Abstract
Dyes are colored compounds which are visible even at trace concentrations. Due to their recalcitrance and esthetic persistence, certain methods are unable to effectively eliminate them. So far, adsorptive treatment using activated carbons (ACs) is one of the most successful methods. In this study, we have employed orange peel (OP) as a cost-effective alternative to the expensive coal- and coir-based precursors to synthesize ACs for cationic methylene blue (MB) and anionic methyl orange (MO) dye adsorption. The pre-carbonized OP was activated via H2SO4, NaOH, KOH, ZnCl2, and H3PO4 to study the effects of activation reagents on dye removal efficiencies and mechanisms. Among several isotherm models employed to fit the adsorption data, the Langmuir and Sips models sufficiently estimated the maximum equilibrium uptakes close to the experimental values of 1012.10 ± 29.13, 339.82 ± 6.98, and 382.15 ± 8.62 mg/g, for ZnCl2-AC (MO), ZnCl2-AC (MB), and KOH-AC (MB), respectively. The adsorption mechanisms were suggested to involve electrostatic binding, pi–pi interactions, hydrogen bonding, and electron donor–acceptor reactions. Consequently, more than 99% removal efficiency was achieved from a laboratory organic wastewater sample bearing ~ 35 mg/L of MB. The results thus suggest that the synthesized ACs from agricultural waste have the tendencies to be applied to real dye wastewater treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dyes including methylene blue (MB) and methyl orange (MO) are high-intensity colored compounds with pronounced visibilities even at very low concentrations, which make them undesirable when present in effluents (Adegoke and Bello 2015; Vijayaraghavan and Yun 2007; Kelm et al. 2019). Most industries, especially the textile, lithography, coating, pulp mill, and cosmetic industries, use dyes and pigments to color a wide range of products. It is estimated that an average of over 700,000 tons of more than 100,000 different kinds of commercially available dyes are produced on yearly basis (Li et al. 2013). The wastewaters discharged from these industries are thus laden with dyes and pigments which range between 5 and 1500 mg/L mean concentrations and tend to contaminate any water bodies that they come into contact with (Kim et al. 2015; Li et al. 2013).
Dye contamination of water resources has both esthetic and health effects as it may affect the photosynthetic activities of aquatic living things and cause cancer in most biological species including human beings (Adegoke and Bello 2015; Ngulube et al. 2017). This is because some dyes and their degraded products or intermediaries are not easily biodegradable. The removal of dyes from contaminated effluents is thus a major environmental concern that requires utmost attention. However, the decolorization of dye-contaminated effluents is problematic due to the difficulty in treating such wastewaters by some conventional treatment methods (Adegoke and Bello 2015; Vargas et al. 2011). The most commonly used treatment methods for dye wastewaters include biological oxidation and chemical precipitation techniques, which are expensive and require high concentrations of dyes for effective treatment. Adsorptive treatment has been identified as one of the best alternatives owing to its simplicity, low-cost, environmental benignity, and the ability to function effectively even at very low dye concentrations (Li et al. 2013; Vargas et al. 2011). Moreover, several classes of adsorbents including agricultural, microbial, synthetic polymer, carbon, clay, and zeolite-based adsorbents are available to choose from (Kim et al. 2015; Li et al. 2013; Sarkar et al. 2016; Vijayaraghavan and Yun 2007).
Adsorption using activated carbons (ACs) is one of the most widely known options; however, a major issue confronting its patronage is the high costs of commercial ACs which are usually sourced from coal, coir, and petroleum residues (Girods et al. 2009; Tan et al. 2008; Yagsi 2004; Yahya et al. 2015). Recent studies have suggested the use of agro-waste-derived ACs as cheaper alternatives. This proposal has received considerable acceptance owing to the ready availability and abundance of agro-waste precursors such as fruit peels, shells, nuts, and seeds (Vargas et al. 2011; Yahya et al. 2015). However, physicochemical characterizations providing sufficient insights into the biomass conversion processes and dye adsorption mechanisms have been seldom discussed in details. Moreover, most of the reported studies were limited to evaluating simulated solutions, leaving a wide gap between idealization and actualization. Understating the mechanisms involved in the biomass conversion and dye binding processes, coupled with full knowledge of the adsorption conditions, is believed to aid practical applications of adsorbents. Adegoke and Bello in their recent review of dye sequestration using agro-wastes as adsorbents emphasized that characterization of the adsorbents, adsorption conditions, and uptake mechanisms are highly desirable to design and carry out pilot-scale operations in order to study and check their feasibility at industrial level (Adegoke and Bello 2015). They also suggested that the low-cost alternative agro-waste adsorbents should be investigated using dye effluents from industries for their actual efficiency to be realized.
Fruits are agricultural crops grown and consumed all over the world, especially in the tropical and subtropical regions, where they are consumed as fresh (cut) fruits or food complements in desserts, salads, gelatins, fruit cocktails, jam, and juice combinations (Armando et al. 2001; Foo and Hameed 2012). The global annual productions of orange and banana, for example, are estimated by the Food and Agriculture Organization (FAO) at approximately 64 and 95.6 million metric tons, respectively (Foo and Hameed 2012; Spreen 2010). The peels of these fruits occupy nearly half of the total fruit weights. However, a lot of these wastes are indiscriminately disposed of unprocessed, and only few recycling or reuse attempts are made (Albarelli et al. 2011; González-Montelongo et al. 2010). Therefore, it is important to consider reprocessing and reutilizing the fruit peels in other to add values to them and reduce their environmental effects (Foo and Hameed 2012). One of such recycling options is to employ them as precursor materials for the production of ACs for water treatment applications such as dye wastewater treatment.
