Abstract
Many bacteria produce polyhydroxyalkanoates (PHAs) when exposed to stressful conditions. It is a known fact that PHAs have the potential to replace petrochemical-based plastics as they are biodegradable, biocompatible, and thermoprocessible materials. The study focusses on producing PHA from microbes isolated from polluted environments and pomegranate peels were utilized as a unique carbon source. This was done to ensure reduction in the cost of the substrate that has not yet been reported as a substrate for PHA production. A comparative study was also done with Cupriavidus necator, the reference strain. Out of many bacterial strains, isolated, eight of these were found to have ability to produce PHA. Pomegranate peel was substituted as carbon source in the medium and all bacterial isolates along with reference strain were used to test their ability to produce PHA from waste. Cupriavidus necator, the reference strain, yielded 71% PHA. Bacillus halotolerans DSM8802 yielded 83% at 1:1:: C:N ratio at 72 h.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bio-based plastics have received tremendous attention in recent years because of their high molecular weight, biodegradability, non-toxicity, thermoplasticity, biocompatibility, elastomeric properties and because of their production from renewable carbon sources. The research in the packaging industry develops and also promotes the use of bioplastics as they prove helpful in reducing waste disposal and are better substitutes for petroleum which is a non-renewable resource. The issue of a large quantity of waste generated due to the non-degradable food packaging materials has ignited the need for research on biopolymers (Ruban 2009; Souza et al. 2010; Azeredo et al. 2012; Gabor et al. 2012). The petrochemical-based plastic materials are the most difficult to manage waste on planet earth because the fact that they are non-biodegradable but have a wide range of applications in households, containers, packaging films, etc. (Zaid et al. 2015; Hawas et al. 2016). Currently various alternative materials are being developed based on natural polymers due to increase in the price of petrochemicals, problems related to their disposal and raising environmental concerns (Farris et al. 2009). A wide array of microorganisms belonging to Gram-positive or Gram-negative and also those belonging to Halobacteriaceae family of the Archaea are known to produce polyhydroxyalkanoates, which are simple macromolecules and belong to the family of biopolyesters that occur naturally and combination of monomers is very much possible which dictates the physical properties of the polymer (Prabhu et al. 2010; Tembadmani et al. 2015). These polymers are produced under unfavorable or stressful conditions, such as non-optimal pH, limited phosphorus, nitrogen, and oxygen. Further, these are stored as food/energy reserves in the presence of excess carbon. Although there are many known PHA polymers, some of the best-known representatives are polyhydroxyalkanoate (PHB) and polyhydroxybutyrate-co-hydroxyvalerate (PHBV) (Kumari and Dhingra 2013). Since it is synthesized naturally, the degradation happens by existing soil microbes which possess depolymerase enzyme complex (Aburas 2016). The blends made out of the polymers have the properties similar to most of the synthetic plastics (Gomaa 2014). Currently, production cost of materials made up of PHA is a challenge as it very expensive and hence widespread research is being carried out to incorporate the inexpensive carbon sources as the raw materials or by improving the yield by applying the knowledge of synthetic biology and genetic engineering (Chanprateep et al. 2010). Our approach towards reducing the effective cost of PHAs is to utilize fruit waste, such as pomegranate peel, which has never been tested as a carbon source for the production of PHAs.
Materials and methods
The bacterial cultures were selected and isolated from polluted land-fills soil, Kalyan, Maharashtra, and Effluent water treatment plants, REVA University, Bangalore. The bacterial isolates were further screened for their ability to produce PHA. Further biochemical testing and growth optimization studies were performed according to protocol explained in Rayasam and Kumar (2019).
Carbohydrate estimation of the raw materials
The inexpensive raw material used as a carbon source in our study is pomegranate peel. Total carbohydrate estimation of selected raw material was done using Phenol–sulphuric acid method (Dubois et al. 1956; Krishnaveni et al. 1984).
C:N ratios
Various ratios of pomegranate peel were used as a substitute for glucose as a carbon source with yeast extract as nitrogen supplement (C:N ratios 1:1, 2:1, 3:1 and 4:1). The amount of PHA produced was quantified at different optimum incubation periods for respective bacterial isolate (Kumar et al. 2009).
Preparation of media for obtaining the Dry Cell Weight (DCW) and PHA production
Medium with nutrient broth components except carbon source was prepared. The raw material (pomegranate peel as per ratio) was added with the yeast extract as the nitrogen source. The Cupriavidus necator, the reference strain, was also used to compare PHA production values in comparison to our isolates. The isolate was subjected to the same parameters as ours. Medium was prepared for each ratio with the desired standardized pH for particular bacterial isolate and autoclaved. The inoculum was prepared and inoculated with actively growing bacterial cells. The flasks were incubated for four consecutive days. The amount of biomass obtained and PHA accumulation was studied after sample withdrawal every 24 h till 4 days. The first step involved centrifugation of culture media to obtain the biomass as a precipitate. After complete drying, the DCW was obtained in g/L.
