Abstract
The utilization of microalgae for wastewater treatment represents an attractive opportunity for wastewater valorization through the use of the produced biomass. Five strains of microalgae were isolated from municipal wastewater and grown in autoclaved and non-autoclaved effluent at 30 °C and 150 μmol photons m−2 s−1 to study biomass production, nutrient removal, and the biochemical composition of the biomass. All strains reached high biomass productivity (35.6 to 54.2 mg dry weight L−1 day−1) within 4 days of batch culturing. In this period, ammonium-N and phosphate were reduced by more than 60 and 90 %, respectively. The high growth rate (0.57 to 1.06 day−1) ensured a rapid removal of nutrients and thereby a short retention time. By the fourth day of cultivation, the algal biomass contained 32 % protein, but only 11 % lipids and 18 % carbohydrates. It was found that the biomass was a suitable raw material for biogas production by anaerobic digestion. Biodigestion of obtained biomass was simulated by employing the Aspen HYSYS modeling software, resulting in methane yields comparable to those found in the literature. The elemental analysis of the algal biomass showed very low concentrations of pollutants, demonstrating the potential of use of the digestate from biodigestion as a bio-fertilizer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global annual freshwater consumption was estimated to be around 3700 billion m3 in 2014 (Bank W. 2016) with most of the used water becoming wastewater. Wastewater from human activities usually is classified as domestic or industrial sewage, or agricultural and aquacultural effluents. Without proper treatment, the release of excess N and P form these effluents may lead to eutrophication and ecosystem damage in downstream watersheds (Moree et al. 2013). In order to reduce the problems caused by the release of wastewater into water bodies, the use of effluents for productive purposes has been studied worldwide. Integrated management of wastewater treatment plants with reuse practices can help to prevent the pollution of water bodies by nutrients, while providing productive activities (Vasquez-Montiel et al. 1996).
Photosynthetic microorganisms use N and P as nutrients, with their growth depending on environmental conditions, such as light, temperature, pH, salinity, and CO2 content. Many species of microalgae are able to effectively grow in wastewaters and, therefore, have the potential to play an important role in phycoremediation, particularly during the final (tertiary) treatment phase of wastewater (Bohutskyi et al. 2015a). Several studies show high efficiency in the removal of contaminants from different kinds of wastewater by microalgae (e.g., Doria et al. 2012; Posadas et al. 2014; Liu et al. 2016; Zhang et al. 2016).
One of the potential methods to reduce the costs of algal mass cultivation is to integrate wastewater treatment with algal biomass production. Benefiting from the abundance of nutrients, cultivation of microalgae in wastewater substantially reduces the need for chemical fertilizers and their burden on the life cycle of algae (Clarens et al. 2010). Although tertiary wastewater treatment associated with biodiesel production has been studied previously (Zhou et al. 2011; Craggs et al. 2012; Mahapatra et al. 2013; Jia et al. 2016), few studies have analyzed the effects of wastewater on the biochemical composition of microalgae throughout their growth cycle (Kiran et al. 2014). In order to achieve successful exploitation of the potential of microalgae, a good understanding of the physiology, biochemistry, and ecology of microalgae is essential. The proximate composition of algal cells can also be manipulated by nutrient concentrations and the forms of the nutrients supplied (Borowitzka 2016). Such information may be important for potential commercial applications of microalgae biomass, such as aquaculture feeds, agricultural fertilizer, or as a source of high-value chemicals such as pharmaceuticals and of special chemicals (Borowitzka 2013).
Recently, many studies have been using native microalgae isolated from wastewater, because they perform better when cultured in wastewater than most other species acquired from culture collections (Liu et al. 2012; Park et al. 2012; Mahapatra et al. 2013; Samori et al. 2013; Lynch et al. 2015). For instance, N and P removal rates by species isolated from wastewater were found to be 10 to 25 times higher than by species acquired from culture collections (Jiménez-Pérez et al. 2004). Native species cultivated in wastewater also had higher growth rates, biomass productivities, and lipid content (Zhou et al. 2011; Cheng and Tian 2013).
This work evaluates the utilization of native microalgae for simultaneous production of biomass and for the reduction of N and P levels in a municipal wastewater. Using the Environmental Sanitation Experimental Center of the Federal University of Rio de Janeiro (Rio de Janeiro, Brazil) as a model, we isolated five native microalgae from the stabilization lagoon of this plant. We determined growth rate, biomass yield, and nutrient removal efficiency of these microalgal strains using the sewage as a culture medium and determined the biochemical composition of their biomass in order to test the potential for biotechnological use of the generated feedstock. Anaerobic digestion (AD) of the obtained biomass was evaluated by process simulation for the strains with the highest specific growth rate and biomass productivity in the non-autoclaved effluent.
Material and methods
Wastewater pretreatment
This study was performed with effluent collected from the stabilization lagoon of the Environmental Sanitation Experimental Center of the Federal University of Rio de Janeiro (Rio de Janeiro, Brazil). The sampled wastewater was filtered through membranes with distinct porosities to remove large solid particles (5.0 μm), eukaryotes (0.45 μm), and prokaryotes and their resistant cysts (0.22 μm). After filtration, the effluent was autoclaved at 121 °C for 45 min. The autoclaved effluent (AE) was used to evaluate growth and removal of N and P for cultures without bacterial contamination. Tests were also conducted with effluent that was filtered as described above, but not autoclaved (NAE). After pretreatment, both effluent media were stored at 4 °C until use for algal cultivation.
Microalgae isolation
Uniclonal strains of five microalgae were isolated from the stabilized lagoon of the Environmental Sanitation Experimental Center of the Federal University of Rio de Janeiro according to Li (2002), using a flow cytometer equipped with a fluorescence-activated cell sorter—FACS (Beckman Coulter MoFlo cytometer). The strains were identified by optical microscopy based on cell morphology.
