Abstract
Carbohydrates are more likely to be found in lipid-extracted microalgal biomass, which can be used to produce bioethanol with different pretreatment methods. The objective of this study was to prepare lipid-extracted biomass of Scenedesmus sp. grown in anaerobically digested brewery effluent for bioethanol production. Pretreatments such as autoclave, microwave, oven, and water bath heating with alkalis (NaOH and KOH), acids (HCL and H2SO4), and H2O as hydrolytic agents, as well as optimization of an effective pretreatment method for carbohydrate and reducing sugar extractions, were used. Bioethanol was produced from lipid-extracted microalgal hydrolysate under optimum conditions. The findings showed that the highest contents of carbohydrates (222.59 ± 3.16 mg/g) and reducing sugar (150.52 ± 5.57 mg/g) were obtained using microwave pretreatment with HCl, whereas the lowest contents of carbohydrates (34.48 ± 1.36 mg/g) and reducing sugar (30.85 ± 3.22 mg/g) were obtained in water bath heating with H2O. After optimizing the main parameters of microwave pretreatment, the highest carbohydrate and reducing sugar contents were increased by 24.72% and 27.92%, respectively, at the optimum conditions. The maximum bioethanol yield of 0.1 g/g lipid-extracted microalgal biomass with a fermentation efficiency of 94.84% was obtained at a fermentation time of 24 h. This study demonstrated that lipid-extracted biomass of microalgae obtained from wastewater has a high potential for bioethanol production and, consequently, the development of microalgae-based biorefineries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The issues of energy security and greenhouse gas emission due to the utilization of fossil fuels have led to the development of alternative energy sources to satisfy the demands of energy in the world. Biofuels are considered an alternative to reduce dependence on fossil fuels, and they are derived from different types of biomass [1]. Among biomass, microalgae are recently perceived as a potentially renewable source for the production of biofuel due to their features such as higher photosynthetic efficiency, faster growth rate, and higher biomass production compared to other conventional biofuel sources [2]. Moreover, they are able to utilize available nutrients in wastewater and produce biomass, which can be converted into biofuels [3].
The growth of microalgae in wastewater has been suggested as a cost-effective method for biomass production with wastewater management and biofuel production [4]. Several previous studies have been undertaken to produce biomass for lipid and carbohydrate production from microalgae in wastewater. For instance, Mercado et al. [5] reported a maximum carbohydrates of 27% and lipids of 50% from Scenedesmus sp. grown in anaerobically digested (AD) dairy wastewater. Ansari et al. [6] found 35.1% carbohydrates and 30.8% lipids from Scenedesmus obliquus grown in raw aquaculture wastewater. Karpagam et al. [7] achieved a lipid content of 231.8 mg/L from Scenedesmus sp. grown in vegetable waste extract–treated growth media. These studies showed that microalgae store a substantial amount of lipids and carbohydrates when they are grown in wastewater.
Moreover, the use of lipid-extracted microalgal biomass (LEMB) for biofuel production or other applications can reduce the cost of biofuel production [8]. In previous studies, lipid-extracted biomass was called de-oiled or defatted biomass or residual biomass [9,10,11]. It was reported that LEMB has a higher carbohydrate yield than whole microalgal biomass (WMB) [11]. Therefore, the utilization of LEMB for carbohydrate and bioethanol production might promote the biorefinery concept. The production of bioethanol from microalgae can be done through either a step-by-step method or an integrated method. The step-by-step method involves first lipid extraction from microalgae and then bioethanol production from LEMB, whereas the integrated method involves simultaneous carbohydrate and lipid extraction from microalgal biomass [12].
Bioethanol production from microalgae involves steps such as microalgae cultivation, pretreatment, hydrolysis, fermentation, and distillation. Among these steps, pretreatment is an important one because it is used to enhance the release of fermentable sugars from microalgal biomass or affect the efficiency of bioconversion [13]. Pretreatment methods have commonly been employed for bioethanol production from various feedstocks, including microalgae. For instance, Manmai et al. [14] employed chemical and biological pretreatments on sunflower stalks and obtained higher reducing sugar, total sugar, and bioethanol with chemical pretreatment. Yirgu et al. [15] used microwave, autoclaved, oven, and water bath heating pretreatments of the whole microalgal biomass and obtained the highest reducing sugar using the microwave pretreatment. Like WMB, LEMB also requires pretreatment to modify the structure of internal carbohydrates and enhance the extraction of fermentable sugar. The pretreatment methods such as sonication, ultrasonic, chemical, and enzymatic and their combinations have been employed to extract reducing sugar from LEMB for the production of bioethanol [9, 11, 16].
The lipid-extracted biomass of Scenedesmus sp. after growing in synthetic medium and/or wastewater has been investigated for carbohydrate extraction with different pretreatments for bioethanol production. For example, Pancha et al. [9] achieved a maximum saccharification yield of 44% with 0.40 g bioethanol/g glucose from de-oiled Scenedesmus sp. biomass obtained from synthetic medium. Thangam et al. [17] obtained a maximum reducing sugar yield of 11.2% with a bioethanol yield of 10.48 g/L from the LEB of Scenedesmus sp. grown in domestic wastewater. Scenedesmus sp. can be used for lipid production from WMB, and carbohydrate and bioethanol production from lipid-extracted biomass after growing in a synthetic medium or wastewater. Most of the previous studies have used a synthetic medium for microalgae growth for bioethanol production from LEMB [9,10,11]. In addition, bioethanol production from the whole biomass of Scenedesmus has received significant attention. However, the utilization of LEB of Scenedesmus sp. after growing in wastewater for bioethanol production has rarely been reported.
