Abstract
The Brazilian ethanol industry is very relevant in the global market; however, vinasse is generated in significant amounts and its management has become costly for distilleries. In this study, the aim was to evaluate vinasse as basal culture medium for P(3-hydroxybutyrate) (PHB) biosynthesis. Two bacterial strains were evaluated, a sucrose-consuming halophilic strain, Halomonas sp. HG03; and Ralstonia eutropha L359PCJ, which used glycerol from vinasse as carbon source. Firstly, shake flask bioprocesses analyzed cellular growth and PHB biosynthesis in vinasse-based media: in natura and concentrated vinasses were both evaluated in volumetric dilutions of 50% and 75% in mineral medium. Increasing vinasse concentration improved cellular growth rather than PHB accumulation for both bacteria. In vinasse-based treatments, Halomonas sp. HG03 had PHB content between 19.6 and 75.2% and R. eutropha L359PCJ, 48.4–68.5%. Further experiments in CSTR bioreactors used concentrated vinasse-based medium and R. eutropha L359PCJ had PHB content of 66.3%, concentration of residual cell dry weight (rCDW) = 9.4 g L−1, PHB = 18.6 g L−1, YX/S = 0.16 grCDW gGLYCEROL−1, YP/S = 0.32 gPHB gGLYCEROL−1, and 0.25 gPHB Lh−1. Halomonas sp. HG03 had PHB content of 42.2%, rCDW = 10.2 g L−1, PHB = 7.4 g L−1 and YX/S = 0.22 grCDW gSUCROSE−1, YP/S = 0.16 gPHB gSUCROSE−1 and 0.14 gPHB Lh−1. Finally, cost reductions by concentrated vinasse-based medium were evaluated. As glycerol source for PHB production by R. eutropha L359PCJ, vinasse reduced overall production costs by 22.2%. Unit production costs between US$ 2.8 and 5.4 kgPHB−1 were determined for scenarios that combined vinasse-based medium with high cell density culture and improvements of productivity. The investment payback times ranged from 1.6 to 4.5 years.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Bioplastics are a growing market due to increasing investments and expanding policies on sustainability around the world. By 2025, the global market of polyhydroxyalkanoates (PHAs) is expected to grow about 14% and become one of the main sectors in the bioplastics market, along with polylactates. Poly(3-hydroxybutyrate) (PHB), a short-chain polymer, is one of the main PHAs with industrial applications [25, 26].
PHAs are naturally synthesized by many microorganisms, including bacteria and archaeas. Unbalanced nutritional conditions such as highly available carbon source and limited nitrogen, sulfur, or phosphate source trigger the PHAs biosynthesis: carbon is assimilated, the actual cellular growth ceases, and the cell enlarges due to accumulation of PHAs granules that serve as energetic storage. In hostile environments, PHA producers may have survival advantages because they become more resilient to starvation. In industry, the main producers are bacterial strains of the species Ralstonia eutropha (also known as Cupriavidus necator, Alcaligenes eutrophus, or C. eutrophus), Pseudomonas spp., Aeromonas spp., Bacillus spp., and, more recently, bioprocesses with Escherichia coli and Halomonas sp. strains have been developed [26, 50].
Production costs have been limiting factors for expansion of the PHAs market and in literature a wide range of production costs may be found. Listewnik et al. [39] described PHB production by a mixed culture with natural gas as carbon source and costs were about US$ 15 kgPHB−1. Posada et al. [51] described costs between US$ 1.9 and 2.4 kgPHB−1 for a process with pure glycerol (98%) as carbon source by a C. necator strain. Indeed, unit production costs are variable and strongly affected by production scale, feedstocks’ prices, bioprocess configuration, and the microorganism’s efficiency.
PHAs production in biorefineries could reduce production costs because bioprocesses could share equipment, other facility-related costs (steam generation, water recycling, piping, electrical, labs, etc.), and lower capital investments would be needed. Besides, biorefineries usually recycle resources, that is, by-products from one process are inputs for another, which is quite advantageous for wastes management in addition to lower dependency on external feedstocks.
Integrating bioprocesses comprises many complex factors, which may be technical, such as process parameters, conditions, and resources demand. Some factors may be economical, namely capital investments, operational costs, and strategical planning, which must ultimately aim to competitiveness. Thus, even for early-stage technologies, the use of bioprocess simulation tools is decisive in evaluating technical and economical bottlenecks [49].
SuperPro Designer® is widely used in bioprocess simulations for many purposes, including agroindustrial wastes valorization for food application [30], diversification of bioproducts from sugarcane [71], and reducing production costs in bioprocesses [31].
In parallel, the Sucroenergetic Sector in Brazil is a great producer of sucrose and ethanol. Vinasse is the most important industrial waste produced by sucroenergetic activities: after ethanol is distilled from fermented broth, all the remaining material is characterized as vinasse and the production ratio is about 10–15 LVINASSE LETHANOL−1. In general, the polluting potential is very high (COD between 21,000 and 34,000 mgO2 L−1) and important concentrations of carbon compounds and mineral salts may be found in vinasses [6, 21].
Due to its nutritional value, vinasse has been widely used as fertilizer in sugarcane fields, however, such practice has become environmentally risky and a financial burden for ethanol companies. Previous studies associated vinasse fertirrigation with soil salinization; superficial and groundwater contamination by sulfates, nitrates, and metals; and greenhouse gases emissions by degradation of carbon compounds [13, 48, 65].
Economically, transporting vinasse to sugarcane fields has variable costs, which may be about US$ 0.5–14 m−3 of vinasse and because the production ratio (LVINASSE LETHANOL−1) is very high, costs may be significant. Whether a large ethanol mill (400,000 m3ETHANOL per season) uses vinasse (10–15 LVINASSE LETHANOL−1) exclusively for fertirrigation, estimated transportation costs could be US$ 1.8–82 million every year [44, 61].
In order to decrease vinasse volumes, some ethanol distilleries employ multi-effect evaporators and the in natura vinasse (2–4°Brix) is concentrated up to 8–60°Brix. Although it is very energy consuming, there is an increasing interest in the technology because transportation becomes more practical, good-quality water may be recovered and nutrients in vinasse are concentrated, as well [12]. Besides, the legislation CETESB P4.231/2005 in the São Paulo State regulates the volume of vinasse to be used in fertirrigation [15].
One of the main nutrients in vinasses is glycerol, a byproduct from Saccharomyces cerevisiae metabolism during alcoholic fermentation so the yeast cell integrity is not unsettled by increasing concentrations of ethanol. Once vinasse is concentrated, glycerol content becomes more important and that carbon source is not yet exploited by the Sucroenergetic Sector as it is wasted in fertirrigation [53].
In this study, we aimed to investigate the potential of integrating PHB and ethanol bioprocesses by using concentrated vinasse as basal medium for PHB biosynthesis. Firstly, two bacterial strains were evaluated: a glycerol-consuming strain of Ralstonia eutropha and a sucrose-consuming halophilic strain of Halomonas sp.
Halomonas sp. HG03 is a sucrose-consuming bacterium and it is adapted for growing in NaCl concentrations up to 100 g L−1, which makes it an interesting candidate for concentrated vinasse-based media while using a carbon source as cheap as sucrose [11, 42]. Besides, it can be cultivated in non-sterile conditions and PHB could be synthesized by a robust production process.
On the other hand, previous studies investigated crude glycerol from biodiesel as carbon source for PHB production by R. eutropha strains [14, 28, 29], as well as molasses [1, 19]. Both are concentrated forms of carbon sources. However, to our knowledge, no previous study evaluated the use of concentrated vinasse as glycerol source.
Finally, SuperPro Designer® software was used to simulate large scale bioprocesses with concentrated vinasse-based culture medium. Multiple technical and economic scenarios were analyzed, and production costs could be estimated and vinasse potential as nutrients source for PHB biosynthesis was assessed.
2 Material and methods
2.1 Microorganisms
The halophilic strain Halomonas sp. HG03 is derived from Halomonas HG01, a PHB-producing bacterium first isolated from salines in Peru [11, 42]. Before experiments, Halomonas sp. HG03 cells were activated in halophilic nutrient broth (L−1: beef extract, 3.0 g; peptone, 5.0 g; NaCl, 100 g. The glycerol-consuming strain Ralstonia eutropha PHB−4 LFM035-9 pBBR1MCS-2::phaPCJ (conveniently named as R. eutropha L359PCJ) was activated in Lysogeny broth (LB) and all experimental procedures had the final concentration of 0.25 μg L−1 of kanamycin sulfate. Both strains were cultivated in their respective culture medium at 30 °C for 24 h and cultures were used as inoculum for experimental procedures.
2.2 Vinasse
Concentrated vinasse (18°Brix) was obtained from a distillery that produces ethanol from sugarcane molasses in São Paulo State, Brazil. In our laboratory, vinasse was further concentrated to 34.4°Brix and kept at 4 °C. Whenever necessary, 34.4°Brix vinasse was diluted in distilled water into concentrations of 3.4°Brix and 7°Brix.
Before all analytical procedures and shake flasks experiments, vinasse was filtered in vacuum filtration system, which consisted of a Kitassato flask, Büchner funnel, and qualitative paper filter. Prior to all experimental procedures, vinasse had pH set to 6.8 < pH < 7.8 with NaOH 10 M solution, followed by autoclave sterilization, and at last it was added to sterile culture media.
In the laboratory, the in natura form of vinasse (3.4°Brix) had pH measured (pH = 4.3) and its chemical composition was analyzed and compared with previous studies in literature (Table 1).
