Abstract
Polyhydroxyalkanoate (PHA) synthesised by microbial strains has properties (biocompatible, non-toxic, and biodegradable) that make it appropriate as an environment-friendly plastic component. This research evaluated the cheap carbon substrates (molasses, olive oil, and their mixture) of industrial waste as an alternative to costly ones for the manufacturing enhancement of mcl-PHA by C. necator. The strain was cultured in both nutrient and mineral media with and without nitrogen. mcl-PHA content was found to be 24.33%, 18.66%, and 40% with molasses, olive oil, and a mixture of both substrates, respectively. The chromatographic technique, GCMS, was utilized to confirm the types of PHA monomers (3HB and HHx). The maximum PHA content produced was 2.03 g/L using a combination of substrates compared to molasses (1.41 g/L) and olive oil (1.12 g/L). Morphology exhibits pseudo-spherical granules with comparatively consistent distribution by SEM. FTIR spectroscopy was used to detect PHA presence rapidly. In conclusion, C. necator DSM 428 cultivated on a mixture of substrates is proficient in manufacturing mcl-PHA along with scl-PHA; the type of PHA monomer depends upon the selection of substrate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
PHAs are emerging and significant biopolymers compared to petroleum-based plastics. Conventional plastics caused major issues like soil infertility, environmental pollution, and non-biodegradability [1]. Substitution of traditional plastics by PHAs has gained considerable importance regarding ecological aspects. The European exploitation of bio-based plastics was approximately 50 K to 100 K tons in 2007 and is anticipated to consolidate 2–6 Mt by 2025 [2]. The recently reported market operation of PHA-based commodities was 10,000 Mt in 2013 to 34,000 Mt in 2018 [3]. PHAs are polyesters of 3-, 4-, 5-, and 6-alkanoic acids, manufactured by many strains and serve as carbon and energy storing constituents. The constituent’s characteristics are of great interest because of the compatibility, biodegradability, renewable behaviour, and eco-friendly aspects [4]. The degradation rate of PHA is 3–9 months, which is most furious compared to petroleum plastics. Microorganisms convert them into CO2 and H2O utilizing their PHA depolymerase [5]. To date, over 150 various hydroxy alkanoic acids/PHA monomers are reported in the literature, these monomeric units are responsible for the physical and chemical characteristics of PHA [6]. Higher than 90 kinds of microbes are being used to accumulate the polyester in the shape of granules [7, 8].
PHAs are specified in 3 different categories, short, medium, and long chains based on the presence of C-atoms in their lateral chains [9]. Scl-PHAs, such as Poly (3HB) and Poly (3HV), have fewer than five carbon atoms, whereas mcl-PHAs, such as Poly (3HHx), Poly (3HO), and Poly (3HD), have five to fourteen carbon atoms. Lcl-PHAs, on the other hand, have more than fourteen carbon atoms. Though lcl-PHAs are produced but are infrequent, less reported, and less examined [10]. PHA exhibits the following characteristics: crystal clear, rigid, and flexible. Scl-PHAs are majorly hard, firm, and fragile, while they possess lesser capabilities to shape. Compared to scl-PHA, mcl-PHA is of great interest because of its elasticity, flexibility and higher tendency to mould [11]. Physico-chemical characteristics of PHA polymers can be improved by altering and blending their exteriors. Alteration can be accomplished via mixing the PHA polymer with additional specific types of polymers along with enzymes [12].
PHAs, particularly poly (3-hydroxybutyrate), were revealed in B. megaterium by Lemoigne in 1925 [13]. The production of PHA would be fascinating concerning both ecological as well as economic points of analysis due to the simple access of carbon sources, like corn, starch, molasses, whey, vegetable oil, agricultural wastes, and wheat [14,15,16]. Molasses is mainly intended for the manufacturing of PHA, especially responsible for the synthesis of PHB (scl-PHA), oils like olive oil, sunflower oil, sesame seed oils, canola and other fatty acids are majorly responsible for the mcl-PHA production separately [17, 18]. Mixtures of these substrates are used to enhance the productivity of PHA and to accumulate various varieties of PHA in microbes. Genetically engineered microbes are highly regarded for producing a great yield of PHA compared to native species, although genetic engineering demands additional costs than natural bacterias [19, 20]. Their consumption is now introverted because of the greatest prices of growth. Consumption of organic agro-waste materials, like molasses and olive oil, as an alternative to costly carbon sources, was used for PHA production [21, 22].
In earlier studies, PHAs have been produced using a pure culture of C. necator DSMz 545 with a volatile fatty acids rich, anaerobically digested olive mill wastewater [23, 24]. In another study, PHA polymer was produced using a mixture of glucose and acetate from C. necator DSMz428 [25]. The PHA production was also reported using a mixture of olive oil mill effluent with glucose from mutant B. cereus FA11 [26]. The choice of bacteria has a significant influence on PHA formation. C. necator is a very well microbe for producing scl-PHA; however, it may also produce mcl-PHA when given certain nutrients. [27]. C. necator was selected because this strain accumulated PHA at 80–90% dry cell weight. This study evaluated the effect of olive oil from industrial waste as an additional carbon source with molasses on the thermochemical characteristics of short and mcl-PHAs during the growth phase in C. necator. This is the first attempt to use olive oil with molasses to accumulate PHAs from the pure culture of C. necator DSMz 428. This investigation aims to eliminate the petrochemical plastics by replacing economical, eco-friendly new biopolymer expectedly mcl-PHA.