Therefore, this study aimed to pretreat lipid-extracted biomass of Scenedesmus sp. obtained after growing in UASB (up-flow anaerobic sludge blanket) brewery effluent for carbohydrate and bioethanol production. The LEMB was pretreated via microwave, autoclave, oven, and water bath heating with the combination of acids and alkalis hydrolytic agents to select the effective pretreatment and hydrolytic agent. Moreover, optimization of the main variables using a one-variable-at-a-time approach was performed for carbohydrate and reducing sugar extractions from LEMB. Bioethanol was produced from reducing sugar obtained under optimum conditions.
2 Materials and methods
2.1 Microalgae cultivation in wastewater
The local microalga Scenedesmus sp. was cultivated in AD brewery effluent after being isolated from a water sample of Lake Ziway, Ethiopia. BBM (Basal Bold Medium) [18] was used for the isolation and inoculum preparation processes. The isolation of Scenedesmus sp. was carried out succeeding the techniques in Andersen and Kawachi [19] and identified on the basis of morphology features explained in Bellinger and Sigee [20] and Shubert and Gärtner [21]. AD brewery effluent (hereafter, brewery effluent) was kindly provided by St. Gorge Brewery Industry, found in Addis Ababa, Ethiopia. It was collected after the UASB reactor and first filtered through Whatman filter paper (No. 1) before the cultivation of microalgae. The COD (chemical oxygen demand), TN (total nitrogen), and TP (total phosphorus) concentrations of the brewery effluent samples were 399.58 ± 24.14, 53.42 ± 6.19, and 50.00 ± 2.64 mg/L, respectively. The cultivation was performed both in brewery effluent and BBM (as control) in a batch mode with 10% of the inoculum [22] and 1600 mL working volume in 2-L conical flasks as a photobioreactor. The cultivation conditions and biomass collection method were reported in our previous study [15].
2.2 Lipid extraction
The extraction of total lipids from the microalgal biomass was carried out according to the modified methods of Bligh and Dye [23]. The procedures used in this study were reported in Yirgu et al. [15]. The biomass leftover after lipid extraction was carefully collected and dried at 60 °C in an oven, and then stored until the analysis of carbohydrates, reducing sugars, and proteins, as well as bioethanol production.
2.3 Pretreatment of microalgal biomass
A combination of both chemical and thermal pretreatments was performed for the extraction of carbohydrates from LEMB and WMB. The LEMB and WMB were pretreated in an autoclave (Model, DIXONS and ST3028), oven heating (Model, GX65B), microwave (Milestone SK-10 and SK-12, Italy), and water bath heating (DK-98-II) with acids (HCl and H2SO4), alkalis (NaOH and KOH), and H2O as hydrolytic agents, as reported in Yirgu et al. [15]. After pretreatment, the supernatant was then separated using centrifugation after cooling and neutralizing, and then taken for carbohydrate and reducing sugar content determination. After selecting an effective pretreatment method with a hydrolytic agent, optimization of main operational parameters was employed using a single parameter at a time approach.
2.4 Bioethanol production
Bioethanol was produced from the hydrolysates of LEMB obtained at optimum conditions. The fermentation process was performed with Saccharomyces cerevisiae (commercial baker's yeast), which was first activated and prepared using Luria Broth (LB) medium according to Harun et al. [24]. Fermentation was performed with a 50 mL working volume in 125-mL conical flasks containing the hydrolysate of LEMB with fermentation nutrients (0.1 g ammonium chloride (NH4Cl), 0.2 g potassium dihydrogen phosphate (KH2PO4), and 0.5 g yeast extracted) [25]. The mixture was first adjusted to a pH of 5 [26] and then sterilized at 121 °C for 20 min in an autoclave. Ten percent of pre-cultured Saccharomyces cerevisiae yeast was added under aseptic conditions in the flasks to inoculate the sterilized hydrolysate solution [27]. Then, the flasks were placed on a shaker incubator (ZHWY-103B, China) at 150 rpm and 30 °C for 3 days. Bioethanol was determined after distillation within an interval of 24 h.
2.5 Analytical methods and calculations
2.5.1 Biomass production
The local microalgae growth was daily measured by optical density (OD) at 680 nm [28] using a UV/Vis spectrophotometer (Jenway, model 6705). The dry cell weight (DCW) for biomass yield estimation was determined according to the APHA method [29] for the total suspended solid. The linear relationship between OD680 and dry cell weight was obtained as follows: DCW = 0.95*OD680 − 0.037 (R = 0.990, P < 0.05). The biomass productivity of local Scenedesmus sp. was calculated using Eq. (1) [22].
where PB is the biomass productivity, and Xt and X0 are biomass concentrations at time tt and at an initial time t0, respectively.
2.5.2 Lipid and protein contents and productivities
The total lipid content after extraction and separation was determined using the gravimetric method. The total lipid content (LC) and lipid productivity (PL) were determined according to Eqs. (2) and (3), respectively, [30].
where LC is the lipid content, WLE is the weight of lipid extracted, and WWMB is the weight of whole microalgal biomass
where PL is the biomass productivity, LC is the lipid content, and Pb is the biomass productivity.
The total protein contents in lipid-extracted microalgal biomass were calculated based on the amount of TN, which was determined according to Kjeldahl’s method as stated in the AOAC [31], and the procedures used in this method were reported by Yirgu et al. [15]. Protein productivity Pp over the cultivation time was determined according to Eq. (4) [6].
where PP is the protein productivity, PB is the biomass productivity, and PC is the protein content.
2.5.3 Carbohydrate determination
The carbohydrate contents in WMB and LEMB were analyzed using a phenol–sulfuric acid method [32]. In brief, 1.0 mL of 5% phenol solution and 5 mL concentrated H2SO4 were added to a test tube containing 2 mL of supernatant. A test tube was vortexed for 1 min and then maintained in a 30 °C water bath. The color of the mixture was then turned to orange, which is the result of the reaction between phenol and carbohydrates. A calibration curve was constructed to determine the amount of carbohydrates using glucose as a standard, ranging from 10 to 80 µg/mL. The carbohydrate content was determined on the basis of absorbance read at 490 nm using a UV/Vis spectrophotometer (Jenway).