2.3 PHB biosynthesis in shake flasks
Mineral medium (MM) was used as control treatment for PHB production [55] (L−1): carbon source, 15.0 g; Na2HPO4, 3.5 g; KH2HPO4, 1.5 g; (NH4)2SO4, 1.0 g; MgSO4.7H2O, 0.2 g; CaCl2.2H2O, 0.01 g; ammonium ferric citrate, 0.06 g; and 1 mL of trace elements solution (L−1: H3BO3 0.3 g; CoCl2.6H2O, 0.2 g; ZnSO4.7H2O, 0.1 g; MnCl2.4H2O, 0.03 g; NaMoO4.2H2O, 0.03 g; NiCl2.6H2O, 0.02 g; CuSO4.5H2O, 0.01 g).
All experimental treatments with R. eutropha L359PCJ used glycerol as carbon source (MMRe); treatments with Halomonas sp. HG03 used sucrose as carbon source and 80 g L−1 of NaCl was added to mineral medium (MMHa).
Cellular growth and PHB biosynthesis were evaluated in vinasse-based media, which consisted of volumetric dilutions of both in natura (3.4°Brix) and concentrated (7°Brix) vinasses in mineral medium.
R. eutropha L359PCJ was cultivated in four vinasse-based treatments and vinasse was a glycerol source, as detailed: 50% (v v−1) of vinasse 3.4°Brix in MMRe (MMReV503) had 5 g L−1 of glycerol; 75% (v v−1) of vinasse 3.4°Brix in MMRe (MMReV753) had 7.5 g L−1 of glycerol; 50% (v v−1) of vinasse 7°Brix in MMRe (MMReV507) had 10 g L−1 of glycerol; 75% (v v−1) of vinasse 7°Brix in MMRe (MMReV757) had 15.0 g L−1 of glycerol. A concentrated glycerol solution (150 g L−1) was used to set final concentration of 15 g L−1 in all treatments, except for MMReV757, which had no external glycerol source added to the medium.
Halomonas sp. HG03 was cultivated in similar treatments: 50% (v v−1) of vinasse 3.4°Brix in MMHa (MMHaV503); 75% (v v−1) of vinasse 3.4°Brix in MMHa (MMHaV753); 50% (v v−1) of vinasse 7°Brix in MMHa (MMHaV507); and 75% (v v−1) of vinasse 7°Brix in MMHa (MMHaV757). A concentrated solution of sucrose (300 g L−1) was used to set a final concentration of 15 g L−1 in all treatments.
In order to ensure proper nutritional conditions, all treatments for R. eutropha L359PCJ and Halomonas sp. HG03 cultures had carbon, nitrogen, and phosphate sources set to the same concentrations as those described above for MM. NaCl concentration was also standardized for all treatments with Halomonas sp. HG03.
Experiments were carried out with initial 6.8 < pH < 7.8, adjusted with NaOH 10 M, inoculum ratio of 5% (v v−1) in 125-mL flasks with 25 mL of culture medium incubated at 30 °C, under 150 rpm agitation for 48 h.
By the end of 48 h, processes were interrupted and cultures were submitted to centrifugation (4500 g, 15 min, 4 °C). Pellets were further used for determination of total cell dry weight (tCDW) and PHB content (% mPHB mtCDW−1); supernatant was further used for quantification of residual concentration of carbon sources and ammonium.
All experiments in shake flasks had treatments conducted in triplicates and analytical procedures were carried out in duplicates. Data were analyzed using ANOVA and means were compared with Tukey’s test (5% probability) by using ExpDes.pt package in RStudio software [25].
2.4 PHB biosynthesis in bioreactors
Experiments were carried out in CSTR bioreactors, model BiostatⓇ Cplus, Sartorius Stedim Biotech, with a 15-L vessel.
R. eutropha L359PCJ and Halomonas sp. HG03 were cultivated under similar conditions and operation mode, expect for specific nutritional requirements, which are specified whenever necessary.
Bioprocesses had inoculum ratio of 10% (v v−1) and initial volume of 6 L. Mineral medium for bioreactor (MMB) had the following composition [55] (L−1: carbon source, 15 g; KH2PO4, 0.65 g; (NH4)2SO4, 3.6; MgSO4.7H2O, 0.31 g; CaCl2.2H2O, 0.01 g; ammonium ferric citrate, 0.15 g; and trace elements solution, 2 mL. Culture medium consisted of MMB diluted in 50% (v v−1) of concentrated vinasse (7°Brix). Except for carbon, nitrogen, and phosphate sources, mineral nutrients were diluted by the addition of concentrated vinasse.
R. eutropha L359PCJ used vinasse as partial glycerol source (10 g L−1) and a concentrated glycerol solution (150 g L−1) was used to set 15 g L−1 of glycerol as final concentration (MMBReV507).
Halomonas sp. HG03 used sucrose as carbon source, which was fully provided by a concentrated solution (300 g L−1), so final sucrose concentration was set to 15 g L−1. Culture medium also had 80 g L−1 of NaCl (MMBHaV507).
Bioprocesses were operated in consecutive batches mode: two initial batches had growth-inducing conditions, followed by batches that induced PHB accumulation. The aim was to achieve higher concentration of residual cell dry weight (rCDW) before imposing nitrogen limitation for PHB accumulation. Besides, we aimed to minimize any osmotic stress during growth phase as a result of higher concentrations of carbon source, salts, and vinasse.
Once carbon source reached limiting concentration (below 5 g L−1), the first batch was considered finished. The second batch was initiated by adding a concentrated solution (10 ×) so initial concentrations (MMBReV507; MMBHaV507) could be restored. Once the second batch reached limiting concentrations of carbon source, growth phase was considered finished and further batches imposed nitrogen limitation, aiming to induce conditions for PHB accumulation.
During accumulation phase, R. eutropha L359PCJ had highly concentrated vinasse (34.4°Brix) and concentrated glycerol solution (300 g L−1) as the sole nutrients sources. Vinasse supplied 10 g L−1 of glycerol and a concentrated solution (300 g L−1) provided 5 g L−1 of glycerol. These materials were added in variable volumes, so final concentration of glycerol could be kept about the same for all batches (15 g L−1).
Halomonas sp. HG03 had sucrose fully provided by a concentrated solution (600 g L−1) so the final concentration was similar for all batches (15 g L−1). Highly concentrated vinasse (34.4°Brix) was added as well, so the initial volumetric dilution of 50% of concentrated vinasse could be kept. No other nutrients were added during accumulation phase.
Bioprocesses with both R. eutropha L359PCJ and Halomonas sp. HG03 were carried out at 30 °C and pH was automatically controlled at 6.8 < pH < 7.8 (H2SO4 1 M/NaOH 2 M). The air inflow was set to 3 Lpm (0.5 vvm) and agitation speed to 300 rpm. Bioreactors were set to keep saturation of dissolved oxygen at 40% by controlling both air inflow and agitation speed.
2.5 Analytical methods
Organic acids (acetic acid, succinic acid, lactic acid) and carbohydrates (glucose, fructose, sucrose, glycerol) were analyzed by liquid chromatography (HPLC) in a Dionex® system (Ultimate 3000, Thermo Fisher Scientific Inc., Waltham, MA, USA) with Aminex HPX-87H Bio-Rad column and RefractoMax 520 detector. Ionic forms of calcium, potassium, sodium, and ammonium in vinasse were determined by ionic chromatography using Metrohm 930 Compact system, with Metrosep C4 250/4.0 column. Amino acids were quantified by using the Phenomenex “EZ: Faast (™) Family of Amino Acids Analysis Kit,” for GC-FID determination [4].
Residual ammonium in experimental samples was determined by using Orion™ Ion-Specific Electrode kit for ammonia analysis (Thermo Fisher Scientific Inc., Waltham, MA, USA). Sample volumes of 3.0 mL were used and NaOH 10 M solution was used to alkalize samples and make ammonium available for detection, as procedure recommended by the manufacturer.
Cell dry weight was determined by lyophilization of cells, so PHB extraction could be performed next. Sample volumes of 10 mL were centrifuged, resuspended in saline solution (0.85% m v−1), next washed in the same solution, and centrifuged. The remaining pellets were then submitted to lyophilization overnight. Resulting dry mass was measured and cell dry weight was calculated in order to determine tCDW (g L−1).
The same dry mass samples were further used for PHB extraction by propanolysis method [57]. Propyl esters were next quantified by gas chromatography Agilent 7890A with HP-5 column (5% diphenyl-95%-dimethyl-polisiloxane, 30 m, 320 mm, 0.25-μm film thickness). Volume samples of 1.0 μL were analyzed after split injection (1:20) using a flame-ionization detector. Injector and detector temperatures were, respectively, 250 °C and 300 °C. Helium (0.8 mL min−1) was the carrier gas and a quantification program was set to 100 °C 1 min, 210 °C 15 min (8 °C min−1), up to 240 °C 1 min. Benzoic acid (40 mg mL−1) was used as internal standard.
PHB cellular content (mPHB mtCDW−1) was determined as the ratio of PHB concentration in tCDW. The concentration of rCDW was then obtained by subtracting PHB mass from tCDW.
In spite of vinasses being previously filtered, specific procedures were performed to ensure that insoluble solids from vinasse would not overestimate dry weight measures. Before bacterial inoculation, all vinasse-based culture media were submitted to dry cell weight analyses, following the same procedures described above. Vinasse-based media were analyzed in triplicates, the resulting dry mass was determined and subtracted from dry cell weight measures in bioprocesses samples.