The isolated algae were maintained as axenic cultures and are available at the Laboratório de Estudos Aplicados em Fotossíntese (LEAF-Culture Collection, Institute of Chemistry, Federal University of Rio de Janeiro, Brazil), where they were cataloged as strain LEAF614 (Scenedesmus sp1), LEAF615 (Scenedesmus sp2), LEAF616 (Desmodesmus sp.), LEAF735 (Chlorella sp1), and LEAF736 (Chlorella sp2).
Microalgal culturing
The native microalgae strains were batch-cultured photoautotrophically in 3-L Erlenmeyer flasks containing 1.7 L of AE or NAE. Cultures were exposed to lateral illumination (150 μmol photons m−2 s−1 on the outer surface of the vessels) provided by fluorescent lamps (Philips 23 W, white light), and a 12:12-h photoperiod. Culture flasks were maintained on a rotatory shaker adjusted at 126 rpm (Lab-Line Instruments Inc.) at 30 ± 2 °C. Four independent biological replicates were tested for each species in each type of effluent. Since the strains had distinct sizes, we used different inoculation density in order to achieve similar initial dry mass for all cultures and attain the stationary growth phase in the same period of time. Thus, the initial cell density was 5.0 × 104 cells mL−1 for Scenedesmus sp1 and Scenedesmus sp2, 8.0 × 104 cells mL−1 for Desmodesmus sp., 1.5 × 105 cells mL−1 for Chlorella sp1, and 3.0 × 104 cells mL−1 for Chlorella sp2.
Growth monitoring and kinetics growth parameters
Daily, 4 h after the beginning of the light period, 30 mL aliquots of algal suspension were filtered (Whatman GF/F) for analysis of cell biomass. Biomass was determined gravimetrically by obtaining the weight difference of the fiber filter dried at 65 °C to a constant weight before and after filtering microalgae cultures, according to Zhu and Lee (1997).
The maximum specific growth rate (μmax) was estimated from the linear coefficient of the equation modeling the exponential portion of the growth curve, that corresponds, in most of the cases, to the period of time comprised between the first and third days of cultivation: μ max = (ln (X 2 )−ln (X 1 ))/(t 2 –t 1 ), where X 2 and X 1 correspond to cell biomass (mg L−1) at, respectively, time t 2 and time t 1 (days).
Nutrient removal and pH determination
For these analytical assays, the concentrations of nitrogen and phosphorus in the culture medium were determined in the filtrates obtained from samples collected for biomass determination. The concentration of ammonia/ammonium was spectrophotometrically determined using the Koroleff reaction (1969). Orthophosphate concentration was determined spectrophotometrically at 885 nm after reaction with ammonium molybdate (Murphy and Riley 1962). The percentage of nutrient removal was calculated as
where C 0 and C i are the concentrations of N or P at the beginning and on day i of the treatment.
The same analyses were carried out on controls, which were maintained in the absence of microalgae in order to analyze the loss of ammonia through volatilization.
The pH of the culture media was measured, in the same filtrate used for nitrogen and phosphorus determinations, with an Incibras pH-1400 potentiometer (São Paulo, Brazil) coupled to an Analyser 2A13-HC electrode (São Paulo, Brazil).
Assays for biomass composition
To analyze the biomass composition of Scenedesmus sp2 and Desmodesmus sp., batch cultures were grown in 9-L glass carboys containing 5.5 L of NAE (three independent biological replicates per species). Cultures were maintained at 30 °C in an incubator (Forma Scientific Inc., USA), with constant magnetic stirring. The cultures were gassed with filtered atmospheric air, at flow of 3.5 L min−1, supplied by aquarium pumps.
The biochemical composition of both species grown in NAE was examined in the early (fourth day) and in the late (ninth day) stationary growth phase. Cells were centrifuged at 10,000×g for 10 min at room temperature (Sorvall RC5B, Thermo Fisher Scientific). Pellets were frozen at −20 °C and freeze-dried. After weighing, the material was stored in desiccators until further biochemical analysis.
Analytical methods
Nitrogen, hydrogen, carbon, and oxygen concentrations in lyophilized biomass were determined using a CHNO analyzer (Perkin-Elmer 2400). Samples were completely combusted and reduced to the elemental gases CO2, H2O, and N2 for CHN analyses. The oxygen content was determined by pyrolyzing samples at 1000 °C, and the resulting products of reaction containing oxygen were converted to carbon monoxide. Total protein content was obtained by assuming a protein:N ratio of 4.78 (Lourenço et al. 2004). Carbohydrate contents were extracted in 80 % H2SO4 according to Myklestad and Haug (1972). Carbohydrate concentration was determined spectrophotometrically at 485 nm (Shimadzu, UV-1800 model) by the phenol-sulfuric acid method (Dubois et al. 1956) using glucose as standard. Total lipids were extracted according to Folch et al. (1957) and quantified gravimetrically after solvent evaporation. Before lipid determination, the algal pellet was sonicated for 20 min to promote cell disruption.
Ash content was determined according to the Association of Analytical Communities—AOAC (1990). For this, crucibles containing the samples were placed in a muffle furnace at 450 °C until a constant weight was reached and then weighed.
Samples were digested with aqua regia (HNO3:HCl, 1:3, v/v) and were subsequently subjected to inductively coupled plasma optical emission Spectrometry (ICP-OES) (Optima 7000 DV Perkin Elmer) according to Carmo et al. (2000) to determine total phosphorus, potassium, sodium, calcium, magnesium, aluminum, barium, iron, cadmium, lead, chromium, nickel, copper, zinc, vanadium, selenium, and manganese.
Potential of biogas production
Picardo et al. (2013) proposed model molecules to represent microalgae as a lump of lipids, carbohydrates and proteins in process simulators. Based on their approach, this work employs Aspen HYSYS (ASPENTECH Inc.) to simulate AD as an equilibrium reactor (Gibbs reactor) to obtain the maximum theoretical yield of CH4 by employing the Peng-Robinson equation of state. Experimental values obtained for the strains presenting the best performance in terms of specific growth rate and biomass productivity in the non-autoclaved effluent were used as inputs to the process simulation. The adopted assumptions for process simulation are described in Online Resource 1.
