Abstract
A total of 20 different strains were isolated, purified and screened for polyhydroxyalkanoate (PHA) production. PHA-producing strains were screened by Nile blue staining and confirmed by Sudan Black B staining. Strain 1.1 was selected for further analysis due to its high PHA production ability. PHA production was optimized and time profiling was calculated. PHA production on various different cheap carbon sources, i.e., sugar industry waste (fermented mash, molasses, spent wash) and corn oil, was compared. Cell dry weight and PHA content (%) were calculated and compared. The 12.53 g/L is the CDW of bacterial strain when grown in medium containing corn oil. It was found that corn oil at 12.53 g/L medium can serve as a carbon source for bacterial growth, allowing cells to accumulate PHA up to 35.63 %. The PhaC gene was amplified to confirm the genetic basis for the production of PHAs. Moreover, 16S rRNA gene sequence analysis showed that strain 1.1 belongs to Pseudomonas species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Increased growth of the Earth’s human population has led to a drastic accretion of non-degradable waste materials; of these, plastic waste is most harmful to the environment (Guillet 2004). Degradation of conventional waste is very slow, and produces very harmful toxins during this process (Gross and Kalra 2002). Consumption of petrochemical-based plastics is linked directly with diminishing oil resources. The world’s population consumes almost 150 t plastic every year each.
Bacteria have the ability to produce bioplastics in the form of polyhydroxyalkanoates (PHAs) (Chen 2009). PHAs are 3-hydroxy fatty acid monomers that form linear, head-to-tail polyesters. PHA is usually produced as a polymer of 103–104 monomers, which accumulates as inclusions of 0.2–0.5 μm in diameter. PHA synthesis inside bacteria occurs when the organism is experiencing nutrient imbalance, such as more carbon with reduce phosphorus, nitrogen and oxygen (Anderson and Dawes 1990). The monomer composition, physiochemical properties, size and structure vary depending on the type of bacterium producing the PHA (Ha and Cho 2002).
The fact that these bioplastics are biodegradable and can be produced from renewable resources gives them an edge over conventional plastics. Bacteria can degrade their own PHAs at a high rate (3–9 months) by their own secreted PHA depolymerases (Jendrossek 2001). The only drawback of these PHAs is their high production costs. However, bacteria can be grown on different media easily depending on their availability (Jiang et al. 2008), so researchers have tested a wide range of renewable carbon sources for use in PHA production. The use of different waste materials for PHA biosynthesis is a good strategy as production is cost efficient, and disposal problems are also overcome (Koller et al. 2005).
For PHA production, molasses originating from paper mill wastewater (Bengtsson et al. 2008), sugar cane molasses (Albuquerque et al. 2007), activated sludge (Hu et al. 2006), food waste (Rhu et al. 2003) and plant oils, i.e., olive oil effluent (Beccari et al. 2009) and palm oil (Marsudi et al. 2008) have been used. Other agriculture wastes from the sugar industry, like spent wash and fermented mash, have also shown great potential for use as carbon sources. Fukui and Doi (1998) succeeded in producing PHA from Alcaligenes eutrophus H16 and its recombinant strains by using olive oil, corn oil, palm oil and oleic acid as carbon substrate, generating PHA content of up to 81 % when grown for 72 h. High PHA productivity (0.68 g L−1 h−1) was also achieved when corn oil hydrolysate was used as carbon source in fed batch cultures of Pseudomonas putida KT2442, yielding cell densities of up to 109 g /L (Shang et al. 2008).
In the present study, we screened PHA-producing bacterial strains from carbon-rich sources and nutrient imbalanced places, and evaluated the feasibility of using these strains for PHA production by using cheaper carbon media, i.e., corn oil, molasses, fermented mash and spent wash. The goal of the current study was to lessen the cost of PHA production by replacing commercially available pure carbon sources with inexpensive sugar industry wastes, as a unique adjunct to work already studying waste carbon sources like palm oil, olive oil, wastewater sludge, vegetable oils, and starch, etc.
Materials and methods
Isolation and purification of bacterial strains
Six different samples were collected from different localities in Pakistan and Oman (oil well and oil pipe line samples from Oman; soil samples from a bus workshop, agriculture field at Punjab University, canal sediment soil from Lahore; and water sample from a freshwater spring from Sakardu in the northern hilly area of Pakistan). The samples were collected in sterile glass containers, tapered tightly, processed for physical parameters, i.e., temperature and pH, and stored at −20°C for further analyses.
