Abstract
Halomonas boliviensis LC1 is able to accumulate poly(β-hydroxybutyrate) (PHB) under conditions of excess carbon source and depletion of essential nutrients. This study was aimed at an efficient production of PHB by growing H. boliviensis to high cell concentrations in batch cultures. The effect of ammonium, phosphate, and yeast extract concentrations on cell concentration [cell dry weight (CDW)] and PHB content of H. boliviensis cultured in shake flasks was assayed using a factorial design. High concentrations of these nutrients led to increments in cell growth but reduced the PHB content to some extent. Cultivations of H. boliviensis under controlled conditions in a fermentor using 1.5% (w/v) yeast extract as N source, and intermittent addition of sucrose to provide excess C source, resulted in a polymer accumulation of 44 wt.% and 12 g l−1 CDW after 24 h of cultivation. Batch cultures in a fermentor with initial concentrations of 2.5% (w/v) sucrose and 1.5% (w/v) yeast extract, and with induced oxygen limitation, resulted in an optimum PHB accumulation, PHB concentration and CDW of 54 wt.%, 7.7 g l−1 and 14 g l−1, respectively, after 19 h of cultivation. The addition of casaminoacids in the medium increased the CDW to 14.4 g l−1 in 17 h but reduced the PHB content in the cells to 52 wt.%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biopolyesters, the biodegradable polymers produced by several microorganisms from renewable resources, have been considered as good candidates to substitute petroleum-derived plastics (Lee 1996b; Steinbüchel and Füchtenbush 1998). These biopolymers, also called polyhydroxyalkanoates (PHAs), are stored intracellularly by several microorganisms as carbon and energy reservoir. The most commonly studied PHA, polyhydoxybutyrate (PHB), is known to possess physical properties similar to those of polyethylene, and has potential applications as disposable bulk material in packing films, containers, or paper coatings, amongst others (Lee 1996a; Reddy et al. 2003).
There are only a few bacterial species considered to be good candidates for large-scale production of PHB. These organisms are pseudomonads and Wautersia eutropha (formerly called Ralstonia eutropha), which produce PHB in the presence of excess carbon source and the depletion of a nutrient element (Lee 1996a); other well-known species are Alcaligenes latus, Azotobacter vinelandii UWD, and recombinant Escherichia coli, which do not require nutrient limitation for PHB synthesis (Lee et al. 1994; Lee 1996a). The former two strains are able to produce the polymer during their active growth phase. Furthermore, both A. vinelandii and E. coli can be grown in complex nitrogen medium, and yet lead to high cell densities and polymer contents (Page and Cornish 1993; Lee and Chang 1995).
As the efficiency and economics of the manufacturing process of PHB are determined by the carbon source, fermentation process, and downstream processing of the polymer, the development of cultivation conditions for microorganisms that allow high PHA content and productivity from cheap and renewable carbon sources is important (Lee 1996a; Choi and Lee 1999). Thus, A. latus and A. vinelandii UWD can utilize sucrose as carbon source to produce PHB, implying that inexpensive substrates such as raw sugar, beets, or cane molasses can also be utilized. Both strains are able to store 70–83 wt.% PHB of their cell dry weight (CDW), and reach amongst the highest cell densities reported so far (Page and Cornish 1993; Wang and Lee 1997). On the other hand, the extremely halophilic archaeon Haloferax mediterranei is able to store large amounts of PHA (67 wt.%) from starch; however, it also produces an extracellular polysaccharide, which might interfere with purification of the polymer, and requires high concentration of salts (25% w/v) for optimum PHA production (Lillo and Rodriguez-Valera 1990; Rodriguez-Valera and Lillo 1992).