2.5.4 Reducing sugar determination
The procedures of the DNS method with minor modifications were used to determine reducing sugar content [33]. Briefly, an equal amount of hydrolysate of microalgal biomass and DNS reagent (1 mL each) was mixed in capped test tubes and heated in boiling water (95 °C). Eight milliliters of distilled water was added to the test tubes after cooling using running tap water to room temperature. The calibration curve was constructed using d-glucose as a standard, ranging from 0.1 to 0.3 mg/mL with R2 = 0.9951 in the test range. The amounts of reducing sugar were determined according to the absorbance read at 540 nm using a UV/Vis spectrophotometer (Jenway).
2.5.5 Bioethanol determination
The bioethanol concentration was estimated according to the procedures provided by Crowell and Ough [34] using the potassium dichromate method. Briefly, the distilled samples of bioethanol (2 mL) and the acidic potassium dichromate reagent (10 mL) were mixed in a test tube and then heated in a water bath for 2 h at 60 °C. The bioethanol concentration was determined by measuring absorbance at 600 nm using a UV/Vis spectrophotometer. A calibration curve was prepared using absolute ethanol solution as standard [35], ranging from 1 to 3 mg/mL which provided R2 of 0.997. The bioethanol yield kinetics were determined according to Manmai et al. [14] using the following Eqs. (5), (6), (7), and (8).
2.6 Data analysis
All experiments in this study were performed in triplicate, except for the fermentation process (duplicate), and findings were presented as the mean ± standard deviation. Paired sample t-tests were used for the comparison of biomass production, total lipids, and protein production using Microsoft Excel 2013. One-way ANOVA with the Tukey post hoc test using R-software was performed to compare carbohydrate and reducing sugar yields with different pretreatments. The results are significant at a significant level of 95% (P < 0.05).
3 Results and discussion
3.1 Biomass production
The local microalga Scenedesmus sp. used in the present study was selected on the basis of its proven capability to grow in different wastewaters and to accumulate relatively high amounts of lipids and carbohydrates in its biomass. Scenedesmus sp. was cultivated in BBM (as a control) and brewery effluent until maximum biomass was obtained, which was on the18th day. Figure 1 depicts the biomass production of Scenedesmus sp. in BBM and brewery effluent over the cultivation period. The maximum biomass production and productivity obtained in BBM were 1.26 ± 1.05 and 93.30 mg/L/d, and in AD brewery effluent, they were 1.05 ± 0.10 g/L and 64.33 mg/L/d, respectively. It is clearly observed that the biomass production and productivity obtained in BBM were higher than those attained in AD brewery effluent. This attribution might be due to the balanced nutrient and mineral composition in BBM rather than AD brewery effluent for microalgae growth. The biomass production obtained in AD brewery effluent was comparable with the maximum result achieved by Ferreira et al. [36] and Marchão et al. [37] using Scenedesmus obliquus in brewery effluent. Diniz et al. [38] and Ansari et al. [22] obtained a maximum biomass production of 0.445 and 0.258 g/L using Scenedesmus sp. in institutional and municipal wastewater, respectively, which are lower than this study. Likewise, both of these studies reported lower biomass productivity using Scenedesmus sp. than in this study. However, Ferreira et al. [36] reported higher biomass productivity than this study. As a result, this study demonstrated that the brewery effluent has great potential for the production of microalgal biomass for biofuel feedstock.
3.2 Lipid and protein extraction from wastewater-grown Scenedesmus sp.
The microalgal biomass of Scenedesmus sp. obtained from AD brewery effluent had a lipid content of 13.67 ± 0.31% and a lipid productivity of 8.79 ± 0.20 mg/L/d. The lipid contents of local Scenedesmus sp. were categorized under the moderate lipid content range of microalgae, which is about 10 to18% [39]. The lipid and protein contents and productivities of Scenedesmus sp. in different wastewaters are presented in Table 1. Scenedesmus sp. achieved a similar lipid content when it grew in institutional wastewater [22], municipal wastewater [38], and brewery effluent (this study). Nayak et al. [40], Gupta et al. [41], and Thangam et al. [17] reported different values of lipid contents from Scenedesmus sp. grown in domestic wastewater. Dairy wastewater [5] offered a better lipid accumulation in Scenedesmus sp. compared to the other wastewaters except the domestic wastewater used by Thangam et al. [17]. The lipid productivity found in this study was similar to that reported by Ansari et al. [22] using institutional wastewater but greater than that reported by Diniz et al. [38] using municipal wastewater. However, it was lower than those achieved in domestic wastewater [17, 40] and dairy wastewater [5]. The differences in lipid content and productivity of Scenedesmus sp. grown in different wastewaters may be due to the availability of nutrients in the wastewater and cultivation conditions (temperature, pH, light intensity, photoperiod, etc.).
The total protein content found in LEMB was 53.98 ± 0.08%, with a productivity of 34.72 ± 0.05 mg/L/day. This showed that the total protein obtained in LEMB was 8.41% higher than that obtained in WMB, as reported in our previous study [15]. Likewise, protein productivity achieved on the basis of proteins from LEMB was 9.18% higher than that obtained on the basis of WMB. As a result, the use of lipid-extracted microalgal biomass is a sustainable approach to extracting more protein than the whole microalgal biomass. The accumulation of proteins on the biomass of Scenedesmus sp. varied with wastewater streams (Table 1). The protein content obtained in this study was higher than those achieved by Diniz et al. [38], Ansari et al. [6], and Gupta et al. [41] in domestic, aquaculture, and municipal wastewater, respectively. However, protein productivity calculated from the data reported by Mercado et al. [5] was higher than in this study. Ansari et al. [8] found that the lipid-extracted biomass of Scenedesmus sp. grown in BG11 has a higher protein content than WMB, which is similar to that attained in this study. As a result, brewery effluent seemed to be suitable for the accumulation of more total proteins in the cells of Scenedesmus sp.