Samples were enriched by culturing in enriched medium (yeast extract 5 g/L, tryptone 8 g/L, NaCl 2.5 g/L) and 50 μl pre-culture was spread on PHA detection agar [PDA; glucose 20 g/L, (NH4)2SO4, 2 g/L, KH2PO4 13.3 g/L, MgSO4⋅7H2O 1.2 g/L, citric acid 1.7 g/L, trace elements solution 10 mL/L and agar 15 g/L] containing Nile blue stain (0.5 μg/mL) (Spiekermann et al. 1999). After overnight incubation, plates were observed under UV light. Colonies producing fluorescence were purified and the isolates were further confirmed by Sudan Black B staining (Lee and Choi 2004).
Selection of the most suitable strain
Out of 20 different bacterial strains, only strain 1.1 was chosen for further experimental work as this strain was able to utilize all the carbon sources tested when supplied one by one in minimal medium; maximum cell density and PHA accumulation was achieved with corn oil as carbon source. Strain 1.1 was also optimized for PHA production at various temperatures (20°C, 37°C, 45°C and 55°C) and pH values (3, 5, 7 and 9) (data for other strains not shown here).
PHA production and extraction in shake flask cultures
Carbon sources (corn oil, molasses, fermented mash and spent wash) were diluted, adjusted to pH 7, filtered through 0.45 μm filters and added to PHA production medium broth [(NH4)2SO4, 2 g/L, KH2PO4 13.3 g/L, MgSO4⋅7H2O 1.2 g/L, citric acid 1.7 g/L, trace elements solution 10 mL/L] at 2 % in different flasks. A 10 mL innoculum from a pre-culture with an optical density (OD) of 0.5 at 600 nm of strain 1.1 was added to 90 mL minimal medium with each different carbon source at 2 % in place of glucose in separate flasks with the remaining medium composition as described above. The flasks were incubated at 37°C for more than 3 days at 200 rpm. Samples were collected at 2 h intervals from 0 to 78 h. Cells were collected by centrifuging samples taken at regular intervals and drying at 55°C to constant weight. PHA was extracted from cultures using the sodium hypochlorite method of Ramsay et al. (1990). PHA content was determined by dividing the weight of PHA obtained after extraction by the cell dry weight (CDW) and expressed in terms of percentage content of CDW.
Genomic DNA extraction and amplification of 16S rRNA and phaC genes
Genomic DNA of strain 1.1 was isolated using a Fermentas genomic DNA isolation kit. 16S rRNA gene was amplified using Primus96 (PeQLab) with PCR master mix forward primer 16S-3, CCCGGGAACGTATTCACCG and reverse primer 16S-5, GCYTAAYACATGCAAGTCGA (Rehman et al. 2007). PCR was carried out by denaturing DNA at 110°C for 10 min followed by 30 cycles of amplification (95°C for 2 min, 52°C for 1 min and 72°C for 2 min). The PhaC polymerase gene was amplified using Primus96 (PeQLab) with PCR master mix, forward primer 179–L (5′-ACAGATCAAGTTCTACATCTTCGAC-3′) and the reverse primer 179–R (5′-GGTGTTGTCGTTCCAGTAGAGGATGTC-3′) (Solaiman et al. 2000). PCR was carried out by denaturing DNA at 110°C for 10 min followed by 30 cycles of amplification (95°C for 2 min, 56°C for 1 min and 72°C for 2 min).
Sequencing and phylogenetic analysis of genes
The 16S rRNA gene was sequenced at the Center for Excellence in Molecular Biology, (Pakistan) on a DNA sequencing system (3100/ga3100-1696-013; Applied Biosystems, Foster City, CA) based on the dideoxy DNA sequencing method. Sequence analysis and alignment were carried out using the NCBI BLAST tool. The 16S rRNA gene partial sequence was submitted to the NCBI database under the accession number HM140411.
Results
Isolation and characterization of PHA-producing bacteria
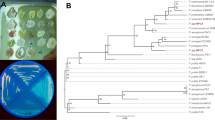
Out of six samples, 20 bacterial strains were isolated. All strains were Nile-blue-positive (Fig. 1), while PHA granules were found in only 14 strains (GS1, 9.1, 1.1, 1.2, GS3, O2, 4, GS6, 2.1, 2.2, GW3, 7, 5, and 6) by Sudan Black B staining. On the basis of these results, 14 strains were found to be PHA producers.