Statistical analysis
Data were analyzed using GraphPad Prism 3.01. The results of growth were analyzed by one-way ANOVA to compare the species in each effluent. The results of biochemical composition were analyzed by Student’s t test to compare two different effluents (AE and NAE) and two sampling days (fourth and ninth day), applying a significance level of α = 0.05 (Zar 1996).
Results and discussion
Five local microalgae were isolated and evaluated for their ability to grow and remove nutrients from the wastewater collected from the stabilized lagoon of the Environmental Sanitation Experimental Center of the Federal University of Rio de Janeiro. We used the effluent as culture medium which was filtered and autoclaved (AE) or just filtered (NAE) to avoid influence of bacteria and other microorganisms on the rate of nutrients removal by each isolated microalgal strain, as well as on the biomass composition of the microalgae, as previously reported (Muñoz and Guieysse 2006, Silva-Benavides and Torzillo 2012; Hernández et al. 2013; Renuka et al. 2013). Given that samples of the effluent were collected over distinct periods of time, a large difference between the initial concentration of N and P in the two types of effluents was observed (Table 1). The AE was collected from May to July 2014 (ammonium-N concentration ranged from 7.17 to 28.20 mg L−1 and phosphate from 0.20 to 6.47 mg L−1). The NAE was collected from September 2014 to January 2015 (ammonium-N concentration ranged from 21.5 to 53.46 mg L−1 and phosphate from 8.14 to 18.3 mg L−1).
Algae growth and wastewater nutrients removal
Figure 1 shows the growth curves of the native microalgae cultured in either AE or NAE at 30 °C and 150 μmol photons m−2 s−1. Chlorella species were unable to grow in the AE, probably due to the degradation of some unknown growth factor by heat. Growth of all other strains was very similar in both types of effluent, with an exponential growth phase of 2–3 days. The stationary phase was reached within 4 days and the onset of the phase could be related to an unfavorable nutrient ratio (N/P ∼3), as well as to low carbon availability due to the initial rapid growth and increased pH values of the culture medium (see below).
Despite the difference in the concentration of N and P in the two culture media used, we did not detect variation of growth of Scenedesmus sp1 and sp2 (p > 0.05) in response to the culture medium (Table 2). Although the specific growth rate of Desmodesmus sp. was also similar in both types of effluent (p > 0.05, Table 2), biomass concentration differed significantly on the ninth day of the experiment (p = 0.0135).
No significant differences in the biomass concentration, productivity, and specific growth rate were found among the microalgae strains in each culture medium (Table 2). The only exception was the specific growth rate of Chlorella sp1, which was significantly higher than that of Chlorella sp2 in the NAE (p = 0.0371).
Although cell division was strongly reduced during the stationary phase, biomass concentration still increased until the late stationary phase (ninth day), reaching maximum average values of 0.26 g L−1 in cultures of Scenedesmus sp2 and Chlorella sp1 in NAE (Table 2). Care must be taken when comparing the values obtained in this study with those in the literature, due to the variation of nutrient concentration in the effluents utilized. Final dry biomass value of other studies ranged from 0.11 to 1.18 g L−1, due to the large variability in the initial concentration of nutrients in wastewater, as well as of growth conditions (Li et al. 2011; Mata et al. 2012).
The specific growth rates of the isolated strains were essentially the same, doubling their biomass daily during the exponential growth phase (Table 2), and indicating that the effluent is a promising culture medium. The specific growth rates recorded in this study (from 0.57 to 1.06 day−1) were among the highest values recorded in the literature, which range from 0.15 to 0.70 day−1 (Li et al. 2011; Liu et al. 2012). Given that the efficiency of wastewater treatment by microalgae is directly related to their growth rates, the high growth rates observed suggest the suitability of these five species for the removal of N and P, and for wastewater treatment with short retention times. Moreover, high specific growth rates resulted in high biomass productivity within the first 4 days of culture (Table 2).
Figure 2 depicts ammonium-N and phosphate removal, as well as pH variation, during growth of the native strains in each type of effluent. For the AE, Scenedesmus sp1 and sp2 showed similar ammonium-N removal. Both species removed about 64 % ammonium-N in the first 4 days of culture and removal reached about 70 % after 9 days. For Desmodesmus sp., 61 % of ammonium-N were removed during 8 days of culture (ammonium-N decreased from 13.1 to 5.09 mg L−1). In the NAE, Desmodesmus sp. showed a removal rate of 65 % of ammonium-N in the first 4 days (from 36.8 to 12.8 mg L−1), reaching 68 % after 9 days. In the same effluent, Scenedesmus sp2 removed ammonium-N faster than Scenedesmus sp1. By the fourth day, the ammonium-N removal efficiency by cultures of Scenedesmus sp2 was 71 % (the nitrogen fraction decreased from 37.4 to 10.9 mg L−1), while Scenedesmus sp1 removed only 60 % of ammonium-N (from 40.3 to 15.9 mg L−1). In the first 4 days of culture in the NAE, the ammonium-N removal efficiency by Chlorella sp2 was 61 % (from 34.1 to 13.3 mg L−1) and, after 9 days, about 68 % of the initial ammonium-N concentration had been removed. Chlorella sp1 showed the lowest ammonia-N removal efficiency. During 4 days of culture, this strain removed only 46 % of nitrogen, but reached 73 % removal at the end of the 9 days of cultivation.
