reaction mixture that produces 1 μmol of product per 1 min. Figures 3, 4, and 5 compare the cellulase enzyme activities of seven thermophilic fungal isolates. According these results, two isolates (numbers 3 and 4) were selected for fermentation and bioethanol production phase. These two fungal isolates showed the highest endoglucanase activity among seven isolates. Exoglucanase, and FPase activities of them placed in the second rank. Although fungal isolate 6 indicated the highest levels of exoglucanase and FPase activities, its endoglucanase activity was weak. We forecasted that isolates 3 and 4 would be more successful in hydrolyzing of pretreated wheat straw because of primary and important role of endoglucanase enzymes. Endoglucanases are the first enzymes that attack to cellulose fibers. These enzymes cut internal glucoside bonds and create new ends on cellulose fibers which could be utilized by exoglucanase enzymes and result in more degradation of cellulose [29]. By increasing the cellulase activities of thermophilic fungal isolates, the hydrolysis of pretreated wheat straw and amounts of produced glucose would be increased. Most studies utilize industrial enzymes that are pure and high in concentration. We applied the enzymes secreted by isolated thermophilic fungi for assessing the probability of utilizing these enzymes in bioethanol production process and reducing the cost of process.
CMCase activities of thermophilic fungal isolates. Endoglucanase (CMCase) activities of seven thermophilic fungal isolates were measured at optimal pHs and temperatures. The levels of enzyme activity represent as mean ± SEM. Different letters indicate the significant differences between CMCase activities of fungal isolates at 0.05 level (least significant differences test). SEM, standard error of the mean
Exoglucanase activities of thermophilic fungal isolates. Exoglucanase (avicelase) activities of seven thermophilic fungal isolates were measured at optimal pHs and temperatures. The levels of enzyme activity represent as mean ± SEM. Different letters indicate the significant differences between avicelase activities of fungal isolates at 0.05 level (least significant differences test). SEM, standard error of the means
Total cellulase activities of thermophilic fungal isolates. Total cellulase (FPase) activities of seven thermophilic fungal isolates were measured at optimal pHs and temperatures. The levels of enzyme activity represent as mean ± SEM. Different letters indicate the significant differences between FPase activities of fungal isolates at 0.05 level (least significant differences test). SEM, standard error of the means
3.3 Molecular identification of fungal isolates
Genomic DNA was extracted from isolates 3 and 4 with the highest cellulase activities and used to amplify the ITS region (600 bp) by polymerase chain reaction method (Fig. 6). The nucleotide BLAST of the DNA fragments resulted in identifying these isolates as Aspergillus fumigatus (isolate 3) and Rhizomucor pusillus (isolate 4). Both isolates indicated 100% identity with registered sequences in NCBI database and also BOLD system software related to Aspergillus fumigatus and Rhizomucor pusillus (Fig. 7). Phylogenetic tree categorized isolate 3 with known Aspergillus fumigatus and isolate 4 with known Rhizomucor pusillus. Related species were classified in distinct groups (Fig. 8).
Phylogeny of thermophilic fungal isolates. Phylogenetic tree of Aspergillus fumigatus and Rhizomucor pusillus isolates from cow manure samples was constructed based on maximum likelihood method, Tamura–Nei model, and 5000 replications using ITS sequences. A. fumigatus and R. pusillus isolates showed as Fungus YU-3 and Fungus YU-4, respectively. Rozella allomycis was utilized as the out group
Aspergillus fumigatus is a cellulase-producing filamentous fungus. Various strains of this fungus grew at the range of 30–55°C [30, 31]. Thermostable and thermophilic cellulases were extracted from A. fumigatus. Mehboob et al. [32] examined the optimum conditions for producing endoglucanase enzyme by A. fumigatus fungus. They earned the maximum amounts of the enzyme after 72 h, at 55°C, pH 5.5, and 70% moisture level. The CMCase enzyme showed the highest activity at 55°C and pH 4.8. Another study reported a thermophilic CMCase enzyme from A. fumigatus that had the optimal activity at 80°C and pH 4 [28]. A thermostable endoglucanase enzyme was obtained from A. fumigatus JCM 10253 which showed the highest activity at 50°C and pH 6. Some cations such as Ni+2, Fe+2, Cu+2, Mn+2, Mg+2, and Ca+2 increased the enzyme activity [33]. We cultured A. fumigatus fungus at 45°C and pH 4, while optimum temperature and pH of its cellulase enzymes were determined 50°C and pH 4.5.