2 Materials and methodology
2.1 Strain
C. necator ATCC17699 was purchased from the international collection DSMZ. C. necator stores polyhydroxybutyrate (PHB), a biopolymer with intracellular carbon and energy storing ability [28].
2.2 Media
Two kinds of culture media were utilized for the growth of bacterial strains: nutrient media and mineral media. Lineup of NM is as follows: yeast extract (2 g/L), beef extract (1 g/L), peptone (5 g/L), glucose (10 g/L), NaCl (5 g/L), KH2PO4 (3.6 g/L), and Na2HPO4 (14.4 g/L). By the addition of 15 g/L of agar to the same content, a nutrient agar medium was formed. MM was prepared using following constituents: KH2PO4 (3.6 g/L), Na2HPO4.12H2O (14.4 g/L), NaCl (0.015 g/L), MgSO4.7H2O (0.5 g/L), CaCl2.2H2O (0.05 g/L), FeSO4.7H2O (0.009 g/L), (NH4)2SO4 (3 g/L), trace elements (1 mL), carbon substrate; molasses (10 g/l), olive oil (10 g/l), and pH (7.4). MnSO4.H2O (1 g/L), CuCl2.2 H2O (0.2 g/L), CoCl2.6H2O (0.5 g/L), ZnCl2 (0.1 g/L), Na2MoO4.2 H2O (0.04 g/L), H3BO3 (0.5 g/L), and NiCl2 (0.05 g/L) made up the trace element solution. For every medium sequentially, the ingredients were combined in baffled Erlenmeyer flasks. After using 2 N NaOH to get the pH to 7.4, flasks were closed using cotton as well as aluminium paper. The culture medium was sanitised at 121 °C for 15 min. To avoid becoming wet throughout this sterilisation procedure, specimen flasks were wrapped in aluminium foil and then preserved. To produce a mineral medium without nitrogen, only (NH4)2SO4 was not added to the above-described components of the mineral medium.
Molasses and olive oil were collected from the sugarcane molasses producing industry and oil refining industry, respectively. It was reacted with H2SO4 solution (0.75 wt%, pH 1.1), and heated for 15 min at 100 °C, 115 °C, and 130 °C. This solution was acidic. 5 M NaOH solution was used to neutralize this solution, and pH was maintained at 7.0. The solution was filtered and autoclaved for 15 min at 121 °C. The lyophilized strain’s development kinetics were carried out according to the previously reported procedure [29]. For these investigational situations, dry cell weight (DCW), as well as PHAs development in pellets, was concluded.
2.3 Effect of different carbon sources on the developmental behaviour of bacterial strains
The influence of seed age on microbial development, as well as PHA formation in bacterial cells, was evaluated with (10 g/L) glucose. Two percent (v/v) inoculum size was transported into a 100-mL mineral medium and incubated at 30 °C, 250 rpm for different time intervals (8–72 h). Specimens were analysed for biomass as well as for the presence of PHA. These experiments revealed that 48 h is suitable for developing PHA pellets. After 48 h, these pellets achieved stable status along with the highest PHA formation. A consequence of various carbon sources on development as well as PHA formation was taken out with glucose (10 g/L), molasses (10 ml/L), olive oil (10 ml/L), and a mixture of molasses and olive oil (5 + 5 ml/L). Flasks were incubated at 30 °C and 250 rpm. The biomass and PHA were examined in culture media after 48 h.
2.4 Optimization of statistical investigation
It took two phases to optimise the media components for PHA production. The initial phase involved identifying the elements that significantly impact PHA development. The second stage looked at these elements’ ideal values. A total of eight factors studied were glucose, treated molasses, crude olive oil, a blend of treated molasses and crude olive oil, KH2PO4/Na2HPO4 (with buffer and without buffer), initial pH, seed age, agitation speed. Next, cell dry weight (CDW) was assessed by measuring the mass provided as follows: The specimens (10 ml of culture) were centrifuged for 15 min at 4 °C and 10,000 rpm. Reconstituted microbial cells were centrifuged after being deposited in distilled water. The cleaned granules were dried in an oven at 90 °C until they reached a consistent weight. Optical density (OD) was measured at a wavelength of 600 nm to assess the growth of cells in the fermentation media.
2.5 Extraction method
In this extraction technique, comparatively fewer chemicals are needed than in solvent extraction. The self-floatation of pellet remnants is the technique used in this process. This process involves fusing pellets in chloroform at 30 °C for 72 h, followed by overnight incubation at room temperature to allow pellet residues to self-float. According to this method, the PHAs were up to 98% pure and 85% effective (w/w), respectively [30]. Additional advantages of this method include lower costs, simpler handling, and less waste of the recovered polymer.