Ammonium-N and phosphate concentrations and pH values in autoclaved (a, c, e) and non-autoclaved (b, d, f) effluent during growth experiments of Scenedesmus sp1 (white circle), Scenedesmus sp2 (black circle), Desmodesmus sp. (black triangle), Chlorella sp1 (black square), and Chlorella sp2 (white square). Controls (black diamond) were maintained in the absence of microalgae to verify the stability of nutrient concentrations and pH during the experiment. Values are means of four (n = 4) independent biological replicates ± SD. The horizontal dashed lines correspond to the limit for ammonium-N concentration in discharged effluents imposed by the Brazilian legislation (Brazilian National Environmental Council-CONAMA enacted Resolution No. 430/2011)
The highest removal of ammonium-N was verified in cultures of Scenedesmus sp2 and Desmodesmus sp. in NAE, once there was a higher consumption of nitrogen in less time (4 days). Figure 2 shows that the ammonium-N concentrations in the wastewater after cultivation of microalgae were always below the maximum allowed (20 mg L−1) by the Brazilian legislation (Brazilian National Environmental Council—CONAMA—Resolution No. 430/2011) to be discarded in water bodies.
Besides biological assimilation, ammonium disappearance from culture medium can be promoted by ammonia volatilization and/or oxidation to nitrates (Zimmo et al. 2003; Bohutskyi et al. 2015a). The latter process should not be present because both type of effluents were previously filtered through membranes of 0.22 μm porosity to remove prokaryotes and the isolated microalgae were maintained in axenic conditions. On the other hand, ammonia (NH3) is relatively volatile and can be removed from solution through mass transfer from the water surface to the atmosphere. Thus, loss of NH4 +-N by volatilization depends on the proportion of NH4 + and NH3 in the solution, which is pH and temperature dependent (Erickson 1985). In our experiments, a progressive increase in the pH of algal cultures was observed due to the photosynthetic activity of microalgae and concomitant CO2 uptake (Garcia et al. 2006). Although the pH of the algal cultures was around 10.0–10.5 by the third day of cultivation, loss of NH4 +-N by ammonia volatilization should not be significant in comparison with the amount required to support the algal growth between the third and fourth day of cultivation. Note that essentially there is no decrease of NH4 +-N during the stationary growth phase, showing that loss of ammonia by volatilization is minimal. On the other hand, given that virtually all (>90 %) of the inorganic carbon is present in the form of carbonate ions at a pH above 10, microalgal growth probably was carbon limited after the fourth day of culturing. After the fourth day, pH remained unchanged during the stationary growth phase.
Phosphate was entirely depleted in both types of effluent during the exponential growth phase, suggesting that this nutrient could limit microalgal growth (Fig. 2). As observed for the removal of ammonium-N, the phosphate uptake by Scenedesmus sp2 was not affected by autoclaving the effluent. This strain removed about 90 % phosphate in the first 3 days of growth (from 4.17 to 0.39 mg L−1 and from 10.6 to 0.67 mg L−1 in AE and NAE, respectively). After 4 days of growth, a high phosphate removal (89 %) from AE by Scenedesmus sp1 was observed, and even higher depletion of phosphate (97 %) was observed for the NAE. The phosphate removal by Desmodesmus sp. was 68 % (from 4.50 to 1.43 mg L−1) and 96 % (from 10.3 to 0.38 mg L−1), after 4 days in AE and NAE, respectively. In the same period of time, Chlorella sp1 removed about 94 % phosphate (from 11.4 to 0.71 mg L−1) from NAE, whereas Chlorella sp2 showed the lowest phosphate removal (about 87 %).
It has been shown that changes in pH induced by photosynthesis can cause phosphate precipitation in seawater, and that up to 20 % of the phosphate in the culture medium was precipitated at pH approximate to 9 (Olsen et al. 2006). In our case, however, no precipitate was observed in the algal cultures, even at pH values above 10, probably due to low phosphate concentrations in the culture medium as a consequence of algal uptake. In the control experiments, the phosphate concentration remained essentially the same for 9 days in the absence of cells in pH values next of 9.0 (Fig. 2e, f).
We observed that phosphate and ammonium-N removal was greater and faster than those reported in other studies using municipal wastewater (Li et al. 2011; Samori et al. 2013), suggesting that the algae strains used in this study are well adapted for nutrient removal from domestic sewage. The high specific growth rate and the high consumption of N and P in a short interval of time (up to 4 days) suggest the suitability of these five microalgae for the wastewater treatment (nutrient removal), ensuring short retention times for this treatment.
Isolation and screening of novel native algae allow the selection of strains adapted to specific climate, water chemistry, and other selective conditions, such as pH, temperature, and salinity. In line with our results, it has been reported (Bohutskyi et al. 2015a) that several studies found that local isolates of chlorophytes, phylogenetically clustered as Chlorella and Scenedesmus, showed the best growth in wastewater.
In this study, although the specific growth rate, productivity, and biomass concentration were similar for the five strains analyzed, Scenedesmus sp2 and Desmodesmus sp. appear to have a great ability to removal nitrogen and phosphorus (more than 65 % of ammonium-N and 95 % phosphate).
Furthermore, in the experimental conditions used in this study, the results for growth and nutrient removal indicate that the cultivation of the Desmodesmus sp. and Scenedesmus sp2 in NAE can be limited to 4 days since, after this time, biomass productivity declines and the ammonium-N and phosphate removal does not substantially increase when cells are cultivated for 9 days. However, culturing microalgae for less than 4 days is not recommended because the removal of ammonium-N and phosphate were below 60 and 90 %, respectively.
Gross biochemical composition and potential for biogas production of the biomass
The biochemical composition of the dry biomass of Scenedesmus sp2 and Desmodesmus sp. grown in NAE, at 30 °C and 150 μmol photons m−2 s−1, was determined to evaluate the incorporation wastewater nutrients into algal biomass. Although we found that a cultivation of 4 days was ideal for the removal of ammonium-N and phosphate, we also analyzed the biochemical composition of biomass on the ninth day of culture to verify if accumulation of compounds, such as carbohydrates and lipids, would be influenced under nitrogen or phosphorous deficiency (Hu et al. 2008).