3.3 Carbohydrate extraction
3.3.1 Effect of pretreatment on carbohydrate extraction
Carbohydrates were extracted from WMB and LEMB using microwave, autoclave, oven, and water bath heating pretreatments with acid and alkali hydrolytic agents to identify the effective pretreatment (Fig. 2a–d). Results showed that microwave pretreatment provided higher carbohydrates from WMB and LEMB with all hydrolytic agents compared to autoclave, oven, and water bath heating pretreatments. The highest carbohydrate yields in LEMB and WMB were obtained using HCl, followed by H2SO4, NaOH, KOH, and H2O. Moreover, the LEMB provides higher carbohydrate contents than those obtained from the WMB in all pretreatment methods. The highest carbohydrate content obtained from LEMB was 222.59 ± 0.89 mg/g using microwave with HCl, whereas the lowest was 81.90 ± 2.21 mg/g using water bath heating with H2O. The highest and lowest carbohydrate contents obtained from LEMB are 7.16% and 33.12% higher, respectively, than those achieved from WMB. Furthermore, the carbohydrate yield obtained in a microwave using HCl differed significantly (P < 0.05) from that obtained using the other hydrolytic agents (H2SO4, NaOH, KOH, and H2O).The results obtained in this study also showed that alkaline pretreatment was less effective for carbohydrate extraction compared to acid pretreatment. The higher carbohydrate yield obtained in LEMB could be explained due to the organic solvent used for lipid extraction weakening the structural integrity of the cell wall by dissolving the cellulose [42]; therefore, this enhances the digestibility of the LEMB. Lee et al. [43] and Lee et al. [11] conducted a study to extract carbohydrates from LEMB and WMB for bioethanol production and reported that LEMB has a higher carbohydrate yield than WMB of Dunaliella tertiolecta and Chlorella sp., respectively. Moreover, these two studies found that HCl is more effective for releasing carbohydrates and reducing sugar than H2SO4 and the results are higher than those obtained in this study. However, Ansari et al. [44] and Vardon et al. [45] reported a lower carbohydrate yield in LEMB than WMB of Scenedesmus obliquus and Scenedesmus sp., respectively. Furthermore, they achieved a lower carbohydrate content from LEMB when compared to those obtained in this study.
3.3.2 Effect of pretreatment on reducing sugar extraction
Like carbohydrates, the LEMB released a higher amount of reducing sugar in all pretreatment methods than the WMB (Fig. 3a–d). Pretreatment with HCl released a higher reducing sugar in microwave pretreatment while H2SO4 released a higher reducing sugar in autoclave, oven, and water bath heating pretreatments. The highest reducing sugar content obtained in LEMB was 159.19 ± 1.05 mg/g in microwave pretreatment using HCl, while the lowest reducing sugar content achieved was 30 ± 3.22 mg/g in water bath heating with H2O. The highest reducing sugar content obtained in LEMB is 7.77% higher than that obtained from WMB. Microwave pretreatment was more effective with HCl, whereas autoclave, oven, and water bath heating were more effective with H2SO4 for reducing sugar extraction. Alkaline pretreatment in this study released a higher reducing sugar from LEMB than WMB; however, the results were lower than acid pretreatment. Ansari et al. [8] reported a reducing yield of 12.37–19.51% from the LEB of Scenedesmus obliquus using autoclave pretreatment with H2SO4. Additionally, Thangam et al. [17] found a maximum reducing sugar yield of 112 mg/g from the LEB of Scenedesmus sp. grown in domestic wastewater using heating at 120 °C with H2SO4. However, Pancha et al. [9] obtained the highest reducing sugar yield (29.35%) from LEB of Scenedesmus sp. with HCl compared to H2SO4, HNO3, H3PO4, NaOH, and KOH. Furthermore, they obtained a lower reducing sugar yield with alkaline (NaOH or KOH) pretreatment, which concurred with this study. A lower sugar yield with alkaline pretreatment was also reported by Hernández et al. [46] compared to acid pretreatment. The lower sugar yield using alkaline agents for microalgal biomass hydrolysis is predominantly because of the degradation of sugar at high pH and alkaline agents which mostly hydrolyze fiber polymer compared to complex sugar [9, 46].
3.3.3 Effects of operational parameters on carbohydrate extraction
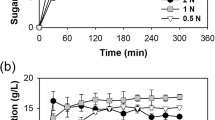
The optimizations of carbohydrate and reducing sugar extractions were carried out in microwave for four main operational parameters: acid concentrations, temperature, microwave power, and extraction time. The weight of LEMB (5% (w/v)) was constant throughout the optimization of the above variables with a single parameter at a time approach. The results obtained from the optimization process are illustrated in Fig. 4a–d. Figure 4a shows the effect of acid concentrations ranging from 0.1 to 6 N on carbohydrate and reducing sugar extractions from LEMB at 1000 W and 120 °C for 15 min. The carbohydrate and reducing sugar yields increased with acid concentration and reached maximum at the concentrations of 1 N and 2 N, respectively. After these acid concentrations, both carbohydrate and reducing sugar contents decreased and reached the lowest at 6 N. Therefore, the acid concentrations of 2 N for carbohydrates and 1 N for reducing sugar were considered optimal acid concentrations. The contents of 247.86 ± 1.30 and 165.075 ± 2.19 mg/g were found at the optimum acid concentration of 2 and 1 N for carbohydrates and reducing sugar, respectively. The carbohydrate contents achieved at 1 N and 2 N differed significantly (P < 0.05) from those attained at concentrations of 0.1, 0.5, 3, 4, 5, and 6 N. However, the reducing sugar contents found at 1 N and 2 N did not differ significantly. The decrease in sugar content with increasing acid concentration may be attributed to monosaccharide degradation into sugar degradation products such as furfural [47].