2.6 Fourier Transform-Infrared Spectroscopy (FTIR)
For analyzing the implementation of an IR spectrum, a subsequent process [31] was utilized from earlier laboratory studies [32]. FTIR was used to identify the chemical composition of the extracted PHA. Through centrifugation (3300 g, 7 min), cells were extracted from 10 mL of fermentation media. They were washed twice with 5 mL of sterile saline (9 g/L NaCl). To obtain an adequate concentration for specific assays, the provided cells were dispersed in an appropriate volume of physiological water. For the FTIR analysis, exactly 5 µl of these saturated bacterial solutions were used (400 to 4000 cm−1).
2.7 Estimation of molasses by 3,5-dinitrosalicylic acid
The procedure to verify the sugar concentration of reducing sugars was adopted from the previous studies [33]. By heating the bacterial culture extracted at regular time intervals along with 3,5-dinitrosalicylic acid (DNS), which produced a red-brown product. Since this response is immediate, this approach was used instead of Benedict’s test. The spectrometer calculated the coloured complex’s content at 540 nm of wavelength. A calibration graph was made employing known sugar quantities and was used to detect the sugar content of an unknown specimen. Therefore, the concentration of an unknown solution is determined by diluting a known quantity solution [33].
2.8 Estimation of olive oil by HPLC
The tyrosol-to-syringic acid (internal standard) was revealed as explained in previous studies [34, 35], and total olive oil concentrations were estimated. The method is as follows: Polar components were extracted in methanol/water 80/20 with a 1/10 (w/w) oil/solvent proportion as explained after 1.00 g of EVOO was treated with a known quantity of syringic acid as the standard solution. Following centrifugation, the polar portion (top layer) was gathered, concentrated in a Savant speed-vac to a volume of roughly 0.5 mL, and afterwards dissolved to a volume of 1 mL using trifluoroacetic acid at a concentration of 0.1% (TFA). RP-HPLC was used to isolate one-tenth of the polar extract using only a module HP 1100 chromatographer with a diode array detector. Using a thermostatic oven, a C-18 reversed-phase column measuring 250 2.1 mm in diameter and 4 m in particle size served as the solid phase. A 5–65% gradient of the active ingredient (solvent B: acetonitrile/TFA 0.1%) was applied during physical separation at a constant volume of 0.2 mL min−1, followed by 5 min of isocratic elution at 5% B. The percentage B was raised to 100% following 65 min. In HPLC-grade water, solvent A contained 0.1% TFA. HPLC ChemStation software version A.07.01 incorporated peaks while discharges were observed at = 280 nm wavelengths.
2.9 Gas chromatography-mass spectrometry
Ten milligrams of lyophilized granules were solubilized in 2 mL of a 15% sulphuric acid in methanol solution with 1.0 ml chloroform at − 80 °C and 10 mbar pressure. For 10 min, the tube was swirled at room temp. Two millilitres of dichloromethane with 0.3 g/L of standard solution methyl benzoate were injected. The solution was heated in a reactor for 240 min at 100 °C before being put on ice for 10 min to end the reaction. The tube was filled with two millilitres of demineralized water to differentiate the organic and aqueous portions. Phases were isolated using centrifugation at 2800 g for 5 min after 1 min of mixing. After recovering the lower organic layer, the leftover sulphuric acid was neutralised by 0.4 g of anhydride Na2CO3. After that, the sample was vortexed as well as centrifuged at 2800 g for 5 min while being dehydrated with 0.4 g of anhydrous MgSO4. Subsequently, the organic layer was filtered using nylon filter paper with pore sizes of 0.2 and 0.45 mm. The GC–MS experiment utilized the 1.0 L Sample. The protocol was adapted from previous studies carried out on PHA production from C. necator [36].
2.10 Scanning electron microscopy (SEM)
Using a SEM, the microstructure, as well as surface morphology of specimens, was examined. The sample jar containing the PHA samples was sealed with carbon adhesive tape. Containers holding specimens were moved into the TESCAN VEGA 3 LM SEM. The PHA compounds were screened using a 5kv voltage that was applied.
3 Results and discussion
3.1 Cell growth summary of C. necator DSM 428
Cultivation was accomplished using glucose for the synthesis of biopolymer. Firstly, the cause of seed time on pellet development, as well as P(3HB) formation, was examined. The pellet development along with P(3HB) growth was gained in 24 h (Fig. 1). There was a slight reduction in the quantity of P(3HB) for extended incubation time. The examined development of P(3HB) in addition to pellet concentration was less because of carbon source reduction as well as the production of intracellular by-products, which affected hindrance. Comparison of progress curves showed that exponential phases in RM were reached in about 4–5 h for all strains, while after 8 h, most of the strains entered the stationary growth phase. Growth was relatively slow in MM.