Recently, we reported the production of PHB by a moderately halophilic bacterium Halomonas boliviensis LC1 from various carbon sources, including volatile fatty acids, mono-/disaccharides (Quillaguamán et al. 2006), and starch hydrolysate containing a mixture of maltooligosaccharides (Quillaguamán et al. 2005). In contrast to the culture requirements of extremely halophilic archaea, sodium chloride concentrations of 0.5 and 4.5% (w/w) provided the highest cell densities and PHB accumulation in the case of H. boliviensis (Quillaguamán et al. 2006). The polymer content obtained was in the range of 55 to 88 wt.%; however, the cell concentration was low (up to 2 g l−1), hence resulting in a low volumetric productivity of PHB. Although some improvements in PHB accumulation and cell mass concentration were reached by the addition of some nutrients, major enhancements in the PHB yield were attained when oxygen limitation was induced in a fermentor (Quillaguamán et al. 2005). This paper reports the optimization of culture conditions with an aim to improve the biomass concentration of H. boliviensis LC1 during PHB production, utilizing sucrose as the polymer precursor.
Materials and methods
Bacterial strain and maintenance
Halomonas boliviensis LC1T (=DSM 15516T) was maintained at 4°C on solid HM medium (Quillaguamán et al. 2004), containing (%, w/v) NaCl, 4.45; MgSO4×7H2O, 0.025; CaCl2×2H2O, 0.009; KCl, 0.05; NaBr, 0.006; peptone, 0.5; yeast extract, 1.0; glucose, 0.1; and granulated agar, 2.0. The pH of the medium was adjusted to 7.5 using 3 M NaOH.
Culture composition
Halomonas boliviensis was grown in Mineral Salts (MS) medium containing (%, w/v) NaCl, 4.5; MgSO4×7H2O, 0.190; CaCl2×2H2O, 0.065; KCl, 0.375; and NaBr, 0.02. Sucrose (Merck, Whitehouse Station, NJ, USA) and yeast extract (Merck) were added to the medium in varying amounts for different experiments.
Factorial design
Halomonas boliviensis was grown at 30°C in 60 ml of MS medium supplemented with 0.1% (w/v) yeast extract and 1% (w/v) sucrose in 250-ml flasks with rotary shaking at 200 rpm for 13 h (OD600 of 1.20 ± 0.05). Subsequently, 15 ml of the resulting culture broth was inoculated in 1-l Erlenmeyer flasks containing 235 ml of MS medium with 1% (w/v) sucrose, and combination of nutrients according to Table 1. The pH was initially adjusted to 7.5 using 3 M NaOH. Cultures were incubated at 35°C with shaking at 200 rpm and samples withdrawn at defined time intervals for determination of CDW and PHB content.
PHB production in a fermentor using stepwise addition of sucrose
For PHB production in a fermentor, H. boliviensis was first grown in 1-l flasks containing 100 ml of MS medium supplemented with 0.5% (w/v) yeast extract and 1% (w/v) sucrose, at 30°C with shaking at 200 rpm for 13 h (CDW of 2.3 ± 0.1 g l−1, OD600 ≈ 1.5). This culture was used to inoculate a 2-l fermentor vessel (Voyager, Luton, UK) with 1.4 l of MS medium containing 1% (w/v) sucrose. A solution containing MS medium supplemented with 100% (w/v) sucrose was intermittently added to the fermentor to provide excess carbon source to the cells. Antifoam was added when needed. The pH of the medium was initially adjusted to 7.7 with concentrated NH4OH, and was maintained at this value by using 5 M HCl/NaOH. The air inflow rate and agitation speed were initially set to 1.0 l min−1 and 700 rpm for all fermentations, and were increased up to 4.0 l min−1 and 1,100 rpm, respectively, when a decrease from the initial dissolved oxygen concentration (i.e., 85%) was detected. The effect of various nutrients such as sucrose, KH2PO4, and NH4OH were studied under these conditions.