We incubated R. pusillus fungus onto wheat straw at 45°C and acidic pH for obtaining cellulase enzymes. These enzymes indicated the highest activities at 45°C and pH 4.5–5.5.
3.4 Components of wheat straw and PHP pretreatment
3.4.1 Untreated wheat straw contents
The main components of wheat straw are cellulose, hemicellulose, and lignin. Amounts of these ingredients (of pretreated and untreated wheat straw) were determined using ADF and NDF methods. NDF method detected lignin, cellulose, and hemicellulose, and other components with a lower percentage were separated from wheat straw. The three main ingredients compose 71.38–72.22% of wheat straw. ADF value can vary from 44 to 48% depending on the type of wheat straw and growing conditions. The ADF value was 46.64 to 47.68% in our study. This value was in agreement with some other studies [34]. Wheat straw lignin, cellulose, and hemicellulose contents were calculated as 8.38, 38.59, and 24.7%, respectively (Table 4). The main part of wheat straw is cellulose, and the ultimate goal of pretreatment is to separate this part from the wheat straw for hydrolysis and conversion to glucose. The final ash content of wheat straw was about 5.64–5.886%.
3.4.2 Confirming efficient wheat straw pretreatment by physicochemical method
Lignin and hemicellulose act as inhibitors for the function of cellulase enzymes. Therefore, they were removed from the wheat straw structure during the pretreatment phase, and the only remaining material was cellulose, as far as possible. As a result, the cellulase enzymes would easily affect cellulose and provide the required glucose sugar for the fermentation phase. To confirm the functionality of pretreatment methods (grinding and PHP) on wheat straw, the percentage of residual cellulose was determined using ADF and NDF methods after wheat straw pretreatment. It was found that high percentages of lignin and hemicellulose was removed from the structure of wheat straw and the pretreated wheat straw mainly contained cellulose (88.91%) (Table 4).
Wheat straw pretreatment using this method was very efficient because cellulose content of wheat straw increased from about 38.5 to 88.9%, while lignin and hemicellulose contents reduced markedly (from about 31 to 11%). Wheat straw used in this study contained lower amounts of lignin than other straws, and therefore, cellulose separation was probably done more efficiently. Rice straws of different Chinese rice varieties possessed 14–28% lignin, while the wheat straw was applied in the present study was composed of about 9% lignin [35]. The lignin and silica in straws are a barrier to chemical pretreatment. High amounts of wood is composed of lignin (up to 40% lignin), and it causes high hardness for penetration and pretreatment. Rice straw usually has more lignin than wheat straw (5–24% for rice straw and 10–30% for wheat straw), and additionally, there is a significant amounts of silica in rice straw. It is expected that rice straw requires a longer pretreatment process, and the percentage of obtained cellulose is lower in comparison with wheat straw [9, 36, 37]. Other methods, including alkaline pretreatment or acid pretreatment, also separate 80 to 90% of cellulose from wheat straw, but PHP method can maintain the cellulose up to 95%. In acidic or alkaline methods, the bonds between lignin and cellulose are reversible, and the separation of lignin is not done very effectively. On the contrary, PHP method separates lignin and hemicellulose from the solid tissue of cellulose efficiently and remains the cellulose with a very low lignin content [9].