Secondly, the effect of agitation rate on cell development, as well as the P(3HB) synthesis, was also determined by optical density. Both the cell development and P(3HB) formation had a growing tendency at the agitation rate of 250 rpm, as shown in Fig. 2. Cell autolysis occurred for the agitation rate of greater than 250 rpm, and the productivity started to decrease. Thirdly, the effects of the consumption of substrates were determined using optical activity. The consumption of molasses and olive oil was determined separately for the cell development plus P(3HB) formation. In this research, C. necator DSMZ 428 was used to generate the PHA using a mixture of both substrates (molasses and olive oil) for the first time. The effect of consumption of a mixture of both substrates was determined by the growth rate of PHB. Figure 3 depicts that the quantity of molasses is maximum at 0 and decreases as the time (h) increases.
The quantity of olive oil was also maximum at the starting time, but it was reduced as the time (h) increased. The effect of olive oil at the growth rate was the same as described for the molasses but the amount of PHB produced is less compared to molasses given in Fig. 4. The consequence of the addition of olive oil with molasses showed good improvement in biopolymer formation. The highest P(3HB) formation was gained at 48 h incubation, 30 °C, and an agitation speed of 250 rpm Fig. 5. Forty percent of PHA content was produced using a mixture of both substrates Table 1, which was good enough compared to a single substrate. Such as in another study conducted on C. necator DSM 545 using Sodium glutamate produced 33% PHA through extraction procedure [37]. Several other studies showed similar results that using a single substrate produced relatively less amount of PHA, 38% with waste glycerol [36], 25% with glucose [38], 34% with lignin [22], and even 23% with volatile Fatty acids [39].
3.2 Analytical techniques used for characterization of microbial PHA
3.2.1 FTIR spectroscopy used for identification of PHA monomer
FTIR was used for the existence of PHA in microbial cells and to identify the monomers based on functional groups present in the samples. C. necator produced high content of PHA using three types of substrates. This strain produced high PHA content using molasses compared to olive oil. The maximum PHA polymer was produced with a mixture of molasses and olive oil compared to the PHA content produced using these substrates separately. PHA production was examined at different time intervals to check the production rate by these substrates. C. necator produced the maximum PHA height at 48 h with a mixture of both substrates. The spectrum of C. necator DSM 428 developed for 24 h in MM with a mixture of both molasses and olive oil as a substrate shows the presence of a PHA major peak at a wavenumber of 1740 cm−1. Assessment of PHA growth for different time intervals using different carbon sources employing the value of peak at 1740 cm−1 using FTIR is given in Fig. 6.The infrared region consequent carbonyl band of ketones and esters is 1750–1730 cm−1 [40]. The spectrum of C. necator DSM 428 represents the peaks at 1732–1738 cm−1 for PHB presence and at 1739-1740 cm−1 represents the presence of mcl-PHA [32]. A PHA major peak in the region 1750—1730 cm−1 was detected in the spectrum of C. necator DSM 428 developed for 24 h in MM with substrates.
3.2.2 Determination of PHA type produced using gas chromatography-mass spectrometry
PHA content and monomer composition within the bacterial cells were also monitored using GS-MS, as given in Table 2. Mass spectra of methyl 3-hydroxy alkanoates have shown the existence of a particular MS fragment (m/z = 103) that characterizes the molecular size of the fundamental PHA monomer (3HB). Retention times were examined by injecting pure standards (3HB, 3HHx). A comparison of retention times of these standard monomers was prepared with the retention times of these two monomers existing in the samples to authenticate the identification of the presence of the monomer. In the GC–MS chromatograms of the samples, one monomer of scl-PHA (C4: 3HB) and one monomer particular to mcl-PHA were distinguished. GC–MS chromatogram showed PHA monomer peaks of C. necator DSM 428 grown in the MM for 24 h using a mixture of molasses and olive oil as a carbon source. Two different peaks at retention times of 27.53 and 31.51 min indicated 3HB and 3HHx methyl esters in the PHA sample, respectively. C. necator is very well known for the production of short-chain length PHA such as PHB and PHV [41]. In this study, it was the first time observed the production of PHA. It might be due to utilization of a mixture of two complex substrates. Hence, it is proposed that utilization of mineral medium without nitrogen caused degradation of PHB, and energy generated as a result was used to produce mcl-PHA and initiate its metabolic pathway. This specific monomer of mcl-PHA from irrelevant sources and mixture and this shift among PHB and mcl-PHA with the same source have been reported for the first time. GC–MS chromatogram of C. necator DSM 428 cultivated in MM using a mixture of both substrates (treated molasses and crude olive oil) as carbon source is shown in Fig. 7.
Concentrations of PHA produced after the extraction method
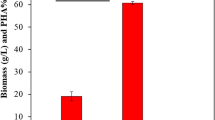
The obtained results showed that adding olive oil with molasses enhanced the PHA concentration to 2.03 g/L in comparison to using molasses 1.41 g/L and olive oil 1.12 g/L, respectively.
3.2.3 Scanning electron microscope (SEM)
The microstructure shows a reasonable spongy material and tiny granules, which are consistent as well as have a powerful affinity to shape multigrain cumulations. Morphology illustrates granules, which are pseudo-spherical in structure along with reasonably homogeneous distribution (Fig. 8).