Batch cultivations in a fermentor
Halomonas boliviensis was first grown under the conditions described above in 1-l flasks containing 150 ml of MS medium supplemented with 0.5% (w/v) yeast extract and 2.5% (w/v) sucrose. After 13 h of growth (CDW of 1.5 ± 0.17 g l−1), the culture was used to inoculate a 2-l fermentor vessel (Voyager) containing 1.35 l of MS medium with 2.5% (w/v) sucrose and 1.5% (w/v) yeast extract. The pH of the medium was maintained at 7.7 by using 5 M HCl/NaOH. The air inflow rate and agitation speed were initially set as described above. Subsequently, air inflow was increased to 4.0 l min−1, while agitation speed was modified to 800, 900, and 1,000 rpm, respectively, for different experiments to investigate the effect of oxygen limitation on cell concentration and PHB accumulation by H. boliviensis.
The effect of supplementing the medium with 0.2% (w/v) of casaminoacids and whey was also investigated, while maintaining an agitation speed of 900 rpm.
Quantitative analysis
CDW was determined by centrifuging 3 ml of the culture samples at 2,000×g for 15 min, followed by washing the pellet with distilled water and drying it at 75°C until constant weight was obtained. PHB quantification was performed by the method of Law and Slepecky (1961), according to which the dried pellets containing intracellular PHB were hydrolyzed using concentrated sulfuric acid for 1 h to obtain crotonic acid, which was quantified by measuring absorbance at 235 nm. Analysis was performed in triplicates in all cases.
Residual cell mass (RCM) concentration was calculated as the difference between cell concentration (CDW) and PHB concentration, while PHB content (wt.%) was obtained as the percentage of the ratio of PHB concentration to cell concentration (CDW) as defined by Lee et al. (2000).
Results
Factorial design studies on PHB production by H. boliviensis
To find optimum conditions for cell growth and PHB accumulation by H. boliviensis, the influence of various nutrients and all their possible combinations in the MS culture medium were investigated by a factorial design as indicated in Table 1. Sucrose was used as the carbon source because of its ready availability from renewable agricultural feedstocks. Yeast extract and ammonium sulfate were used as organic and inorganic nitrogen sources, respectively. The results of the factorial design experiments are depicted in Fig. 1a,b. It was observed that increasing the yeast extract concentration from 0.05 to 0.15% (w/v) led to an increase in cell concentration, but the levels of PHB showed a negative trend. Increasing the phosphate concentration at a fixed yeast extract concentration showed an increase in both cell mass and PHB content only in cases when the medium was supplemented with ammonium sulfate. The highest cell mass of 3.57 g l−1 was achieved in the medium with 0.2% (w/v) phosphate, 0.15% (w/v) yeast extract, and 0.15% (w/v) ammonium sulfate, which provided PHB accumulation of 69 wt.% (Fig. 1a,b). Reducing the yeast extract concentration in the medium to 0.05% (w/v) increased the PHB content to 78 wt.%, but the cell mass was decreased to 3 g l−1.
Influence of the amount of phosphate and organic and inorganic nitrogen, assayed as indicated in Table 1, on a PHB accumulation and b RCM by H. boliviensis LC1
Effect of yeast extract and phosphate on PHB production by H. boliviensis
Additional investigations on the increments of yeast extract and phosphate concentrations were performed in a fermentor in which pH and dissolved oxygen were controlled. In all fermentations, aliquots of 100% (w/v) sucrose in MS medium were intermittently added to keep excess carbon source in the medium. The maximum cell mass and PHB accumulation were obtained after 24 h of cultivation. Table 2 shows that while maintaining the yeast extract concentration at 1% (w/v), increase in phosphate levels from 0.15 g resulted first in a slight increase and then a decrease in both cell mass and PHB content. On the other hand, increasing the yeast extract amount while maintaining low phosphate concentration provided the highest cell mass of 12 g l−1 and PHB content of 44 wt.%. In all cases, inorganic nitrogen (NH4OH) was added initially to adjust the pH.