Tabka et al. [38] utilized wheat straw, one of the most abundant and cheap lignocellulosic biomass for bioethanol production. They performed acid pretreatment and steam blasting to make cellulose available for enzymatic digestion. These pretreatments partially destroyed the cell wall polysaccharides and decomposed the lignocellulosic fibers. This pretreatment recovered 51% of wheat straw cellulose, and then, 81% of cellulose was decomposed into glucose. Tsegaye et al. [39] applied alkaline pretreatment of wheat straw using different concentrations of sodium hydroxide to remove lignin and release polysaccharides. They tried to eliminate lignin and reduce the carbohydrate lost during the pretreatment phase. They prepared an enzymatic mixture by culturing microorganisms that had been isolated from termites. It was resulted in simple sugar production from pretreated wheat straw and eventually led to bioethanol production. The maximum conversion of polysaccharides to glucose and xylose was 83.68%. Alkaline hydrogen peroxide method is another pretreatment method of wheat straw that exploits hydrogen peroxide and sodium hydroxide under mild temperature and pressure conditions. This procedure caused the efficient separation of lignin and easy hydrolysis of biomass. At the next step, enzymatic hydrolysis of cellulosic compounds and fermentation of glucose resulted in ethanol production with an approximate concentration of 31.1 g/L [10]. Another study carried out an alkaline and hydrothermal pretreatment on wheat straw and removed efficiently lignin, that it subsequently increased the effect of the enzymes on the cellulose substrate with less barriers and obtained glucose and xylose were fermented to 18.6% bioethanol [40].
3.4.3 Confirming efficient wheat straw pretreatment by FTIR spectrum
Changes in the main components of wheat straw (lignin, hemicellulose, and cellulose) were analyzed for each of them by observing the appearance or disappearance of adsorption bands in FTIR spectrum. Absorption bands between 3100 and 3500 cm−1 were assigned to lignin vibration. For untreated straw, high lignin vibrations, large and wide bands were observed, which were related to O–H tensile. After pretreatment of wheat straw, vibration and band intensity was decreased. The specialized vibrations of lignin, which were caused by the aromatic ring, disappeared in 1508 cm−1 at the pretreated spectrum. However, these vibrations were quite evident at the untreated spectrum. Peak 1245 cm−1, which was related to the hemicellulose structure and tensile C-O, was completely removed from the band after pretreatment. Band 13 for pretreated wheat straw in 1157 cm−1 corresponded to the glucose tensile ring for cellulose. In practice, this band appeared after pretreatment of straw for cellulose. Band 1064 or 1052 cm−1 also corresponded to cellulose. Loss of hemicellulose bands meant the removal of a high percentage of hemicellulose in the straw structure, which caused releasing of cellulose from the wheat straw matrix. Efficient pretreatment of wheat straw was confirmed by FTIR spectral method via reducing the intensity of hemicellulose and lignin peaks at the spectrum (Fig. 9).
FTIR spectrum of wheat straw. FTIR spectrum of A untreated wheat straw and B pretreated wheat straw are presented. Grinding and phosphoric acid plus hydrogen peroxide (PHP) methods were utilized for wheat straw pretreatment. The intensity of lignin and hemicellulose peaks in this spectrum has been reduced
3.5 Ethanol production phase
Hydrolysates (containing glucose as the carbon source of yeast cells) were obtained from enzymatic hydrolysis of pretreated wheat straw. Hydrolysates were concentrated five times by heating and evaporating the extra water. Then 1 g of peptone water (as the nitrogen source of yeast cells) was added to every 50 mL hydrolysate. Saccharomyces cerevisiae yeast cells were added into these hydrolysates for fermentation and bioethanol production. Figure 10 showed decreasing the glucose concentrations during the fermentation stage. It was observed that the highest amounts of glucose were consumed during the first 48 h. Glucose consuming rate was relatively high until 72 h of fermentation and then glucose concentration decreased with a gentle rate to 120 h (Fig. 10) [41]. The highest ethanol production occurred during the first 72 h of the yeast fermentation, according to high consumption of glucose in this period. It seemed that ethanol production process was stopped after 72 h and ethanol concentrations were approximately unchanged. The highest amounts of ethanol produced were measured 5.31% (24.02 g/L) and 4.05% (21.88 g/L) for hydrolysates 4 (the 96-h yeast cultivations) and 3 (the 72-h yeast cultivations), respectively (Fig. 11) [41]. It was considered that the control samples (yeast cells cultivated in YPD culture medium) significantly produced ethanol lower (approximately 3 and 4 times) than hydrolysates 3 and 4. Hydrolysates 3 and 4 were obtained from pretreated wheat straw by A. fumigatus and R. pusillus cellulase enzymes, respectively.