2.6 In silico analyses of PHB production by R. eutropha L359PCJ in concentrated vinasse-based culture medium using SuperPro Designer®
SuperPro Designer ® v.9.5 was used in all technical and economical simulations in this study. A base scenario was designed and named SC1, which did not simulate the use of concentrated vinasse as nutrients source, meaning that MMB components were fully purchased. Scenarios that simulated vinasse-based culture medium described nutritional conditions of MMBReV507, that is, vinasse was a partial carbon source in addition to an external glycerol source (2:1). The bioprocess flowsheet is detailed in Fig. 1.
PHB bioprocess was simulated by a stoichiometric model based on methods described elsewhere [2, 10, 18, 32, 37, 49].
The global stoichiometric equations R1 and R2 described bioprocesses in seed fermentation (unit procedure P-11) and fermentation (unit procedure P-1), respectively. R1 used experimental yields of glycerol into biomass (YX/S grCDW gGLYVEROL−1) and product (YP/S gPHB gGLYCEROL−1) determined during growth phase, and R2 used global YX/S and YP/S (growth and accumulation phases). Other culture medium components, such as nitrogen, phosphate, and other salts, were considered in stoichiometric equations, as well. According to the composition of MMB medium, all nutrients had their concentrations normalized to glycerol, which resulted in their mass coefficients (Supplementary Materials, Table S1).
The main parameters for bioprocess simulation in unit procedures seed fermentation and fermentation are summarized in Supplementary Materials, Table S2.
Analyses assumed the fermentation procedure was carried out with high cell density cultures. Additionally, we simulated different PHB productivities, which were calculated from experimental data as described below.
Firstly, calculations considered growth phase and accumulation phase separately. For growth phase, experimental data of μmáx (h−1) and Xi (g L−1) were used so simulated time ranges for growth phase (tX-Si) could be determined for any given cell density (Xf) (g L−1), as described by Eq. 1. The base scenario SC1 assumed Xf = 30 g L−1 and ti = 0 h; tf = tX-Si (h).
Next, we considered that cells (Xf) reached the end of growth phase with the PHB content we obtained experimentally, so tCDW concentration and PHB titer at the end of growth phase were calculated.
For accumulation phase, PHB titers (P g L−1) at the beginning (Pi) and at the end (Pf) of accumulation phase were calculated. Pi was the same as the PHB titer at the end of growth phase. Pf was calculated using the experimental data of PHB content at the end of accumulation phase. Equation 2 details the calculation of PHB titers (P g L−1) for any PHB content (% mPHB mtCDW−1) and cell density (X = Xf g L−1).
Next, the time range of accumulation phase (tPHB-Si) was calculated by Eq. 3:
It was considered that rCDW concentration in accumulation phase did not vary, so X in Eq. 3 was replaced by Xf (g L−1) from Eq. 1.
Experimental data of specific biosynthesis velocities (μP) (g gh−1) were determined along the process and the maximum μP value was determined during the accumulation phase (μP = 0.09 g gh−1). Thus, the calculation of time range (tPHB-Si) only considered μP data that were observed after the maximum μP occurred and three values of μP were selected to describe optimistic (μP = 0.07 g gh−1), realistic (μP = 0.05 g gh−1), and pessimistic (μP = 0.02 g gh−1) scenarios of specific biosynthesis velocity, which impact PHB productivity directly. Then, ti was set to 0 h and tf = tPHB-Si (h) was calculated.
Finally, simulated global productivities (PpG-Si g Lh−1) were calculated by taking into consideration time ranges for both growth phase (tX-Si) and accumulation phase (tPHB-Si), as well as the PHB titer Pf (g L−1), as detailed in Eq. 4.
The model was designed for operation in batch mode and the annual capacity was set to 10,000 tons of PHB, which was determined based on three specific requirements for the integration of PHB and bioethanol processes:
-
The model had the annual campaign set to 240 days year−1 (5760 operational hours), which corresponds to the time range of bioethanol campaign and sugarcane harvest;
-
Vinasse demand would be compatible with large ethanol distilleries that produce 393,780–590,670 m3 of ethanol annually (10–15 LVINASSE LETHANOL−1), providing 59,067 tons of glycerol annually (in natura vinasse with 10 g L−1 of glycerol, Table 1);
-
Concentrated vinasse had no transportation or processing costs, so the theoretical model simulated a distillery that already had operating multi-effect evaporators, which means the model does not evaluate vinasse concentration.
Clearly, a shorter annual campaign imposes challenges regarding facility use and capital investment. So, in this study, productivities played a major role in techno-economic evaluation.
The model consisted of three sections: (i) The upstream section included culture medium preparation and seed fermentation. (ii) The bioprocess section consisted of fermentation in production reactors, followed by transfer out of PHB-rich medium into a storage tank. (iii) The downstream section was based on previously described processes of PHB recovery by surfactant-hypochlorite digestion [2, 18, 37]. According to authors, the method is economically viable, it has low environmental impacts in comparison to other extraction methods, and the global yield is up to 95%. In this study, downstream process yield was set to 90%. It comprised the addition of surfactant solution (1% m v−1) followed by incubation at 25 °C, 1 h. Next, hypochloride solution (NaOCl 30% m v−1) is added in flow-through and aqueous phase is separated by centrifugation. Finally, the material is washed with water (1:1), concentrated by centrifugation and spray-dried. The final product was PHB ≥ 99% m m−1.
Economical evaluations were performed for a 15-year period, annual interest rate of 6%, and selling price of US$ 10 kgPHB−1 [37]. Direct fixed cost (DFC) was calculated based on equipment purchase costs (Supplementary Materials, Tables S3 and S4) [37] and other facility-related costs (installation, engineering, instrumentation, electrical, piping, yard improvement, construction indirect, contractor fee, and contingency) were calculated by a Lang factor of 3.5 [73] and a loan interest rate on DFC of 10.25% was used [24].
2.7 Sensitivity analyses
Two sets of analyses were performed. First, the model was evaluated by different glycerol prices and the use of concentrated vinasse as partial source of glycerol (2:1) and minerals. A second set of analyses aimed to investigate how improvements in PHB productivity and higher cell densities could impact techno-economic feasibility.
According to Stracke et al. [69], crude glycerol from biodiesel processes in Brazil might have prices ranging from US$ 200 to 400 ton−1, depending on location, purification degree, and other factors. Thus, glycerol prices in scenarios SC1, SC2, and SC3 were respectively US$ 400 ton−1, US$ 300 ton−1, and US$ 200 ton−1; other components of MMB were fully purchased and vinasse was not employed in these scenarios.
Next, each glycerol price was evaluated combined with concentrated vinasse-based medium. So scenarios SC4, SC5, and SC6 simulated, respectively, glycerol purchased for US$ 400 ton−1, US$ 300 ton−1, and US$ 200 ton−1 as well; however, due to vinasse, final costs with glycerol in culture medium were eventually reduced to a third: US$ 133 ton−1, US$ 100 ton−1, and US$ 67 ton−1, respectively. Costs with mineral nutrients for culture medium were also reduced: except for phosphate and nitrogen sources, and all other mineral nutrients were diluted by vinasse (MMBReV507) and their costs were reduced by 50%.
The second set of analyses simulated concentrated vinasse-based medium for all scenarios: glycerol was purchased for US$ 400 ton−1 and final costs with glycerol and minerals in culture medium were reduced as described above.
Three cell densities were evaluated, Xf = 30 g L−1, Xf = 40 g L−1, and Xf = 50 g L−1, and each value of Xf was combined with experimental values of μP (g gh−1), which resulted in optimistic, realistic, and pessimistic scenarios of PHB productivities (PpG-Si) (scenarios SC7-SC15).
3 Results and discussion
3.1 PHB biosynthesis in shake flasks
Increasing volumetric dilutions of both in natura and concentrated vinasses had positive effect on cellular growth.
In Halomonas sp. HG03 cultures, rCDW concentration in MMHaV757 was statistically the highest among treatments. MMHaV507 had rCDW concentration statistically similar to that in MMHaV753. As for the lowest concentration of vinasse in culture medium (MMHaV503), rCDW concentration was not significantly different from that in control treatment MMHa (Fig. 2).
In R. eutropha L359PCJ cultures, all vinasse-based treatments showed a significant increase in cellular growth. Both in natura vinasse-based treatments MMReV503 and MMReV753 had statistically different concentrations of rCDW in comparison to control MMRe. The use of concentrated vinasse in MMReV507 treatment did not contribute significantly to increase rCDW in comparison to in natura vinasse treatments. MMReV757, however, resulted in the statistically highest concentration of rCDW (Fig. 3).
Quantification of residual ammonium showed that no detectable concentration was found in any treatment at t = 48 h. Both Halomonas sp. HG03 and R. eutropha L359PCJ depleted the main nitrogen source in control and vinasse-based treatments, so unbalanced nutritional conditions for PHB accumulation were met.
PHB cellular content decreased as vinasse concentration in culture media increased. In this study, the highest PHB content was determined for R. eutropha L359PCJ in MMRe (84.1 ± 3.6%), which was statistically higher than those determined for in natura vinasse-based treatments MMReV503 and MMReV753: 68.5 ± 4.9% and 65.1 ± 2.3%, respectively. PHB content in treatment MMReV507 (60.8 ± 4.0%) did not differ from that in MMReV753, but treatment MMReV757 had the lowest PHB content among R. eutropha L359PCJ treatments (48.4 ± 1.3%).