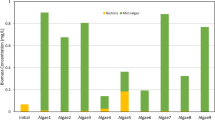
Table 3 shows that, after 4 days growth, the algal biomass contained large amounts of protein (31–34 %) and about 10–12 % of lipids, similar to those reported by Bellou et al. (2014). Although many microalgae accumulate neutral lipids under unfavorable growth conditions, such as nitrogen or phosphorus deficiency (Roopnarain et al. 2014), this was not observed in the present study, as previously found by Palmucci et al. (2011). In our study, the lipid content remained at the same level or even decreased after 5 days under phosphorus limitation (Table 3). In addition, proteins were significantly reduced after 4 days growth, probably due to the low levels of nitrogen. Thus, in accordance with the results for nutrient removal and biomass productivity, we recommend the harvesting of biomass on the fourth day of culturing, before lipids and proteins begin to decrease.
Information on the chemical composition of algal biomass is crucial to determine its potential application. For instance, due to their high heating value, the content of lipids and carbohydrates is an important indicator of whether a microalgal strain has the potential to be used as a feedstock for biofuel production. Both strains used in the current study contained low percentages of lipids and carbohydrates, which makes them unfeasible for biodiesel and bioethanol production. In addition to the low heating value, the high ash content makes this biomass unfeasible to be used in thermochemical processes such as pyrolysis and gasification (Wang et al. 2015). The high ash content is not convenient for thermochemical processes because it demands additional purification treatment of the final product (Ali et al. 2015). However, the low amounts of lipids (lower than 40 %) and carbohydrates make both microalgal biomasses suitable for AD, since this process can convert all fractions of organic matter, including lipids, proteins, carbohydrates, and nucleic acids to biofuel (Sialve et al. 2009; Bohutskyi and Bouwer 2013).
Although the potential of biogas production from AD of microalgal biomass is recognized (Bahr et al. 2014), it has received much less attention than the production of liquid biofuels derived from microalgae. Nevertheless, increased interest has occurred in the recent years (Murphy et al. 2015) due to the advantages of AD over alternative technologies. From a process engineering view, the increased interest in AD results from the fact that this technology does not require that biomass is dried before processing, as drying is a major bottleneck of microalgal-based renewable energy due to the large amount of energy it requires. In fact, in high-solid sludge digestion, methanogenic activity has been observed to decrease from 100 to 53 % when the moisture content decreased from 96 to 90 % (Mata-Alvarez et al. 2000). Furthermore, AD does not require pure cultures, nor is a specific fraction of the biomass an essential production objective (Murphy et al. 2015). Opposite to biodiesel, which requires high lipid contents (favored by nutritional stress and consequently reduced growth rate), selecting a particular microalgae species for biogas production should mainly target high specific growth rates. Furthermore, in industrial terms, AD of solid wastes can be seen as a mature technology (Mata-Alvarez et al. 2000).
Although AD is apparently the best strategy for energy production when the content in non-polar lipids is less than 40 % of dry biomass (Samori et al. 2013), enhancement of methane yield requires technological advances (Tan et al. 2015). Cultivation of microalgae requires the use of low energy intensive processing and substitution of process inputs (e.g., nutrients) by wastes. Hence, coupling wastewater treatment to biogas production has potential for positive economic outcomes (Bellou et al. 2014), but requires investigation of operational parameters to maximize performance.
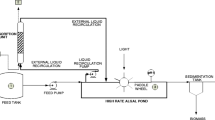
Based on the experimental results reported in Tables 2 and 3, inputs to the process simulation were specified from modeling of growth and densification steps (Fig. 3), with the process premises adopted shown in Online Resource 1. The biomass obtained from wastewater treatment (step 1) is fed to a tubular photobioreactor (PBR, step 2), with a volume to area ratio (V/A) of 100 m3 m−2, and a residence time adjusted to increase cell population yielding outlet concentration (set in this study to 5.32 g L−1), corresponding to a biomass productivity in the range 100–130 g m−2 day−1. The product from the PBR has its humidity adjusted to 96 % (step 3) and sent to anaerobic digestion (step 4, AD). AD is modeled as a perfectly mixed continuous flow-stirred tank reactor (CSTR) with HRT of ∼30 days and volumetric organic load of 1.4 g VS L−1 day−1, as reported in Bahr et al. (2014). The process simulation scheme using model molecules (Picardo et al. 2013) is shown in Fig. 4, and simulation results are presented in Table 4.
Utilization of microalgae for removal of N and P from wastewater coupled to biogas production. Q flow rate (L day−1), C biomass concentration (g L−1), W water removal flow rate (L day−1), V digestor volume of anaerobic digestor (L), HRT hydraulic retention time (days), Bv volumetric organic loading rate (g VS L−1 day−1), Q* feed to anaerobic digestor (L day−1), VS volatile solids (g L−1). Abbreviations and formulas presented in this figure are explained in Online Resource 1
Note that the conceptual design of the process dewatering in Fig. 3 was only applied when unavoidable since it is an energy-intensive operation that reduces the overall energy performance of the process. Nevertheless, it is recognized that the digestate is rich in nutrients and may contribute with macronutrients necessary for biomass growth, as previously reported (Bohutskyi et al. 2015b). Therefore, digestate utilization for nutrient supply should be explored. Hence, this dewatering stage is represented as a dashed box (step 5), and its use should be evaluated on economic grounds, beyond the scope of this work.