Influence of oxygen limitation and complex nitrogen-source supplementation on PHB production
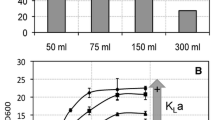
Our earlier studies have shown that the yield of PHB in H. boliviensis is improved under conditions of oxygen limitation (Quillaguamán et al. 2005). Oxygen limitation was thus induced in the fermentor by setting different agitation speeds (Fig. 2). The medium was supplemented with 25 g l−1 sucrose in accordance with other reports using carbohydrate concentration in the range of 20–30 g l−1 for optimum cell growth and PHB accumulation in various microorganisms (Page 1992; Lee et al. 1994; Wang and Lee 1997). At agitation speeds of 800, 900, and 1,000 rpm, respectively, the profiles of cell mass and PHB content with time were nearly similar, reaching a maximum at 17–19 h of cultivation, followed by a slight decrease (Fig. 2a,b). The maximum PHB yield of 54 wt.% was obtained in fermentations with agitation at 900 rpm (Fig. 2a). At the lower agitation speed, the dissolved oxygen in the medium was drastically reduced to about 4% with respect to air saturation, resulting in a reduction in the amount of the polymer (50 wt.%). PHB synthesis rate was increased at 1,000 rpm (reaching 54 wt.% in 17 h) but with a decrease in cell concentration, as seen in the decline of the RCM (Fig. 2b).
PHB accumulation (a); RCM (open symbols) and CDW (filled symbols) (b), and dissolved oxygen (c) during cultivations of H. boliviensis LC1 in MS medium supplemented with 2.5% (w/v) sucrose and 1.5% (w/v) yeast extract in a fermentor. Oxygen limitation was induced for different experiments by changing the agitation speed from 700 rpm to 800 (circles), 900 (squares), and 1,000 rpm (triangles). In all cases, 0.15 g \({\text{PO}}^{{3 - }}_{4} \) was added as indicated in Table 2
While keeping an agitation speed of 900 rpm, the effect of additional supplementation of casaminoacids and whey as nitrogen sources was examined. Addition of 0.2% (w/v) of casaminoacids resulted in an increase of CDW to 14.4 g l−1 in 17 h, but the polymer content in the cells was reduced to 52 wt.%. On the other hand, the addition of milk whey hindered both CDW and PHB accumulation, to a maximum of 12 g l−1 and 42 wt.%, respectively, in 19 h. Comparison of yeast extract and casaminoacids as nitrogen sources for cultivation of H. boliviensis in shake flasks showed that, when used individually at low concentrations (0.2% w/v), both allowed accumulation of comparable amounts of PHB, i.e., 25–30 wt.%, whereas, at a higher casaminoacids concentration (0.5% w/v), cell metabolism was directed mainly to cell growth, resulting in only 11 wt.% PHB in comparison to 47 wt.% with the same concentration of yeast extract.
Discussion
Investigations on PHB production by moderate halophiles have been recently initiated with studies on H. boliviensis, which is able to accumulate significant amounts of the polymer (50–88 wt.%) when grown on different carbon sources (Quillaguamán et al. 2005, 2006). The polymer productivity is, however, limited by the low cell densities of the organism during the cultivations.
In this work, the effects of different nutrients and cultivation conditions were examined with an aim to improve the production of PHB by H. boliviensis. A high concentration of yeast extract was necessary for high cell growth, but combination with a low phosphate concentration seemed to be necessary for increasing the formation of PHB (Fig. 1, Table 2). Indeed, yeast extract was able to provide enough nitrogen supply for the cells. It is usually expected that the consumption of NH3 by the cells would lead to a reduction in the pH of the medium, which is thus regulated by adding NH4OH (Lageveen et al. 1988; Kim et al. 1994). In our experiments, however, a reduction in pH was only detected after 5 h of cultivation, during the initial stages of the polymer accumulation by the organism, as has been reported earlier (Quillaguamán et al. 2005).
