Abstract
Releasing wastewater containing organic dyes into water bodies generates a variety of hazards for humans and marine life. Thus, developing effective adsorbents to remove organic dyes from wastewater is critical. Herein, a mixture of the orange peel (OP) and watermelon rind (WR) wastes was converted into a mesoporous activated carbon (OPWRAC) via microwave-induced ZnCl2. Several analytical techniques such as XRD, N2 adsorption–desorption isotherms, FTIR, pHpzc, and SEM–EDX were applied to characterize the physicochemical properties of OPWRAC. Subsequently, the adsorptive efficiency of OPWRAC was comprehensively explored towards the removal of two structurally different organic dyes, namely, crystal violet (CV) and methylene blue (MB). The operational adsorption conditions such as OPWRAC dose (0.04–0.1 g) coded as (A), solution pH (4–10) coded as (B), and contact time (10–60 min) coded as (C) were statistically optimized using the response surface methodology-Box-Behnken design (RSM-BBD). The adsorption isotherm data for CV and MB dyes agree with the Freundlich model, and the kinetic data can be explained by the pseudo-second-order model. Thus, OPWRAC displays remarkable adsorption capacity for capturing CV (137.8 mg/g) and MB (200.7 mg/g). The tailored adsorption mechanism of CV and MB by the OPWRAC indicates the involvement of several types of electrostatic forces, π-π stacking, pore filling, and H-bonding. The output of this research shows the feasibility of converting the mixture of OP and WR into promising activated carbon with potential application for capturing two structurally cationic dyes from an aqueous environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Water pollution is a globally observed phenomenon due to its negative effects on the ecosystem and human health. Water pollution is generated from discharging effluents related to industrial activities into freshwater without treatment [1]. Organic dyes (e.g., methylene blue (MB), crystal violet (CV)) are one the significant constituents of industrial effluents and considered one of the common water pollutants due to their harmful effects on the environment and human health, for instance, rapid heartbeat, nausea, mental confusion, shortness of breath, skin irritation, and diminishing photosynthesis [2, 3]. Therefore, it is crucial that organic dyes are removed from industrial wastewater. Organic dyes are typically removed from water using a variety of techniques, including ion exchange [4], electrochemical degradation [5], photocatalysis [6], adsorption [7], and coagulation/flocculation [8]. Most of these procedures are hindered by secondary waste, cost, or difficulty. The adsorption method has been identified as the optimum way to separate these contaminants owing to its amazing properties such as non-susceptibility to contaminants, reusability, lower waste production, low cost, and high efficiency [9].
Activated carbon (AC) has special characteristics, for instance, porosity, greater surface area, adsorption ability, plenty of functional groups, and thermal stability [10]. These characteristics make it an effective adsorbent in adsorption techniques to remove organic dyes [11]. However, commercial AC has a number of drawbacks, such as producing from nonrenewable precursors and high costs, which limit its applicability [12]. The availability of affordable and sustainable precursors is itself a major obstacle to the production of AC. In order to prepare AC from plentiful, affordable, ecological, and sustainable precursors, research efforts have increased [13]. Due to its renewability, availability, economic efficiency, and nontoxicity, the production of AC from lignocellulosic materials constitutes a viable option [14]. Numerous earlier literatures have reported the generation of AC using different lignocellulosic precursors, for instance, mango peels and seed wastes [15], Aesculus hippocastanum L. biomass [16], orange peel [17], hawthorn kernel [18], and Althaea officinalis L. [19].
Typically, a number of methods, including traditional pyrolysis and microwave radiation, can be used to create AC from lignocellulosic biomass [20]. The microwave heating approach used in this study has several benefits over conventional heating, including a large amount of desired product, improved heating efficiency, process control, and consistent heat distribution [21]. In the synthesis of AC, precursors are impregnated with activating chemicals (e.g., ZnCl2, H3PO4, KOH, and K2CO3), and carbonization and activation take place concurrently [22]. Among various chemical activators, ZnCl2 promotes fabricating AC with outstanding properties like pore volume, smaller pore size, and higher surface area, increasing the carbon yield, and plentiful reactive groups for the uptaking of pollutants [23]. Lately, generating AC from a variety of precursors using ZnCl2 as an effective chemical agent has been reported and the resultant AC has been successfully used to remove contaminants such as methylene blue dye [24], tetracycline hydrochloride [25], Cr (VI) ion [23], nitrate [26], and malachite green dye [27].
The goal of this study is to produce high-surface-area activated carbon (hereinafter, termed OPWRAC) from sustainable and affordable precursors (orange peels (OP), watermelon rind (WR)) via microwave-induced ZnCl2 activation. The generated OPWRAC was used to remove CV and MB dyes from aqueous systems. Box-Behnken design (BBD) was selected to establish the optimal conditions for CV and MB adsorption onto the OPWRAC surface. Kinetic and isotherm models were used to simulate the empirical adsorption data of the CV and MB dyes. A probable mechanism for the CV and MB adsorption on OPWRAC was outlined.