Figure 4b shows the effect of microwave power ranging from 600 to 1400 W on carbohydrate and reducing sugar extractions with a fixing temperature of 120 °C, an acid concentration of 1 N for reducing sugar and 2 N for carbohydrates, and an extraction time of 15 min. The maximum carbohydrate content obtained was 247.96 ± 1.54 mg/g at a microwave power of 1200 °C, which differed significantly (P < 0.05) from those achieved at 600, 800, 1000, and 1400 W. The maximum reducing sugar content was also determined to be 167.92 ± 2.79 at 1200 W, which did not differ significantly (P > 0.05) from the value obtained at 1400 W but did differ significantly (P < 0.05) from the reducing sugar contents obtained at 600, 800, and 1000 W. Therefore, the optimal value of microwave power for carbohydrate and reducing sugar extraction was 1200 W.
Figure 4c displays the effect of temperatures ranging from 80 to 180 °C on carbohydrate and reducing sugar extraction. The other parameters were fixed at 1200 W, 2 N for carbohydrates/1 N for reducing sugar, and a 15 min extraction time. The maximum carbohydrate content of 276.96 ± 2.13 mg/g and the reducing sugar content of 181.27 ± 2.49 mg/g were obtained at 140 °C. These carbohydrate and reducing sugar values were significantly different from those found at temperatures of 80 °C, 100 °C, 120 °C, 160 °C, and 180 °C. Hence, the optimum temperature for carbohydrate and reducing sugar extraction was 140 °C.
Figure 4d illustrates the effect of extraction time ranging from 5 to 30 min on carbohydrate and reducing sugar extraction from LEMB. The variables acid concentration, microwave power, and temperature were held constant at 2 N (for carbohydrates)/1 N (for reducing sugar), 1200 W, and 140 °C, respectively. As shown in Fig. 4d, the carbohydrate and reducing sugar yields from LEMB increased with extraction time, reaching a maximum at 20 min but decreasing after this time. At 20 min, the carbohydrates of 277.24 ± 0.98 mg/g and the reducing sugar of 192.54 ± 1.37 mg/g were obtained from LEMB. The total carbohydrate content as well as reducing sugar content obtained at 20 min significantly differed from those obtained at 5, 10, 15, 25, and 30 min. Hence, the extraction time of 20 min was taken as the optimal value for carbohydrate and reducing sugar extraction.
Finally, the optimum conditions obtained for carbohydrate and reducing sugar extractions from LEMB were found to be the combination of 2 N, 1200 W, 140 °C, and 20 min and 1 N, 1200 W, 140 °C, and 20 min, respectively. At optimum conditions, the carbohydrate content of 277.24 ± 0.98 mg/g (27.72%) and reducing sugar of 192.54 ± 1.37 mg/g (19.25%) were obtained from the LEB of Scenedesmus sp. According to Demirbas [48], Scenedesmus sp. contains between 21 to 52% of carbohydrates, which includes the results of this study. Therefore, this study found that microwave-assisted acid hydrolysis has the potential to improve carbohydrates and reducing sugar released from lipid-extracted microalgal biomass.
3.4 Bioethanol production from LEMB
Microalgal carbohydrates are not readily fermentable due to the fact that they are mostly found as starch in chloroplasts and as cellulose in the cell walls. The process of solvent extraction with the microwave pretreatment method used in this study enhanced the breakdown of the complex structure of polysaccharides in microalgae. In the present study, bioethanol was produced from the hydrolysate of LEMB obtained at optimum conditions using the yeast Saccharomyces cerevisiae. The bioethanol yield kinetic parameters and reducing sugar reduction during the fermentation period are provided in Table 2. As shown in Table 2, the concentration of bioethanol was increased and reached a maximum at a 24 h fermentation time, whereas the concentration of reducing sugar was decreased. The decrease in reducing sugar over the 24 h fermentation period demonstrated that the Saccharomyces cerevisiae yeast utilized the LEMB hydrolysate as a substrate. The highest bioethanol concentration, bioethanol yield, bioethanol productivity, and fermentation efficiency were found to be 6.04 g/L, 0.1 g/g LEMB, 0.084 g/L/d, and 94.84% at the fermentation time of 24 h, respectively. The bioethanol yield and fermentation efficiency obtained from LEMB were increased by 25% and 7.5%, respectively, compared to the results obtained from the whole microalgal biomass which were reported in our previous study [15]. The high fermentation efficiency obtained in this study probably indicated that the hydrolysis of LEMB does not form fermentation inhibitors, which are common in lignocellulosic biomass [11]. This could be one of the advantages of using LEMB for bioethanol production compared to lignocellulosic biomass. Moreover, the production of bioethanol from the biomass left after lipid extraction may enhance the biorefinery concept through coupling with biodiesel production.