A number of studies had already demonstrated the use of raw, carbonized, and activated agro-derived biomass for water decontamination, including dye adsorption. For example, cotton stalk was evaluated under three conditions, i.e., without treatment, H2SO4- and H3PO4-treatments, which saw the acid-treated samples exhibit adsorption affinities for MB (Deng et al. 2011). Moreover, coconut coir carbon activated with ZnCl2 (Sharma et al. 2010), marine algae carbon activated with H3PO4 (Salima et al. 2013), and flamboyant pod carbon activated with NaOH (Vargas et al. 2011) showed improved adsorptions of MB, malachite green, and safranine O. Just recently, a nitrogen-doped activated mesoporous carbon aerogel prepared from chitosan was evaluated for MO adsorption from aqueous solution (Jiang et al. 2019). As stated earlier, despite the many reports, there still remain some knowledge gaps regarding the factors driving the activation processes for producing high-performing activated carbons, the effects of activation reagents, and mechanisms involved in the binding of target pollutants. In addition, only few studies have adequately demonstrated practical application potentials of the prepared activated carbons (Yagub et al. 2014; Pessôa et al. 2019). Thus, in this study, we have selected orange peel (OP) as a representative agro-waste precursor, along with five different kinds of activation agents, viz. H2SO4, NaOH, KOH, ZnCl2, and H3PO4. The different activation agents were employed in order to test their effects on MB and MO adsorption capacities of the synthesized ACs. Moreover, several instrumental characterizations including FE-SEM, FTIR, XRD, and XPS were conducted to study the biomass conversion and dye adsorption mechanisms. For practical exploration, the synthesized ACs were eventually evaluated for adsorption of actual laboratory MB dye-containing organic wastewater, and the dye removal characteristics were thoroughly studied.
Materials and methods
Materials and reagents
The MB dye (CAS 7220-79-3; C16H18ClN3S.3H2O; MW 373.9 g/mol; mp 190 °C), MO dye (CAS 547-58-0; C14H14N3NaO3S; Mw 327.33 g/mol; mp > 300 °C), ZnCl2, and H3PO4 were purchased from Sigma-Aldrich Korea Ltd. (Yongin, South Korea). The KOH, NaOH, H2SO4, and HCl were obtained from Daejung Chemicals and Metals Co., Ltd. (Gyeonggi-do, South Korea). The sun-dried fruit peel precursor, OP, was secured from Ghana and stored in an oven dryer at 70 °C to maintain constant weight until use. Double distilled water (DW) was used throughout the study for washing and dilution and was obtained from a Direct-Q UV Millipore dispenser, Merck Millipore.
Synthesis of ACs
The OP biomass was washed with DW to wipe off adhering particles, and subsequently dried in an oven (Vision Scientific Co., Ltd.). It was then pulverized into course granules using a Philips blender (Allan Technology (Huizhou) Co., Ltd., China) and sieved through two layers of meshes, viz. 2 mm and 0.35 mm size meshes (standard testing sieves, Chung Gye Sang Gong SA., Seoul, South Korea). Those particles that passed through the 2-mm mesh but retained on the 0.35-mm mesh were selected and used for the synthesis of the ACs. About 6 g of the biomass was weighed into five crucibles and pre-carbonized in an automated furnace at 400 °C for 1 h under continuous N2 flow. To evaluate the effects of different activation agents, the pre-carbonized precursors were impregnated with KOH, NaOH, H2SO4, H3PO4, and ZnCl2 at fixed ratios of 2:1 (precursor: reagent) for 3 h. After that, the impregnated samples were filtered and dried in an oven dryer for ~ 24 h. The dried samples were placed back into the furnace for activation at 800 °C for 1 h under N2 atmosphere. Consequently, the ACs were rinsed with DW, oven-dried for 24 h, and labeled according to the respective activation agents.
Characterization
The physicochemical characteristics of the ACs were examined via instrumental characterization. The surface morphologies were viewed on a field emission scanning electron microscope, FE-SEM (SUPRA 40VP, Carl Zeiss, Germany). N2 adsorption isotherm and pore size distribution measurements were performed on a BELSORP-max BET equipment at 77 K. Crystallinity studies were conducted using an XRD (multipurpose high-performance X-ray diffractometer, X’pert Powder, PANalytical, the Netherlands) with a Cu-Kα radiation source. In addition, functional groups analyses were performed with a Fourier transforms infrared spectrometer (PerkinElmer spectrophotometer: Spectrum GX, FTIR System) by employing the KBr disk technique in the wavelength range from 4000 to 400 cm−1. Moreover, an AXIS-NOVA spectrometer (Kratos Analytical, Ltd., UK) with monochromatic Al Kα (1486.71 eV of photons) as the X-ray source was used to study the atomic bonding states of the prevailing elements. Lastly, pH point of zero charge (pHpzc) of the selected best two ACs was measured using the pH drift method described by Sarkar et al. 2019. The results pointed that ZnCl2-AC and KOH-AC would show neutrality at pH 6.7 and 9.9, respectively (data not shown).
Adsorption evaluations
To examine potential application of the synthesized ACs in dye wastewater treatment, batch adsorption experiments were conducted using MB and MO as model dye pollutants. Firstly, single-point adsorption tests were carried out to check the adsorption capacities of the various AC samples. Next, two best-performed ACs were selected for characterization and further adsorption studies including isotherm and kinetics. The stock solutions (~ 1200 mg/L) were first prepared and then diluted into working solutions. Approximately 10 mg of the AC samples was weighed into 50-mL falcon tubes, and 20 mL of the dye solutions was added for adsorption. The contents were placed in a multishaking incubator (HB-201MS-2R, Hanbaek, Korea) set to 120 rpm and 25 ± 2 °C for 24 h. The initial concentrations for the isotherm experiments were 0, 50, 100, 200, 400, 700, and 1100 mg/L at pH ranging from 7 to 8 for MB and 3 and 7 for MO adsorption (Mahmoudi et al. 2014). For the kinetic experiments, 300 mL of 100 mg/L dye solutions was filled into glass beakers tightly covered with aluminum foils and placed on multipurpose magnetic stirrers with rod-type stirring bars. AC dozes of 0.1 or 0.05 g were then weighed and added to the solutions and a timer was immediately set. Approximately 1 mL of each solution was drawn from the bulk at varying time intervals for 24 h, centrifuged at 12000 rpm, diluted and analyzed using a UV spectrophotometer (UV-2550, Shimadzu, Kyoto, Japan) at 664 and 505 nm for MB and MO, respectively. The equilibrium uptakes, q, were calculated from the mass balance expression in Eq. (1).
where Ci and Ce are the initial and equilibrium dye concentrations (mg/L), V is the volume of dye (L), and M is the dry mass of AC (g).