2 Materials and methods
2.1 Materials
The fruit biomass wastes orange peel (OP) and watermelon rind (WR) as a precursor of activated carbon were collected from fruit bars in Shah Alam city, Selangor state, Malaysia. For the adsorption study, the stranded solutions (1000 mg/L) for CV (λmax, 584 nm; MW, 408 g/mol; R&M Chemicals) and MB (λmax, 661 nm; MW, 319.86 g/mol; R&M Chemicals) were prepared and adjusted as needed. In all experiments, analytical-grade chemicals and reagents from R&M Chemicals, Malaysia, including hydroxide sodium (NaOH) pellets, zinc chloride (ZnCl2), sodium chloride (NaCl) powder, and hydrochloric acid (HCl) were supplied without additional purification.
2.2 OPWRAC preparation
The fruit biomass wastes (OP and WR) were thoroughly cleaned with hot distilled water to remove dust and other unwanted substances. Prior to the drying process by an oven, the samples (orange peel and watermelon rind) were left few days under sunlight for air-drying process. Then, the drying process using an oven was carried out at 100 °C for 24 h to remove the remaining moisture and to ensure a perfect drying process for the precursors. After that, the OPWR was crashed into powder form and sieved to uniform particles size (2 mm). In the activation process, 1 g of the precursor (OPWR, the mixing ratio of OP and WR was 1:1) and 2 g of chemical activator (ZnCl2) were mixed at a fixed impregnation ratio of 1:2 (this mixing ratio was predetermined to be the optimum mixing ratio). In this regard, it was proven in the literatures that the best impregnation mixing ratio of biomass wastes such as carrot juice pulp and pomegranate peel [7] and mango peels and seeds [15] with ZnCl2 chemical activator was 1:2 by using a microwave activation technique. Subsequently, the OPWR/ZnCl2 system was then heated for 24 h at 100 °C inside an oven to achieve complete drying. Thus, the activated OPWR was transferred to a specially designed microwave oven (SAMSUNG ME711K, 20 L) before being subjected to the microwave irradiation (800 W) for 20 min under pure N2 (99.99%) condition with a fixed flow rate of 100 mL/min (pre-identified as the optimum activation settings). After that, distilled water was used to wash the resultant OPWRAC to neutralize the pH of the solution. For drying purpose, the produced OPWRAC was placed inside an oven for 24 h at 100 °C. Then, the OPWRAC was sieved using 250-μm sieve to obtain a uniform particle size (250 μm), and it was then kept in a sealed container for subsequent use.
2.3 Characterization
The OPWRAC’s surface physical properties, such as its specific surface area and pore volume, were analyzed using the equipment (Micromeritics, ASAP 2060). Scanning electron microscopy (SEM, Zeiss Supra 40 VP) and energy dispersive X-ray (EDX) examination were used to characterize the surface topologies and the mineral composition of the OPWRAC before and after the adsorption of CV and MB. Using an X-ray polycrystal diffractometer (XRD, PANalytical X’Pert PRO), the crystalline nature of OPWRAC was examined in the 10–90° scanning range. All samples were examined between 4000 and 450 cm−1 using Fourier transform infrared (FTIR) spectra acquired from an FTIR spectrometer (Perkin-Elmer, Spectrum RX I) to characterize fundamental groups of OPWR and OPWRAC before and after the adsorption of CV and MB. A point of zero charge (pHpzc) method was employed to identify the charge of the OPWRAC surface [28].
2.4 Experimental design
For the optimization process, a Box-Behnken design (BBD) was used together with quadratic programming to determine the optimal operational conditions for CV and MB removal by OPWRAC within 3 input parameters (OPWRAC dose, solution pH, and time). The software Design Expert (13.0, Stat-Ease, USA) was used to construct the adsorption study and statistically interpret of the results. Table 1 illustrates 3 ranges (i.e., − 1, 0, and + 1) of explored factors, namely, OPWRAC dose (A), solution pH (B), and time (C). The preliminary tests were used to determine the coded values of the parameters as well as the parameters to be employed in the adsorption optimization. The second-degree polynomial employed for fitting the components (independent factors and MB removal) of the model is illustrated in Eq. (1).
where Y is reply; \({\beta }_{i}\) is linear impact; \({\beta }_{ij}\) is interaction impact; \({\beta }_{0}\) is intercept; and \({X}_{i}\) and \({X}_{j}\) are independent variables. The data of developed models along with responses (CV removal (%) and MB removal (%)) are given in Table 2. The adsorption study of the CV and MB was carried out by adding a specific dose of adsorbent to Erlenmeyer flasks containing 100 mL of the dye solution before being placed inside a thermostatic water bath shaker at a fixed shaking speed of 110 strokes per min. Then, adsorbent-free liquids were produced by passing the mixtures in a 0.45-μm Nylon syringe filter. The concentration of the CV and MB during the treatment process was spectrophotometrically assessed at λmax = 661 nm and at λmax = 584 nm, respectively, using a spectrophotometer (HACH DR 3900). Then, the dye removal efficiency (R, %) of CV and MB dyes was determined by using Eq. (2).
where \({C}_{o}\) (mg/L) and \({C}_{e}\) (mg/L) represent the concentrations of the adsorbates (CV and MB) in their initial and equilibrium states, respectively.