Bioethanol production from LEB of different microalgae in other studies is provided in Table 3. Dhandayuthapani et al. [16] reported a maximum yield of bioethanol (0.087 g/g LEMB) from Chlorella sorokiniana grown in sterilized municipal wastewater using ultrasonic pretreatment. Lee et al. [43] studied chemo-enzymatic saccharification for bioethanol production from LEB of Dunaliella tertiolecta grown in a synthetic medium, and they found a maximum reducing sugar of 42% and bioethanol of 0.14 g/g from LEMB. Lee et al. [11] attained a maximum yield of bioethanol (0.16 g/g LEMB) from Chlorella sp. grown in a synthetic medium. Chng et al. [49] obtained a maximum of 0.26 g/g bioethanol yield from the LEB of Scenedesmus dimorphus grown in a synthetic medium without any pretreatment. The bioethanol yields obtained from LEMB in most previous studies were higher than that found in this study, except that reported by Dhandayuthapani et al. [16]. On the other hand, the bioethanol yield obtained in this study was higher than that achieved from whole microalgal biomass. Yu et al. [25] and Sivaramakrishnan and Incharoensakdi [50] found a maximum bioethanol yield of 0.076 g/g biomass from Scenedesmus sp. and Chlorella sp., respectively. Reyimu and Ozçimen [51] reported a maximum bioethanol yield of 0.04 g/g bioethanol yield from the whole biomass of Nannochloropsis oculata grown in municipal wastewater. However, most of the previous studies reported utilizing a synthetic medium for microalgae growth and subsequently producing bioethanol. This may add an extra cost to the production of bioethanol and other biochemical compounds from microalgae. Therefore, the application of wastewater as a growth medium for microalgae like in the present study is more attractive in order to reduce bioethanol production costs.
3.5 Mass balance in bioethanol production from microalgal biomass
The overall mass balance of lipid and bioethanol production was analyzed from 1 kg of whole microalgal biomass. Figure 5 demonstrates the overall mass balance diagram of bioethanol production from lipid-extracted biomass. From 1 kg of WMB, 0.135 kg of lipids and 0.865 g of LEMB can be obtained. The LEMB contains 0.466 kg of proteins, 0.240 kg of carbohydrates (0.173 kg of reducing sugar and 0.067 kg of non-reducing sugar), and 0.159 kg of others. The hydrolysate containing reducing sugar (0.173 kg) was fermented using Saccharomyces cerevisiae at 30 °C for 72 h. The fermentation process can convert the reducing sugar with 94.84% fermentation efficiency and produce 0.086 kg of bioethanol from 0.865 kg of LEMB, indicating a 0.1 g bioethanol yield/g LEMB. Moreover, the lipid content may be enhanced in the combination of solvent extraction with the pretreatment methods and used for biodiesel production. Therefore, local Scenedesmus sp. has great potential for a sustainable approach to developing microalgae-based biorefineries.
4 Conclusion
The results of the present study demonstrated the feasibility of the production of bioethanol from LEB of local microalga grown on AD brewery effluent. Results showed that microwave pretreatment with HCl was the most effective pretreatment method for carbohydrate and reducing sugar extraction. The maximum yields of reducing sugar and carbohydrates obtained from LEMB were 159.19 and 222.5 mg/g, which were increased by 20.94% and 19.74% after optimization, respectively. The maximum bioethanol yield achieved was 0.1 g/g LEMB with a fermentation efficiency of 94.84%. The results found in this study suggested that LEMB is a promising biomass for bioethanol production with the appropriate pretreatment method and that it has great potential for protein production. Moreover, the utilization of wastewater as a growth medium is a cost-effective and eco-friendly approach for carbohydrate and bioethanol production. Furthermore, the lipid production from local microalgae can be enhanced through pretreatment methods and then, it can be used for biodiesel production.
Data availability
The data sets used in this study are available from the corresponding author on reasonable request.
References
Velazquez-lucio J, Rodríguez-jasso RM, Colla LM, Sáenz-galindo A, Cervantes- DE, Aguilar CN et al (2018) Microalgal biomass pretreatment for bioethanol production: a review. Biofuel Res J. 17:780–91. https://doi.org/10.18331/BRJ2018.5.1.5
Mahmoud EA, Farahat LA, Abdel ZK, Fatthallah NA, Salah RA, Din E (2015) Evaluation of the potential for some isolated microalgae to produce biodiesel. Egypt J Pet 24(1):97–101. https://doi.org/10.1016/j.ejpe.2015.02.010
Tripathi R, Gupta A, Thakur IS (2019) An integrated approach for phycoremediation of wastewater and sustainable biodiesel production by green microalgae, Scenedesmus sp. Renew Energy 135:617–625. https://doi.org/10.1016/j.renene.2018.12.056
Ummalyma SB, Sirohi R, Udayan A, Yadav P, Raj A, Sim SJ et al (2022) Sustainable microalgal biomass production in food industry wastewater for low-cost biorefinery products: a review. Phytochem Rev. https://doi.org/10.1007/s11101-022-09814-3
Mercado I, Xavier Á, Verduga M, Cruz A (2020) Scenedesmus sp. cultivated in the wastewater of the dairy industry. Processes. 8(1458):1–19
Ansari FA, Singh P, Guldhe A, Bux F (2017) Microalgal cultivation using aquaculture wastewater : integrated biomass generation and nutrient remediation. Algae 21:169–177. https://doi.org/10.1016/j.algal.2016.11.015