3.3 Statistical analysis for biomass production
PHA content 6.27%, 24.33%, and 10% values were given by using molasses. After that \(X-\overline{X }\) calculated which were − 9.26, − 8.8, and − 5.53 for 6.27%, 24.33%, and 10% respectively. Then, \({\left(X-\overline{X }\right)}^{2}\) values 85.74, 77.44, and 30.58 were calculated. Then standard deviation was calculated using this formula \(\sqrt{\frac{\sum {\left(X-\overline{X }\right)}^{2}}{N} =}0.892\). An error was calculated by using this formula \(\frac{0.892}{\sqrt{3}}=\pm 0.51.\) Then, PHA content 1.25%, 18.66%, and 2% values were given by using olive oil. The mean value was calculated for PHA content with olive oil, which was 18.66. After that \(X-\overline{X }\) calculated which were − 6.05, 11.36, and − 5.3 for 1.25%, 18.66%, and 2% respectively. \(\Sigma X-\overline{X }\) value 64.57 was calculated for olive oil substrate. Standard deviation and error values were 0.892 and \(\pm\) 0.51, respectively. After that, PHA content 8.76%, 40%, and 16.5% values were given using molasses and olive oil mixture. The mean value was calculated for PHA content with a mixture of molasses and olive oil which was 40. After that \(X-\overline{X }\) calculated which were − 12.99, 18.25, and − 5.25 for 8.76%, 40%, and 16.5% respectively. \(\Sigma X-\overline{X }\) value 175.23 was calculated for a mixture of molasses and olive oil substrate. Standard deviation and error values were 1.47 and \(\pm\) 0.84, respectively. Table 3 illustrates the mean, standard deviation and error values.
Statistical analysis by ANOVA
All experimental fermentation processes were examined/accomplished in triplicate. Optimization of PHA production was carried out using statistical analysis, all data from repetitive quantities were statistically matched as well as estimated by applying the ANOVA. The relationship between means was evaluated through the F-test \(\left(\alpha =0.05\right).\) Table 4 and Table 5 depict the analysis of variance at significance level \(\left(\alpha =0.05\right)\), and analysis of variance F(2,6) = 6.66, p < 0.05 respectively.
Correction term:
Sum of squares of total:
Sum of squares among groups:
Sum of squares within groups:
Mean of the sum of squares among groups:
Mean of the sum of squares within groups:
Null hypothesis:
Alternative hypothesis:
Significance level:
Calculated F > F (table: \(\alpha =0.05\))
So, analysis of variance F(2,6) = 6.66, p < 0.05.
Hence, with 95% confidence, there is a significant effect of seed age on the CDW% of PHA.
C. necator is usually used specifically to produce PHAs consisting of 3HB monomers abundantly, by using carbon substrates like molasses as well as other vegetable oils. Various researches have confirmed the production of pure PHB by using vegetable oils [42]. Dennis et al. [43] confirmed that the C. necator synthase could take C6 carbon sources. Fewer findings have also recognized bigger monomeric components by veg-oils as substrates [44].
Optimization of physical and chemical factors is vital for achieving a high/maximum PHA production of PHAs. Various kinds of substrates were used for the PHA manufacturing like molasses, olive oil, and a mixture of these substrates. The addition of olive oil with molasses showed good improvement by using optical density (OD) on biopolymer formation. OD evaluated the effects of seed age on cell growth as well as the impact of agitation speed on pellet developments. The quantity of molasses and olive oil was high at the starting point but reduced as the time (h) increased. Effect of the amount of olive oil and molasses same at the growth rate, but the amount of PHB produced is less from olive oil compared to molasses. PHA content % CDW produced using various carbon substrates like molasses, and olive oil, as well as a mixture of both substrates, was 24.33%, 18.66% and 40%, respectively.
The main aim of this research was to make the PHB along with mcl-PHA (HHx) using molasses and olive oil from C. necator DSM 428. The amount of nitrogen also impacts the development of PHA, the present quantity of nitrogen in the medium has a good effect on the cell shifting. Nitrogen-deficient media is required to enhance the dry cell weight (CDW%) of PHA rather than cell shifting. Cell shifting is developed by the media having a considerable nitrogen amount. Highly deficient media has been revealed to increase the cell weight of PHB monomers in C. necator. In this study, using three different kinds of substrates (molasses, olive oil, and a mixture of both), this development achieved the main objective of this research work by gaining the HHx monomer and PHB using mineral media with a mixture of mixture molasses and olive oil by C. necator. Additionally, a drop in the medium's ultimate pH during fermentation due to bacterial cells transitioning from the exponential phase to the stationary phase was investigated. According to Philip et al., low pH levels prevent PHAs from degrading in an unbuffered media [45]. Buffers are used in media to maintain pH. Culture media turns acidic without buffers after 15 h of culture, and PHA production is inhibited.