2.5 Adsorption study of CV and MB by OPWRAC
The adsorption study of the MB and CV dyes onto the surface of OPWRAC was carried out in batch mode at the following operational conditions: OPWRAC dosage (0.1 g), solution pH (10), and time (35 min). These operational conditions were found to be the best conditions to achieve the highest removal of CV (91.6%) and MB (94.1%), according to the BBD model (run no. 4 in Table 2). Thus, the adsorption equilibrium experiments were conducted with these optimum input variables throughout a range (20–200 mg/L) of starting CV and MB concentrations. The same methodology outlined in the Sect. 2.4 was used to carry out batch adsorption testing for MB and CV dyes. The following mathematical formula (3) was applied to calculate the equilibrium adsorption quantity (qe, mg/g) of the OPWRAC:
where W (g) represents the OPWRAC's weight and V (L) refers to the volume of the dye solution.
3 Results and discussion
3.1 Characterization of OPWRAC
The surface area and porosity of the OPWRAC are necessary aspects in the adsorption of CV and MB dyes. The specific surface area and pore properties of OPWRAC are stated in Table 3. According to calculations, OPWRAC’s total pore volume and specific surface area are 0.49 cm3/g and 661.3 m2/g, respectively. This finding (total pore volume) indicates the formation of pores during the activation procedure with the chemical agent ZnCl2 [23]. According to IUPAC [29], the OPWRAC has a mesoporous structure with pores ranging in size from 2.0 to 50 nm, as demonstrated by the findings of the mean pore diameter (4.57 nm). The N2 adsorption/desorption isotherms of the OPWRAC are displayed in Fig. 1 together with the related BJH (Barrett, Joyner, Halenda) pore size distributions (insert). The isotherms show that the OPWRAC has a mesoporous structure since they match the type-IV isotherm [29]. Additionally, the BJH desorption curve demonstrated that the OPWRAC had a broad pore width distribution in the mesoporous region. The hysteresis loop seen in Fig. 1 could be categorized as type H4, revealing the presence of mesopores in the OPWRAC structure [30]. The fact that ZnCl2 and its hydrates had minuscule particle sizes may help to explain why most of the holes created in the OPWRAC prepared with the ZnCl2 agent were quite small [31]. In this respect, high surface area and porous activated carbon materials were produced from biomass wastes such as carrot juice pulp and pomegranate peel (1202.2 m2/g) [7] and mango peels and seeds (1151.6 m2/g) [15] using microwave-assisted ZnCl2 activation. According to the above data, the obtained OPWRAC possesses a great degree of porosity, a significant pore volume, and a sizable specific surface area; it provides a considerable number of active sites which may bind with CV and MB molecules effectively.
In order to figure out the phase structure of OPWRAC, XRD analysis was utilized. The XRD pattern of OPWRAC is presented in Fig. 2. A broad peak at 2θ of 24° and a sharp peak at 2θ of 44.2° in the XRD pattern of OPWRAC were associated with the (002) and (100) reflection planes of the graphitic structure of OPWRAC, respectively [32]. It can also be demonstrated that OPWRAC displays significant peaks at 2θ = 31.9° (100), 34.5° (002), 36.3° (101), 56.7° (110), and 66.5° (112), which are related to the action of an activator (ZnCl2) during the production of OPWRAC and correlate to ZnO (JCPDS file no. 01–079-0205) [33].
To gain knowledge about the functional groups present in the OPWR, OPWRAC, OPWRAC-CV, and OPWRAC-MB, FTIR spectroscopic analysis was employed. In Fig. 3, the FTIR spectra of OPWR, OPWRAC, OPWRAC-CV, and OPWRAC-MB are exhibited. The OPWR spectrum (Fig. 3a) exhibits absorption bands in the 3300–3800 cm−1 range, which is ascribed to the O–H stretching vibrations of the cellulose, hemicellulose, pectin, and lignin [34]. Various peaks were also noticed at 2923 cm−1, 2341 cm−1, 1650 cm−1, 1450 cm−1, and 1027 cm−1, which are ascribed to the C-H vibrations in –CH3 and -CH2- groups, alkyne group (C≡C), carbonyl C = O groups, stretching vibration of C = C band, and C-O stretching, respectively [34, 35]. Comparing the FTIR spectra of OPWRAC to OPWR reveals modifications or shifts in the strength of several peaks, demonstrating the decomposition of surface groups throughout the carbonization and activation steps. The FTIR spectra of OPWRAC-CV (following CV adsorption on the OPWRAC surface) and OPWRAC-MB (following MB adsorption on the OPWRAC surface) showed spectra that were relatively similar to OPWRAC with significant alterations in several bands, demonstrating that the OPWRAC’s functional groups participated in the adsorption of CV and MB.