Results and discussion
Evaluation of MB and MO dye adsorption capacities
Figure 1 shows the MB and MO dye adsorption results of the ACs prepared with different activation agents, as evaluated through single-point adsorption studies. From the figure, it could be seen that all the ACs exhibited considerable adsorption affinities towards the dyes. However, ZnCl2-AC showed the best affinities for both dyes, while KOH-AC and H2SO4-AC also showed comparable uptakes as ZnCl2-AC, for MB and MO, respectively. Overall, the orders of uptakes were ZnCl2-AC > KOH-AC > H3PO4-AC > NaOH > H2SO4-AC for MB, and ZnCl2-AC > H2SO4-AC > KOH-AC > NaOH > H3PO4-AC for MO. The differences in the adsorption trends could be due to the different mechanisms and degrees of activations by the individual activation agents. According to the literature, carbon activation using metal hydroxides involves etching of the carbon frameworks by redox reactions, formation of H2O, CO2 and carbon gasification reactions, and eventual formation of metallic residues that intercalate into the carbon lattices (Abechi et al. 2013; Foo and Hameed 2012; Wang and Kaskel 2012; Zequine et al. 2017). The intercalation causes expansion of the carbon lattices and hence increases the porosity of the ACs. Moreover, the activation processes of acids are said to proceed by initiation of bond cleaving which leads to dehydration, liberation, and elimination of various light and volatile substances, thereby yielding partial aromatization and hence carbonization (Khaled et al. 2009). Thus, the higher uptakes of the prepared ACs could be attributed to the reaction mechanisms described above. Following the preliminary results, the ZnCl2-AC and KOH-AC were characterized and used for detailed adsorption studies.
Characteristics of the ACs
Morphological and textural characterizations
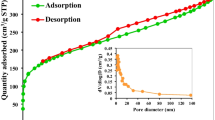
Figure 2 presents the FE-SEM images of the ZnCl2-AC and KOH-AC and shows that they possess porous surface morphologies. The micro-pores of the ZnCl2-AC were, however, more visible on the surface. The surface morphologies experienced slight alterations after the dye adsorption. The surface of the KOH-AC became slightly fluffy, whereas that of the ZnCl2-AC became wave-like with limited visibility of the earlier visible numerous micro-pores. This could be due to coverages of the adhering dyes on and within the porous surfaces. In both AC materials, interconnected micro-channels were seen as probable migration routes for facilitating rapid adsorption (Sarkar et al. 2019). From the N2 adsorption curves shown in Fig. 3a, the ZnCl2-AC exhibited a higher BET surface area and N2 adsorption capacity (1439.50 m2/g, 0.331 cm3 STP/g) than the KOH-AC (1370.76 m2/g, 0.283 cm3 STP/g), an obvious validation of its higher degree of micro-porosity as evident from Fig. 2 and the inset of Fig. 3a.
Crystallinity and functional group analyses
The crystallinity of an AC is helpful for determining its structural or backbone orientation and degree of graphitization. The KOH-AC and ZnCl2-AC samples showed broad humps at 2θ = 24° and 43°, which are characteristics of the cellulosic backbone of the pristine biomass and graphitic structures of the carbon frameworks, respectively (Fig. 3b) (Bediako et al. 2017, Bediako et al. 2015, Chen et al. 2010a, b, Huang et al. 2011, Nekouei et al. 2016, Sun et al. 2008). Besides, a sharp and high-intensity graphite-like peak appeared at 28.9° in the spectrum of the KOH-AC, an indication that a higher degree of graphitization had occurred in its structure (Chen et al. 2010a, b; Huang et al. 2011; Nekouei et al. 2016; Sun et al. 2008). The peak, however, experienced drastic reduction in intensity after the MB dye adsorption. In addition, several low-intensity amorphous-like peaks were observed which may be due to edge defects in the carbon structures (Lagerge et al. 1999).
Furthermore, distinct peaks mainly belonging to the hydroxyl, amine, carboxyl, and carbonyl functional groups were observed in the FTIR spectra of the samples (Fig. 3c). That is, the strong stretching vibrational peaks emerging around 3451 cm−1 were attributed to overlapping of the O–H and N–H bands (Li et al. 2013; Tang et al. 2017). The bands at ca. 1637 cm−1 were due to asymmetric stretching vibrations of the C=O groups (Bediako et al. 2015; Bediako et al. 2016c; Tang et al. 2017). In addition, the bands at 1446, 1218, and 1004 cm−1 were assigned to the presence of O–H, C–N, and C–O stretching vibrations, respectively (Bankar et al. 2010; Bediako et al. 2016c; Liu et al. 2016). Finally, the stretch of bands between 890 and 517 cm−1 reflects C–S bending vibrations and N–H deformation of amines (Bediako et al. 2015; Memon et al. 2008). The presence of vast varieties of functional groups was not unexpected owing to the nitrogen environment employed in the synthesis, coupled with the complex composition of the orange peel biomass precursor.
Adsorption studies
Adsorption kinetics
Adsorption kinetics is helpful for determining the phenomenological coefficients that characterize the transport of adsorbates within adsorbents (Azizian 2004; Bediako et al. 2016a). It is crucial to define the rate of adsorption from the bulk medium onto the surfaces of the adsorbents. Figure 4 a shows the adsorption kinetics of MB and MO adsorption. The adsorption rates were swifter at the beginning of the adsorption process owing to the ready availability of binding sites but became quite sluggish towards equilibrium, due to exhaustion and lack of easy access to the binding sites (Anirudhan et al. 2012; Bediako et al. 2016b). The KOH-AC required about 6 h to reach adsorption equilibrium for MB, while the ZnCl2-AC required a little over 20 h to achieve same for MB but took a relatively shorter time of only 4 h for MO. The continuous rising nature of the data points for MB adsorption by the ZnCl2-AC likely delayed its equilibrium time. Thus, since the dyes have similar dimensions (Xu et al. 2018), the differences in reaching the adsorption equilibrium could be due to adsorption affinity differences by the ACs and the mechanisms involved, rather than size difference of the dyes.
The pseudo-first-order (Bhattacharya and Venkobachar 1984) and pseudo-second-order (Ho and McKay 1999) models were used to fit the kinetics data, and the model equations are given by Eq. (2) and Eq. (3), respectively.
where qt is the uptake (mg/g) at time, t; q1 and q2 are the uptakes at equilibrium (mg/g); and k1 and k2 are the pseudo-first-order and pseudo-second-order rate constants, respectively.