Optimization of growth and PHA production for strain 1.1
Strain 1.1 was selected due to its highest PHA production ability and was optimized in terms of growth and PHA production under various conditions of temperature and pH. When grown at pH 7 for 24 h, growth of strain 1.1 gave OD values of 1.311 at 20°C, 2.56 at 37°C, and 0.71 at 45°C and 0.021 at 55°C. Growth at 37°C for 24 h gave OD values of 0.01 at pH 3, 0.05 at pH 5, 2.56 at pH 7 and 0.61 at pH 9. These results indicate the maximum growth of strain 1.1 can be obtained at pH 7 and 37°C. Strain 1.1, when grown on PHA-accumulating medium at 37°C and pH 7 for 52 h showed increasing patterns as shown in Fig. 2. PHA production was concomitant with bacterial growth and became almost static after 22 h as shown in Fig. 3, indicating that growth and PHA accumulation happen in parallel.
Cell dry weight and PHA production by strain 1.1 on medium supplemented with spent wash, fermented mash, molasses and corn oil
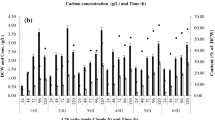
CDW and PHA production by strain 1.1 was analyzed for different carbon sources after growth for 24, 48, and 72 h, at pH 7 and 37°C. CDW of 8.56, 7.98 and 6.83 g/L and PHA content (%) of 25.46, 16.45 and 14.36 while growing on medium supplemented with spent wash (Fig. 4a), CDW of 7.02, 6.52 and 6.23 g/L and PHA content (%) of 23.56, 15.69 and 13.98 with fermented mash (Fig. 4b), CDW of 10.54, 9.62 and 9.05 g/L and PHA content (%) of 20.63, 18.79 and 16.45 with molasses (Fig. 4c), and finally CDW with corn oil in medium, of 12.53, 10.68 and 9.81 g/L and PHA content (%) of 35.63, 29.45 and 26.47 (Fig. 4d) were obtained. It seems that corn oil and molasses are easily used up by the strain to produce relatively high cell densities as compared to spent wash and fermented mash (Fig. 5a) but the PHA contents were found to be far better with corn oil than with the other three carbon sources (Fig. 5b).
Ribotyping and PCR amplification of PhaC and 16S rRNA gene
PhaC gene of length 540 bp was amplified from strains 1.1, Y and GS2 (Fig. 6). The DNA sequence of the 16S rRNA gene was aligned with previously published sequences of representative bacterial genes in the database, revealing that strain 1.1 had 99 % homology with Pseudomonas putida strain R2-240 EU834357.1, Pseudomonas sp. RW4P2 AM911655.1, and most other Pseudomonas sp. (Fig. 7).
Discussion
Recent literature has clearly highlighted that the use of renewable carbon sources for the production of PHAs can greatly reduce the production costs of this useful biopolymer (Kim 2000; Akaraonye et al. 2010). Thus, we explored the potential of sugar industry wastes for use as low-cost substrates for bacterial media. Samples were collected from carbon-rich and nutrient-deficient locations. Oil wells and canal sediments are considered as rich sites for organic carbon. Fresh water springs contain a huge diversity of bacteria.
In order to produce PHA on large scale, selection of a cheap medium is very important. The use of PHA as a constituent in conventional synthetic plastic has been compromised because of its current high production cost (Bucci et al. 2005; Chen et al. 2001), stimulating the search for cheap carbon sources and nutritional supplements in order to lower costs (Albuquerque et al. 2007; Mazur et al. 2009). In this study, we selected different industrial wastes as media to minimize PHA production cost, and report a novel approach for small-scale PHA production. Molasses, spent wash and fermented mash represent industrial waste from the sugar industry. Their high sugar content means they have the potential to be used as carbon source in PHA media. Corn oil is also high in nutrition in terms of high carbon number fatty acids. In our study, maximum PHA content was observed in the first 24 h: 25.46 % with spent wash, 23.56 % with fermented mash, 20.65 % with molasses, and 35.63 % with corn oil. Thereafter, production starts decreasing with time while biomass remains relatively high until 72 h (Fig. 4). These results were comparable to those of Khardenavis et al. (2009), who obtained PHB up to 31 % by utilizing molasses spent wash as carbon source. Using spent wash, biomass production remained stable until 40 h, while PHA showed almost the same pattern as for molasses (Fig. 4c). The higher PHA accumulation seen with fermented mash and spent wash may be due to balanced sugar and nitrogen availability because a lot of sugar is used during the fermentation process when fermented mash is being produced industrially. For fermented mash, biomass (7.02 g/L) and PHA content (23.56 %) were higher in the first 24 h and started decreasing (to 6.23 g/L CDW and 13.98 % PHA) after 72 h (Fig. 4b) as was also the case with other carbon sources (Fig. 5).