As for Halomonas sp. HG03, no statistical difference was found between control MMHa (81.7 ± 3.8%) and MMHaV503 (75.2 ± 2.7%). In other treatments, as vinasse concentration increased PHB content decreased significantly: 56.3 ± 3.1% (MMHaV753), 50.7 ± 6.9% (MMHaV507) and 19.6 ± 3.7% (MMHaV757).
Our results for PHB content were consistent with those reported by other authors that cultivated Halomonas sp. strains. According to literature, different culture conditions and carbon sources might result in PHB contents between 44 and 90% [36, 58, 62]. More specifically, shake flasks bioprocesses that used sucrose as carbon source showed PHB contents between 44 and 75% and rCDW concentrations between 1.1 and 1.6 g L−1 [36, 54, 68]. In our study, in natura and concentrated vinasse-based treatments (MMHaV503, MMHaV753, and MMHaV507) had PHB content (50.7–75.2%) consistent with those found in literature but rCDW concentrations (1.1–2.3 g L−1) were higher than those reported elsewhere.
In shake flasks cultures, with glycerol as carbon source, R. eutropha strains were described by other authors with rCDW concentrations between 0.66 and 2.2 g L−1, and PHB cellular content from 57 to 65% [14, 27, 72].
Similarly to our results with Halomonas sp. HG03, increasing vinasse in culture media improved R. eutropha L359PCJ cellular growth and rCDW concentrations (2.2–4.1 g L−1) were higher than those described elsewhere.
PHB biosynthesis is not growth related; however, efficient cellular growth is just as important because product titer is related to both PHB content and cellular density. In literature, producer microorganisms are reported to accumulate PHB up to 90% of their cellular weight [50]. Thus, improving product titer would be limited by cell density.
In this study, vinasse-based media showed potential as cheap media for improving cellular density by Halomonas sp. HG03 and R. eutropha L359PCJ. Product yield in treatment MMHaV507 decreased significantly in comparison to control, but it was balanced with YX/S (Table 2). R. eutropha L359PCJ, on its turn, was generally more efficient in converting substrate into product, especially in concentrated vinasse-based media. In MMReV507, YX/S was over three times higher than that in control treatment; YP/S was 15% lower than control and it did not differ from those determined in in natura vinasse-based treatments (Table 3).
Treatments with the highest concentration of vinasse did not show satisfactory results of PHB production, particularly by Halomonas sp. HG03. YP/S in MMHaV757 was 72% lower than that in MMHa; and MMReV757 showed YP/S 34.5% lower than control. Those results actually indicate that concentrated vinasse was not inhibitory to bacteria and the low product synthesis is likely due to unfavorable nutritional conditions for PHB accumulation, which were not unbalanced enough to trigger PHB metabolism efficiently. The use of high dilutions of concentrated vinasse would not necessarily be unviable for PHB production by those bacteria, but further studies would be needed.
Given the vinasse composition (Table 1), amino acids might have been used as nitrogen sources and/or building blocks by Halomonas sp. HG03 and R. eutropha L359PCJ. No previous literature was found about the amino acids composition of vinasses from the Brazilian bioethanol process. Most likely, yeast cells are the main source of amino acids in vinasse because they may lyse along the fermentation process, so cellular components are released in culture medium and remain in vinasse. Deeper comprehension of optimal medium composition for high PHB synthesis by these bacterial strains would be valuable information for vinasse-based bioprocesses.
Other authors also investigated the use of vinasse-based media for PHAs biosynthesis and some of them evaluated the use of in natura vinasse as carbon source, without supplementing any external source. Zanfonato et al. [74] used in natura vinasse as glycerol source (4.5 g L−1) for PHB production by C. necator DSM 545. Bhattacharyya et al. [5] and Pramanik et al. [52] investigated the use of in natura vinasses as carbon sources for PHAs production by archaeal strains, aiming to exploit organic acids as substrates.
However, in natura vinasses usually are diluted carbon sources and product titers are low. So, other authors studied the use of sugarcane molasses added to vinasse-based media in order to supply higher concentrations of carbon sources [1, 19, 47].
Cheaper feedstocks are a major interest in large scale bioprocesses in general. Biotechnological processes might have carbon sources accounting for up to 50% of total operational costs [22, 50]. As a consequence, efforts have focused on valorization of agroindustrial wastes through new technologies, aiming to eco-friendly solutions and profitable processes.
To our knowledge, many halophilic strains have been extensively studied in complex wastewaters and agroindustrial by-products [16, 17, 40], but no study on concentrated vinasse-based media has been found. These robust microorganisms have great potential for many biotechnological processes and still, there is little information about their productive capacity in sugarcane-derived by-products.
Moreover, many studies described the use of crude glycerol, a by-product from biodiesel process, as carbon source for PHAs production by some microbial strains: C. necator DSM 545 [14],C. necator DSM 7237 [33],Paracoccus denitrificans, C. necator JMP 134 [43],Burkholderia sacchari DSM 17,165 [59],C. eutrophus B-10646 [72]. As far as we know, no previous study described the use of concentrated vinasse as glycerol source, as it is presented in this study with R. eutropha L359PCJ.
Despite lower PHB production by R. eutropha L359PCJ in MMReV757 treatment, it is important to emphasize that such process had zero cost with carbon source acquisition. MMReV507 had an important reduction of costs with carbon source, since vinasse provided two thirds of glycerol. Moreover, results were particularly interesting because PHB production in MMReV507 was competitive with those in our control treatment and those reported elsewhere [14, 27, 72].
3.2 PHB biosynthesis in bioreactors
R. eutropha L359PCJ and Halomonas sp. HG03 were both cultivated in bioreactors using culture media with 50% (v v−1) of concentrated vinasse.
R. eutropha L359PCJ was cultivated for 74 h and maximum growth velocity was µmax = 0.255 h−1 between t = 0 h and t = 7.15 h. Other authors previously cultivated R. eutropha strains with crude glycerol as carbon source and maximum growth velocities between 0.11 and 0.15 h−1 were described [14, 72]. Zanfonato et al. [74] used in natura vinasse as glycerol source and µmax = 0.21 h−1 was determined.
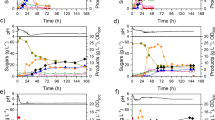
At t = 26 h, the second batch was initiated, which supplied all nutrients from culture medium (MMBReV507). At t = 35 h the third batch supplied concentrated vinasse (34.4°Brix) and glycerol solution as the sole nutrients source, which initiated the PHB accumulation phase. Further batches were initiated at t = 40 h, t = 50 h, and t = 57 h. In total, six batches were carried out (Fig. 4).
By the end of the growth phase (second batch) ammonium had been depleted, tCDW concentration was 15.6 g L−1 and PHB content was 21.4% (3.3 g L−1 of PHB). Previous studies also reported PHB content around 20% by R. eutropha strains at the end of growth phases [35]. During growth phase, conversion yields of glycerol into biomass (rCDW) and PHB were, respectively, 0.52 g g−1 and 0.14 g g−1 (Fig. 5).
By the end of accumulation phase (t = 74 h), the global conversion yields (growth and accumulation phases) were YX/S = 0.16 grCDW gGLYCEROL−1, YP/S = 0.32 gPHB gGLYCEROL−1 and the final concentration of tCDW was 28.0 g L−1, while PHB content was 66.3% (18.6 g L−1 of PHB and 9.4 g L−1 of rCDW). PHB productivity was 0.25 g Lh−1.
In shake flasks bioprocesses, R. eutropha L359PCJ had PHB content of 60.6 ± 4.0% and conversion yields were YX/S = 0.23 ± 0.05 grCDW gGLYCEROL−1 and YP/S = 0.34 ± 0.03 gPHB gGLYCEROL−1 (MMReV507 treatment). Thus, product yield in bioreactor process was consistent with those in shake flasks experiments and PHB content improved. Moreover, PHB production using concentrated vinasse as glycerol source was competitive with previous studies that cultivated R. eutropha strains with glycerol as carbon source: PHB content of 38–65%; rCDW concentration of 7.4–16.8 g L−1; PHB concentration of 6.7–26 g L−1; YP/S = 0.30–0.34 gPHB gGLYCEROL−1; and productivities between 0.16 and 1.52 g Lh−1 [9, 14, 33, 64].
Halomonas sp. HG03 was cultivated for 53 h and maximum growth velocity was µmax = 0.09 h−1 between t = 20 h and t = 27 h. At t = 22 h, the second batch was initiated by providing all nutrients of MMBHaV507. At t = 28 h, the third batch was initiated and a solution of sucrose and concentrated vinasse was the sole nutrients source. A fourth batch was carried out, which started at t = 44 h. Glycerol was not significantly consumed by Halomonas sp. HG03 because the bacterial strain does not metabolize glycerol efficiently, so accumulation along the process was expected (Fig. 6).
Although residual ammonium reached limiting concentrations at t = 32.5 h, rCDW showed increasing concentration until t = 44 h, which suggests that amino acids from vinasse might have been used as nitrogen source and/or building blocks and enabled Halomonas sp. HG03 to grow (Fig. 7). Previous studies described bioprocesses by Halomonas sp. strains that were cultivated in complex media, such as hydrolysates or amino acid-enriched media, and continued cellular growth was observed as well, even though the main nitrogen source had been depleted [58, 63].