SEM–EDX analysis was used to analyze the morphological characteristics and elemental constitution of OPWRAC, OPWRAC-CV, and OPWRAC-MB. The findings of the SEM images and EDX assessments for OPWRAC, OPWRAC-CV, and OPWRAC-MB are illustrated in Fig. 4a–c. According to Fig. 4a, the surface of OPWRAC is uneven, diverse, and rough. The results of the EDX analysis of OPWRAC show that C, O, and N elements are present. After the adsorption of CV (Fig. 4b) and MB (Fig. 4c), the morphological structure of OPWRAC was dramatically changed into a smoother one in line with the adsorption of CV and MB species on the OPWRAC surface. C, O, and N are present in the EDX data for OPWRAC-CV, whereas C, O, N, and S are present in the EDX data for OPWRAC-MB. The S element’s existence is linked to the composition of the MB dye.
3.2 Statistical evaluation
ANOVA test was used to analyze the connection between the predicted and empirical data and the finesse of the derived second-order mathematical model. The outcomes of the ANOVA for MB removal are mentioned in Table S1. The F-values of the CV removal and MB removal were 102.27 and 110.55, respectively, stating the possibility of employing the model for assessing the relation between the analyzed and experimental data [36]. The models’ relevance and the fact that the calculated and observed removal data of CV and MB dyes are fully consistent were outlined by the high R2 values of CV dye removal (R2 = 0.99) and MB dye removal (0.99). The developed models are also logical because the deviations between the adjusted R2 (0.98 for both CV and MB removal models) and predicted R2 (0.95 for CV removal model and 0.96 for MB removal model) values were 0.03 and 0.02, respectively [37]. The expected models’ validity was confirmed by the negligible p values (lack of fit), which were 0.7641 and 0.6982 for CV removal and MB removal, respectively [37]. The characteristics of the proposed model are presumed to be statistically relevant if p ˂ 0.05 and vice versa. The following codes A, B, C, AC, A2, B2, and C2 are considered statistically influencing CV removal, whereas the following codes A, B, C, AB, BC, A2, and C2 are considered statistically influencing MB removal. By estimating the most significant input variables, the quadratic Eqs. (4, 5) of the CV removal and MB removal were created.
The normal probability of the CV removal and MB removal models, respectively, is shown as graphs in Fig. S1a and b. The dots (Fig. S1a and b) appear to be tightly fitted with the single line, highlighting that the model and the ANOVA findings are adequate and acceptable [38]. It also can be concluded from Fig. S1(a, b) that the residuals are independent. The diagrams show that the relationship between the predicted and actual values of CV and MB dye removal are outlined in Fig. S1c and d. The strong correlation between the observational outcomes (CV removal and MB removal) and those that are theoretically estimated is clearly seen in Fig. S1c and d, demonstrating the validity of the constructed models from the statistical perspective.
3.3 Dual effects of variables
3D response surface curves were generated to evaluate the influence of the input variables and to identify the key interactions between the input variables on the CV and MB removal processes. The dual effect of the OPWRAC dose and time on the CV removal rate is shown in Fig. 5a (3D plot), while the pH (7) was maintained constant. The results shown in Fig. 5a show that increasing the OPWRAC dose from 0.04 g to 0.1 g improved the removal efficiency of MB. Thus, this trend is caused by excessive surface area and effective adsorption sites of OPWRAC with higher OPWRAC doses. The magnitude of CV removal increased with higher time (60) according to Fig. 5a. This is a result of the CV molecules needing enough time to pass into the OPWRAC pores and bind on the OPWRAC surface and reaching to the equilibrium time.
The dual effect of the OPWRAC dose and pH on the MB removal rate is shown in Fig. 5b, while the time (35 min) was maintained constant. From the findings displayed in Fig. 5b, the adsorption effectiveness of MB was greatly enhanced at pH 10. In this regard, the pHpzc characteristic of OPWRAC and the preferred type (anionic or cationic) of the MB to OPWRAC surface may be used to analyze the influence of pH on MB adsorption. The pHpzc of OPWRAC was 6.9, as shown in Fig. 5c. As a result, at a high basic pH value (10) above pHpzc, the OPWRAC surface and MB dye molecules are negatively and positively charged. As a result, as shown in the formula below, this amplifies OPWRAC’s attention to binding positively charged MB molecules and enhances MB dye absorption (6).