PHA is produced using molasses, olive oil, and the mixture of molasses and olive oil from C. necator confirmed by FTIR analysis. This strain produced high PHA with molasses compared to the olive oil. Good yield of PHA polymer produced by the mixture of both substrates compared to the PHA content produced by using these substrates separately. A spectrum of C. necator DSM 428 represents the peaks at 1732–1738 cm−1 for PHB presence and at 1739–1740 cm−1 represents the presence of mcl-PHA. PHA major peak in the region 1750—1730 cm−1 was detected in the spectrum of C. necator DSM 428 developed for 24 h in MM with substrates. The spectrum of the strain, when it produced PHA, consisted of three peaks at 1060, 1085, and 1101 cm−1, while the spectrum consisted of only one peak at 1085 cm−1 when the cells did not store PHA. Two more peaks at 1185 and 1260 cm−1 were also observed in the polysaccharide region in the spectrum of the cells producing PHA. Polysaccharide peaks have been observed at different positions in spectra of strains producing PHA. These different positions of the same peak considered the type of PHA, like; 1260 cm−1 corresponded to PHB whereas, 1160 cm−1 indicated mcl-PHA existence in C. necator [46, 47].
GC–MS chromatogram showing PHA monomer peaks of C. necator DSM 428 grown in a MM for 24 h using a mixture of both substrates (molasses + olive oil) as carbon source. Two different peaks at retention times of 27.53 and 31.51 min indicated 3HB and 3HHx methyl esters in the PHA sample, respectively [48]. The considerably great (p < 0.05) yield of PHAs (40%) was achieved at 250 rpm, pH 4.7, as well as 30 °C. The manufacturing of PHAs was carried out with C. necator for 24 h to regulate an appropriate harvesting period. C. necator produced optimal quantities of 77% of PHAs in continuous fermentation [49]. The PHAs synthesis was carried out at different seed ages to gain its optimum content. The best possible yield of PHAs (40%) was gained at 24 h older inoculum of C. necator at pH 7.4, 30 °C as well as 250 rpm. In other research, PHA (8%) was produced by the same strain using rapeseed oil with propanol in fed-batch fermentation [50]. The molasses, olive oil, and mixture of both were individually added to the medium to estimate their influence on PHAs content. A considerably (p < 0.05) content of PHAs 24.33%, 18.66%, and 40% was achieved by molasses, olive oil, and a mixture of both respectively in MM after 24 h of incubation at pH 7, 30 °C, and 250 rpm.
4 Conclusion
This research evaluated the results of cheap carbon substrates of industrial waste as an alternative to costly ones for production enhancement of mcl-PHA, specifically. The strain was cultured in nutrient and mineral media with substrates at different experimental conditions. PHA content was found to be 24.33%, 18.66%, and 40% with molasses, olive oil, and a mixture of both substrates, respectively. The chromatographic technique, GC–MS, was utilized to confirm the types of PHA monomers (3HB and HHx). Morphology exhibits granules that are pseudo-spherical in structure with comparatively consistent distribution by Scanning Electron Microscopy. Biopolymer concentration 2.03 g/L is produced using a mixture of olive and molasses compared to the separately used substrates. The strain is capable of producing PHA from even complex sources and waste materials. It also permits bacterial growth even in nitrogen-deficient conditions and is also associated with an unusual PHA metabolism. Future research is required, specifically to understand how nitrogen contributes to the unanticipated metabolic change for unrelated substrates.
References
Chidambarampadmavathy K, Karthikeyan OP, Heimann K (2017) Sustainable bio-plastic production through landfill methane recycling. Renew Sustain Energy Rev 71:555–562
Napathorn S et al (2010) Biosynthesis and biocompatibility of biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Polym Degrad Stab 95(10):2003–2012
Amache R et al (2013) Advances in PHAs production. Chem Eng Trans 32:931–936
Jendrossek D (2009) Polyhydroxyalkanoate granules are complex subcellular organelles (carbonosomes). J Bacteriol 191(10):3195–3202
Jendrossek D, Handrick R (2002) Microbial degradation of polyhydroxyalkanoates. Annu Rev Microbiol 56:403–32
Steinbüchel A, Hein S (2001) Biochemical and molecular basis of microbial synthesis of polyhydroxyalkanoates in microorganisms. Adv Biochem Eng Biotechnol 71:81–123. https://doi.org/10.1007/3-540-40021-4_3
Zinn M, Witholt B, Egli T (2001) Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv Drug Deliv Rev 53:5–12
Salma S et al (2021) Polyhydroxyalkanoates: next generation natural biomolecules and a solution for the world’s future economy. Int J Biol Macrol 166:297–321