Changes in glucose concentrations during the yeast fermentation phase. The curve shows that the highest levels of glucose are consumed during the first 48 h of Saccharomyces cerevisiae fermentation. Samples 3 and 4 were hydrolysates of pretreated wheat straw hydrolysis by Aspergillus fumigatus and Rhizomucor pusillus cellulase enzymes. Yeast cells were cultivated in YPD culture medium as the control sample
Ethanol production using Saccharomyces cerevisiae cultivation in wheat straw hydrolysates. Hydrolysates containing glucose were obtained from enzymatic hydrolysis of pretreated wheat straw by thermophilic fungal cellulase enzymes. The orange line (hydrolysate 4) shows the highest ethanol production at 96 h. The blue line (hydrolysate 3) indicates the highest ethanol concentration at 72 h. The gray line also is the control sample (cultivation of yeast cells in YPD culture medium) that produces the highest level of ethanol at 96 h
In this process, thermophilic fungal cellulase enzymes were used to hydrolysis cellulose and convert it to glucose. Most studies have been utilized industrial enzymes for this phase, whereas these enzymes are expensive and probably not economical for ethanol production. Therefore, produced enzymes by isolated thermophilic fungi were consumed in the present study. The main part of culture medium of these fungi was wheat straw, which was cheap and available. Peptone water was added to the hydrolysates which increased ethanol production significantly. Bioethanol (5.3 and 4% or 24 and 21 g/L) was produced markedly more than some previous methods [41, 42]. Several fungi, such as white-rot fungi, have been reported to produce enzymes that are capable of hydrolyzing cellulose and hemicellulose. The production of bioethanol from wheat straw was conducted using Aspergillus cereus for microbial hydrolysis and Saccharomyces cerevisiae for fermentation. After microbial hydrolysis and fermentation of pretreated wheat (10 g/L), ethanol (3 g/L) was obtained [43].
4 Conclusion
Isolated thermophilic fungi including Aspergillus fumigatus and Rhizomucor pusillus from animal manure produced efficient cellulase enzymes onto the pretreated wheat straw medium. Wheat straw pretreatment by grinding and PHP methods was very effective because the cellulose of lignocellulosic materials was maintained up to 88.91% and this cellulose was converted to glucose using fungal cellulase enzymes. Hydrolysates were obtained from cellulase enzyme activities of Aspergillus fumigatus and Rhizomucor pusillus resulting in 4.3% and 5% (v/v) or 21.88 and 24.02 g/L ethanol production, respectively. It seems that utilizing thermophilic cellulase enzymes and wheat straw is a promising economic process for bioethanol production.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Basile A, Dalena F (2017) Methanol: science and engineering. Elsevier
Sarangi PK, Nanda S, Mohanty P (2018) Recent advancements in biofuels and bioenergy utilization. Springer, Berlin
Qiu J, Tian D, Shen F, Hu J, Zeng Y, Yang G, Zhang Y, Deng S, Zhang J (2018) Bioethanol production from wheat straw by phosphoric acid plus hydrogen peroxide (PHP) pretreatment via simultaneous saccharification and fermentation (SSF) at high solid loadings. Bioresour Technol 268:355–362
Ray RC, Ramachandran S (2018) Bioethanol production from food crops: sustainable sources, interventions, and challenges. Academic Press, Elsevier
Hasanly A, Khajeh Talkhoncheh M, Karimi Alavijeh M (2018) Techno-economic assessment of bioethanol production from wheat straw: a case study of Iran. Clean Technol Environ Policy 20(2):357–377
Koppram R, Tomás-Pejó E, Xiros C, Olsson L (2014) Lignocellulosic ethanol production at high-gravity: challenges and perspectives. Trends Biotechnol 32(1):46–53