The pseudo-first-order model defines the adsorption interaction between the solid ACs and dye solution based on the adsorption capacity of the ACs by assuming that one dye molecule will occupy only one adsorption site (Bediako et al. 2016a; Boparai et al. 2011). The pseudo-second-order model, on the other hand, examines the chemisorption kinetics of the ACs from the liquid dye solution (Azizian 2004; Ho 2006). From the model parameters summarized in Table 1, it could be observed that both but the pseudo-second-order model provided a better representation of the data in most instances, which is consistent with the results obtained by some other authors for dye adsorption (Li et al. 2013; Ofomaja 2007; Sarkar et al. 2015; Mahmoudi et al. 2014). The proximity in the estimated coefficient of correlation, R2 values for both kinetic models thus suggest that a combination of chemical and electrostatic interactions were likely involved in the adsorption process and that there were strong interactions between the dyes and functional groups present on and within the AC samples (Bediako et al. 2016c; Boparai et al. 2011; Qi et al. 2013).
To understand the diffusion mechanisms involved in the adsorption process, the kinetic data were further examined with the intra-particle diffusion (IPD) model which is represented by Eq. (4).
where ki is the intra-particle diffusion rate constant, and Ci is the intercept representing the thickness of the boundary layer (Kim et al. 2015; Li et al. 2013). The IPD model parameters given in Table 2 were obtained from the plot of qt versus t0.5. According to this model, the adsorption process is assumed to be associated with IPD if the regression of the above plot is linear, and if the regression line passes through the origin, then IPD becomes the sole rate-controlling step of the reaction (Kim et al. 2015; Li et al. 2013; Won et al. 2013). The plot of qt versus t0.5 showed multilinear characteristics for the MB adsorption by KOH-AC, revealing that three steps were involved in the adsorption process (Fig. 4b). The first sharp portion is the external surface adsorption or instantaneous adsorption stage where the adsorption was swifter with higher uptake (larger slope), the second subdued section is the gradual adsorption or transition stage, and the third section is the final equilibrium phase where intra-particle diffusion controlled the rate of adsorption (Bediako et al. 2019a; Li et al. 2013; Won et al. 2013).
Conversely, the MB adsorption by ZnCl2-AC exhibited a single linear phase passing through the origin and suggested IPD as the sole rate-limiting step. Since the IPD mechanism involves time-dependent transport of dye from the bulk surface into the inner cavities of the AC adsorbents, the slower MB adsorption rate of the ZnCl2-AC could be understood. For the MO adsorption, however, the ZnCl2-AC showed two phases of instantaneous external adsorption, followed by the final equilibrium phase. This swift switch from instantaneous to equilibrium adsorption explains the reason why the MO adsorption was the fastest to reach equilibrium. Moreover, the dimensions of the MB are approximated as 0.17 wide × 0.76 high × 0.33 nm thick (Arias et al. 1999), and those of MO are 0.18 wide × 0.76 high × 0.60 nm thick (Xu et al. 2018). These size dimensions make it possible for easy penetration of the dyes into the AC pores which are about the mean size 0.7–1.5 nm.
Adsorption isotherms
Adsorption isotherm is important in adsorption studies because it helps to describe the interactive behaviors of adsorbents and adsorbates, as well as estimating the maximum adsorption capacities (Li et al. 2014; Park et al. 2018). By definition, it represents the amount of species bound onto the surface of adsorbents as a function of the remaining species in solution (Bediako et al. 2016c). The results of the adsorption isotherm experiments as fitted to several adsorption models as shown in Fig. 5. Both the KOH-AC and ZnCl2-AC showed similar adsorption trends for MB and MO. In other words, the isotherm data points followed the usual adsorption phenomenon where the adsorption capacity increases with increasing initial concentration and reaches a near plateau-like shape at equilibrium (Liang et al. 2009; Pillai et al. 2013).
To statistically analyze the results, the experimental data were exported from excel to Sigma Plot software (version 12.0, SPSS, USA) and fitted via the Langmuir (Langmuir 1918), Freundlich (Freundlich 1906), Redlich–Peterson (R–P) (Bediako et al. 2017), Temkin (Dada et al. 2012), and Sips (Nethaji et al. 2013) isotherm models which are expressed by Eq. (5) through Eq. (9).
where qm is maximum uptake at equilibrium (mg g−1), b is the coefficient corresponding to the affinity between the adsorbent and adsorbate, qe is the equilibrium uptake (mg g−1), and Ce is the equilibrium concentration of adsorbate (mg L−1).
where K and n are the Freundlich constants denoting the relative adsorption capacity and adsorption intensity, respectively. The Langmuir model depicts a monolayer adsorption phenomenon of adsorbates onto adsorbents at specific homogeneous sites, while the Freundlich model describes both monolayer and multilayer adsorption onto heterogeneous surfaces (Bediako et al. 2016a; Pillai et al. 2013).
where KRP and aRP are the Redlich–Peterson isotherm constants (L/mg) and βRP is the Redlich–Peterson model exponent which values range between 0 and 1. The model tends to follow the Langmuir model when the R–P constant is 1 or close to 1 and assumes the Freundlich model when R–P constant is (zero) 0 or close to zero (Bediako et al. 2017).
where AT is the Temkin isotherm equilibrium binding constant (L/g), bT is the Temkin isotherm constant, R is the universal gas constant (8.314 J/mol/K), and T is temperature at 298 K.
where KS (L/mg) represents the affinity constant and nS indicates the surface heterogeneity. When nS approaches unity, the Sips isotherm assumes the Langmuir isotherm and predicts adsorption at homogeneous sites. Conversely, deviation of the nS value away from unity suggests heterogeneous surface adsorption.
As can be seen from the model parameters displayed in Table 3, all models except the Temkin model fitted the isotherm data with very high R2 values exceeding 0.90. Particularly, the Sips and Langmuir models predicted the maximum equilibrium uptakes quite well, thus depicting mixed monolayer and multilayer adsorptions onto the surfaces of the ACs. This is however not unexpected because it is a common characteristics of activated carbons (Tran et al. 2017). Furthermore, the ACs exhibited stronger affinity and adsorption intensity for MB than MO as can be observed from the initial slopes of the isotherm curves in Fig. 5 and the model parameters in Table 3. The better MB affinity of the ACs did not necessarily translate into higher equilibrium uptakes because adsorption is a time-dependent process. Moreover, a higher equilibrium MO uptake was obtained at pH 3 than obtained at pH 7. This was due to the pHpzc of the ZnCl2-AC which is 6.7. It presupposes that the surface of the AC would be positively charged at pH 3 and, hence, could effectively interact with the negatively charged MO, leading to a higher adsorption through electrostatic binding.