Halomonas sp. HG03 culture in CSTR bioreactor using concentrated vinasse-based culture medium MMBHaV507: monitoring of tCDW (blue); rCDW (purple); PHB concentration (g L−1) (green); PHB content (% m m−1) (red); consumed total sugars (sucrose, glucose, and fructose) (g L−1) (yellow); reactor volume (L) (gray)
During the first batch, at t = 17.5 h, PHB content was 28.8%, and it showed little variation until the end of the second batch at t = 26 h (28.5%). By the end of the fourth batch, at t = 53 h, PHB content finally reached 42.2% and tCDW concentration was 17.6 g L−1 (PHB = 7.4 g L−1 and rCDW = 10.2 g L−1). Globally (growth and accumulation phases), conversion yields of sucrose into PHB and rCDW were, respectively, YP/S = 0.16 gPHB gSUCROSE−1, and YX/S = 0.22 grCDW gSUCROSE−1, and PHB productivity was 0.14 gPHB Lh−1.
In comparison to results obtained in shake flasks, the conversion yield of sucrose into rCDW reached an expected value, since it had been determined as YX/S = 0.23 ± 0.08 grCDW gSUCROSE−1 (MMHaV507 treatment). However, the consecutive batches operation mode was not successful in achieving higher product yield and PHB content, which had been, respectively: YP/S = 0.23 ± 0.02 gPHB gSUCROSE−1 and 50.7 ± 6.9% (MMHaV507 treatment). Further investigation on Halomonas sp. HG03 nutritional requirements is needed and such information could be valuable for improving bioprocess strategies for PHB production with vinasse-based medium.
Other authors cultivated halophilic strains with sugars (glucose, sucrose, molasses) as carbon sources and the described results were PHB contents between 52–55%, PHB = 0.6–7.5 g L−1, rCDW = 0.5–6.9 g L−1, and PHB productivities = 0.14–0.16 gPHB Lh−1 [46, 56, 62].
In this study, PHB production by Halomonas sp. HG03 was less attractive than that by R. eutropha L359PCJ: Halomonas sp. HG03 had lower PHB content, lower PHB titer, and its efficiency in converting substrate into product was lower, as well. Moreover, R. eutropha L359PCJ consumed glycerol from concentrated vinasse efficiently, which was further analyzed in silico by simulating large scale bioprocesses in SuperPro Designer®.
3.3 In silico analyses of PHB production by R. eutropha L359PCJ in concentrated vinasse-based medium
Experimental data of conversion yields during growth phase (YX/S = 0.52 grCDW gGLYCEROL−1; YP/S = 0.14 gPHB gGLYCEROL−1) were used in the global stoichiometric reaction R1 that defined inoculum production in seed fermentation.
The stoichiometric reaction R2 in production reactor was global, meaning that experimental yields corresponded to both growth and accumulation phases (YX/S = 0.16 grCDW gGLYCEROL−1; YP/S = 0.32 gPHB gGLYCEROL−1) and defined PHB and rCDW biosynthesis in Fermentation. The base scenario SC1 had PpG-Si = 1.1 g Lh−1 (X = 30 g L−1; experimental μP = 0.05 g gh−1; experimental PHB content of 21.4% at the end of growth phase; experimental PHB content of 66.3% at the end of accumulation phase; tX-Si + tPHB-Si = 51.7 h) ( Supplementary material, Table S2).
Efforts in improving productivity, especially in large scale processes, are important for reducing occupancy time of the production reactor (Supplementary material, Fig. S1). Besides, shorter batch times demand lower capital investments because fewer equipment units are needed in order to achieve an annual production demand, and maintenance costs might be reduced as well.
Table 4 details batch size, number of batches per year, production costs, capital investment, and profitability indicators in base scenario SC1. As a consequence of the shorter campaign in our model (240 days year−1), batch size was 91.8 tonPHB, which may be relatively large in comparison to the study by Leong et al. [37], who described a PHB process with an annual capacity of 9000 tonPHB year−1 and a 330-day campaign. The authors simulated batch size of 56.3 tonPHB and 146 batches per year.
Raw materials had a very important share in overall production costs (48.6%). Among those, glycerol was the most important and accounted for 30.1% of total production costs. Facility-dependent costs were just as important (49.1%), which indicated that optimal use of equipment played an important role in the model. As production reactors are the major equipment, efforts in improving productivity and final product titer are a common approach in industrial biotechnology processes, so equipment can be used more efficiently and capital investment becomes more attractive [73].
Utilities costs are mostly composed by energetic resources such as power, steam, high pressure steam, and cooling water, which were some of the least relevant in this study. Similarly to experimental procedures, medium sterilization for R. eutropha L359PCJ culture was considered in the model (steam-in-place operation). So, according to these results, medium sterilization might not represent an economical burden, although a non-sterile process would bring advantages nevertheless, since operation would be simplified.
Further analyses aimed to simulate glycerol price variations estimate how concentrated vinasse might contribute for reduction of costs and investigate the impact that improved productivities and higher cell density cultures might have on production costs.
3.4 Sensitivity analyses
Scenarios SC1-SC6 evaluated two different approaches for reducing costs with glycerol acquisition and their impact over production costs. Scenarios SC1, SC2, and SC3 reduced costs by purchasing glycerol at lower prices: US$ 400 ton−1, US$ 300 ton−1, and US$ 200 ton−1, respectively. As a result, annual costs reduced 8% as glycerol price decreased by US$ 100 ton−1. On the other hand, scenarios SC4, SC5, and SC6 reduced costs by using concentrated vinasse as partial glycerol source (2:1). Thus, scenario SC4 had costs reduced by 22.2% in comparison to SC1 (US$ 400 ton−1); SC5 reduced costs by 18.4% in comparison to SC2 (US$ 300 ton−1); and SC6 had costs 14% lower than those in SC3 (US$ 200 ton−1) (Fig. 8). Concisely, reduction costs by concentrated vinasse were higher for scenarios that simulated higher glycerol prices.
Unit production costs (US$ kgPHB−1) and annual production costs discriminated by raw materials (minerals, glycerol and other raw materials), facility, and other general production costs in scenarios with mineral medium MMB (SC1, SC2, SC3) and scenarios with concentrated vinasse-based medium (SC4, SC5, SC6)
Unit production cost ranged from US$ 5.1 kgPHB−1 to US$ 4.3 kgPHB−1 in scenarios SC1–SC3, and US$ 3.9 kgPHB−1 to US$ 3.7 kgPHB−1 in scenarios that simulated vinasse-based media, SC4–SC6. Figure 8 shows that unit costs were not strongly affected by glycerol prices in scenarios that used vinasse as partial glycerol source, which means the PHB process could be less vulnerable to external factors such as glycerol suppliers, market prices of feedstocks, or other external variables.
Besides glycerol, facility-dependent costs were also very relevant for PHB process (Fig. 8). So productivities and product titers were evaluated in scenarios SC7–SC15.
Optimistic (µP = 0.07 g gh−1), realistic (µP = 0.05 g gh−1), and pessimistic (µP = 0.02 g gh−1) productivities (PpG-Si) were calculated for different scenarios of high cell density (Xf): 30 g L−1, 40 g L−1, and 50 g L−1 (Table 5).
The simulated productivities (PpG-Si) ranged between 0.7 and 2.3 g Lh−1, which were consistent with those experimentally described elsewhere for R. eutropha strains using glycerol as carbon source [8, 14, 72].
Figure 9 discriminates glycerol and facility-dependent costs among annual production costs for base scenario SC1 and scenarios SC7–SC15, as well as unit production costs (US$ kgPHB−1).
Unit costs for scenarios SC7–SC15 ranged from US$ 2.8 kgPHB−1 to US$ 5.4 kgPHB−1 and all scenarios had lower annual costs than SC1, except for the pessimistic scenario SC9.
Scenarios SC1 and SC8 were technically similar because both had PpG-Si = 1.1 g L−1, Xf = 30 g L−1, and Pf = 59 g L−1, but SC8 simulated concentrated vinasse-based medium and its annual costs were 22.2% lower. As expected, optimistic productivities decreased production costs, and SC7 had costs 3% lower than those in SC8 and 24% lower than those in SC1. The pessimistic productivity in scenario SC9 increased annual costs by 7% in comparison to SC1 and 37.5% in comparison to SC8.
Optimistic productivity for cellular density of 40 g L−1 (SC10) decreased annual costs by 11% in comparison to the realistic scenario (SC11), and a pessimistic productivity (SC12) increased those costs by 34%. As for cellular density of 50 g L−1, a similar analysis determined annual costs 9% lower for SC13 (optimistic) and 39% higher for SC15 (pessimistic) in comparison to SC14 (realistic).
According to our results, increasing cellular density could be an important approach to decrease unit production costs (Fig. 10). Given that PHB content was invariable among scenarios (66.3%), cellular density determined product titers and concisely, pessimistic scenarios became less disadvantageous as cellular density increased due to higher product titers.
Scenario SC15 had a pessimistic productivity (PpG-Si = 1.1 g Lh−1), but the highest cell density (X = 50 g L−1), the highest PHB titer (98.4 g L−1), and the unit production cost was US$ 4.3 kgPHB−1. Scenario SC9, on the other hand, had a pessimistic productivity (PpG-Si = 0.7 g Lh−1), the lowest cell density (X = 30 g L−1), the lowest PHB titer (59 g L−1,) and the unit production cost was US$ 5.4 kgPHB−1, which made it a less competitive scenario than SC15 and base scenario SC1 (US$ 5.1 kgPHB−1). Similar interpretation can be made for optimistic and realistic productivities (Figs. 9 and 10). Thus, both productivity and cellular density were relevant for reducing production costs.