Chauvigné-Hines LM, Anderson LN, Weaver HM, Brown JN, Koech PK, Nicora CD, Hofstad BA, Smith RD, Wilkins MJ, Callister SJ, Wright AT (2012) Suite of activity-based probes for cellulose-degrading enzymes. J Am Chem Soc 134(50):20521–20532
Gao J, Weng H, Zhu D, Yuan M, Guan F, Xi Y (2008) Production and characterization of cellulolytic enzymes from the thermoacidophilic fungal Aspergillus terreus M11 under solid-state cultivation of corn stover. Bioresour Technol 99(16):7623–7629
Wan X, Tian D, Shen F, Hu J, Yang G, Zhang Y, Deng S, Zhang J, Zeng Y (2018) Fractionating wheat straw via phosphoric acid with hydrogen peroxide pretreatment and structural elucidation of the derived lignin. Energy Fuels 32(4):5218–5225
Yuan Z, Wen Y, Li G (2018) Production of bioethanol and value added compounds from wheat straw through combined alkaline/alkaline-peroxide pretreatment. Bioresour Technol 259:228–236
Nielsen NS, Stubbs TL, Garland-Campbell KA, Carter AH (2019) Rapid estimation of wheat straw decomposition constituents using near-infrared spectroscopy. Agronomy 9(8):462
Hart TD, De Leij FAAM, Kinsey G, Kelley J, Lynch JM (2002) Strategies for the isolation of cellulolytic fungi for composting of wheat straw. World J Microbiol Biotechnol 18(5):471–480
Michielse CB, Hooykaas PJ, Van Den Hondel CA, Ram AF (2008) Agrobacterium-mediated transformation of the filamentous fungus Aspergillus awamori. Nat Protoc 3(10):1671–1678
Nevalainen H, Kautto L, Te’o J (2014) Methods for isolation and cultivation of filamentous fungi. In: Paulsen IT, Holmes AJ (eds) Environmental microbiology. Humana Press, Totowa, pp 1–13
Sazci A, Erenler K, Radford A (1986) Detection of cellulolytic fungi by using Congo red as an indicator: a comparative study with the dinitrosalicyclic acid reagent method. J Appl Bacteriol 61(6):559–562
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Zhang YH, Hong J, Ye X (2009) Cellulase assays. Biofuels 581:213–231
Takó M, Kotogán A, Krisch J, Vágvölgyi C, Mondal KC, Papp T (2015) Enhanced production of industrial enzymes in Mucoromycotina fungi during solid-state fermentation of agricultural wastes/by-products. Acta Biol Hung 66(3):348–360
Sambrook J, Russell DW (2006) Alkaline agarose gel electrophoresis. Cold Spring Harb Protoc 1:2006
Taherzadeh MJ, Gustafsson L, Niklasson C, Lidén G (2000) Inhibition effects of furfural on aerobic batch cultivation of Saccharomyces cerevisiae growing on ethanol and/or acetic acid. J Biosci Bioeng 90(4):374–380
Caputi A, Ueda M, Brown T (1968) Spectrophotometric determination of ethanol in wine. Am J Enol Vitic 19(3):160–165
Intasit R, Cheirsilp B, Suyotha W, Boonsawang P (2021) Synergistic production of highly active enzymatic cocktails from lignocellulosic palm wastes by sequential solid state-submerged fermentation and co-cultivation of different filamentous fungi. Biochem Eng J 173:108086
Olajuyigbe FM, Ogunyewo OA (2016) Enhanced production and physicochemical properties of thermostable crude cellulase from Sporothrix carnis grown on corn cob. Biocatal Agric Biotechnol 7:110–117
El-Nahrawy S, Metwally M, El-Kodoos A, Rizk Y, Belal ESB, Shabana SA, El-Refai IM (2017) Optimization of culture conditions for production of cellulase by Aspergillus tubingensis KY615746 using rice straw waste. Env Biodivers Soil Secur 1(2017):177–189
Javanmard A, Matin MM, Rouhani H, Mashreghi M, Bahrami AR (2017) Investigating cellulase producing potential of two Iranian Thermoascus aurantiacus isolates in submerged fermentation. J Genet Resour 3(2):87–97
Garbin AP, Garcia NF, Cavalheiro GF, Silvestre MA, Rodrigues A, Paz MF, Fonseca GG, Leite RS (2021) β-glucosidase from thermophilic fungus Thermoascus crustaceus: production and industrial potential. An Acad Bras Cienc 93(1):e20191349
Gu X, Lu H, Zhang L, Meng X (2021) A Thermophilic GH5 endoglucanase from Aspergillus fumigatus and its synergistic hydrolysis of mannan-containing polysaccharides. Catalysts 11(7):862