Kunasundari, Sudesh (2011) Isolation and recovery of microbial polyhydroxyalkanoates. Express Polym Lett 5(7):620–634
Wang S et al (2016) Modification and potential application of short-chain-length polyhydroxyalkanoate (SCL-PHA). Polymers 8:273
Vibhuti S, Rutika S, Reena G (2021) Polyhydroxyalkanoate (PHA): properties and modifications. Polymers 212. https://doi.org/10.1016/j.polymer.2020.123161
Ahmad MG, Muhammad HA, Mohamad SMA (2015) Modification of polyhydroxyalkanoates (PHAs). RSC Green Chem 30:141–182
Chee JY et al (2010) Bacterially produced polyhydroxyalkanoate (PHA): converting renewable resources into bioplastics. App Microbiol Microb Biotechnol 2:1395–1404
Ojumu TV, Yu J, Solomon BO (2004) Production of polyhydroxyalkanoates, a bacterial biodegradable polymer. Afr J Biotechnol 3(1):18–24
Aramvash A (2016) Effective enhancement of hydroxyvalerate content of PHBV in Cupriavidus necator and its characterization. Int J Biol Macromol 87:397–404
Wong AL, Chua H, Yu PHF (2000) Microbial production of polyhydroxyalkanoates by bacteria isolated from oil wastes. Appl Biochem Biotechnol 84–86:843–857. https://doi.org/10.1385/ABAB:84-86:1-9:843
Arikawa H, Matsumoto K, Fujiki T (2017) Polyhydroxyalkanoate production from sucrose by Cupriavidus necator strains harboring csc genes from Escherichia coli W. Appl Microbiol Biotechnol 101(20):7497–7507. https://doi.org/10.1007/S00253-017-8470-7
Munawar KMM, Simarani AK (2016) Bioconversion of mix free fatty acids to poly-3-hydroxyalkanoates by Psoudomonas putida BET001 and modelling of fermentation in shake flask. Electron J Biotechnol 19:50–55
Sindhu R et al (2013) Pentose-rich hydrolysate from acid pretreated rice straw as a carbon source for the production of poly-3-hydroxybutyrate. Biochem Eng J 78:67–72
Volova T, Sapozhnikova K, Zhila N (2020) Cupriavidus necator B-10646 growth and polyhydroxyalkanoates production on different plant oils. Int J Biol Macromol 164:121–130
Chaudhry WN et al (2011) Screening for polyhydroxyalkanoate (PHA)-producing bacterial strains and comparison of PHA production from various inexpensive carbon sources. Ann Microbiol 61:623–629
Li M, Wilkins M (2020) Fed-batch cultivation and adding supplements to increase yields of polyhydroxybutyrate production by Cupriavidus necator from corn stover alkaline pretreatment liquor. Biores Technol 299:122676
Gonzalo A et al (2013) Production of polyhydroxyalkanoates by Cupriavidus necator from treated olive mill wastewater. Environ Eng Manag J 12(S11):97–100
Li M, Wilkins M (2020) Flow cytometry for quantitation of polyhydroxybutyrate production by Cupriavidus necator using alkaline pretreated liquor from corn stover. Bioresour Technol 295
SharifzadehBaei M, Najafpour GD, Younesi H, Tabandeh F, Eisazadeh H (2009) Poly(3-hydroxybutyrate) Synthesis by Cupriavidus necator DSMZ 545 Utilizing Various Carbon Sources. World Appl Sci J 7:157–161
Masood F, Abdul-Salam M, Yasin T, Hameed A (2017) Effect of glucose and olive oil as potential carbon sources on production of PHAs copolymer and tercopolymer by Bacillus cereus FA11. 3 Biotech 7(1). https://doi.org/10.1007/S13205-017-0712-Y
Pohlmann A et al (2006) Genome sequence of the bioplastic-producing ‘Knallgas’ bacterium Ralstonia eutropha H16. Nat Biotechnol 24(10):1257–1262. https://doi.org/10.1038/NBT1244
Verlinden RAJ, Hill DJ, Kenward MA, Williams CD, Radecka I (2007) Bacterial synthesis of biodegradable polyhydroxyalkanoates. J Appl Microbiol 102(6):1437–1449. https://doi.org/10.1111/J.1365-2672.2007.03335.X
Salma S et al (2013) Impact of carbon source and variable nitrogen conditions on bacterial biosynthesis of polyhydroxyalkanoates: evidence of an atypical metabolism in Bacillus megaterium DSM 509. J Biosci Bioeng 116(3):302–308
Ibrahim MHA, Steinbüchel A (2009) Poly(3-hydroxybutyrate) production from glycerol by Zobellella denitrificans MW1 via high-cell-density fed-batch fermentation and simplified solvent extraction. Appl Environ Microbiol 75(19):6222–6231. https://doi.org/10.1128/AEM.01162-09
Amiel C (2000) Potentiality of Fourier Transform Infrared Spectroscopy (FTIR) for discrimination and identification of dairy Lactic acid bacteria. Lait 80(4):445–459
Salma S et al (2021) New model development for qualitative and quantitative analysis of microbial polyhydroxyalkanoates: a comparison of Fourier Transform Infrared Spectroscopy with Gas Chromatography. J Biotechnol 329:38–48
Baei MS, Najafpour GD, Younesi H, Tabandeh F, Eisazadeh H (2009) Poly (3-hydroxybutyrate) synthesis by Cupriavidus necator DSMZ 545 utilizing various carbon sources. World Appl Sci J 7(2):157–161. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Poly+%283-hydroxybutyrate%29+synthesis+by+Cupriavidus+necator+DSMZ+545+utilizing+various+carbon+sources.+World+Appl+Sci+J+7%282%29%3A157%E2%80%93161&btnG=