The dual effect of the pH and time on the MB removal rate is shown in Fig. 5d, while the OPWRAC dose (0.07 g) was maintained constant. Figure 5d demonstrates that the OPWRAC’s adsorption effectiveness of the MB dye was very close at different adsorption times (10–60 min). This observation is related to the fact that the OPWRAC’s active sites were mainly occupied at 35 min.
3.4 Adsorption study
The adsorption capacity of the OPWRAC is influenced by changing the initial concentration (20–200 mg/L) of the CV and MB solutions, as shown in Fig. 6a and b. During this experiment, 0.1 g of OPWRAC adsorbent was used, and the solution pH was set at 10. As a result of increasing the initial CV and MB concentration from 20 to 200 mg/L, the adsorption capacity of OPWRAC was dramatically increased (16.83 to 133.98 mg/g for CV; 13.72 to 158.16 mg/g for MB). This observation can be assigned to the relatively high concentration gradient, which boosts dye mobility inside the OPWRAC’s internal pores and causes the dye to transfer to the effective surface of the OPWRAC [39].
3.5 Adsorption kinetics
The scale-up and technological development of adsorptive removal of CV and MB utilizing OPWRAC benefit greatly from the data given by the kinetic analysis. The investigational adsorption data were used to assess kinetic models such as pseudo-first order [40] and pseudo-second order [41]. Table 4 shows the formulae for each of these models, whereas Table 5 offers the kinetic parameter data. It was found that the relatively high R2 values (see Table 5) appeared in the PSO model rather than the PFO model and the calculated values of qe for the PSO kinetic model were remarkably similar to the experimental values of qe, indicating that the PSO model can reasonably explain the adsorption of CV and MB on the OPWRAC. This result denotes that CV and MB adsorption is attributed to the chemisorption process resulting from the interactions between the cationic groups of CV and MB with the anionic groups of OPWRAC [42].
3.6 Adsorption isotherms
Adsorption isotherms were used to assess the nature of interfacial contact between the CV/MB molecules and OPWRAC surface and the OPWRAC’s maximal adsorptive capacity. The adsorption of CV and MB on OPWRAC was explored in-depth with the Langmuir [43], Freundlich [44], and Temkin [45] isotherms. The formula for every one of these models is described in Table 4. As indicated in Table 7 and Fig. 7a and b, parameters and diagrams of isotherm models were generated and reported. The Freundlich model is well-matched with the empirical adsorption data of CV and MB based on the R2 values depicted in Table 6. This finding illustrates that the CV and MB molecules were adsorbed as a multilayer on the heterogeneous surface of the OPWRAC [46]. Additionally, 137.8 mg/g and 200.7 mg/g are the determined adsorption capacities of OPWRAC towards CV and MB, respectively. The reported adsorption capacities of OPWRAC and other adsorbents for CV and MB adsorption are shown in Table 7. The findings demonstrate the possibility and applicability of converting the fruit biomass wastes into mesoporous activated carbon (OPWRAC) with highly preferable adsorptive capacity towards various cationic dyes.
3.7 Tailored adsorption mechanism of CV and MB dyes
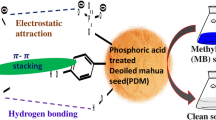
The key interactions listed in the literature as leading to the adsorption of organic dyes on activated carbons include electrostatic interaction, pore-filling, π-π interaction, and hydrogen bonding [55]. These interactions may all be involved in the adsorption process of CV and MB by OPWRAC as shown in Fig. 8. The electrostatic interactions between the CV and MB cations and the negatively charged surface of the OPWRAC considerably accelerated the CV and MB adsorption process at a pH higher than the pHpzc of the OPWRAC, indicating that this parameter constituted a feasible path for CV and MB adsorption onto the OPWRAC. The interaction of nitrogen atoms of adsorbates (CV and MB) with free hydrogen atoms of adsorbent (OPWRAC) produces H-bonding interactions, which contribute to the adsorption of the CV and MB dyes. Furthermore, based on BET and SEM examinations of the OPWRAC, which supports its mesoporous structure, CV and MB molecules have molecular sizes of 1.4 nm and 1.43 nm [32, 56], respectively, and may potentially occupy the mesopores in the OPWRAC structure. Lastly, CV and MB contain an aromatic backbone that interacts with OPWRAC’s hexagonal structure to make π-π stacking.