As was previously noted, the RCM of H. boliviensis was approximately constant during polymer accumulation, indicating that PHB is synthesized during the stationary phase of cell growth (Fig. 2b; Quillaguamán et al. 2006). It is thus likely that the depletion of nutrient(s) in yeast extract limits the cell growth and induces PHB accumulation in the cells. Improvements in cell density and inhibitory effect on PHB accumulation obtained on supplementation of the medium with casaminoacids suggest that the depleted components from the yeast extract are restored and could involve one or more amino acids. Amino acids and their derivatives—glutamate, ectoine, and glycine betaine—are essential to maintain cell osmolality and turgor in moderate halophiles to cope with water stress caused by high salt concentrations in their environment (Oren 1999; Kunte 2005). Such compounds are to be synthesized by moderate halophiles at high-energy costs for the cells unless they are provided in the medium (Oren 1999). Yeast extract contains amino acids and glycine betaine (Oren 1999), and could therefore have a positive effect on the growth of H. boliviensis (Table 2). It is not common to utilize high amounts of complex nitrogen sources, as they hinder the process of PHB production. Two exceptions are A. vinelandii UWD and recombinant E. coli. The former produces PHB coupled to the cell growth, and the addition of peptone and yeast extract increases the polymer content but not the final cell mass (Page and Cornish 1993). Yet, both organisms are considered as good candidates for industrial PHA production. On the other hand, the use of large amounts of yeast extract or other complex N sources in the medium leads to increased production costs. Hence, identifying the component(s) coupled to the PHB synthesis in H. boliviensis may allow the use of a suitable, cheaper nitrogen source at an appropriate concentration.
In the case of H. boliviensis, adequate carbon and complex nitrogen sources and oxygen depletion during the process are important for optimal cell growth and polymer production (Fig. 2). As compared with previous studies (Quillaguamán et al. 2005, 2006), the cell mass was increased about sevenfold, hence making it a competitive source of PHB. Table 3 shows that the polymer and cell concentrations attained by H. boliviensis were in the range of the best polymer producers reported so far in batch systems.
It may also be noted that the highest PHB production levels have been attained in fed-batch fermentations (Lee 1996b), e.g., a volumetric productivity of up to 5.13 g l−1 h−1 was obtained when A. latus was cultured with sucrose as the polymer precursor (Wang and Lee 1997). Hence, studies are ongoing to investigate other cultivation strategies and alternative media compositions for further improvements in cell mass and PHB content by H. boliviensis, and also effective and environmentally benign downstream processing methods for obtaining the pure polymer in high yields.
References
Choi J, Lee SY (1999) Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl Microbiol Biotechnol 51:13–21
Doi Y, Tamaki A, Kunioka M, Soga K (1988) Production of copolyesters of 3-hydroxybutyrate and 3-hydroxyvalerate by Alcaligenes eutrophus from butyric and pentanoic acids. Appl Microbiol Biotechnol 28:330–334
Heinzle E, Lafferty RM (1980) A kinetic model for growth and synthesis of poly-β-hydroxybutyric acid (PHB) in Alcaligenes eutrophus. Eur J Appl Microbiol Biotechnol 11:8–16
Kim BS, Lee SC, Lee SY, Chang HN, Chang YK, Woo SI (1994) Production of poly(3-hydroxybutyric acid) by fed-batch culture of Alcaligenes eutrophus with glucose concentration control. Biotechnol Bioeng 43:892–898