Comparison of adsorption performances and practical adsorption evaluation
A compilation of recently reported adsorbents, mostly from agro-waste-derived carbons is summary in Table 4. Like in the present study, most of the previous reports performed detailed characterization and textural analysis of the prepared adsorbents, conducted the adsorption experiments at defined environmental conditions (such as pH, temperature, time, and stirring/shaking speed), and examined the experimental data with several isotherm and kinetic models. However, only a handful of researchers performed thermodynamic and regeneration studies. Ceteris paribus, the adsorption performances of the synthesized ACs, especially for MO, far outweighed most of the reported works, which suggests that the prepared ACs are superior competitors for application.
To test applicability of the ACs in treating dyes on industrial scale, an organic wastewater sample containing MB was collected from the Environmental Biotechnology National Research Laboratory in Chonbuk National University. After initial analysis using UV, the concentration of MB was determined to be ~ 35 mg/g. Subsequently, the ZnCl2-AC and KOH-AC were separately contacted with measured volumes of the organic wastewater sample. After few hours, the blue-colored solutions became colorless to the unaided eye (Fig. 6), indicating complete removal of the MB dye. After UV analysis, the removal efficiencies were found to be greater than 99% for each of the two ACs.
XPS analysis and mechanisms of adsorption
XPS is an important characterization tool for identifying the presence and composition of elements and bond types in materials (Dambies et al. 2001; Zhou et al. 2005). The results from XPS could provide key information on the adsorption mechanisms of the dye molecules. Thus, using the KOH-AC and MB as representative carbon and dye, respectively, the high resolution core-level spectra of the prevailing C, N, and S atoms were deconvoluted into separate peaks as displayed in Fig. 7. Before the deconvolution, each spectrum was calibrated to the C1s peak at 284.6 eV (Wagner 1979). The S2p spectra showed no prevailing S peaks prior to the MB adsorption, indicating that no sulfur groups were initially present on the surface of the AC. After adsorption, however, a pair of peaks corresponding to the S2p3/2 and S2p1/2 orbitals of S with a distance of 1.2 eV apart was observed (Liu et al. 2013; Squarcialupi et al. 2002). These observed S peaks were assigned to the sulfur atom in the MB structure and indicated that the adsorbed dye was stabilized on the AC. Furthermore, a huge bump of doped N atom was seen in the spectrum of the AC before adsorption; however, the intensity had increased significantly after adsorption. Similar to the S peaks, the increase in intensity likely resulted from the N atom contained in the structure of the MB dye (Patel et al. 2015). Thus, the presence of the S and N peaks following the MB dye adsorption signified effective binding and retention of the dye on the surface of ACs.
In the case of the deconvoluted C1s spectra, several bond peaks were observed. These peaks include C–C/C=C, C–N, C–O, C=O/O–C=O, and pi–pi* excitation (Dementjev et al. 2000; Wang et al. 2018). Noteworthily, the intensity of the C–N peak had significantly increased, and a new peak corresponding to C–S/C=S had emerged after adsorption (Wagner 1979). Moreover, the pi–pi* excitation peak had not only shifted to a lower binding energy state from 293.15 to 292.10 eV but had also experienced a significant reduction in intensity. The above observations pointed to the adsorption process likely involving mechanisms such as pi–pi interactions, electrostatic attractions, non-electrostatic hydrogen bond formations, and electron donor–acceptor reactions (Song et al. 2019; Vargas et al. 2011). The pi–pi interactions were possible between the aromatic ring of the MB and the aromatic structure of the graphite-like layers of the ACs. As already discussed under the XRD characterization, the ACs exhibited graphitic carbon peaks and thus graphitic behavior. Moreover, electrostatic attractions between the N atom of the MB dye and the anionic functional groups such as the carboxyl groups on the ACs was possible because the N atoms are reported to assume positive charges at pH ~ 8 where the adsorption experiments were conducted (Bediako et al. 2019b; Sarkar et al. 2019). In addition, the hydroxyl group of AC and terminal nitrogen of MB dye could undergo a non-electrostatic hydrogen bond formation reaction, while the carbonyl oxygen of the AC and aromatic ring of the MB dye would engage in an electron donor–acceptor process (Moreno-Castilla 2004; Vargas et al. 2011). The above-discussed interactions are also expected for the MO adsorption, except that the electrostatic interaction would be between the deprotonated sulfonate groups and positively charged nitrogen groups on the ACs (Mahmoudi et al. 2014; Fraga et al. 2018). By these mechanisms, the effective adsorption and retention of the adsorbed dyes on the ACs could be well comprehended.
Conclusions
Orange peel ACs were synthesized via activation with H2SO4, NaOH, KOH, ZnCl2, and H3PO4. ZnCl2-AC showed the highest MB and MO adsorption capacities, with a faster kinetics of MO adsorption of 4 h. KOH-AC, on the other hand, displayed a faster MB adsorption equilibrium (6 h) than the ZnCl2-AC (~ 20 h). The adsorption capacities obtained with the prepared ACs were higher than most recently reported values. Based on the characterization results, the adsorption mechanisms were proposed to include electrostatic attractions, pi–pi interactions, hydrogen bond formations, electron donor–acceptor reactions, and intra-particle diffusion. Finally, the ACs functioned effectively when applied to MB scavenging from a laboratory organic wastewater, where more than 99% removal efficiency was obtained. Therefore, this work not only does suggest agro-waste orange peel as an AC precursor in lieu of commercial ACs but also provides backgrounds for understanding the adsorptive interactions leading to effective removal of dyes from polluted waters.