Extraction of polyhydroxyalkanoates
The extraction of PHA was performed using the chloroform extraction method. The pellet which was obtained after drying was suspended in 4% sodium hypochlorite solution and kept at 37 °C for 2 h for complete digestion of cellular components. It was then centrifuged to collect PHA granules and the supernatant was discarded. Mixture was washed twice with 10 ml distilled water and centrifuged again. Granules in the sediment were washed twice with a solution of acetone: methanol: diethyl ether (1:1:1). Further, the polymer granules were dissolved in boiling chloroform and were evaporated by air-drying, to yield dry powder of PHA which was then weighed to obtain the amount of extracted PHA (g/L) (Jacquel et al. 2008; Chee et al. 2010; Santhanam and Sasidharan 2010; Marjadi and Dharaiya 2011; Shivakumar 2012; Chaitanya et al. 2014).
Quantification of polyhydroxyalkanoates
Quantification of the percentage PHA accumulation was calculated by applying the following formula (Du et al. 2001; Preethi et al. 2012).
Analysis of variance
Experimental results were processed using analysis of variance (ANOVA). This method was used for testing the effect of considered parameters (four different hours of incubation) on the PHA accumulation. By performing the analysis of variance, it can be decided which independent factor dominates over the other and the percentage contribution of that particular independent variable. This analysis is carried out for a significance level of α- 0.05, i.e. for a confidence level of 95%.
PCR amplification and 16s rRNA gene sequence studies
Genomic DNA isolation and 16s rRNA sequence studies using PCR amplification were performed as explained in Rayasam and Kumar 2019 (Rayasam and Kumar 2019; Girija et al. 2013).
Nucleotide BLAST and phylogenetic tree
The nucleotide BLAST was performed to check the similarity of the bacterial isolates with the bacterial isolates’ database already available on NCBI portal. The 16s rRNA gene sequences of all the bacterial isolates were submitted to GenBank and the accession numbers were procured and phylogenetic tree was constructed to determine how related the bacterial isolates are to each other.
Results
Selection and screening of bacteria
Out of the total isolated bacterial isolates, 8 of them demonstrated to produce PHA using Sudan Black B staining procedure on nutrient agar and also on minimal agar media.
Biochemical testing and growth parameters
The bacterial isolates were biochemically characterized to ensure that each of one of our isolates was different from the other and a set of temperature, pH, carbon and nitrogen sources were provided to all the bacterial isolates with variation in incubation time before the optimum growth conditions for each one of them could be decided. The results of the biochemical tests and optimum growth parameters are shown in Table 1.
Carbohydrate estimation of the raw materials
The Total Carbon Content was found to be 10%, as indicated, referred as per Zhu and Liu (2013).
Biomass and PHA production using pomegranate peels
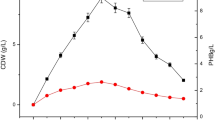
All the bacterial isolates were grown in media containing the pomegranate peels and nitrogen source in respective ratios to estimate biomass and PHA production. Significant results are mentioned hereafter. The maximum amount of biomass produced by Bacillus halotolerans DSM8802 was 31 g/L at 4:1:: C:N ratio at 48 h at which the amount of PHA produced was 3 g/L. At 1:1:: C:N ratio at 48 h, 22 g/L of biomass and 2.5 g/L of PHA production were observed. The highest amount of PHA production of 5.5 g/L was observed at 2:1 and 3:1:: C:N ratios at 48 h during which 18.5 g/L and 10 g/L of biomass production were observed, respectively (Online Resource 1). Bacillus siamensis LFS1715 produced maximum biomass of 8.5 g/L at 4:1:: C:N ratio at 48 h during which 2.5 g/L of PHA production was obtained. The highest PHA production by the bacteria was observed at 3:1:: C:N ratio at 72 h during which 3 g/L of PHA was produced from 6 g/L of biomass (Online Resource 1). Bacillus halotolerans KSI1507 produced the highest amount of biomass as compared to all other bacteria. It produced 74 g/L of biomass and could convert 27 g/L of it into PHA at 1:1::C:N ratio at 24 h. Again, at 24 h at 2:1:: C:N ratio, the bacterium produced 67 g/L of biomass out of which 34.5 g/L was converted into PHA. During the same incubation time, the bacterium produced the highest amount of PHA of 36.5 g/L from 67 g/L of biomass at 4:1:: C:N ratio (Online Resource 1). Geobacillus stearothermophillus TKR1707 could produce 9.5 g/L of biomass in 2:1::C:N ratio at 24 h at which 4.5 g/L of PHA was produced. 