Profitability indicators are strongly influenced by production costs. Coherently, optimistic scenarios had the most attractive indicators and scenario SC13 (Xf = 50 g L−1; optimistic; PpG-Si = 2.3 gPHB Lh−1; Pf = 98.4 g L−1) had the lowest unit production cost (US$ 2.8 kgPHB−1), the lowest payback time (1.6 years), and the highest internal rate of return (IRR) (46.6%). Because higher density cultures had impact on PHB titer and unit production costs, more attractive profitability indicators were expected (Table 6).
In this study, it is important to highlight that productivity was a special interest because facility use is particularly important in biorefinery designs. However, efforts on improving productivity are just as important as achieving high product titers. As it is detailed in Fig. 11, scenarios that did not simulate optimistic productivities but simulated higher cell densities — and higher PHB titers — demanded fewer production bioreactors for the same annual demand of 10,000 tons of PHB.
At last, base scenario SC1 (Xf = 30 g L−1; PpG-Si = 1.1 gPHB Lh−1; Pf = 59 g L−1) and scenarios SC9 (Xf = 30 g L−1; pessimistic; PpG-Si = 0.7 gPHB Lh−1; Pf = 59 g L−1) and SC13 (Xf = 50 g L−1; optimistic; PpG-Si = 2.3 gPHB Lh−1; Pf = 98.4 g L−1) were further analyzed for other glycerol prices: cheaper crude glycerol, US$ 200 ton−1 and industrial grade pure glycerol, US$ 855 ton−1 (Table 7).
Considering the lower glycerol price (US$ 200 ton−1), scenario SC1 was more attractive than SC9, which was the least interesting scenario from the technical point of view due to its pessimistic productivity. However, once a very high glycerol price (US$ 855 ton−1) was simulated, SC9 became more competitive (US$ 6.0 kgPHB−1) than SC1 (US$ 6.8 kgPHB−1) despite the lower productivity. The use of concentrated vinasse as partial glycerol source was important to keep glycerol costs under an acceptable share in SC9 (18% of total production costs) and unit production cost increased US$ 0.8 once glycerol price raised from US$ 200 ton−1 to US$ 855 ton−1, while scenario SC1 had an increase of US$ 2.5 in unit cost.
SC13, on its turn, was the most attractive scenario from both economic and technical points of view since it had the highest productivity and high PHB titer. Despite the very high glycerol price (US$ 855 ton−1), SC13 was still more competitive than most scenarios in this study, except for SC10 (optimistic; Xf = 40 g L−1; purchasing price US$ 400 ton−1; US$ 3.1 kgPHB−1) and SC14 (realistic, Xf = 50 g L−1; purchasing price US$ 400 ton−1; US$ 3.1 kgPHB−1).
Although many factors do affect PHB production costs, such as microbial strain, PHB content, type, quality and logistics of feedstocks, and production capacity, our results were consistent with those previously described by other authors. Leong et al. [37] described US$ 5.8–6.1 kgPHB−1 for a production system that used pure glycerol as carbon source (US$ 500 tonGLYCEROL−1) and production capacity of 9000 tonPHB year−1. Levett et al. [38] described US$ 3.2–6.8 kgPHB−1 for PHB production that used methane as the carbon source and the annual production capacity was 100,000 tonPHB year−1. Van-Wegen, Ling, and Middleberg [70] evaluated PHB production by an E. coli strain, annual capacity of 4300 tonPHB year−1, and glucose was evaluated as the sole carbon source (US$ 5.6 kgPHB−1) or combined with dairy whey (US$ 2.7–3.6 kgPHB−1).
Unfortunately, the unit production costs we determined in this study are still not competitive with those of petroleum-derived polypropylene, US$ 1.1 kg−1 [34]. Technological advances are certainly needed, as well as a deeper comprehension on how PHAs fit in the global market. Their life cycle is not the same as those petroleum-derived products and many policies have been focusing on that aspect [7].
Still, it is noteworthy that our simulation model considered an industrial facility entirely dedicated to the PHB process regarding equipment purchase and other secondary costs. No facility sharing between PHB and bioethanol processes was actually simulated in this study, such as centrifuges, tanks, steam, chilled water, and cooling water generation. Once specific information on facility sharing between bioprocess were included, capital investment and some operational costs in the model could be become more attractive. In this study, the integration between processes focused particularly in vinasse recycle.
Finally, profitability indicators are highly variable among projects, depending on their technical specificities, geographic location, and markets they aim to. Payback time, however, is one of the most general indicators and, at some level, it allows the comparison among a variety of projects.
Previous studies used SuperPro Designer® as a tool for studying valorization of other agroindustrial wastes (whey wastes, syrups, and sugarcane molasses) through biocommodities manufacturing and payback times between 3 and 5.5 years were estimated [3, 30, 71].
Therefore, our results indicated that concentrated vinasse might be a competitive feedstock as glycerol source for PHB production. Experimentally, vinasse provided very satisfactory conditions for R. eutropha L359PCJ growth, no inhibitory effect was observed, glycerol from vinasse was efficiently consumed, and PHB production was consistent with those based on crude glycerol processes. In silico, the relevance of high cell density cultures for productivity and product titer was assessed. Further research is certainly needed in order to develop an optimized process, but there is great potential for competitive PHB production based on concentrated vinasse.
4 Conclusion
Concentrated vinasse has proven to be a potential substrate for producing PHB at lower costs. Different volumetric dilutions of in natura and concentrated vinasse affected cellular growth positively and the higher concentrations of vinasse in culture media indicated that nutritional conditions induced cellular growth over PHB accumulation, which may be very attractive for high cell density cultures.
R. eutropha L359PHB could efficiently consume glycerol from vinasse and biosynthesize PHB and no inhibitory effects were observed in CSTR bioreactor cultivation. Production yields, PHB content and final titer were comparable to those reported for PHB production based on other glycerol sources. Although Halomonas sp. HG03 showed satisfactory PHB biosynthesis, further investigation on the bacterium’s nutritional requirements would be beneficial for PHB biosynthesis in vinasse-based media.
Finally, in silico analyses assessed production costs of vinasse-based processes. The use of concentrated vinasse as nutrients source might reduce costs by 22.2% and higher cellular densities were relevant for improving profitability indicators.
Data availability
All the needed data are provided in the manuscript.
Abbreviations
- COD:
-
Chemical oxygen demand
- CDW:
-
Cell dry weight
- CSTR:
-
Continuous stirred-tank reactor
- DFC:
-
Direct fixed costs
- IRR:
-
Internal rate of return
- NPV:
-
Net present value
- PHB:
-
Poly(3-hydroxybutyrate)
- PHAs:
-
Polyhydroxyalkanoates
- rCDW:
-
Residual cell dry weight
- tCDW:
-
Total cell dry weight
- ROI:
-
Return on investment
References
Acosta-Cárdenas A, Alcaraz-Zapata W, Cardona-Betancur M (2018) Sugarcane molasses and vinasse as a substrate for polyhydroxyalkanoates (PHA) production. Dyna 85:220–225. https://doi.org/10.15446/dyna.v85n206.68279
Akiyama M, Tsuge T, Doi Y (2003) Environmental life cycle comparison of polyhydroxyalkanoates produced from renewable carbon resources by bacterial fermentation. Polym Degrad Stab 80:183–194. https://doi.org/10.1016/S0141-3910(02)00400-7
Anaya-Reza O, Lopez-Arenas T (2017) Comprehensive assessment of the l-lysine production process from fermentation of sugarcane molasses. Bioprocess Biosyst Eng 40:7. https://doi.org/10.1007/s00449-017-1766-2
Badawy AAB (2012) The EZ: Faast family of amino acids analysis kits: application of the GC-FID kit for rapid determination of plasma tryptophan and other amino acids. Methods Mol Biol 828:153–164. https://doi.org/10.1007/978-1-61779-445-2_14
Bhattacharyya A, Pramanik A, Maji SK, Haldar S, Mukhopadhyay UK, Mukherjee J (2012) Utilization of vinasse for production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) by Haloferax mediterranei. Dig AMB Express. https://doi.org/10.1186/2191-0855-2-34
European Bioplastics (2020) Dynamic growth: global production capacities of bioplastics 2020–2025. https://docs.european-bioplastics.org/conference/Report_Bioplastics_Market_Data_2020_short_version.pdf. Accessed 24 May 2021
Blunt W, Levin D, Cicek N (2018) Bioreactor operating strategies for improved polyhydroxyalkanoate (PHA) productivity. Polymers 10:11. https://doi.org/10.3390/polym10111197
Bormann EJ, Roth M (1999) The production of polyhydroxybutyrate by Methylobacterium rhodesianum and Ralstonia eutropha in media containing glycerol and casein hydrolysates. Biotech Lett 21:1059–1063
Canizales L, Rojas F, Pizarro CA, Caicedo-Ortega NH, Villegas-Torres MF (2020) SuperPro Designer®, user-oriented software used for analyzing the techno-economic feasibility of electrical energy generation from sugarcane vinasse in Colombia. Processes 8:9. https://doi.org/10.3390/pr8091180
Cardinali-Rezende J, Nahat RATPS, Moreno CWG, Farfán CRC, Silva LF, Taciro MK, Gomez JGC (2016) Draft genome sequence of Halomonas sp. HG01, a polyhydroxyalkanoate-accumulating strain isolate from Peru. Dig Genome Announc. https://doi.org/10.1128/genomeA.01598-15.