Jalak J, Kurašin M, Teugjas H, Väljamäe P (2012) Endo-exo synergism in cellulose hydrolysis revisited. J Biol Chem 287(34):28802–28815
Sui M, Rong J, Zhang Y, Zhang F, Li J (2020) Study on enzymatic characteristics of cellulose produced by Aspergillus fumigatus and optimization of enzyme production medium. J Phys Conf Ser 1549(3):032066
Miyazawa K, Umeyama T, Hoshino Y, Abe K, Miyazaki Y (2022) Quantitative monitoring of mycelial growth of Aspergillus fumigatus in liquid culture by optical density. Microbiol Spectr 10(1):e00063-e121
Mehboob N, Asad MJ, Asgher M, Gulfraz M, Mukhtar T, Mahmood RT (2014) Exploring thermophilic cellulolytic enzyme production potential of Aspergillus fumigatus by the solid-state fermentation of wheat straw. Appl Biochem Biotechnol 172(7):3646–3655
Saroj P, Narasimhulu K (2022) Biochemical characterization of thermostable carboxymethyl cellulase and β-glucosidase from Aspergillus fumigatus JCM 10253. Appl Biochem Biotechnol 194:2503–2527
Castro LPM, Trejo-Aguilar BA, Osorio GA (1997) Thermostable xylanases produced at 37 C and 45 C by a thermotolerant Aspergillus strain. FEMS Microbiol Lett 146(1):97–102
Janmohammadi H, Taghizadeh A, Yasan P, Shojaa J, Nikkhah A (2014) Determining nutritive value of alfalfa hay and wheat straw from east Azerbaijan province. Iran J Vet Res 6(1):45–53
Chen C, Deng X, Kong W, Qaseem MF, Zhao S, Li Y, Wu AM (2021) Rice straws with different cell wall components differ on abilities of saccharification. Front bioeng biotechnol 8:624314
Alinia R, Zabihi S, Esmaeilzadeh F, Kalajahi JF (2010) Pretreatment of wheat straw by supercritical CO2 and its enzymatic hydrolysis for Sugar production. Biosyst Eng 107:61–66
Do NH, Pham HH, Le TM, Lauwaert J, Diels L, Verberckmoes A, Do NH, Tran VT, Le PK (2020) The novel method to reduce the silica content in lignin recovered from black liquor originating from rice straw. Sci Rep 10:21263
Tabka MG, Herpoël-Gimbert I, Monod F, Asther M, Sigoillot JC (2006) Enzymatic saccharification of wheat straw for bioethanol production by a combined cellulase, xylanase and feruloyl esterase treatment. Enzyme Microb Technol 39(4):897–902
Tsegaye B, Balomajumder C, Roy P (2019) Alkali pretreatment of wheat straw followed by microbial hydrolysis for bioethanol production. Environ Technol 40(9):1203–1211
Sun S, Zhang L, Liu F, Fan X, Sun RC (2018) One-step process of hydrothermal and alkaline treatment of wheat straw for improving the enzymatic saccharification. Biotechnol Biofuels 11:137
Wilkins MR, Suryawati L, Maness NO, Chrz D (2007) Ethanol production by Saccharomyces cerevisiae and Kluyveromyces marxianus in the presence of orange-peel oil. World J Microbiol Biotechnol 23(8):1161–1168
Hjersted JL, Henson MA (2009) Steady-state and dynamic flux balance analysis of ethanol production by Saccharomyces cerevisiae. IET Syst Biol 3(3):167–179
Jha VK, Pranay K, Prasad B (2018) An optimised combination approach of physicochemical methods for separate hydrolysis and fermentation (SHF) pretreatment of wheat straw for high production of bio-alcohol. Curr J Appl Sci Technol 31(3):1–13
Author information
Authors and Affiliations
Contributions
Conceptualization: Ghaedi, Javanmard
Methodology: Valamonfared, Javanmard, Bagherinasab
Data analysis: Valamonfared, Javanmard
Writing, original draft preparation: Valamonfared
Writing, review, and editing: Javanmard, Ghaedi
Supervision: Ghaedi, Javanmard
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Valamonfared, J., Javanmard, A.S., Ghaedi, M. et al. Bioethanol production using lignocellulosic materials and thermophilic microbial hydrolysis. Biomass Conv. Bioref. 14, 16589–16601 (2024). https://doi.org/10.1007/s13399-023-03980-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-03980-w