Methane yield is 11.5 % higher for Scenedesmus sp2 than for Desmodesmus sp. Furthermore, as a consequence of its higher growth rate, the total area of photobioreactor is reduced by 9.6 % with respect to the total area required by Desmodesmus sp. cultivation. Finally, the simulation results for methane yield (maximum theoretical yield calculated by Gibbs reactor model) from microalgal biomass without pretreatment are in line with those recently reported (Bohutskyi et al. 2015b), which are in the lower bound (0.3 sm3 CH4 kg−1 VS) of the yield range of 0.3–0.8 sm3 CH4 kg−1 VS expected for AD of microalgae (Gonzalez-Fernandez et al. 2015). It is worth noting that most of the studies on AD of microalgal biomass use distinct pretreatment methods to enhance biomass biodegradability. These techniques include thermal treatment or hydrolysis (acid, alkaline, or enzymatic), promoting cleavage of cellular components (biopolymers) into shorter carbon chain molecules, which are more promptly degradable, favoring the digestion yield. In the current study, the simulated results are based on original metabolic pool composition (i.e., without biomass compositional modifications) since any additional operation will impact capital and operational expenditures (CAPEX and OPEX), negatively influencing economic performance.
It should be noted that depending on the studied microalgae, nearly 30–60 % of N and P can be removed from microalgal biomass during AD (Bohutskyi et al. 2015b, 2015c) Thus, these remaining macronutrients in the digestate can be further used as bio-fertilizer. Furthermore, the high ash content recorded in the species may also contribute to the supply of mineral elements into the soil. Algal biomass contains nitrogen and phosphorus in organic and inorganic forms that release slowly when biomass is used as bio-fertilizer, unlike chemical fertilizers, which are quickly released and largely lost by evaporation and rain (Mulbry et al. 2006). In this context, microalgae offer the potential to recover nutrients from waste streams and subsequently use the microalgal biomass as a sustainable slow-release fertilizer (Coppens et al. 2016).
The elemental analysis of the algal biomass (Online Resources 2 and 3) confirms its use as bio-fertilizer, since it contains macronutrient (N, P, K, Ca, Mg, and S) and several micronutrients, such as Cu, Fe, Mn, and Zn, necessary for the growth of plants. According to the concentration of N, P, and K, the biomass of Scenedesmus sp2 and Desmodesmus sp. equals NPK fertilizer 6.5/2.2/0.6 and 7.3/2.5/0.8. On the other hand, concentrations of Pb, Cd, Cr, Ni, and Se were below the limits specified in the Normative instructions 27/ 2006 of the Ministry of Agriculture, Livestook and Supply (Ministério da Agricultura, Pecuária e Abastecimento), showing the convenience of the use this algal biomass as a fertilizer.
The use of bio-fertilizer can be an alternative to the shortage of phosphorus reserves that may impact future food production (Louro et al. 2012). The known rich-rock phosphate reserves are limited and its replacement cycle of nature is extremely slow. Considering the current phosphate consumption, it anticipates a shortage of these reserves over a period of about 100 years (Gilbert 2009). The use of algal biomass grown in wastewater as bio-fertilizer allows the recycling of nutrients (especially phosphorus) and may also contribute to decrease the chance of environmental eutrophication.
Conclusions
This work shows that the non-autoclaved effluent collected from a stabilization lagoon is able to support the growth of native microalgae, affording high growth rates and biomass yield. Satisfactory removal of N and P (more than 65 and 95 %, respectively) was obtained in short retention times (4 days). The biomass obtained from wastewater treatment was simulated to be increased in a tubular photobioreactor in order to be used for anaerobic digestion. This process was simulated by employing the Aspen HYSYS modeling software and the outcome these simulations shows that methane yield (0.26–0.29 sm3 CH4 kg−1 VS) is comparable to those found in the literature without biomass pretreatment. In addition, the high content of ash (14–20 %) in the biomass, containing very low amounts of heavy metals, makes the digestate of this biomass suitable for use as bio-fertilizer.
References
Ali SAM, Razzak SA, Hossain MM (2015) Apparent kinetics of high temperature oxidative decomposition of microalgal biomass. Bioresour Technol 175:569–577
AOAC (1990) Official methods of analysis, 15th edn. Association of Official Analytical Chemists, Washington
Bahr M, Díaz I, Dominguez A, Sánchez AG, Muñoz R (2014) Microalgal-biotechnology as a platform for an integral biogas upgrading and nutrient removal from anaerobic effluents. Environ Sci Technol 48:573–581
Bellou S, Baeshed MN, Elazzazy AM, Aggeli D, Sayegh F, Aggelis G (2014) Microalgal lipids biochemistry and biotechnology perspectives. Biotechnol Adv 32:1476–1493
Bohutskyi P, Bouwer E (2013) Biogas production from algae and cyanobacteria through anaerobic digestion: a review, analysis, and research needs. In: Lee JW (ed) Advanced biofuels and bioproducts. Springer, New York, pp. 873–975
Bohutskyi P, Liu K, Nasr LK, Byers N, Rosenberg JN, Oyler GA, Betenbaugh MJ, Bouwer EJ (2015a) Bioprospecting of microalgae for integrated biomass production and phytoremediation of unsterilized wastewater and anaerobic digestion centrate. Appl Microbiol Biotecnol 99:6139–6154
Bohutskyi P, Chow S, Ketter B, Adams KJ, Betenbaugh MJ, Bouwer EJ (2015b) Prospects for methane production and nutrient recycling from lipid extracted residues and whole Nannochloropsis salina using anaerobic digestion. Appl Energy 154:718–731
Bohutskyi P, Ketter B, Chow S, Adams KJ, Betenbaugh MJ, Allnutt T, Bouwer EJ (2015c) Anaerobic digestion of lipid-extracted Auxenochlorella protothecoides biomass for methane generation and nutrient recovery. Bioresour Technol 183:229–239
Borowitzka MA (2013) High value products from microalgae—their development and commercialization. J Appl Phycol 25:743–756
Borowitzka MA (2016) Algal physiology and large-scale outdoor cultures of microalgae. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Cham, pp. 601–652
Brazilian National Environmental Council (Conselho Nacional de Meio Ambiente - CONAMA) Resolution 430/2011. IOP Publishing PhysiscsWeb http://www.mmagovbr/conama. Acessed 15 Jan 2016
Carmo CAFS, Araújo WS, Bernardi ACC, Saldanha MFC (2000) Métodos de análise de tecidos vegetais utilizados na embrapa solos. Embrapa Solos. Rio de Janeiro. Circular Técnica n° 6. ISSN 1517–5146.