4 Conclusion
A mesoporous activated carbon (OPWRAC) was successfully produced from lignocellulosic biomass (OP and WR) wastes via microwave-induced ZnCl2 activation at microwave irradiation (800 W) for 20 min with a constant ratio of impregnation (1 g OPWR:2 g ZnCl2). The BBD model identified that the combinations of the OPWRAC dose (0.1 g), pH (10), and time (35 min) resulted in the highest removal of CV (91.6%) and MB (94.1%). The adsorption process of CV and MB dyes was multilayer adsorption, and it happens via the chemisorption route, according to the kinetic and equilibrium data of CV and MB. Thus, OPWRAC shows remarkable adsorption capacity towards both CV (137.8 mg/g) and MB (200.7 mg/g). Furthermore, the CV and MB adsorption by the OPWRAC is controlled by several mechanisms, comprising electrostatic forces, π-π stacking, pore filling, and H-bonding. This study reveals that the OPWRAC synthesized from OP and WR has the possibility to be a promising adsorbent for the treatment of dye-containing effluents.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Wang Z, Zhang Y, Kang S, Yang L, Shi H, Tripathee L, Gao T (2021) Research progresses of microplastic pollution in freshwater systems. Sci Total Environ 795:148888–148899
Tian T, Liu M, Li Y, Han J, Ren L, Lorenz H, Chen J (2022) β-Cyclodextrin carbon-based nanoparticles with a core–shell–shell structure for efficient adsorption of crystal violet and bisphenol A. Particuol 62:88–97
Temel F, Turkyilmaz M, Kucukcongar S (2020) Removal of methylene blue from aqueous solutions by silica gel supported calix [4] arene cage: investigation of adsorption properties. Eur Polym J 125:109540–109551
Zaharia MM, Vasiliu AL, Trofin MA, Pamfil D, Bucatariu F, Racovita S, Mihai M (2021) Design of multifunctional composite materials based on acrylic ion exchangers and CaCO3 as sorbents for small organic molecules. React Funct Polym 166:104997–105012
de Toledo WDMC, Pinheiro RA, Trava-Airoldi VJ, Corat EJ (2022) Development of boron-doped diamond (BDD) deposited on carbon nanotubes (CNT) to form BDD/CNT structures relevant for electrochemical degradation. Diamond Relat Mater 127:109159–1109170
Jawad AH, Nawi MA (2012) Characterizations of the photocatalytically-oxidized cross-linked chitosan-glutaraldehyde and its application as a sub-layer in the TiO2/CS-GLA bilayer photocatalyst system. J Polym Environ 20(3):817–829
Suhaimi A, Abdulhameed AS, Jawad AH, Yousef TA, AlDuaij OK, ALOthman ZA, Wilson LD (2022) Production of large surface area activated carbon from a mixture of carrot juice pulp and pomegranate peel using microwave radiation-assisted ZnCl2 activation: an optimized removal process and tailored adsorption mechanism of crystal violet dye. Diamond Relat Mater 130:109456–109467
Ihaddaden S, Aberkane D, Boukerroui A, Robert D (2022) Removal of methylene blue (basic dye) by coagulation-flocculation with biomaterials (bentonite and Opuntia ficus indica). J Water Process Eng 49:102952–102964
Reshadi MAM, Bazargan A, McKay G (2020) A review of the application of adsorbents for landfill leachate treatment: focus on magnetic adsorption. Sci Total Environ 731:138863–138878
Koyuncu F, Güzel F, İnal İIG (2022) High surface area and supermicroporous activated carbon from capsicum (Capsicum annuum L.) industrial processing pulp via single-step KOH-catalyzed pyrolysis: production optimization, characterization and its some water pollutants removal and supercapacitor performance. Diamond Relat Mater 124:108920–108933
Sultana M, Rownok MH, Sabrin M, Rahaman MH, Alam SN (2022) A review on experimental chemically modified activated carbon to enhance dye and heavy metals adsorption. Clean Eng Technol 6:100382–100396
Jahan K, Singh V, Mehrotra N, Rathore K, Verma V (2021) Development of activated carbon from KOH activation of pre-carbonized chickpea peel residue and its performance for removal of synthetic dye from drinking water. Biomass Conv Bioref https://doi.org/10.1007/s13399-021-01938-4.