9 g/L of biomass production was observed at 4:1:: C:N ratio at 72 h out of which 4 g/L was converted to PHA. Maximum PHA found to produce by the bacterium was 6.5 g/L at 1:1::C:N ratio at 72 h during which 8.5 g/L of biomass was produced (Online Resource 2). Bacillus subtilis RVR2988 produced 8.5 g/L of biomass at 1:1 and 4:1:: C:N ratios at 24 h at which 2.5 g/L and 3.5 g/L of PHA production were observed, respectively. Highest PHA production by Bacillus subtilis RVR2988 was 5 g/L from at 3:1:: C:N ratio at 72 h and 4:1:: C:N ratio at 96 h during which 7.5 g/L and 7 g/L of biomass production were observed, respectively (Online Resource 2). The maximum amount of biomass production by Bacillus siamensis RET2912 was 8.5 g/L from which 1.5 g/L was converted into PHA at 4:1:: C:N ratio at 48 h. 4 g/L of PHA production was observed at 3:1:: C:N ratio at 24 h and 48 h during which 7.5 g/L and 5.5 g/L of biomass production were observed, respectively (Online Resource 2). Bacillus velezensis BTR2015 produced a maximum 6 g/L of biomass from which it could convert 4.5 g/L into PHA at 4:1:: C:N ratio at 24 h which was also the maximum amount of PHA produced by the bacterium (Online Resource 3). Staphylococcus aureus ESR1315 produced 6 g/L of biomass at 3:1::C:N ratio at 24 h during which 3 g/L of PHA was produced. The maximum PHA produced by the bacteria was 3 g/L at 2:1 and 3:1:: C:N ratios at 24 h and 3:1 and 4:1:: C:N ratios at 72 h (Online Resource 3). Cupriavidus necator, the reference strain, produced maximum biomass of 4.5 g/L at 4:1::C:N ratio at 48 h during which 2.6 g/L was converted into PHA. 4 g/L of biomass was produced at 2:1:: C:N ratio at 72 h and 4:1:: C:N ratio at 96 h and during these time periods, 2.4 g/L and 2 g/L of PHA were produced, respectively (Online Resource 3).
Extraction and quantification of polyhydroxyalkanoates
Before the start of the extraction procedure, optimized growth conditions were provided to each bacterial isolate with the addition of pomegranate peel as the carbon source. The biomass production and PHA production were analyzed in four different C:N ratios for 96 h with the intermittent withdrawal of the sample at every 24 h. Then, the amounts of biomass and PHA yield were used to quantify the percentage of PHA accumulation by each bacterial isolate. The best results obtained by all the bacterial isolates are as follows: Bacillus halotolerans DSM8802 accumulated 83% of PHA at 1:1:: C:N ratio at 72 h (Online Resource 1), Bacillus siamensis LFS1715 accumulated 75% of PHA at 2:1:: C:N ratio at 72 h (Online Resource 1), Bacillus halotolerans KSI1507 accumulated 67% of PHA at 2:1:: C:N ratio at 72 h (Online Resource 1), Geobacillus stearothermophilus TKR1707 accumulated 76% PHA at 1:1:: C:N ratio at 72 h (Online Resource 2), Bacillus subtilis RVR2988 accumulated 71% PHA at 4:1:: C:N ratio at 96 h (Online Resource 2), Bacillus siamensis RET2912 accumulated 73% of PHA at 3:1:: C:N ratio at 48 h (Online Resource 2), Bacillus velezensis BTR2015 accumulated 75% of PHA at 4:1:: C:N ratio at 24 h (Online Resource 3), Staphylococcus aureus ESR1315 accumulated 67% PHA at 2:1:: C:N ratio at 48 h and 3:1:: C:N ratio at 72 h (Online Resource 3). Cupriavidus necator, the reference strain, accumulated 71% of PHA at 3:1, 4:1:: C:N ratios at 24 h and 72 h, respectively (Online Resource 3). It was also observed that when the amount of biomass was less, the corresponding PHA production was high and vice versa. The detailed graphical comparison between the amount of biomass production and PHA accumulation during each hour of incubation is given below (Figs. 1, 2, 3 and 4).