References
Abechi SE, Gimba CE, Uzairu A, Dallatu YA (2013) Preparation and characterization of activated carbon from palm kernel shell by chemical activation. Res J Chem Sci 3:54–61
Adegoke KA, Bello OS (2015) Dye sequestration using agricultural wastes as adsorbents. Water Resour Ind 12:8–24
Albarelli JQ, Rabelo RB, Santos DT, Beppu MM, Meireles MAA (2011) Effects of supercritical carbon dioxide on waste banana peels for heavy metal removal. J Supercrit Fluid 58:343–351
Anirudhan TS, Fernandez NB, Mullassery MD (2012) Removal of Cd(II) ions from aqueous solution using a cation exchanger derived from banana stem. J Chem Technol Biotechnol 87:714–722
Arias M, López E, Nuñez A, Rubinos D, Soto B, Barral MT, Díaz-Fierros F (1999) Adsorption of methylene blue by red mud, an oxide-rich byproduct of bauxite refining. In: Berthelin J., Huang P.M., Bollag JM., Andreux F. (eds) Effect of mineral-organic-microorganism interactions on soil and freshwater environments. Springer, Boston, 361–365
Armando N, Spreen TH, Jauregui C (2001) The citrus industry of Cuba: 1994-1999. Forthcoming international working paper, Food and Resource Economics Department, University of Florida, Gainesville
Auta M, Hameed BH (2012) Modified mesoporous clay adsorbent for adsorption isotherm and kinetics of methylene blue. Chem Eng J 198-199:219–227
Azizian S (2004) Kinetic models of sorption: a theoretical analysis. J Colloid Interf Sci 276:47–52
Bankar A, Joshi B, Kumar AR, Zinjarde S (2010) Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids Surf A Physicochem Eng Asp 368:58–63
Bediako JK, Wei W, Kim S, Yun Y-S (2015) Removal of heavy metals from aqueous phases using chemically modified waste Lyocell fiber. J Hazard Mater 299:550–561
Bediako JK, Kim S, Wei W, Yun YS (2016a) Adsorptive separation of Pb(II) and Cu(II) from aqueous solutions using as-prepared carboxymethylated waste Lyocell fiber. Int J Env Sci Tec 13:875–886
Bediako JK, Wei W, Yun Y-S (2016b) Conversion of waste textile cellulose fibers into heavy metal adsorbents. J Ind Eng Chem 43:61–68
Bediako JK, Wei W, Yun Y-S (2016c) Low-cost renewable adsorbent developed from waste textile fabric and its application to heavy metal adsorption. J Taiwan Inst Chem Eng 63:250–258
Bediako JK, Reddy DHK, Song M-H, Wei W, Lin S, Yun Y-S (2017) Preparation, characterization and lead adsorption study of tripolyphosphate-modified waste Lyocell fibers. J. Env. Chem. Eng. 5:412–421
Bediako JK, Park SW, Choi J-W, Song M-H, Yun Y-S (2019a) High-performance and acid-tolerant polyethylenimine-aminated polyvinyl chloride fibers: fabrication and application for recovery of platinum from acidic wastewaters. J. Environ. Chem. Eng. 7:102839
Bediako JK, Sarkar AK, Lin S, Zhao Y, Song M-H, Choi J-W, Cho C-W, Yun Y-S (2019b) Characterization of the residual biochemical components of sequentially extracted banana peel biomasses and their environmental remediation applications. Waste Manag 89:141–153
Bedin KC, Martins AC, Cazetta AL, Pezoti O, Almeida VC (2016) KOH-activated carbon prepared from sucrose spherical carbon: adsorption equilibrium, kinetic and thermodynamic studies for methylene blue removal. Chem Eng J 286:476–484
Bhattacharya AK, Venkobachar C (1984) Removal of cadmium (II) by low cost adsorbents. J Environ Eng 110:110–122
Boparai HK, Joseph M, O'Carroll DM (2011) Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Meter. 186:458–465
Chen S, Zhang J, Zhang C, Yue Q, Li Y, Li C (2010a) Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis. Desalination 252:149–156
Chen W, Zhang H, Huang Y, Wang W (2010b) A fish scale based hierarchical lamellar porous carbon material obtained using a natural template for high performance electrochemical capacitors. J Mater Chem 20:4773–4775
Dada AO, Olalekan AP, Olatunya AM, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J. Appl. Chem:338–345
Dambies L, Guimon C, Yiacoumi S, Guibal E (2001) Characterization of metal ion interactions with chitosan by X-ray photoelectron spectroscopy. Colloids Surf A Physicochem Eng Asp 177:203–214
de Paiva TMN, Fraga TJM, Sales DCS, Carvalho MN, da Motta Sobrinho MA (2018) Anomalocardia brasiliana shellfish shells as a novel and ecofriendly adsorbent of Nylosan Brilliant Blue acid dye. Water Sci Technol 78:1576–1586
Dementjev AP, de Graaf A, Naumkin AV, van de Sanden MCM, Serov AA (2000) X-ray photoelectron spectroscopy reference data for identification of the C3N4 phase in carbon-nitrogen films. Diam Relat Mater 9:1904–1907
Deng H, Lu J, Li G, Zhang G, Wang X (2011) Adsorption of methylene blue on adsorbent materials produced from cotton stalk. Chem Eng J 172:326–334
Oliveira EHCD, Marques Fraga DMS, da Silva MP, TJM F, Carvalho MN, de Luna Freire EMP, Ghislandi MG, da Motta Sobrinho MA (2019) Removal of toxic dyes from aqueous solution by adsorption onto highly recyclable xGnP® graphite nanoplatelets. J Environ Chem Eng 7
Foo KY, Hameed BH (2012) Preparation, characterization and evaluation of adsorptive properties of orange peel based activated carbon via microwave induced K2CO3 activation. Bioresour Technol 104:679–686
Fraga TJM, dos Santos Marques Fraga DM, da Silva TC, Carvalho MN, da Motta Sobrinho MA (2018) Adsorption of reactive dyes onto thermally treated waste from aluminum lamination. Water Prac Technol 13:629–641
Freundlich HMF (1906) Über die Adsorption in Lösungen. Z Phys Chem A:385–470
Girods P, Dufour A, Fierro V, Rogaume Y, Rogaume C, Zoulalian A, Celzard A (2009) Activated carbons prepared from wood particleboard wastes: characterisation and phenol adsorption capacities. J Hazard Mater 166:491–501