Gayathiri M, Pulingam T, Lee KT, Sudesh K (2022) Activated carbon from biomass waste precursors: factors affecting production and adsorption mechanism. Chemosphere 294:133764–133776
Hoang AT, Kumar S, Lichtfouse E, Cheng CK, Varma RS, Senthilkumar N, Nguyen XP (2022) Remediation of heavy metal polluted waters using activated carbon from lignocellulosic biomass: an update of recent trends. Chemosphere 302:134825–134848
Razali NS, Abdulhameed AS, Jawad AH, ALOthman ZA, Yousef TA, Al-Duaij OK, Alsaiari NS (2022) High-surface-area-activated carbon derived from mango peels and seeds wastes via microwave-induced ZnCl2 activation for adsorption of methylene blue dye molecules: statistical optimization and mechanism. Mol. 27(20):6947–6965
Isinkaralar K, Gullu G, Turkyilmaz A (2022) Experimental study of formaldehyde and BTEX adsorption onto activated carbon from lignocellulosic biomass. Biomass Conver Bio https://doi.org/10.1007/s13399-021-02287-y
Jimoh OS, Ibrahim AO, Bello OS (2022) Metformin adsorption onto activated carbon prepared by acid activation and carbonization of orange peel. Int J Phyto https://doi.org/10.1080/15226514.2022.2064815
Zhen XT, Yu YL, Shi MZ, Zhu SC, Yan TC, Yue ZX, Cao J (2022) Activated carbon derived from hawthorn kernel waste for rapid adsorption of fungicides. Surf Interfaces 28:101700–101713
Isinkaralar K (2022) High-efficiency removal of benzene vapor using activated carbon from Althaea officinalis L. biomass as a lignocellulosic precursor. Environ Sci Pollut Res 29:66728–66740
Rashidi NA, Chai YH, Ismail IS, Othman MFH, Yusup S (2022) Biomass as activated carbon precursor and potential in supercapacitor applications. Biomass Conver. Bio https://doi.org/10.1007/s13399-022-02351-1
Hijab M, Parthasarathy P, Mackey HR, Al-Ansari T, McKay G (2021) Minimizing adsorbent requirements using multi-stage batch adsorption for malachite green removal using microwave date-stone activated carbons. Chem Eng Process-Process Intensif 167:108318–108329
Şahin Ö, Saka C, Ceyhan AA, Baytar O (2015) Preparation of high surface area activated carbon from Elaeagnus angustifolia seeds by chemical activation with ZnCl2 in one-step treatment and its iodine adsorption. Sep Sci Technol 50(6):886–891
Luo X, Cai Y, Liu L, Zeng J (2019) Cr (VI) adsorption performance and mechanism of an effective activated carbon prepared from bagasse with a one-step pyrolysis and ZnCl2 activation method. Cellulose 26(8):4921–4934
Yağmur HK, Kaya İ (2021) Synthesis and characterization of magnetic ZnCl2-activated carbon produced from coconut shell for the adsorption of methylene blue. J Mol Struct 1232:130071–130083
Cai Y, Liu L, Tian H, Yang Z, Luo X (2019) Adsorption and desorption performance and mechanism of tetracycline hydrochloride by activated carbon-based adsorbents derived from sugar cane bagasse activated with ZnCl2. Molecules 24(24):4534–4551
Nassar H, Zyoud A, El-Hamouz A, Tanbour R, Halayqa N, Hilal HS (2020) Aqueous nitrate ion adsorption/desorption by olive solid waste-based carbon activated using ZnCl2. Sustain Chem Pharm 18:100335–100344
Jayabalakrishnan RM, Maheswari M, Boomiraj K, Oumabady S (2021) Coconut shell derived ZnCl2 activated carbon for malachite green dye removal. Water Sci Technol 83(5):1167–1182
Dalvand A, Nabizadeh R, Ganjali MR, Khoobi M, Nazmara S, Mahvi AH (2016) Modeling of Reactive Blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: optimization, reusability, kinetic and equilibrium studies. J Magnet Magnet Mater 404:179–189
Sing KS (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem 57(4):603–619
Sayğılı H, Güzel F (2016) High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: process optimization, characterization and dyes adsorption. J Clean Prod 113:995–1004
Goncalves M, Castro CS, Boas IK, Soler FC, Pinto EDC, Lavall RL, Carvalho WA (2019) Glycerin waste as sustainable precursor for activated carbon production: adsorption properties and application in supercapacitors. J Environ Chem Eng 7(3):103059–103070
Dao MU, Le HS, Hoang HY, Tran VA, Doan VD, Le TTN, Sirotkin, (2021) A Natural core-shell structure activated carbon beads derived from Litsea glutinosa seeds for removal of methylene blue: facile preparation, characterization, and adsorption properties. Environ Res 198:110481–110493
Hadi S, Taheri E, Amin MM, Fatehizadeh A, Lima EC (2021) Fabrication of activated carbon from pomegranate husk by dual consecutive chemical activation for 4-chlorophenol adsorption. Environ Sci Pollut Res 28(11):13919–13930
Köseoğlu E, Akmil-Başar C (2015) Preparation, structural evaluation and adsorptive properties of activated carbon from agricultural waste biomass. Adv Powder Technol 26(3):811–818
Masoudian N, Rajabi M, Ghaedi M (2019) Titanium oxide nanoparticles loaded onto activated carbon prepared from bio-waste watermelon rind for the efficient ultrasonic-assisted adsorption of Congo red and phenol red dyes from wastewaters. Polyhedron 173:114105–114114
Jawad AH, Abdulhameed AS, Wilson LD, Hanafiah MAKM, Nawawi WI, ALOthman ZA, Rizwan Khan M (2021) Fabrication of Schiff’s base chitosan-glutaraldehyde/activated charcoal composite for cationic dye removal: optimization using response surface methodology. J. Polym. Environ. 29(9):2855–2868