Percentage deviation in PHA accumulation obtained by the bacterial isolates compared to the reference bacterium (Cupriavidus necator)
Bacillus halotolerans DSM8802 demonstrated to accumulate 24% more and 10% more PHA than the reference bacterium at 1:1 and 3:1:: C:N ratios at 72 h. Bacillus siamensis LFS1715 accumulated 25% more PHA than the reference bacterium at 1:1:: C:N ratio at 24 h. Bacillus halotolerans KSI1507 accumulated 2% more amount of PHA at 2:1:: C:N ratio at 24 h. Geobacillus stearothermophilus TKR1707 demonstrated to accumulated 68% and 6% more amount of PHA at 1:1 and 2:1:: C:N ratios at 24 h, respectively, and at 1:1:: C:N ratios at 72 h and 96 h, it demonstrated to accumulate 13% and 8% more PHA, respectively. Bacillus subtilis RVR2988 accumulated 28% more PHA than the reference bacterium at 2:1:: C:N ratio at 24 h. While Bacillus siamensis RET2912 showed 34% more PHA accumulation at 1:1:: C:N ratio at 96 h, 8% increase in PHA at 1:1 and 2:1:: C:N ratios at 24 h, respectively, and 9% increased PHA accumulation at 3:1:: C:N ratio at 48 h was observed. Bacillus velezensis BTR2015 at 24 h demonstrated to accumulated 46% and 6% increased the amount of PHA at 2:1 and 4:1:: C:N ratios, respectively. Staphylococcus aureus ESR1315 accumulated 25% more PHA at 1:1:: C:N ratio, 20% at 2:1:: C:N ratio at 24 h and 12% increase in PHA at 4:1:: C:N ratio at 96 h. All of the above results are tabulated in Online Resource 4.
Analysis of variance
This analysis is carried out for a significance level of α- 0.05, i.e. for a confidence level of 95%. Incubation periods with P value less than 0.05 were considered to have a statistically significant contribution towards the amount of biomass production and PHA accumulation by each bacterial isolate. Online Resources 5 and 6 show the ANOVA results for the biomass production and PHA accumulation, respectively, by each bacterial isolate in four different incubation times.
Nucleotide BLAST and phylogenetic tree
The phylogenetic tree was prepared using neighbor-joining method. The evolutionary distances were found out using Maximum Likelihood method. The software used was MEGAX version 10.0.4 (Fig. 5).
Discussion
PHAs are synthesized by different microorganisms as energy reserves, such as Pseudomonas, Alcaligenes and Algae, in excess of carbon and limitation of nitrogen (Thapa et al. 2018). They are also known to be produced by many Gram-positive, Gram-negative bacteria (Godoy et al. 2003; Srilakshmi and Ramachandra 2012) and Archaea when these microorganisms are subjected to environmentally stressful conditions like limited nutrients (Fernandez et al. 1986). We would like to mention some of the relatable works which are done on similar lines as ours. Shah et al. (2014) in a study illustrated the capability of accumulation of PHA by 12 bacterial isolates. Out of these isolates of Bacillus subtilis, G1S1 showed maximum production of about 0.020 g/L in glucose medium and 0.016 g/L in yeast extract medium. Another report claimed that addition of 6% molasses as carbon source and 1% ethanol, ammonium sulphate and ammonium nitrate as nitrogen sources to the media resulted in accumulation of 54–62% of PHA by Bacillus subtilis (Gomaa 2014). Bacteria could accumulate 37% (w/w) and 23% (w/w) of PHA, respectively, when corn oil and waste vegetable oil were used as substrate (Song et al. 2008). It was found that Bacillus thuringiensis accumulates 72.05% when starch in used as carbon source in the media (Yao et al. 1999; Gowda and Shivakumar 2014). Porwal et al. (2008) studied different Bacillus strains and reported that Bacillus cereus EGU3 could accumulate up to 66% of PHA when glucose was used as carbon source. (Porwal et al. 2008). While in one of the studies, 91% recovery of PHA was reported using Ralstonia eutropha on a large-scale (50L) fermenter with sodium gluconate as the carbon source and the extraction procedure was done by sodium hypochlorite method (Heinrich et al. 2012). Paul and coworkers studied different steps like optimization, purification and characterization using Bacillus cereus isolated from sewage for PHB production. They found mixed fruit pulp (298 µg/ml of PHB) to be the best alternative for the carbon source among various other inexpensive sources utilized (Paul et al. 2017). Kumar et al. (2007) reported use of malic acid as carbon source and that Bacillus sps.256 could yield a copolymer of P(HB-co-HV) with a higher HB content. Fermentation strategies were incorporated, resulted in 54% of PHA content with 2.7 g/L of yield. The same bacterial strain yielded 2.5 g/L, which is 52% of PHA (Kumar et al. 2007). On the other hand, review studies reveal that use of inexpensive raw materials like fermented mash, rice straw, wheat straw, tallow, starch, bean curd waste, whey, waste frying oil, sugarcane bagasse, spent mash, vinasse, molasses and spent coffee grounds can yield 8–89% of polymer by various bacteria using different production strategies (Raza et al. 2018). In 2011, Omar and his colleagues tested the PHB accumulation, yield and productivity of Cupriavidus necator CCGUG 52,238 using organic acids from the kitchen waste and reported that under the conditions of batch-fermentation, the bacterium could accumulate highest PHA of about 52.79% of the yield (Omar et al. 2011). A detailed discussion about our results obtained by the selected bacterial isolates of ours is described below.