Karpagam R, Abinaya N, Gnanam R (2021) Assortment of native microalgae for improved biomass and lipid production on employing vegetable waste as a frugal cultivation approach for biodiesel application. Curr Microbiol 78(10):3770–3781. https://doi.org/10.1007/s00284-021-02643-1
Ansari FA, Shriwastav A, Gupta SK, Rawat I, Guldhe A, Bux F (2015) Lipid extracted algae as a source for protein and reduced sugar : a step closer to the biorefinery. Bioresour Technol 179:559–564. https://doi.org/10.1016/j.biortech.2014.12.047
Pancha I, Chokshi K, Maurya R, Bhattacharya S, Bachani P (2016) Comparative evaluation of chemical and enzymatic saccharification of mixotrophically grown de-oiled microalgal biomass for reducing sugar production. Bioresour Technol 204:9–16. https://doi.org/10.1016/j.biortech.2015.12.078
Fetyan NAH, El-Sayed AE-KB, Ibrahim FM, Attia YA, Sadik MW (2021) Bioethanol production from defatted biomass of Nannochloropsis oculata microalgae grown under mixotrophic conditions. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-15758-6
Lee OK, Oh Y-K, Lee EY (2015) Bioethanol production from carbohydrate-enriched residual biomass obtained after lipid extraction of Chlorella sp. KR-1. Bioresour Technol 196:22–27. https://doi.org/10.1016/j.biortech.2015.07.040
Karpagam R, Jawaharraj K, Gnanam R (2021) Review on integrated biofuel production from microalgal biomass through the outset of transesterification route: a cascade approach for sustainable bioenergy. Sci Total Environ. 766:144236. https://doi.org/10.1016/j.scitotenv.2020.144236
Soni VK, Krishnapriya R, Sharma RK. (2021) Algae: biomass to biofuel. In: Basu C, editor. Biofuels and biodiesel. Methods in Molecular Biology 2290: 31–51
Manmai N, Unpaprom Y, Ramaraj R (2020) Bioethanol production from sunflower stalk : application of chemical and biological pretreatments by response surface methodology (RSM). Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00602-7
Yirgu Z, Leta S, Hussen A, Mazharuddin M (2021) Pretreatment and optimization of reducing sugar extraction from indigenous microalgae grown on brewery wastewater for bioethanol production. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01779-1
Dhandayuthapani K, Sarumathi V, Selvakumar P, Temesgen T. (2021) Study on the ethanol production from hydrolysate derived by ultrasonic pretreated defatted biomass of Chlorella sorokiniana NITTS3. Chem Data Collect. 31. https://doi.org/10.1016/j.cdc.2020.100641
Thangam KR, Santhiya A, Sri SRA, Mubarakali D, Karthikumar S, Kumar RS et al (2021) Biorefinery approaches based concomitant microalgal biofuel production and wastewater treatment. Sci Total Environ. 785:147267. https://doi.org/10.1016/j.scitotenv.2021.147267
Nichols HW, Bold HC (1965) Trichosarcina polymorpha. J Phycol 1:34–38
Andersen RA, Kawachi M (2005) Traditional microalgae isolation techniques. In: Andersen RA (ed) Algal culturing techniques. Elsevier/Academic Press, London, UK, pp 83–100
Bellinger EG, Sigee DC. (2010) Freshwater algae: identification and use as bioindicators. John Wiley & Sons, Ltd, UK. p. 187–189
Shubert E, Gärtner G. (2015) Nonmotile coccoid and colonial green algae. In: Wehr JD, Sheath RG, Kociolek JP, editors. Freshwater algae of North America. Elsevier Inc, london, UK. p. 315–73
Ansari AA, Khoja AH, Nawar A, Qayyum M (2017) Wastewater treatment by local microalgae strains for CO2 sequestration and biofuel production. Appl Water Sci 7:4151–4158. https://doi.org/10.1007/s13201-017-0574-9
Bligh EG, Dyer WJ. (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 37
Harun R, Danquah K, Forde GM (2010) Microalgal biomass as a fermentation feedstock for bioethanol production. J Chem Technol Biotechnol 85:199–203. https://doi.org/10.1002/jctb.2287
Yu KL, Chen W-H, Sheen H-K, Chang J-S, Linh C-S, Ong HC et al (2020) Bioethanol production from acid pretreated microalgal hydrolysate using microwave-assisted heating wet torrefaction. Fuel 279:118435. https://doi.org/10.1016/j.fuel.2020.118435
Mahzabin Q, Zhang B, Wang L, Shahbazi A. (2019) A combined pretreatment , fermentation and ethanol-assisted liquefaction process for production of biofuel from Chlorella sp . Fuel. 1–8. https://doi.org/10.1016/j.fuel.2019.116026
Thu M, Choi SP, Lee J, Lee JH, Sim SJ (2009) Hydrothermal acid pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. J Microbiol Biotechnol 19(2):161–166. https://doi.org/10.4014/jmb.0810.578
Lee Y, Chen W, Shen H, Han D, Li Y, Jones HDT et al (2013) Basic culturing and analytical measurement techniques. In: Richmond A, Hu Q (eds) Handbook of microalgal culture: applied phycology and biotechnology. Blackwell Publishing Ltd, Second Edi, pp 37–68
APHA (1999) Standard methods for the examination of water and wastewater, 20th edn. D.C., USA, Washington
Pandey A, Srivastava S, Kumar S. (2019) Sequential optimization of essential nutrients addition in simulated dairy effluent for improved Scenedesmus sp ASK22 growth, lipid production and nutrients removal. 128. https://doi.org/10.1016/j.biombioe.2019.105319
AOAC. (1990) Official methods of analysis. 15th ed. Helrich K, editor. Vol. 1. Virginia, USA: Association of Official Analytical Chemists,INC. 70–74 p
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar use. Anal Chem 31(3):426–428
Crowell EA, Ough CS (1979) A modified procedure for alcohol determination by dichromate oxidation. Am J Enol Vitic 30(1):61–63