Carrilho ENVM, Labuto G, Kamogawa MY (2016) Destination of vinasse, a residue from alcohol industry: resource recovery and prevention of pollution. In: Prasad MNV, Shih K (ed) Environmental Materials and Waste: Resource Recovery and Pollution Prevention. Academic Press, pp 21–43. https://doi.org/10.1016/B978-0-12-803837-6.00002-0.
Cassman NA, Lourenço KS, Carmo JB, Cantarella H, Kuramae EE (2018) Genome-resolved metagenomics of sugarcane vinasse bacteria. Bioenergy Biofuels 11:48. https://doi.org/10.1186/s13068-018-1036-9
Cavalheiro JMBT, Almeida MCMD, Grandfils C, Fonseca MMR (2009) Poly(3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Process Biochem 44:509–515. https://doi.org/10.1016/j.procbio.2009.01.008
CETESB (2005) Stillage – Criteria and procedures for agricultural soil application. https://cetesb.sp.gov.br/wp-content/uploads/2013/11/P4.231_Vinhaça_-Critérios-e-procedimentos-para-aplicação-no-solo-agr%C3%ADcola-3ª-Ed-2ª-VERSÃO.pdf. Accessed 28 March 2022
Chen X, Yu L, Qiao G, Chen GQ (2018) Reprogramming Halomonas for industrial production of chemicals. J Ind Microbiol Biotechnol 45:545–554. https://doi.org/10.1007/s10295-018-2055-z
Chen GQ, Chen XY, Wu FQ, Chen JC (2020) Polyhydroxyalkanoates (PHA) toward cost competitiveness and functionality. Adv Industr Eng Polym Res 3:1–7. https://doi.org/10.1016/j.aiepr.2019.11.001
Choi J, Lee SY (1997) Process analysis and economic evaluation for Poly(3-hydroxybutyrate) production by fermentation. Bioprocess Eng 17:6. https://doi.org/10.1007/s004490050394
Coelho MPM, Correia JE, Vasques LI, Marcato ACC, Guedes TA, Soto MA, Basso JB, Kiang C, Dalsasso RR, Pavan FA, Bordignon SE, Aragão GMF, Poletto P (2019) Polyhydroxybutyrate (PHB) production by Cupriavidus necator from sugarcane vinasse and molasses as mixed substrate. Process Biochem 85:12–18. https://doi.org/10.1016/j.procbio.2019.07.007
Dalsasso RR, Pavan FA, Bordignon SE, Aragão GMF, Poletto P (2019) Polyhydroxybutyrate (PHB) production by Cupriavidus necatorfrom sugarcane vinasse and molasses as mixed substrate. Process Biochemistry. 85:12–18. https://doi.org/10.1016/j.procbio.2019.07.007
Dowd MK, Johansen SL, Cantarella L (1994) Low molecular weight organic composition of ethanol stillage from sugarcane molasses, citrus waste, and sweet whey. J Agri Food Chem 42:283–288. https://doi.org/10.1021/jf00038a011
Eggeling L, Bott M (2015) A giant Market and a powerful metabolism: L-lysine provided by Corynebacterium glutamicum. Appl Microbiol Biotechnol 99:3387–3394. https://doi.org/10.1007/s00253-015-6508-2
España-Gamboa E, Mijangos-Cortes J, Barahona-Perez L, Dominguez-Maldonado J, Hernández-Zarate G, Alzate-Gaviria L (2011) Vinasses: characterization and treatments. Waste Manage Res 29:1235–1250. https://doi.org/10.1177/0734242X10387313
España-Gamboa E, Mijangos-Cortes J, Hernández-Zarate G, Dominguez-Maldonado J, Alzate-Gaviria L (2012) Methane production by treating vinasses from hydrous ethanol using a modified UASB reactor. Biotechnol Biofuels 5:82–90. https://doi.org/10.1186/1754-6834-5-82
Ferreira E, Cavalcanti P, Nogueira D (2014) ExpDes: Na R Package for anova and experimental designs. Appl Math 5:2952–2958. https://doi.org/10.4236/am.2014.519280
Ferreira AG, Azzoni AR, Freitas S (2018) Techno-economic analysis of the industrial production of a low-cost enzyme using E. coli: the case of recombinant beta-glucosidase. Biotechnol Biofuels Bioprod. 11:81. https://doi.org/10.1186/s13068-018-1077-0
Fuess LT, Garcia ML, Zaiat M (2018) Seasonal characterization of sugarcane vinasse: assessing environmental impacts from fertirrigation and the bioenergy recovery potential through biodigestion. Sci Total Environ 634:29–40. https://doi.org/10.1016/j.scitotenv.2018.03.326
Fukui T, Mukoyama M, Orita I, Nakamura S (2014) Enhancement of glycerol utilization ability of Ralstonia eutropha H16 for production of polyhydroxyalkanoates. Appl Microbiol Biotechnol 98:7559–7568. https://doi.org/10.1007/s00253-014-5831-3
Gahlawat G, Soni SK (2017) Valorization of waste glycerol for the production of poly (3-hydroxybutyrate) and poly (3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer by Cupriavidus necator and extraction in a sustainable manner. Biores Technol 243:492–501. https://doi.org/10.1016/j.biortech.2017.06.139
García IL, López JÁ, Dorado MP, Kopsahelis N, Alexandri M, Papanikolaou S, Villar MA, Koutinas AA (2013) Biores Technol 130:16–22. https://doi.org/10.1016/j.biortech.2012.11.088
Gómez JA, Sánchez ÓJ, Correa LF (2020) Techno-economic and environmental evaluation of cheesemaking waste valorization through process simulation using SuperPro designer. Waste Biomass Valoriz 11:11. https://doi.org/10.1007/s12649-019-00833-4
Haas MJ, McAloon AJ, Yee WC, Foglia TA (2006) A process model to estimate biodiesel production costs. Biores Technol 97:4. https://doi.org/10.1016/j.biortech.2005.03.039
Hohenschuh W, Kumar D, Murthy GS (2014) Economic and cradle-to-gate life cycle assessment of poly-3-hydroxybutyrate production from plastic producing, genetically modified hybrid poplar leaves. J Renew Sustain Energy 6:6. https://doi.org/10.1063/1.4901870
Kachrimanidou V, Kopsahelis N, Papanikolaou S, Kookos IK, Bruyn M, Clark JH, Koutinas AA (2014) Sunflower-based biorefinery: Poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production from crude glycerol, sunflower meal and levulinic acid. Biores Technol 172:121–130. https://doi.org/10.1016/j.biortech.2014.08.044
Kachrimanidou V, Ioannidou SM, Ladakis D, Papapostolou H, Kopsahelis N, Koutinas AA, Kookos IK (2021) Techno-economic evaluation and life-cycle assessment of poly(3-hydroxybutyrate) production within a biorefinery concept using sunflower-based biodiesel industry by-products. Biores Technol 326:124711. https://doi.org/10.1016/j.biortech.2021.124711
Kim BS, Lee SC, Lee SY, Chang HN, Chang YK, Woo SI (1994) Production of poly(3-hydroxybutyric acid) by fed-batch culture of Alcaligenes eutrophus with glucose concentration control. Biotechnol Bioeng 43:9. https://doi.org/10.1002/bit.260430908
Kucera D, Pernicová I, Kovalcik A, Koller M, Mullerova L, Sedlacek P, Mravec F, Nebesarova J, Kalina M, Marova I, Krzyzanek V, Obruca S (2018) Characterization of the promising poly(3-hydroxybutyrate) producing halophilic bacterium Halomonas halophila. Biores Technol 256:552–556. https://doi.org/10.1016/j.biortech.2018.02.062
Leong YK, Show PL, Lan JCW, Loh HS, Lam HL, Ling TC (2017) Economic and environmental analysis of PHAs production process. Clean Technol Environ Policy 19:1941–1953. https://doi.org/10.1007/s10098-017-1377-2
Levett I, Birkett G, Davies N, Bell A, Langford A, Laycock B, Lant P, Pratt S (2016) Techno-economic assessment of poly-3-hydroxybutyrate (PHB) production from methane—the case for thermophilic bioprocessing. J Environ Chem Eng 4:4. https://doi.org/10.1016/j.jece.2016.07.033
Listewnik HF, Wendlandt KD, Jechorek M, Mirschel G (2007) Process design for the microbial synthesis of poly-β-hydroxybutyrate (PHB) from natural gas. Eng Life Sci 7:3. https://doi.org/10.1002/elsc.200620193
Mitra R, Xu T, Xiang H, Han J (2020) Current developments on polyhydroxyalkanoates synthesis by using halophiles as a promising cell factory. Microb Cell Fact 19:86. https://doi.org/10.1186/s12934-020-01342-z
Moraes BS, Zaiat M, Bonomi A (2015) Anaerobic digestion of vinasse from sugarcane ethanol production in Brazil: challenges and perspectives. Renew Sustain Energy Rev 44:888–903. https://doi.org/10.1016/j.rser.2015.01.023
Moreno CWG (2015) Produção de Polihidroxialcanoatos (PHA) por Halomonas sp. HG01. Dissertation, University of São Paulo.