Cupriavidus necator, the reference strain
At 2:1:: C:N ratio at 72 h, 60% of PHA accumulation along with 2.4 g/L of PHA was produced from 4 g/L of DCW. The reference bacterium showed 71% of PHA accumulation at multiple parameters like 3:1 and 4:1:: C:N ratios at 24 h and 3:1 and 4:1:: C:N ratios at 72 h during which 3.5 g/l of biomass and 2.5 g/L of PHA were produced, respectively, in all the parameters. Among other bacterial isolates with high rates of accumulation, Geobacillus stearothermophilus R35646 accumulated 84.63% of PHA (Rayasam and Kumar 2018). We in 2019, reported that Bacillus subtilis JCM 1465 accumulated up to 89% and 75% of PHA, onion and orange peel-based media (Rayasam and Kumar 2019).
Bacillus halotolerans DSM8802
This bacterium resulted in 83% of PHA accumulation at 1:1:: C:N ratio at 72 h at which the amount of DCW accumulated was 6 g/L, which accounted for 24% increase in PHA accumulation than that of the reference bacterium under the same parameters. Maximum biomass production of 31 g/L was observed at 4:1:: C:N ratio at 48 h. Significant amount of biomass of up to 3 g/L, was converted to PHA. The maximum PHA production obtained by this isolate was 5.5 g/L at 2:1 and 3:1:: C:N ratios at 48 h. At 3:1:: C:N ratio and 72 h incubation, the bacterium accumulated 78% of PHA. Thus, the above study suggests that the bacterium accumulated a higher amount of PHA if incubated longer which can also be correlated to the late log phase or idiophase production of secondary metabolites.
Bacillus siamensis LFS1715
With 2:1:: C:N ratio at 72 h, the bacterium accumulated 2 g/L of DCW, out of which 1.5 g/L was converted to PHA which accounted for 75% of PHA accumulation (Maximum PHA accumulation by the isolate). Maximum biomass produced was 8.5 g/L at 4:1:: C:N ratio at 48 h which was 89% more than that of the reference bacterium under the same conditions of incubation and C:N ratio.. This bacterium did accumulate 67% of PHA with 3:1:: C:N ratio at 24 h during which amounts of biomass and PHA produced were 3 g/L and 2 g/L, respectively. The bacterium showed a 25% increase in PHA with 1:1:: C:N ratio at 24 h, which was again 20% more than that produced by the reference bacterium. During the same time period, 1.5 g/L of biomass was converted into PHA and 50% of PHA accumulation. Conclusively, incubation of 72 h, 2:1:: C:N ratio was observed to be a suitable ratio for the bacterium to produce PHA.
Bacillus halotolerans KSI1507
The maximum amount of biomass produced by this bacterium was observed with 1:1:: C:N ratio at 24 h during which 27 g/L of PHA was produced from 74 g/L of biomass The maximum PHA accumulation was observed when the amount of biomass produced was 6 g/L (which when compared to reference bacterium was 50% more) with 2:1:: C:N ratio at 72 h with conversion to 4 g/L of PHA which accounted for 67% of PHA accumulation When compared to the reference bacterium, the bacterial isolate showed only 2% more PHA accumulation than the reference bacterium at 2:1:: C:N ratio at 24 h. The above observations suggest that the bacterium could utilize pomegranate peel as a carbon source better than the reference bacterium, but it requires longer incubation time for better production of PHA.
Geobacillus stearothermophillus TKR1707
The bacterium accumulated maximum of 76% of PHA with 1:1:: C:N ratio at 72 h during which 6.5 g/L of PHA was produced, which is also the maximum amount of PHA produced by the bacterium from 8.5 g/l of DCW. This result accounted for 13% increase in PHA accumulation when compared to the reference strain. Maximum biomass production was obtained at 2:1:: C:N ratio at 24 h with 4.5 g/L of PHA from 9.5 g/L of DCW. A significant amount of DCW of 9 g/L has been obtained with 4:1:: C:N ratio at 72 h with 4 g/L of PHA production and 44% PHA accumulation. At 1:1:: C:N ratio and 72 h, 3:1 and 1:1:: C:N ratios and 24 h significant amount of biomass production of up to 8.5 g/L were observed with a maximum 6.5 g/L of PHA production. Finally, these observations suggest that the bacterium could utilize pomegranate peel to produced PHA at a longer interval of time.
Bacillus subtilis RVR2988
The bacterium produced maximum biomass of 8 g/L both with 1:1 and 4:1:: C:N ratios at 24 h. During this time period, 31% and 44% of PHA accumulation were observed. Maximum PHA of 71% was accumulated with 4:1:: C:N ratio at 96 h which was 42% higher than that accumulated by the reference bacterium. With 3:1:: C:N ratio at 72 h, the bacterium produced biomass of 7.5 g/L and could convert 5 g/L into PHA. Also, with 2:1:: C:N ratio at 24 h, the bacterium demonstrated to accumulate 64% of PHA which was 28% higher than the reference bacterium during which it produced 83% more amount of biomass (5.5 g/L) and converted 3.5 g/L into PHA. Hence, the above observations meant that the bacterium could utilize the pomegranate peel as a carbon source in a better way with good amount of PHA at a higher C:N ratio and longer incubation time.