Williams MB, Darwin RH (1950) Colorimetric determination of ethyl alcohol. Anal Chem 22(12):1556–1561
Ferreira A, Ribeiro B, Marques PASS, Ferreira AF, Paula A, Pinheiro HM et al (2017) Scenedesmus obliquus mediated brewery wastewater remediation and CO2 biofixation for green energy purposes. J Clean Prod 165:1316–1327. https://doi.org/10.1016/j.jclepro.2017.07.232
Marchão L, da Silva TL, Gouveia L, Reis A (2018) Microalgae-mediated brewery wastewater treatment: effect of dilution rate on nutrient removal rates, biomass biochemical composition, and cell physiology. J Appl Phycol 30(3):1583–1595. https://doi.org/10.1007/s10811-017-1374-1
Diniz GS, Silva AF, Araújo OQF, Chaloub RM (2017) The potential of microalgal biomass production for biotechnological purposes using wastewater resources. J Appl Phycol 29:821–832. https://doi.org/10.1007/s10811-016-0976-3
Ho S, Chen W, Chang J (2010) Scenedesmus obliquus CNW-N as a potential candidate for CO2 mitigation and biodiesel production. Bioresour Technol 101:8725–8730. https://doi.org/10.1016/j.biortech.2010.06.112
Nayak M, Karemore A, Sen R (2016) Performance evaluation of microalgae for concomitant wastewater bioremediation, CO2 biofixation and lipid biosynthesis for biodiesel application. Algal Res 16:216–223. https://doi.org/10.1016/j.algal.2016.03.020
Gupta SK, Kumar NM, Guldhe A, Ansari FA, Rawat I, Nasr M et al (2018) Wastewater to biofuels : comprehensive evaluation of various flocculants on biochemical composition and yield of microalgae. Ecol Eng 117:62–68. https://doi.org/10.1016/j.ecoleng.2018.04.005
Chng LM, Lee KT, Juinn D, Chan C (2017) Synergistic effect of pretreatment and fermentation process on carbohydrate-rich Scenedesmus dimorphus for bioethanol production. Energy Convers Manag 141:410–419. https://doi.org/10.1016/j.enconman.2016.10.026
Lee OK, Kim AL, Seong DH, Lee CG, Jung YT, Lee JW et al (2013) Chemo-enzymatic saccharification and bioethanol fermentation of lipid-extracted residual biomass of the microalga. Dunaliella tertiolecta Bioresour Technol 132:197–201. https://doi.org/10.1016/j.biortech.2013.01.007
Ansari FA, Ravindran B, Gupta SK, Nasr M, Rawat I, Bux F (2019) Techno-economic estimation of wastewater phycoremediation and environmental benefits using Scenedesmus obliquus microalgae. J Environ Manage 240:293–302. https://doi.org/10.1016/j.jenvman.2019.03.123
Vardon DR, Sharma BK, Blazina GV, Rajagopalan K, Strathmann TJ (2012) Thermochemical conversion of raw and defatted algal biomass via hydrothermal liquefaction and slow pyrolysis. Bioresour Technol 109:178–187. https://doi.org/10.1016/j.biortech.2012.01.008
Hernández D, Riaño B, Coca M, García-gonzález MC (2015) Saccharification of carbohydrates in microalgal biomass by physical, chemical and enzymatic pre-treatments as a previous step for bioethanol production. Chem Eng J 262:939–945. https://doi.org/10.1016/j.cej.2014.10.049
Boonmanumsin P, Treeboobpha S, Jeamjumnunja K, Luengnaruemitchai A, Chaisuwan T, Wongkasemjit S (2012) Release of monomeric sugars from Miscanthus sinensis by microwave-assisted ammonia and phosphoric acid treatments. Bioresour Technol 103:425–431. https://doi.org/10.1016/j.biortech.2011.09.136
Demirbas A (2010) Use of algae as biofuel sources. Energy Convers Manag. 51(12):2738–49. https://doi.org/10.1016/j.enconman.2010.06.010
Chng LM, Chan DJC, Lee KT (2016) (2018) Sustainable production of bioethanol using lipid-extracted biomass from Scenedesmus dimorphus. J Clean Prod 130:68–73. https://doi.org/10.1016/j.jclepro.2016.02.016
Sivaramakrishnan R, Incharoensakdi A (2018) Utilization of microalgae feedstock for concomitant production of bioethanol and biodiesel. Fuel 217:458–466. https://doi.org/10.1016/j.fuel.2017.12.119
Reyimu Z, Ozçimen D (2017) Batch cultivation of marine microalgae Nannochloropsis oculata and Tetraselmis suecica in treated municipal wastewater toward bioethanol production. J Clean Prod 150:40–6. https://doi.org/10.1016/j.jclepro.2017.02.189
Ashokkumar V, Salam Z, Tiwari ON (2015) Chinnasamy S, Mohammed S, Nasir F. An integrated approach for biodiesel and bioethanol production from Scenedesmus bijugatus cultivated in a vertical tubular photobioreactor. Energy Convers Manag 101:778–786. https://doi.org/10.1016/j.enconman.2015.06.006
Kim EJ, Kim S, Choi HG, Han SJ (2020) Co - production of biodiesel and bioethanol using psychrophilic microalga Chlamydomonas sp KNM0029C isolated from Arctic sea ice. Biotechnol Biofuels. 13(20):1–13. https://doi.org/10.1186/s13068-020-1660-z
Acknowledgements
This research was supported by the Center for Environmental Science of Addis Ababa University and Wolaita-Sodo University. The authors of this paper wish to thank BGI Ethiopia for assessing UASB effluent from their wastewater treatment plant.
Author information
Authors and Affiliations
Contributions
ZY, MMK, and TA isolated and identified Scenedesmus sp., designed the study, conducted the experiments, collected and analyzed the data, and wrote the manuscript. SL and AH designed the experiments, supervised the research, analyzed and interpreted the data, and edited the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yirgu, Z., Leta, S., Hussen, A. et al. Pretreatment of lipid-extracted biomass of Scenedesmus sp. grown in wastewater for bioethanol production. Biomass Conv. Bioref. 14, 16867–16878 (2024). https://doi.org/10.1007/s13399-023-03917-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-03917-3