Cheng H, Tian G (2013) Identification of a newly isolated microalga from a local pond and evaluation of its growth and nutrients removal potential in swine breeding effluent. Desalin Water Treat 51:2768–2775
Clarens AF, Resurreccion EP, White MA, Colosi LM (2010) Environmental life cycle comparison of algae to other bioenergy feedstocks. Environ Sci Technol 44:1813–1819
Coppens J, Grunert O, Van Den Hende S, Vanhoutte I, Boon N, Haesaert G, De Gelder L (2016) The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. J Appl Phycol 28:2367–2377
Craggs R, Sutherland D, Campbell H (2012) Hectare-scale demonstration of high rate algal ponds for enhanced wastewater treatment and biofuel production. J Appl Phycol 24:329–337
Doria E, Longoni P, Scibilia L, Iazzi N, Cella R, Nielsen E (2012) Isolation and characterization of a Scenedesmus acutus strain to be used for bioremediation of urban wastewater. J Appl Phycol 24:375–383
Dubois M, Gilles KA, Hamilton JK, Reberts PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Erickson JR (1985) An evaluation of mathematical models for the effect of pH and temperature on ammonia toxicity to aquatic organisms. Water Res 19:1047–1058
Folch J, Lees M, Sloanne-Stanley GH (1957) A simple method for the isolation and purification of total lipid from animal tissue. J Biol Chem 226:497–509
García J, Green BF, Lundquist T, Mujeriego R, Hernández-Mariné M, Oswald WJ (2006) Long term diurnal variations in contaminant removal in high rate ponds treating urban wastewater. Bioresour Technol 97:1709–1715
Gilbert N (2009) The disappearing nutrient. Nature 461:716–718
Gonzalez-Fernandez C, Sialve B, Molinuevo-Salces B (2015) Anaerobic digestion of microalgal biomass: challenges opportunities and research needs. Bioresour Technol 198:896–906
Hernández D, Riaño B, García-González MC (2013) Treatment of agro-industrial wastewater using microalgae-bacteria consortium combined with anaerobic digestion of the produced biomass. Bioresour Technol 135:598–603
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Jia Q, Xiang W, Yang F, Hu Q, Tang M, Chen C, Wang G, Dai S, Wu H, Wu H (2016) Low-cost cultivation of Scenedesmus sp. with filtered anaerobically digested piggery wastewater: biofuel production and pollutant remediation. J Appl Phycol 28:727–736
Jiménez-Pérez V, Sánchez-Castillo P, Romera O, Moreno-Fernández D, Pérez-Martínez C (2004) Growth and nutrient removal in free and immobilized planktonic green algae isolated from pig manure. Enzym Microb Technol 34:392–398
Kiran B, Pathak K, Kumar R, Desmukh D (2014) Cultivation of Chlorella sp IM-01 in municipal wastewater for simultaneous nutrient removal and energy feedstock production. Ecol Eng 73:326–330
Koroleff F (1969) Direct determination of ammonia in natural waters as indophenol blue ICES C M C. Hydr Comm:9
Li WKW (2002) Macroecological pattern of phytoplankton in the northwestern North Atlantic Ocean. Nature 419:154–157
Li Y, ChenY-F CP, Min M, Zhou W, Martinez B, Zhu J, Ruan R (2011) Characterization of a microalga Chlorella sp well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour Technol 102:5138–5144
Liu K, Li J, Qiao H, Lin A, Wang G (2012) Immobilization of Chlorella sorokiniana GXNN 01 in alginate for removal of N and P from synthetic wastewater. Bioresour Technol 114:26–32
Liu C, Subashchandrabose S, Ming H, Xiao B, Naidu R, Megharaj M (2016) Phycoremediation of dairy and winery wastewater using Diplosphaera sp. MM1. J Appl Phycol. doi:10.1007/s10811-016-0894-4
Lourenço SO, Barbarino E, Lavín PL, Marquez UML, Aidar E (2004) Distribution of intracellular nitrogen in marine microalgae calculation of new nitrogen-to-protein conversion factors. Eur J Phycol 39:17–32
Louro CAL, Volschan I Jr, Ávila GM (2012) Sustentabilidade ambiental: estudo sobre o aproveitamento de nutrientes da urina humana para fins agrícolas. Revista eletrônica Sistema & Gestão 7(3):440–447
Lynch F, Santana-Sánchez A, Jämsä M, Sivonen K, Aro E-A, Allahverdiyeva Y (2015) Screening native isolates of cyanobacteria and a green alga for integrated wastewater treatment, biomass accumulation and neutral lipid production. Algal Res 11:411–420
Mahapatra D, Chanakya HN, Ramachandra TV (2013) Euglena sp a suitable source of lipids for potential use as biofuel and sustainable wastewater treatment. J Appl Phycol 25:855–865
Mata TM, Melo AC, Simões M, Caetano NS (2012) Parametric study of a brewery effluent treatment by microalgae Scenedesmus obliquus. Bioresour Technol 107:151–158
Mata-Alvarez J, Macé S, Llabrés P (2000) Anaerobic digestion of organic solid wastes an overview of research achievements and perspectives. Bioresour Technol 74:3–16
Ministry of Agriculture Livestook and Supply (Ministério da Agricultura Pecuária e Abastecimento) Normative instructions 27/2006. IOP Publishing Physiscs Web. http://www.agriculturagovbr. Acessed 15 January 2016
Moree AL, Bensen AHW, Bonwman AF, Willems WJ (2013) Exploring global nitrogen and phosphorus flows in urban wastes during the twentieth century. Glob Biogeochem Cycles 27:836–846
Mulbry W, Kondrad S, Pizarro C (2006) Biofertilizers from algal treatment of dairy and swine manure effluents: characterization of algal biomass as a slow release fertilizer. J Veg Sci 12:107–125
Muñoz R, Guieysse B (2006) Algal-bacterial processes for the treatment of hazardous contaminants: a review. Water Res 40:2799–2815