Bacillus siamensis RET2912
This bacterium accumulated maximum PHA of 73% which was 9% more than the reference bacterium with 3:1:: C:N ratio at 48 h, which led to production of biomass (83% more than the reference bacterium) and 4 g/L of it was converted into PHA. The bacterium demonstrated 89% more amount of biomass than the reference bacterium with 4:1:: C:N ratio at 48 h. At 96 h, with 1:1:: C:N ratio, the bacterium accumulated 67% of PHA which was 34% higher than that accumulated by the reference bacterium. With 1:1 and 2:1:: C:N ratios at 24 h, the bacterium showed 8% increase in PHA accumulation The above data suggest that this bacterium could utilize the pomegranate peel and also accumulates a significant amount of PHA earlier than later.
Bacillus velezensis BTR2015
The maximum amount of PHA that could be obtained by the bacterium was 75% at 4:1:: C:N ratio at 24 h with PHA production of 4.5 g/L and this accounted for 6% increase in PHA accumulation compared to the reference bacterium. With the amount of DCW and PHA obtained with the above-mentioned parameters, the result was maximum. With 2:1:: C:N ratio at 24 h, 73% of PHA accumulation was observed during which 5.5 g/L of biomass and 4 g/L of PHA were obtained. We would like to highlight that in the above-mentioned parameters, throughout our study with the mentioned isolates, we got maximum results with 46% increase in PHA accumulation in comparison to the reference strain (3 g/L of biomass, 1.5 g/L of PHA production and 50% of PHA accumulation were obtained by reference bacterium at 2:1::C:N ratio at 24 h). At 96 h, with 3:1 and 4:1:: C:N ratios, 5 g/L of DCW was obtained in each with 3 g/L and 1.5 g/L of PHA production, respectively. Thus, the above results suggest that this bacterium can utilize pomegranate peel maximally as carbon source and also produce a good amount of PHA even at a shorter interval of time and, hence, holds the potential of commercialization. Though PHA is a secondary metabolite, its production at a shorter interval of time could be a boon for the industry as it can save resources.
Staphylococcus aureus ESR1315
The maximum amount of PHA accumulation obtained by this bacterium was 67% at 2:1:: C:N ratio and 48 h with 2 g/L PHA from 3 g/L DCW. Around this time interval, the reference bacterium too accumulated the same amount of PHA. Further, 67% of PHA accumulation was also obtained at 3:1:: C:N ratio at 72 h during which 4.5 g/L of DCW yielded 3 g/L of PHA. Maximum DCW of 6 g/L was observed at 3:1:: C:N ratio at 24 h of which 3 g/L was converted into PHA which indicates 50% of PHA accumulation. At 3:1:: C:N ratio and 24 h of incubation, DCW produced was 6 g/L PHA accumulation rate of 50%. At 3:1:: C:N ratio and 72 h, amounts of biomass produced by the bacterium were 4.5 g/L and 67% PHA accumulation. At 4:1:: C:N ratio and 72 h, 5.5 g/L of DCW and 55% of PHA accumulation were observed. Thus, the above study suggests that the bacterium could utilize pomegranate peel much better way at 2:1 and 4:1:: C:N ratios when incubated at 24 h to 72 h with the best results obtained at 3:1:: C:N ratio and 72 h.
Conclusion
Pomegranate peel was chosen as a novel carbon source and the bacterial isolates of our study had the potential to produce up to 83% of PHA. This raw material was selected as it is the kitchen waste generated from many households, restaurants, juice corners, etc. Moreover, it is easily available at almost zero cost. All the bacterial isolates have shown considerably good accumulation of PHA as mentioned above. So, if the waste is channelized from different sources to the industries that produce PHAs, then the resulting low-cost PHAs can further be used to manufacture commodities and ultimately substitute the present non-degradable plastic materials from the market.