Excellent results were observed in the case of corn oil. Strain 1.1 showed best growth and biomass production of 12.53 g/L CDW after 24 h using corn oil as a carbon source (Fig. 4d). The results showed that this strain has ability to utilize medium-chain-length fatty acids and give good results. Production of mcl-PHA in a high cell density culture (CDW 109 g/L) utilizing corn oil hydrolysate has already been reported for P. putida KT2442 grown in fed batch culture (Shang et al. 2008), leading to a PHA content of 28.5 %. To obtain medium chain length PHA in high amounts, it is better to provide medium chain fatty acids as carbon source. but these are expensive if used in pure form (Akaraonye et al. 2010). Thus, the use of raw corn oil as carbon source would might help decrease production costs. This is possibly because plant fatty acids serve as good carbon sources as well as being high energy sources as well (Tsuge 2002). Molasses contains a high concentration (12,500 mg/L) of reducing sugars, especially sucrose (Khardenavis et al. 2009) stachyose and raffinose, and is thus a good carbon source for fermentation by microorganisms that possess the appropriate enzymes, i.e., α-galactocidases and β-furanocidases and metabolic pathways to utilize these carbohydrates. Page (1989) reported the production of P(3HB-CO-3HV) at 19 to 22 g polymer by Azotobacter vinelandii grown on beet molasses. Overall, strain development using pure culture techniques can be combined with low value and renewable carbon sources to get more desirable results for the production of high PHA content.
Bacterial PHAs are chromosomally encoded (Antonio et al. 2000) by the PhaCBA operon. PhaC genes of the expected length of 540 bp were amplified from strain 1.1, Y and GS2 as shown in Fig. 6. Kung et al. (2007) succeeded in amplification of the PhaC gene from Pseudomonas and Escherichia coli by using a PCR technique. They suggested that a phenotypic approach, i.e., by using Sudan Black B staining and Nile blue screening, with subsequent confirmation by amplification of the PhaC gene fragment are important methods to confirm the PHA-producing ability of bacterial isolates because Nile blue, being lipophilic, can sometimes detect granules of lipids that differ in nature and composition from PHAs. Sequence analyses predict that strain 1.1 belongs to Pseudomonas sp.
In conclusion, this study not only documents isolation of PHA-producing bacterial strains from a diverse range of soil, water and oil samples, but also demonstrates the utilization of agro-industrial wastes as useful supplements to bacterial media, both reducing the cost of PHA production and removing such waste, which can otherwise cause environmental pollution. Our results indicate corn oil as an excellent supplement medium for PHA production.
Abbreviations
- PHA:
-
Polyhydroxyalkanoate
- PDA:
-
PHA detection agar
References
Akaraonye E, Keshavarz T, Roy I (2010) Production of polyhydroxyalkanoates: the future green materials of choice. J Chem Technol Biotechnol 85:732–743
Albuquerque MGE, Eiroa M, Torres C, Nunes BR, Reis MAM (2007) Strategies for the development of a side stream process for polyhydroxyalkanoate (PHA) production from sugar cane molasses. J Biotechnol 130:411–421
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54:450–472
Antonio RV, Steinbuchel A, Rehm BHA (2000) Analysis of in vivo substrate specificity of PHA synthase from Ralstonia eutropha: formation of novel copolyesters in recombinant Eschericia coli. FEMS Microbiol Lett 182:111–117
Beccari M, Bertin L, Dionisi D, Fava F, Lampis S, Majone M, Valentino F, Vallini G, Villano M (2009) Exploiting olive oil mill effluents as a renewable resource for production of biodegradable polymers through a combined anaerobic–aerobic process. J Chem Technol Biotechnol 84:901–908
Bengtsson S, Werker A, Christensson M, Welander T (2008) Production of polyhydroxyalkanoates by activated sludge treating a paper mill wastewater. Bioresour Technol 99:509–516
Bucci DZ, Tavares LBB, Sell I (2005) PHB packaging for storage of food products. Polym Test 24:564–571
Chen GQ (2009) A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev 38:2434–2446
Chen GQ, Zhang G, Park SJ, Lee SY (2001) Industrial scale production of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate). Appl Microbiol Biotechnol 57:50–55