Mothes G, Schnorpfeil C, Ackermann JU (2007) Production of PHB from crude glycerol. Eng Life Sci 7:475–479. https://doi.org/10.1002/elsc.200620210
NOVACANA (2020) Ranking: As 40 usinas que mais produziram etanol em 2019/20. https://www.novacana.com/n/industria/usinas/ranking-producao-etanol-2019-20-coloca-milho-dianteira-usinas-090720. Accessed 02 Sept 2021
Ortiz-Muniz B, Carvajal-Zarrabal O, Torrestiana-Sanchez B, Aguilar-Uscanga MG (2010) Kinetic study on ethanol production using Saccharomyces cerevisiae ITV-01 yeast isolated from sugar cane molasses. J Chem Technol Biotechnol 85:1361–1367. https://doi.org/10.1002/jctb.2441
Ortíz-Veizán N, Daga-Quisbert J, Perez-Zabaleta M, Guevara-Martínez M, Larsson G, Quillaguamán J (2020) Improvements of poly(3-hydroxybutyrate) production in an air-lift reactor using simple production media. Bioresour Bioprocess 7:1. https://doi.org/10.1186/s40643-020-00308-8
Paula CBC, Paula-Elias FC, Rodrigues MN, Coelho LF, Oliveira NML, Almeida AF, Contiero J (2021) Polyhydroxyalkanoate synthesis by Burkholderia glumae into a sustainable sugarcane biorefinery concept. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2020.631284
Pedro-Escher J, Maziviero GT, Fontanetti CS (2014) Mutagenic action of sugarcane vinasse in the Tradescantia pallida Test System. J Ecosys Econograph. https://doi.org/10.4172/2157-7625.1000145
Petrides, D (2015). Bioprocess design and economics. In Harrison RG, Todd PW, Rudge SR, & Petrides D (eds) Bioseparations Science and Engineering (pp. 11–1–11–83). Oxford University Press
Poltronieri P, Kumar P (2017) Polyhydroxyalcanoates (PHAs) in industrial applications. In: Martínez L, Kharissova O, Kharisov B (eds) Handbook of Ecomaterials. Springer, Cham. https://doi.org/10.1007/978-3-319-48281-1_70-1.
Posada JA, Naranjo JM, López JA, Higuita JC, Cardona CA (2011) Design and analysis of poly-3-hydroxybutyrate production processes from crude glycerol. Process Biochem 46:1. https://doi.org/10.1016/j.procbio.2010.09.003
Pramanik A, Mitra A, Arumugam M, Bhattacharyya A, Sadhukhan S, Ray A, Haldar S, Mukhopadhyay UK, Mukherjee J (2012) Utilization of vinasse for the production of polyhydroxybutyrate by Haloarchula marismortui. Folia Microbiol 57:71–79. https://doi.org/10.1007/s12223-011-0092-3
Quillaguamán J, Delgado O, Mattiasson B, Hatti-Kaul R (2006) Poly(β-hydroxybutyrate) production by a moderate halophile, Halomonas boliviensis LC1. Enzyme Microb Technol 38:1. https://doi.org/10.1016/j.enzmictec.2005.05.013
Quillaguamán J, Muñoz M, Mattiasson B, Hatti-Kaul R (2007) Optimizing conditions for poly(β-hydroxybutyrate) production by Halomonas boliviensis LC1 in batch culture with sucrose as carbon soucr. Appl Microbiol Biotechnol 74:981–986. https://doi.org/10.1007/s00253-006-0754-2
Quillaguamán J, Doan-Van T, Guzmán H, Guzmán D, Martín J, Everest A, Hatti-Kaul R (2008) Poly(3-hydroxybutyrate) production by Halomonas boliviensis in fed-batch culture. Appl Microbiol Biotechnol 78:2. https://doi.org/10.1007/s00253-007-1297-x
Rabelo SC, Costa AC, Rossel CEZ (2015) Industrial waste recovery. In: Santos F, Borém A, Caldas C (ed) Sugarcane: agricultural production, bioenergy and ethanol. Academic Press, pp 365–381. https://doi.org/10.1016/B978-0-12-802239-9.00017-7.
Ramsay BA, Lomaliza K, Chavarie C, Dubé B, Bataille P, Ramsay JA (1990) Production of poly-(beta-hydroxybutyric-Co-hydroxyvaleric) acids. Appl Environ Microbiol 56:7. https://doi.org/10.1128/aem.56.7.2093-2098.1990
Rathi DN, Amir HG, Abed RMM, Kosugi A, Arai T, Sulaiman O, Hashim R, Sudesh K (2012) Polyhydroxyalkanoate biosynthesis and simplified polymer recovery by a novel moderately halophilic bacterium isolated from hypersaline microbial mats. J Appl Microbiol 114:384–395. https://doi.org/10.1111/jam.12083
Riis V, Mai W (1988) Gas chromatography determination of poly-β-hydroxybutyric acid in microbial biomass-esther hydrochloric acid propanolisis. J Chromatogr 445:285–289
Rivera-Terceros P, Tito-Claros E, Torrico S, Carballo S, Van-Thuoc D, Quillaguamán J (2015) Production of poly(3-hydroxybutyrate) by Halomonas boliviensis in na air-lift reactor. Dig J Biol Res-Thessaloniki. https://doi.org/10.1186/s40709-015-0031-6
Rodríguez-Contreras A, Koller M, Dias MMS, Calafell-Monfort M, Braunegg G, Marqués-Calvo MS (2015) Influence of glycerol on poly(3-hydroxybutyrate) production by Cupriavidus necator and Burkholderia sacchari. Dig Biochem Eng J. https://doi.org/10.1016/j.bej.2014.11.007
Romanholo-Ferreira LF, Aguiar MM, Messias TG, Pompeu GB, Queijeiro-Lopez AM, Silva DP, Monteiro RT (2011) Evaluation of sugarcane vinasse treated with Pleurotus sajor-caju utilizing aquatic organisms as toxicological indicators. Ecotoxicol Environ Saf 74:132–137. https://doi.org/10.1016/j.ecoenv.2010.08.042
Santos F, Borém A, Caldas C (2012) Cana-de-açúcar: bioenergia, açúcar e etanol – tecnologias e perspectivas. Ed. Mecenas, Viçosa/MG. ISBN: 978–85–6024–939–8
Sharma K, Srivastava AK, Sreekrishnan TR (2021) Model-based optimization of biopolymer production from glycerol. Chem Biochem Eng Q 1:65–80. https://doi.org/10.15255/CABEQ.2020.1864
Soto MAA, Basso JB, Kiang CH, Genuchten MT (2015) Simulação de fluxo e transporte de íons de vinhaça através da vertente da formação Rio Claro. Águas Subterrâneas 29:162–174. https://doi.org/10.14295/ras.v29i2.28239
Souza JKC, Mesquita FO, Dantas-Neto J, Souza MMA, Farias CHA, Mendes HC, Nunes RMA (2015) Fertirrigação com vinhaça na produção de cana-de-açúcar. Agropecuária Científica no Seminárida. 11:7–12
Sreedevi S, Unni KN, Sajith S, Priji P, Josh MS, Benjamin S (2015) Bioplastics: advances in polyhydroxybutyra research. Adv Polym Sci. https://doi.org/10.1007/12_2014_297.
Stanley A, Kumar HNP, Mutturi S, Vijayendra SVN (2017) Fed-Batch Strategies for production of PHA using a native isolate of Halomonas venusta KT832796 strain. Appl Biochem Biotechnol 184:935–952. https://doi.org/10.1007/s12010-017-2601-6
Stracke MP, Wbatuba BBR, Padilha G, Seibert RM, Schrekker HS (2018) Biodiesel production process: valorization of the crude glycerol constituents. Braz J Dev 4:3401–3416
Van-Wegen RJ, Ling Y, Middelberg APJ (1998) Industrial production of polyhydroxyalkanoates using escherichia coll: an economic analysis. Chem Eng Res Des 76:3. https://doi.org/10.1205/026387698524848
Vieira JPF, Ienczak JL, Costa PS, Rossell CEV, Franco TT, Pradella JGC (2016) Single cell oil production integrated to a sugarcane-mill: conceptual design, process specifications and economic analysis using molasses as raw material. Dig Industr Crops Prod. https://doi.org/10.1016/j.indcrop.2016.05.046
Volova T, Demidenko A, Kiselev E, Baranovskiy S, Shishatskaya E, Zhila N (2018) Polyhydroxyalkanoate synthesis based on glycerol and implementation of the process under conditions of pilot production. Appl Microbiol Biotechnol 103:225–237. https://doi.org/10.1007/s00253-018-9460-0
Warner M (2019) Industrial biotechnology commercialization handbook: how to make proteins without animals and fuels and chemicals without crude oil. Warner Advisors LLC
Zanfonato K, Schmidt M, Quines LK, Gai CS, Schmidell W, Aragão GMF (2018) Can vinasse be used as carbon source for poly(3-hydroxybutyrate) production by Cupriavidus necator DSM545? Braz J Chem Eng 35:901–908. https://doi.org/10.1590/0104-6632.20180353s20170265
Funding
This study was financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES), Finance Code 001.
Author information
Authors and Affiliations
Contributions
MSS: experimental execution, formal analysis, data curation, writing; RAMP: conceptualization, supervision of the study, formal analysis, manuscript review; JLMSR: experimental execution, manuscript review; JGCG: conceptualization, manuscript review; ASB: conceptualization, manuscript review. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Silverio, M.S., Piccoli, R.A.M., dos Reis, J.L.M.S. et al. Techno-economic feasibility of P(3-hydroxybutyrate) bioprocess with concentrated sugarcane vinasse as carbon and minerals source: an experimental and in silico approach. Biomass Conv. Bioref. 14, 2071–2089 (2024). https://doi.org/10.1007/s13399-022-02710-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02710-y