Kutluay S, Temel F (2021) Silica gel based new adsorbent having enhanced VOC dynamic adsorption/desorption performance. Colloids Surf A: Physicochem Eng Asp 609:125848–125862
Marichamy MK, Kumaraguru A, Jonna N (2021) Particle size distribution modeling and kinetic study for coagulation treatment of tannery industry wastewater at response surface optimized condition. J Clean Prod 297:126657–126673
Jawad AH, Abdulhameed AS, Hanafiah MAKM, ALOthman ZA, Khan MR, Surip SN (2021) Numerical desirability function for adsorption of methylene blue dye by sulfonated pomegranate peel biochar: modeling, kinetic, isotherm, thermodynamic, and mechanism study. Korean. J. Chem. Eng. 38(7):1499–1509
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Vet Akad Handl 24:1–39
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Theamwong N, Intarabumrung W, Sangon S, Aintharabunya S, Ngernyen Y, Hunt AJ, Supanchaiyamat N (2021) Activated carbons from waste Cassia bakeriana seed pods as high-performance adsorbents for toxic anionic dye and ciprofloxacin antibiotic remediation. Bioresour Technol 341:125832–125842
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Temkin MI (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta physiochim URSS 12:327–356
Saleem A, Hussain A, Chaudhary A, Ahmad QA, Iqtedar M, Javid A, Muhammad Akram A (2022) Acid hydrolysis optimization of pomegranate peels waste using response surface methodology for ethanol production. Biomass Conv Bioref 12:1513–1524
Oni BA, Olawole OC, Ayeni AO, Sanni SE (2020) African star apples whole seed activated carbon powder as a bio-adsorbent of crystal violet dye removal from aqueous solution. Water Conser Sci Eng 5(1):97–114
Dil EA, Ghaedi M, Ghaedi A, Asfaram A, Jamshidi M, Purkait MK (2016) Application of artificial neural network and response surface methodology for the removal of crystal violet by zinc oxide nanorods loaded on activate carbon: kinetics and equilibrium study. J Taiwan Inst Chem Eng 59:210–220
Yusuff AS, Ajayi OA, Popoola LT (2021) Application of Taguchi design approach to parametric optimization of adsorption of crystal violet dye by activated carbon from poultry litter. Sci African 13:00850–0063
Foroutan R, Peighambardoust SJ, Peighambardoust SH, Pateiro M, Lorenzo JM (2021) Adsorption of crystal violet dye using activated carbon of lemon wood and activated carbon/Fe3O4 magnetic nanocomposite from aqueous solutions: a kinetic, equilibrium and thermodynamic study. Mol 26(8):2241–2260
Tuli FJ, Hossain A, Kibria AF, Tareq ARM, Mamun SM, Ullah AA (2020) Removal of methylene blue from water by low-cost activated carbon prepared from tea waste: a study of adsorption isotherm and kinetics. Environ Nanotechnol Monit Manage 14:100354–100362
da Silva AI, Paranha G, Maia LS, Mulinari DR (2021) Development of activated carbon from pineapple crown wastes and its potential use for removal of methylene blue. J Nat Fibers 1–16.
Misran E, Bani O, Situmeang EM, Purba AS (2022) Banana stem based activated carbon as a low-cost adsorbent for methylene blue removal: isotherm, kinetics, and reusability. Alex Eng J 61(3):1946–1955
Abbas M, Trari M (2020) Removal of methylene blue in aqueous solution by economic adsorbent derived from apricot stone activated carbon. Fibers Polym 21(4):810–820
Mahapatra U, Chatterjee A, Das C, Manna AK (2021) Adsorptive removal of hexavalent chromium and methylene blue from simulated solution by activated carbon synthesized from natural rubber industry biosludge. Environ Technol Innova 22:101427–101408
Wathukarage A, Herath I, Iqbal MCM, Vithanage M (2019) Mechanistic understanding of crystal violet dye sorption by woody biochar: implications for wastewater treatment. Environ Geochem Health 41(4):1647–1661
Funding
The authors extend their appreciation to the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) for funding and supporting this work through Research Partnership Program no. RP-21–09-73. The author (Norah Salem Alsaiari) would like to thank for the support given by Princess Nourah bint Abdulrahman University Researchers Supporting Project Number PNURSP2022R19, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Nurul Afiqah Mohd Hanafi: formal analysis, validation, data curation. Ahmed Saud Abdulhameed: formal analysis, validation, data curation, and writing original draft. Ali H. Jawad: conceptualization, methodology, software, supervision, project administration, writing, reviewing, and editing. Zeid A. ALOthman: formal analysis and validation. Tarek A. Yousef: validation and funding acquisition. O.K. Alduaijd: formal analysis and funding acquisition. Norah Salem Alsaiarie: funding acquisition and validation.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hanafi, N.A.M., Abdulhameed, A.S., Jawad, A.H. et al. Optimized removal process and tailored adsorption mechanism of crystal violet and methylene blue dyes by activated carbon derived from mixed orange peel and watermelon rind using microwave-induced ZnCl2 activation. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03646-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03646-z