Kunte HJ (2005) K+ transport and its role for osmoregulation in a halophilic member of the Bacteria domain: characterization of the K+ uptake systems from Halomonas elongata. In: Gunde-Cimerman N, Oren A, Plenemitas A (eds) Adaptation to life at high salt concentrations in Archaea, Bacteria, and Eukarya. Springer, Berlin Heidelberg New York, pp 289–300
Lageveen RG, Huisman GW, Preusting H, Ketelaar P, Gerrit E, Witholt B (1988) Formation of polyesters by Pseudomonas oleovorans: effect of substrates on formation and composition of poly-(R)-3-hydroxyalkanoates and poly-(R)-3-hydroxyalkenoates. Appl Environ Microbiol 54:2924–2932
Law JH, Slepecky RA (1961) Assay of poly-β-hydroxybutyric acid. J Bacteriol 82:33–36
Lee SY (1996a) Bacterial polyhydroxyalkanoates. Biotechnol Bioeng 49:1–14
Lee SY (1996b) Plastic bacteria? Progress and prospects for polyhydroxyalkanoate production in bacteria. Trends Biotechnol 14:431–438
Lee SY, Chang HN (1995) Production of poly(3-hydroxybutyric acid) by recombinant Escherichia coli strains: genetic and fermentation studies. Can J Microbiol 14(Suppl 1):207–215
Lee SY, Lee KM, Chang HN, Steinbüchel A (1994) Comparison of recombinant Escherichia coli strains for synthesis and accumulation of poly-(3-hydroxybutyric acid) and morphological changes. Biotechnol Bioeng 44:1337–1347
Lee SY, Wong HH, Choi J, Lee SH, Lee SC, Han CS (2000) Production of medium-chain-length polyhydroxyalkanoates by high-cell-density cultivation of Pseudomonas putida under phosphorus limitation. Biotechnol Bioeng 68:466–470
Lillo JG, Rodriguez-Valera F (1990) Effects of culture conditions on poly(β-hydroxybutyric) acid production by Haloferax mediterranei. Appl Environ Microbiol 56:2517–2521
Oren A (1999) Bioenergetic aspects of halophilism. Microbiol Mol Biol Rev 63:334–348
Page WJ (1992) Production of poly-β-hydroxybutyrate by Azotobacter vinelandii UWD in media containing sugars and complex nitrogen. Appl Microbiol Biotechnol 38:117–121
Page WJ, Cornish A (1993) Growth of Azotobacter vinelandii UWD in fish peptone medium and simplified extraction of poly-β-hydroxybutyrate. Appl Environ Microbiol 59:4236–4244
Quillaguamán J, Hatti-Kaul R, Mattiasson B, Alvarez MT, Delgado O (2004) Halomonas boliviensis sp. nov., an alkalitolerant, moderate halophile bacterium isolated from soil around a Bolivian hypersaline lake. Int J Syst Evol Microbiol 54:721–725
Quillaguamán J, Hashim S, Bento F, Mattiasson B, Hatti-Kaul R (2005) Poly(β-hydroxybutyrate) production by a moderate halophile, Halomonas boliviensis LC1 using starch hydrolysate as substrate. J Appl Microbiol 99:151–157
Quillaguamán J, Delgado O, Mattiasson B, Hatti-Kaul R (2006) Poly(β-hydroxybutyrate) production by a moderate halophile, Halomonas boliviensis LC1. Enzyme Microb Technol 38:148–154
Reddy CSK, Ghai R, Kalia V (2003) Polyhydroxyalkanoates: an overview. Bioresour Technol 87:137–146
Rodriguez-Valera F, Lillo JAG (1992) Halobacteria as producers of polyhydroxyalknoates. FEMS Microbiol Rev 103:181–186
Steinbüchel A, Füchtenbush B (1998) Bacterial and other biological systems for polyester production. Trends Biotechnol 16:419–427
Wang F, Lee SY (1997) Poly(3-hydroxybutyrate) production with high productivity and high polymer content by a fed-batch culture of Alcaligenes latus under nitrogen limitation. Appl Environ Microbiol 63:3703–3706
Acknowledgements
The authors want to express their gratitude to the Swedish International Development Cooperation Agency (Sida) for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quillaguamán, J., Muñoz, M., Mattiasson, B. et al. Optimizing conditions for poly(β-hydroxybutyrate) production by Halomonas boliviensis LC1 in batch culture with sucrose as carbon source. Appl Microbiol Biotechnol 74, 981–986 (2007). https://doi.org/10.1007/s00253-006-0754-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0754-2