Fukui T, Doi Y (1998) Efficient production of polyhydroxyalkanoates from plant oils by Alcaligenes eutrophus and its recombinant strain. Appl Microbiol Biotechnol 49:333–336
Gross RA, Kalra B (2002) Biodegradable polymers for the environment. Science 297:803–807
Guillet J (2004) Plastics and environment. In: Scott G (ed) Degradable polymers: principles and applications, 2nd edn. Kluwer, New York, pp 1254–1263
Ha CS, Cho WJ (2002) Miscibility, properties and biodegradability of microbial polyester containing blends. Prog Polym Sci 27:759–809
Hu W, Wang Y, Hua F, Chua H, Sin S, Yu H (2006) Synthesis of poly-hydroxyalkanoates from activated sludge under various oxidation-reduction potentials. Ann Microbiol 56:257–260
Jendrossek D (2001) Microbial degradation of polyesters. Adv Biochem Eng Biotechnol 71:293–325
Jiang Y, Song X, Gong L, Li P, Dai C, Shao W (2008) High poly (bhydroxybutyrate) production by Pseudomonas fluorescens A2a5 from inexpensive substrates. Enzyme Microb Technol 42:167–172
Khardenavis AA, Vaidya AN, Kumar MS, Chakrabarti T (2009) Utilization of molasses spentwash for production of bioplastics by waste activated sludge. Waste Manage 29:2558–2565
Kim BS (2000) Production of poly (3-hydroxybutyrate) from inexpensive substrates. Enzyme Microb Technol 27:774–777
Koller M, Bona R, Braunegg G, Hermann C, Horvat P, Kroutil M, Martinz J, Neto J, Pereira L, Varila P (2005) Production of polyhydroxyalkanoates from agricultural waste and surplus materials. Biomacromolecules 6:561–565
Kung SS, Chuang YC, Chen CH, Chien CC (2007) Isolation of polyhydroxyalkanoates-producing bacteria using a combination of phenotypic and genotypic approach. Lett Appl Microbiol 44:364–371
Lee SY, Choi JI (2004) Polyhydroxyalkanoates: biodegradeable polymer. In: Demain AL, Davies JE, Atlas RM, Cohen G, Hershberger CL, Hu WS, Sherman DH, Willson RC, David JH (eds) Manual of indusrial microbiology and biotechnology, 2nd edn. American Society of Microbiology, Washington, pp 616–624
Marsudi S, Unno H, Hori K (2008) Palm oil utilization for the simultaneous production of polyhydroxyalkanoates and rhamnolipids by Pseudomonas aeruginosa. Appl Microbiol Biotechnol 78:955–961
Mazur LP, da Silva DD, Grigull VH, Garcia MCF, Magalhães TO, Wagner TM, Einloft S, Dullius J, Schneider AL, Pezzin APT (2009) Strategies of biosynthesis of poly (3-hydroxybutyrate) supplemented with biodiesel obtained from rice bran oil. Mater Sci Eng C 29:583–587
Page WJ (1989) Production of poly-β-hydroxybutyrate by Azotobacter vinelandii strain UWD during growth on molasses and other complex carbon sources. Appl Microbiol Biotechnol 31:329–333
Ramsay JA, Berger E, Ramsay BA, Chavarie C (1990) Recovery of poly-3-hydroxyalkanoic acid granules by a surfactant hypochlorite treatment. Biotechnol Tech 4:221–226
Rehman S, Jamil N, Husnain S (2007) Screening of different contaminated environments for polyhydroxyalkanoates-producing bacterial strains. Bioloogia 62:650–656
Rhu D, Lee W, Kim J, Choi E (2003) Polyhydroxyalkanoate (PHA) production from waste. Water Sci Technol 48:221–228
Shang LG, Jiang M, Yun Z, Yan HQ, Chang HN (2008) Mass production of medium-chain-length poly(3-hydroxyalkanoates) from hydrolyzed corn oil by fed-batch culture of Pseudomonas putida. World J Microbiol Biotechnol 24:2783–2787
Solaiman DKY, Ashby RD, Foglia TA (2000) Rapid and specific identification of medium-chain length polyhydroxyalkanoate synthase gene by polymerase chain reaction. Appl Microbiol Biotechnol 53:690–694
Spiekermann P, Rehm BHA, Kalscheuer R, Baumeister D, Steinbuchel A (1999) A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds. Arch Microbiol 17:73–80
Tsuge T (2002) Metabolic improvements and use of inexpensive carbon sources in microbial production of polyhydroxyalkanoates. J Biosci Bioeng 94:579–584
Acknowledgment
We are grateful to the Higher Education Commission (HEC), Pakistan, for providing grants under the Presidential Young Innovator research project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaudhry, W.N., Jamil, N., Ali, I. et al. Screening for polyhydroxyalkanoate (PHA)-producing bacterial strains and comparison of PHA production from various inexpensive carbon sources. Ann Microbiol 61, 623–629 (2011). https://doi.org/10.1007/s13213-010-0181-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-010-0181-6