References
Aburas MMA (2016) Production of poly β-hydroxybutyrate from Pseudomonas putida MAO12 isolated from waste water sample. J Am Sci 12(5):107–112
Azeredo CMH, Miranda EWK, Ribeiro LH, Rosa FM (2012) Nanoreinforced alginate-acerola puree coatings on acerola fruits. J Food Eng 113:505–510
Chaitanya K, Mahmood SK, Rana K, Sunil KN (2014) Biotechnological production of polyhydroxyalkanoates by various isolates: a review. Int J Pharm Sci Invent 3(9):01–11
Chanprateep S (2010) Current trends in biodegradable polyhydroxyalkanoates. J Biosci Bioeng 110(6):621–632
Chee JY, Yoga SS, Lau NS, Ling SC, Abed RMM, Sudesh K (2010) Bacterially produced polyhydroxyalkanoates (PHAs): converting renewable resources into bioplastics. In: Mendez- Vilas A (ed) Applied microbiology and microbial biotechnology. Universiti Sains Penang, Malaysia, Formatex, pp 395–1404
Du G, Chen J, Yu J, Lun S (2001) Continuous production of poly-3-hydroxybutyrate by Ralstonia eutropha in a two-staged culture system. J Biotechnol 88:59–65
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Farris S, Schaich MK, Liu L, Piergiovanni L, Yam LK (2009) Development of polyion-complex hydrogels as an alternative approach for the production of bio-based polymers for food packaging applications: a review. Trends Food Sci Technol 20:316–332
Fernandez-Castillo R, Rodriguez-Valera F, Gonalez-Ramos J, Ruiz-Berraquero F (1986) Accumulation of poly (β- hydroxybutyrate) by Halobacteria. Appl Env Microbiol 51(1):214–216
Gabor D, Tita O (2012) Biopolymers used in food packaging: a review. Acta Universitatis Cibiniensis Series E Food Technol 16(2):3–19
Girija D, Deepa K, Xavier F, Antony I, Shidhi PR (2013) Analysis of cow dung microbiota—a metagenomic approach. Ind J Biotechnol 12:372–378
Godoy F, Vancanneyt M, Martinez M, Steinbüchel A, Swings J, Rehm BH (2003) Sphingopyxis chilensis sp. nov, a chlorophenol-degrading bacterium that accumulates polyhydroxyalkanoates and transfer of Sphingomonas alaskensis to Sphingopyxis alaskensis comb. nov. Int J Syst Evol Microbiol 53:473–477
Gomaa EZ (2014) Production of polyhydroxyalkanoates (PHAs) by Bacillus subtilis and Escherichia coli Grown on Cane Molasses Fortified with ethanol. Braz Arch Biol Tech 51(1):145–154
Gowda V, Shivakumar S (2014) Agrowaste-based polyhydroxyalkanoate (PHA) production using hydrolytic potential of Bacillus thuringiensis IAM12077. Braz Arch Biol Technol 57(1):55–61
Hawas JME-M, El-Banna TE-S, Belal EBA, El-Aziz AAA (2016) Production of bioplastics from some selected bacterial strains. Int J Curr Microbiol App Sci 5(1):10–22
Heinrich D, Madkour MH, Al-Ghamdi MA, Shabbaj II (2012) Large scale extraction of poly (3-hydroxybutyrate) from Ralstonia eutropha H16 using sodium hypochlorite. AMB Express 2(59):1–6
Jacquel N, Lo CW, Wei YH, Wu HS, Wang SS (2008) Isolation and purification of bacterial poly (3-hydroxyalkanoates). Biochem Eng J 39:15–27
Krishnaveni S, Balasubramanian T, Sadasivam S (1984) Sugar distribution in sweet stalk sorghum. Food Chem 15:229–232
Kumar PKA, Shamala TR, Kshama L, Prakash MH, Joshi GJ, Chandrashekar A, Kumari KSL, Divyashree MS (2007) Bacterial synthesis of poly (hydroxybutyrate-co- hydroxyvalerate) using carbohydrate- rich Mahua (Madhuca.sps) flowers. J Appl Microbiol 103:204–209
Kumar T, Singh M, Purohit HJ, Kalia VC (2009) Potential of Bacillus sp. to produce polyhydroxybutyrate from biowastes. J Appl Microbiol 106:2017–2023
Kumari P, Dhingra KH (2013) Isolation and characterization of PHB producing micro-organisms isolated from root nodules of leguminous plants. Int Q J L Sci 8(1):109–113
Marjadi D, Dharaiya N (2011) Isolation, screening and characterization of polyhydroxyalkanoates producing bacteria utilizing edible oil as carbon sources. J Env Res Dev 5(3):764–772
Omar FN, Rahman NAA, Hafid HS, Mumtaz T, Yee PL, Hassan MA (2011) Utilization of kitchen waste for the production of green thermoplastic polyhydroxybutyrate (PHB) by Cupriavidus necator CCGUG 52238. Afr J Microbiol Res 5(19):2873–2879
Paul S, Sasikumar SC, Balakumaran MD (2017) Optimization, purification and characterization of polyhydroxybutyrate (PHB) produced by Bacillus cereus Isolated from sewage. Int J Chemtech Res 10(7):884–904
Porwal S, Kumar T, Lal S, Rani A, Kumar S, Cheema S, Purohit JH, Sharma R, Patel SKS, Kalia CV (2008) Hydrogen and Polyhydroxybutyrate producing abilities of microbes from diverse habitats by dark fermentative process. Bioresour Technol 99:5444–5451