González-Montelongo R, Gloria Lobo M, González M (2010) Antioxidant activity in banana peel extracts: testing extraction conditions and related bioactive compounds. Food Chem 119:1030–1039
Ho Y-S (2006) Review of second-order models for adsorption systems. J Hazard Mater 136:681–689
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Huang W, Zhang H, Huang Y, Wang W, Wei S (2011) Hierarchical porous carbon obtained from animal bone and evaluation in electric double-layer capacitors. Carbon 49:838–843
Jiang X, Xiang X, Peng S, Hou L (2019) Facile preparation of nitrogen-doped activated mesoporous carbon aerogel from chitosan for methyl orange adsorption from aqueous solution. Cellulose 26:4515–4527
Kelm MAP, da Silva Junior MJ, de Barros Holanda SH, de Araujo CMB, de Assis Filho RB, Freitas EJ, Dos Santos DR, da Motta Sobrinho MA (2019) Removal of azo dye from water via adsorption on biochar produced by the gasification of wood wastes. Environ Sci Pollut Res Int 26:28558–28573. https://doi.org/10.1007/s11356-018-3833-x
Khaled A, El Nemr A, El-Sikaily A, Abdelwahab O (2009) Removal of Direct N Blue-106 from artificial textile dye effluent using activated carbon from orange peel: adsorption isotherm and kinetic studies. J Hazard Mater 165:100–110
Kim MH, Hwang C-H, Kang SB, Kim S, Park SW, Yun Y-S, Won SW (2015) Removal of hydrolyzed Reactive Black 5 from aqueous solution using a polyethylenimine–polyvinyl chloride composite fiber. Chem Eng J 280:18–25
Lagerge S, Zajac J, Partyka S, Groszek AJ (1999) Comparative study on the adsorption of cyanide gold complexes onto different carbonaceous samples: measurement of the reversibility of the process and assessment of the active surface inferred by flow microcalorimetry. Langmuir 15:4803–4811
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Li Y, Du Q, Liu T, Sun J, Wang Y, Wu S, Wang Z, Xia Y, Xia L (2013) Methylene blue adsorption on graphene oxide/calcium alginate composites. Carbohydr Polym 95:501–507
Li Y, Sun J, Du Q, Zhang L, Yang X, Wu S, Xia Y, Wang Z, Xia L, Cao A (2014) Mechanical and dye adsorption properties of graphene oxide/chitosan composite fibers prepared by wet spinning. Carbohydr Polym 102:755–761
Liang S, Guo X, Feng N, Tian Q (2009) Application of orange peel xanthate for the adsorption of Pb2+ from aqueous solutions. J Hazard Meter 170:425–429
Liu YT, Zhu XD, Duan ZQ, Xie XM (2013) Flexible and robust MoS2-graphene hybrid paper cross-linked by a polymer ligand: a high-performance anode material for thin film lithium-ion batteries. Chem. Commun. (Camb) 49:10305–10307
Liu Z, Zhan X, Yang M, Yang Q, Xu X, Lan F, Wu Y, Gu Z (2016) A magnetic-dependent protein corona of tailor-made superparamagnetic iron oxides alters their biological behaviors. Nanoscale 8:7544–7555
Mahmoudi K, Hosni K, Hamdi N, Srasra E (2014) Kinetics and equilibrium studies on removal of methylene blue and methyl orange by adsorption onto activated carbon prepared from date pits-a comparative study. Korean J Chem Eng 32:274–283
Memon JR, Memon SQ, Bhanger MI, Memon GZ, El-Turki A, Allen GC (2008) Characterization of banana peel by scanning electron microscopy and FT-IR spectroscopy and its use for cadmium removal. Colloid Surface B 66:260–265
Mokhtari P, Ghaedi M, Dashtian K, Rahimi MR, Purkait MK (2016) Removal of methyl orange by copper sulfide nanoparticles loaded activated carbon: kinetic and isotherm investigation. J Mol Liq 219:299–305
Moreno-Castilla C (2004) Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon 42:83–94
Nekouei F, Kargarzadeh H, Nekouei S, Tyagi I, Agarwal S, Kumar Gupta V (2016) Preparation of nickel hydroxide nanoplates modified activated carbon for malachite green removal from solutions: kinetic, thermodynamic, isotherm and antibacterial studies. Process Saf Environ Prot 102:85–97
Nethaji S, Sivasamy A, Mandal AB (2013) Adsorption isotherms, kinetics and mechanism for the adsorption of cationic and anionic dyes onto carbonaceous particles prepared from Juglans regia shell biomass. Int J Environ Sci Technol 10:231–242
Ngulube T, Gumbo JR, Masindi V, Maity A (2017) An update on synthetic dyes adsorption onto clay based minerals: a state-of-art review. J Environ Manag 191:35–57
Ofomaja AE (2007) Kinetics and mechanism of methylene blue sorption onto palm kernel fibre. Process Biochem 42:16–24
Park SW, Bediako JK, Song M-H, Choi J-W, Lee H-C, Yun Y-S (2018) Facile fabrication of polyacrylic acid-polyvinyl chloride composite adsorbents for the treatment of cadmium-contaminated wastewater. J Env Chem Eng 6:2401–2408
Patel M, Feng W, Savaram K, Khoshi MR, Huang R, Sun J, Rabie E, Flach C, Mendelsohn R, Garfunkel E, He H (2015) Microwave enabled one-pot, one-step fabrication and nitrogen doping of holey graphene oxide for catalytic applications. Small 11:3358–3368
Pessôa TS, LED LF, da Silva MP, Pereira Neto LM, BFd N, TJM F, Jaguaribe EF, Cavalcanti JV, da Motta Sobrinho MA (2019) Açaí waste beneficing by gasification process and its employment in the treatment of synthetic and raw textile wastewater. J Clean Prod 240
Pezoti O, Cazetta AL, Souza IPAF, Bedin KC, Martins AC, Silva TL, Almeida VC (2014) Adsorption studies of methylene blue onto ZnCl2-activated carbon produced from buriti shells (Mauritia flexuosa L.). J Ind Eng Chem 20:4401–4407
Pillai SS, Deepa B, Abraham E, Girija N, Geetha P, Jacob L, Koshy M (2013) Biosorption of Cd(II) from aqueous solution using xanthated nano banana cellulose: equilibrium and kinetic studies. Ecotoxicol Environ Safe 98:352–360
Qi X, Li L, Tan T, Chen W, Smith RL (2013) Adsorption of 1-butyl-3-methylimidazolium chloride ionic liquid by functional carbon microspheres from hydrothermal carbonization of cellulose. Environ Sci Technol 47:2792–2798