International Olive Council (2009) Official method of analysis. Determination of biophenols in olive oil by HPLC. COI/T.20/Doc. No. 29
Siano F, Vasca E, Picariello G (2022) Accurate determination of total biophenols in unfractionated extra-virgin olive oil with the fast blue BB assay. Food Chem 370:130990. https://doi.org/10.1016/J.FOODCHEM.2021.130990
Cavalheiro JMBT, de Almeida MCMD, Grandfils C, da Fonseca MMR (2009) Poly(3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Process Biochem 44:509–515
Berezina N (2013) Novel approach for productivity enhancement of polyhydroxyalkanoates (PHA) production by Cupriavidus necator DSM 545. New Biotechnol 30(2):192–195
Pradhan S, Dikshit PK, Moholkar VS (2018) Production, ultrasonic extraction, and characterization of poly (3-hydroxybutyrate) (PHB) using Bacillus megaterium and Cupriavidus necator. Polym Adv Technol 29(8):2392–2400. https://doi.org/10.1002/PAT.4351
Al Battashi H, Al-Kindi S, Gupta VK, Sivakumar N (2021) Polyhydroxyalkanoate (PHA) production using volatile fatty acids derived from the anaerobic digestion of waste paper. J Polym Environ 29(1):250–259. https://doi.org/10.1007/S10924-020-01870-0
Helm D, Labischinski H, Schallehn G, Naumann D (1991) Classification and identification of bacteria by Fourier-transform infrared spectroscopy. J Gen Microbiol 137(1):69–79
Atlić A, Koller M, Scherzer D, Kutschera C, Grillo-Fernandes E, Horvat P, Chiellini E, Braunegg G (2011) Continuous production of poly([R]-3-hydroxybutyrate) by Cupriavidus necator in a multistage bioreactor cascade. Appl Microbiol Biotechnol 91(2):295–304
Oliveira FC, Freire DMG, Castilho LR (2004) Production of poly(3-hydroxybutyrate) by solid-state fermentation with Ralstonia eutropha. Biotechnol Lett 26(24):1851–1855
Dennis D, McCoy M, Stangl A, Valentin HE, Wu Z (1998) Formation of poly(3-hydroxybutyrate-co-3- hydroxyhexanoate) by PHA synthase from Ralstonia eutropha. J Biotechnol 64(2–3):177–186
Rathinasabapathy A, Ramsay BA, Ramsay JA, Pérez-Guevara F (2014) A feeding strategy for incorporation of canola derived medium-chain-length monomers into the PHA produced by wild-type Cupriavidus necator. World J Microbiol Biotechnol 30(4):1409–1416
Philip S, Sengupta S, Keshavarz T, Roy I (2009) Effect of impeller speed and pH on the production of poly(3- hydroxybutyrate) using Bacillus cereus SPV. Biomacromolecules 10(4):691–699. https://doi.org/10.1021/bm801395p
Kumar P, Ray S, Patel SKS, Lee JK, Kalia VC (2015) Bioconversion of crude glycerol to polyhydroxyalkanoate by Bacillus thuringiensis under non-limiting nitrogen conditions. Int J Biol Macromol 78:9–16. https://doi.org/10.1016/J.IJBIOMAC.2015.03.046
Nygaard D, Yashchuk O, Hermida ÉB (2021) PHA granule formation and degradation by Cupriavidus necator under different nutritional conditions. J Basic Microbiol 61(9):825–834. https://doi.org/10.1002/JOBM.202100184
Saratale GD, Oh M-K (2015) Characterization of poly-3-hydroxybutyrate (PHB) produced from Ralstonia eutropha using an alkali-pretreated biomass feedstock. Int J Biol Macromol 80:627–635
Atlić A et al (2011) Continuous production of poly([R]-3-hydroxybutyrate) by Cupriavidus necator in a multistage bioreactor cascade. Appl Microbiol Biotechnol 91(2):295–304. https://doi.org/10.1007/S00253-011-3260-0
Obruca S, Marova I, Snajdar O, Mravcova L, Svoboda Z (2010) Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Cupriavidus necator from waste rapeseed oil using propanol as a precursor of 3-hydroxyvalerate. Biotechnol Lett 32(12):1925–1932. https://doi.org/10.1007/S10529-010-0376-8
Acknowledgements
Analytical and instrumentation facilities like SEM and FTIR was provided by NTU; Faisalabad is appreciatively acknowledged.
Funding
Higher Education Commission (HEC), Islamabad, Pakistan, supported this work. The authors are grateful to the Government College Women University, Faisalabad, Pakistan, for financial support under the in-house research grant program.
Author information
Authors and Affiliations
Contributions
Conception and design of the study, Salma Shahid, Muhammad Bilal; investigation, Sadia Razzaq; formal analysis and data curation, Robina Farooq; software and validation, Sadia Noreen, Sofia Perveen; writing—review and editing, Salma Shahid, Muhammad Bilal; supervision, Salma Shahid. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent of publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Razzaq, S., Shahid, S., Farooq, R. et al. Sustainable bioconversion of agricultural waste substrates into poly (3-hydroxyhexanoate) (mcl-PHA) by Cupriavidus necator DSM 428. Biomass Conv. Bioref. 14, 9429–9439 (2024). https://doi.org/10.1007/s13399-022-03194-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03194-6