Prabhu NN, Santimano MC, Mavinkurve S, Bhosle SN, Garg S (2010) Native granule associated short chain length polyhydroxyalkanoate synthase from marine derived Bacillus sp. NQ-11/A2. Antonie Van Leeuwenhoek 97:41–50
Preethi R, Sasikala P, Aravind J (2012) Microbial production and polyhydroxyalkanoate (PHA) utilizing fruit waste as a substrate. Res Biotechnol 3(1):61–69
Rayasam V, Kumar T (2018) Banana peel as an inexpensive carbon source for microbial polyhydroxyalkanoates (PHA) production. Int Res J Env Sci 7(1):1–8
Rayasam V, Kumar T (2019) Microbial production of polyhydroxyalkanoates (PHAs) using kitchen waste as an inexpensive carbon source. Biosci Biotechnol Res Asia 16(1):155–166
Raza ZA, Abid S, Banat MI (2018) Polyhydroxyalkanoates: characteristics, production, recent developments and applications. Int Biodeterior Biodegrad 126:45–56
Ruban WS (2009) Biobased packaging-application in meat industry. Vet World 2(2):79–82
Santhanam A, Sasidharan S (2010) Microbial production of polyhydroxyalkanoates (PHA) from Alcaligens spp. and Pseudomonas oleovorans using different carbon sources. Afr J Biotechnol 9(21):3144–3150
Shah RK (2014) Optimization and production of polyhydroxybutyrate (PHB) by Bacillus subtilis G1S1 from soil. Int J Curr Microbiol Appl Sci 3(5):377–387
Shivakumar S (2012) Accumulation of poly (3-hydroxybutyrate) by Microbacterium barkeri DSM 20145. Turk J Biol 36:225–232
Song HJ, Jeon OC, Choi HM, Yoon CS, Park W (2008) Polyhydroxyalkanoates (PHA) production using waste vegetable oil by Pseudomonas sp. Strain DR2. J Microbiol Biotechnol 18(8):1408–1415
Souza SWB, Cerqueira AM, Martins TJ, Casariego A, Teixeira AJ, Vicente AA (2010) Influence of electric fields on the structure of chitosan edible coatings. Food Hydrocoll 24:30–335
Srilakshmi S, Ramachandra Rao VSC (2012) Studies on screening, isolation and molecular characterization of PHB producing Staphylococcus spp. Int J Int Sci Inn Tech Sec B 1(5):24–31
Tembadmani K, Shankar A, D’Souza S, Narvekar M, Rao P (2015) Microbial production of polyhydroxyalkanoates (PHA) from novel sources: a review. Int J Res Biosci 4(4):16–28
Thapa C, Shakya P, Shrestha R, Pal S, Manandhar P (2018) Isolation of polyhydroxybutyrate (PHB) producing bacteria, optimization of culture conditions of PHB Production, Extraction and characterization of PHB. Nepal J Biotechnol 6(1):62–68
Yao J, Zhang G, Wu Q, Chen GQ, Zhang R (1999) Production of polyhydroxyalkanoates by Pseudomonas nitroreducens. Antonie Van Leeuwenhoek 75:345–349
Zaid M, Jamil N (2015) Production and in-vitro evaluation of bioplastics produced by bacteria using Bagasse. In: International conference on chemical, food and environmental engineering (ICCFEE), Dubai (UAE). https://doi.org/10.17758/IAAST.A0115051:11-12
Zhu C, Liu X (2013) Optimization of extraction process of crude polysaccharides from pomegranate peel by response surface methodology. Carbohydr Polym 92:1197–1202
Acknowledgements
We are thankful to Dr. P. Shyama Raju, The Founder and Hon’ble Chancellor of Reva University; Dr. N. Ramesh, the Dean- Training, Placement and Planning and Dr. M. Dhanamjaya, the Registrar of Reva University, Bangalore for constant support and encouragement. We are grateful to Sateesh. G. Muttagi, CEO; Dr. Siddalingeshwar. K. G, Quality Manager and Mr. Babu. S, Business Development Head of Scientific and Industrial Research Center (NABL) Laboratories, Bangalore for providing the lab facilities and for their constant support and assistance. We are grateful to Prof. K.D. Vachhrajani, Head of the Department, Department of Environmental Studies and Prof. H.R. Kataria, Dean, Faculty of Science, The Maharaja Sayajirao University of Baroda for encouragement. We express our immense gratitude towards the GSBTM (Gujarat State Biotechnology Mission) for providing the funding through BIOTHON grant.
Funding
GSBTM, Government of Gujarat, Department of Science and Technology, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rayasam, V., Chavan, P. & Kumar, T. Polyhydroxyalkanoate synthesis by bacteria isolated from landfill and ETP with pomegranate peels as carbon source. Arch Microbiol 202, 2799–2808 (2020). https://doi.org/10.1007/s00203-020-01995-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-01995-9