Salima A, Benaouda B, Noureddine B, Duclaux L (2013) Application of Ulva lactuca and Systoceira stricta algae-based activated carbons to hazardous cationic dyes removal from industrial effluents. Water Res 47:3375–3388
Sarkar AK, Saha A, Panda AB, Pal S (2015) pH triggered superior selective adsorption and separation of both cationic and anionic dyes and photocatalytic activity on a fully exfoliated titanate layer-natural polymer based nanocomposite. Chem Commun (Camb) 51:16057–16060
Sarkar AK, Saha A, Tarafder A, Panda AB, Pal S (2016) Efficient removal of toxic dyes via simultaneous adsorption and solar light driven photodegradation using recyclable functionalized amylopectin–TiO2–Au nanocomposite. ACS Sust Chem Eng 4:1679–1688
Sarkar AK, Bediako JK, Choi J-W, Yun Y-S (2019): Functionalized magnetic biopolymeric graphene oxide with outstanding performance in water purification NPG Asia Materials 11
Sharma YC, Uma SASK, Upadhyay SN (2010) Characterization and adsorption studies of Cocos nucifera L. activated carbon for the removal of methylene blue from aqueous solutions. J Chem Eng Data 55:2662–2667
Song M, Duan Z, Qin R, Xu X, Liu S, Song S, Zhang M, Li Y, Shi J (2019) Simultaneous adsorption of Cd2+ and methylene blue from aqueous solution using xanthate-modified baker’s yeast. Korean J Chem Eng 36:869–879
Spreen TH (2010) Projections of world production and consumption of citrus to 2010. FAO Corporate Document Repository, Economic and Social Development Department. <http://www.fao.org/DOCREP/003/X6732E/x6732e 02.htm>
Squarcialupi MC, Bernardini GP, Atrei A, Rovida G (2002) Characterisation by XPS of the corrosion patina formed on bronze surfaces. J Cult Herit 3:199–204
Sun G, Li X, Qu Y, Wang X, Yan H, Zhang Y (2008) Preparation and characterization of graphite nanosheets from detonation technique. Mater Lett 62:703–706
Tan IAW, Ahmad AL, Hameed BH (2008) Preparation of activated carbon from coconut husk: optimization study on removal of 2,4,6-trichlorophenol using response surface methodology. JHazard Mater 153:709–717
Tang J, Li Y, Wang X, Daroch M (2017) Effective adsorption of aqueous Pb2+ by dried biomass of Landoltia punctata and Spirodela polyrhiza. J Clean Prod 145:25–34
Tran HN, You SJ, Hosseini-Bandegharaei A, Chao HP (2017) Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Res 120:88–116
Vargas AMM, Cazetta AL, Kunita MH, Silva TL, Almeida VC (2011) Adsorption of methylene blue on activated carbon produced from flamboyant pods (Delonix regia): study of adsorption isotherms and kinetic models. Chem Eng J 168:722–730
Vijayaraghavan K, Yun YS (2007) Chemical modification and immobilization of Corynebacterium glutamicum for biosorption of reactive black 5 from aqueous solution. Ind Eng Chem Res 46:608–617
Wagner CD (1979) Handbook of X-ray photoelectron spectroscopy: a reference book of standard data for use in X-ray photoelectron spectroscopy. Physical Electronics Division, Perkin-Elmer Corp
Wang J, Kaskel S (2012) KOH activation of carbon-based materials for energy storage. J Mater Chem 22:23710–23725
Wang X, Liu X, Yuan H, Liu H, Liu C, Li T, Yan C, Yan X, Shen C, Guo Z (2018) Non-covalently functionalized graphene strengthened poly(vinyl alcohol). Mater Des 139:372–379
Won SW, Kim S, Kotte P, Lim A, Yun Y-S (2013): Cationic polymer-immobilized polysulfone-based fibers as high performance sorbents for Pt(IV) recovery from acidic solutions. J. Hazard. Meter. 263, Part 2, 391–397
Xu T, He Y, Qin Y, Zhao C, Peng C, Hu J, Liu H (2018) Facile preparation of porous organic copolymer based on triptycene and crown ether for efficient organic dye adsorption. RSC Adv 8:4963–4968
Yagsi NU 2004: Production and characterisation of activated carbon from Apricot Stones, Middle East Technical University (METU), Ankara pages 19–105 pp
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interf Sci 209:172–184
Yahya MA, Al-Qodah Z, Ngah CWZ (2015) Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: a review. Renew Sust Energ Rev 46:218–235
Yao Y, Bing H, Feifei X, Xiaofeng C (2011) Equilibrium and kinetic studies of methyl orange adsorption on multiwalled carbon nanotubes. Chem Eng J 170:82–89
Zequine C, Ranaweera CK, Wang Z, Dvornic PR, Kahol PK, Singh S, Tripathi P, Srivastava ON, Singh S, Gupta BK, Gupta G, Gupta RK (2017) High-performance flexible supercapacitors obtained via recycled jute: bio-waste to energy storage approach. Sci Rep 7:1174
Zhang Z, Zhang Z, Fernández Y, Menéndez JA, Niu H, Peng J, Zhang L, Guo S (2010) Adsorption isotherms and kinetics of methylene blue on a low-cost adsorbent recovered from a spent catalyst of vinyl acetate synthesis. Appl Surf Sci 256:2569–2576
Zhou D, Zhang L, Guo S (2005) Mechanisms of lead biosorption on cellulose/chitin beads. Water Res 39:3755–3762
Acknowledgments
The authors wish to acknowledge the Chonbuk National University’s Center for University-wide Research Facility (CURF), especially Mr. Kim Seok Jun and Ms. Song Min-kyeong for assisting with the XRD and FE-SEM analyses, respectively. Final appreciations go to Ms. Chin Narae (Soletek Trading Co., Ltd., Soeul, Korea) and Dr. Kim Hyun Sook of Wongwang University (Iksan, Korea) for assisting with the BET and XPS measurements, respectively.
Funding
This study was supported by the Korean Government through the National Research Foundation, NRF (2017R1A2A1A05001207) grant.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bediako, J.K., Lin, S., Sarkar, A.K. et al. Evaluation of orange peel-derived activated carbons for treatment of dye-contaminated wastewater tailings. Environ Sci Pollut Res 27, 1053–1068 (2020). https://doi.org/10.1007/s11356-019-07031-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07031-8