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Murphy JD, Drosg D, Allen E, Jerney J, Xia A, Herrmann C (2015) A perspective on algal biogás. . IEA Bioenergy. http://www.iea-biogas.net/files/daten-redaktion/download/Technical%20Brochures/AD_of_Algae_ebook_end.pdf. Acessed 10 February 2016
Myklestad S, Haug A (1972) Production of carbohydrates by the marine Chaetoceros affinis var willei (Gran) Hustedt I Effect of the concentration of nutrients in the culture medium. J Exp Mar Biol Ecol 9:125–136
Olsen LM, Sakshaug E, Johnsen G (2006) Photosynthesis-induced phosphate precipitation in seawater ecological implications for phytoplankton. Mar Ecol Prog Ser 319:103–110
Palmucci M, Ratti S, Giordano M (2011) Ecological and evolutionaty implications of carbon allocation in marine phytoplankton as a function of nitrogen availability: a Fourier transform infrared spectroscopy approach. J Phycol 47:313–323
Park KC, Whitney C, McNichol JC, Dickinson KE, MacQuarrie S, Skrupski BP, Zou J, Wilson KE, O’Leary SJB, McGinn PJ (2012) Mixotrophic and photoautotrophic cultivation of 14 microalgae isolates from Saskatchewan, Canada: potential applications for wastewater remediation for biofuel production. J Appl Phycol 24:339–348
Picardo MC, Medeiros JL, Monteiro JGM, Chaloub RM, Giordano M, Araújo OQF (2013) A methodology for screening of microalgae as a decision making tool for energy and green chemical process applications. Clean Technol Envir 15:275–291
Posadas E, Bochon S, Coca M, García-González PA, Muñoz R (2014) Microalgae-based agro-industrial wastewater treatment: a preliminary screening of biodegradability. J Appl Phycol 26:2335–2345
Renuka N, Sood A, Ratha SK, Prasanna R, Ahluwalia AS (2013) Evaluation of microalgal consortia for treatment of primary treated sewage effluent and biomass production. J Appl Phycol 25:1529–1537
Roopnarain A, Gray VM, Sym SD (2014) Phosphorus limitation and starvation effects on cells growth and lipid accumulation in Isochrysis galbana U4 for biodiesel production. Bioresour Technol 156:408–411
Samori G, Samori C, Guerrini F, Pistocchi R (2013) Growth and nitrogen removal capacity of Desmodesmus communis and of a natural microalgae consortium in a batch culture system in view of urban wastewater treatment: part I. Water Res 47:791–801
Sialve B, Bernet N, Bernard O (2009) Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotech Adv 27:409–416
Silva-Benavides AM, Torzillo G (2012) Nitrogen and phosphorus removal through laboratory batch cultures of microalga Chlorella vulgaris and cyanobacterium Planktothrix isothrix grown as monoalgal and as co-cultures. J Appl Phycol 24:267–276
Tan J, Wang J, Xue J, Liu S-Y, Peng S-C, Ma D, Chen T-H, Yue Z (2015) Methane production and microbial community analysis in the goethite facilitated anaerobic reactors using algal biomass Fuel. 145:196–201
Vasquez-Montiel O, Horan NJ, Mara DD (1996) Management of domestic wastewater for reuse in irrigation. Water Sci Technol 33:355–362
Wang S, Ru B, Lin H, Sun W, Luo Z (2015) Pyrolysis behaviors of four lignin polymers isolated from the same pine wood. Bioresour Technol 182:120–127
World Bank (2016). World development indicators 2016. http://data.worldbank.org/ Acessed 30 April 2016
Zar JH (1996) Biostatistical analysis, 3rd edn. Prentice Hall Inc, Upper Saddle River
Zhang L, Lu H, Zhang Y, Li B, Liu Z, Duan N, Liu M (2016) Nutrient recovery and biomass production by cultivating Chlorella vulgaris 1067 from four types of post-hydrothermal liquefaction wastewater. J Appl Phycol 28:1031–1039
Zhou W, Li Y, Min M, Hu B, Chen P, Ruan R (2011) Local bioprospecting for high-lipid producing microalgal strains to be grown on concentrated municipal wastewater for biofuel production. Bioresour Technol 102:6909–6919
Zhu CJ, Lee YK (1997) Determination of biomass dry weight of marine microalgae. J Appl Phycol 9:189–194
Zimmo OR, van der Steen NP, Gijzen HJ (2003) Comparison of ammonia volatilization rates in algae and duckweed-based waste stabilization ponds treating domestic wastewater. Water Res 37:4587–4594
Acknowledgments
We are indebted to the Moflo - Unidade multiusuários of the Federal University of Rio de Janeiro for making the flow cytometer available to us and to Dr. R. Pulleri for assistance with the nutrient determination techniques. Dr. A.G. Torres is gratefully acknowledged for providing facilities to determination of the biochemical composition of microalgal biomass. We thank Dr. Dgamar Frisch for the manuscript revision. This research received financial support from the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) through grant E-26/111.973/2012, from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) through grants 404778/2013-5 and 405851/2013-8 and from Fundação Coordenação de Projetos, Pesquisas e Estudos Tecnológicos (Fundação COPPETEC-COPPE/UFRJ). G.S. Diniz was a recipient of a fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 33 kb)
Rights and permissions
About this article
Cite this article
Diniz, G.S., Silva, A.F., Araújo, O.Q.F. et al. The potential of microalgal biomass production for biotechnological purposes using wastewater resources. J Appl Phycol 29, 821–832 (2017). https://doi.org/10.1007/s10811-016-0976-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-016-0976-3