Abstract
Metal corrosion is one of the key challenges for materials scientists. This natural process creates loses in a variety of industries and necessitates enormous efforts to mitigate its effects. Organic coatings are still the most commonly utilised technique for protecting metallic materials against corrosion. They have opened a new field of research for obtaining coatings with better performance, lifetime, and customized features. While they have excellent anticorrosive characteristics, they must be updated by more environment friendly technology. As a result, there is a need to develop new and more cost-effective methods for creating and applying smart and environmentally friendly organic coatings to reduce corrosion. Anticorrosion research and implementations have progressed as a result of the functionality gained from these coatings at the metal-solution interface in harsh conditions. Smart coatings can react quickly to changes in the environment, cure coating flaws, and prevent additional corrosion. They possess better anticorrosion potential than the traditional anticorrosive coatings. This review discusses self-healing, corrosion sensing, anti-fouling, self-cleaning and anti-microbial organic coatings. It also provides a discussion on selected groups of smart anticorrosive organic coatings such as bio-based and water-borne epoxy resins, hyper branched polyesters and waterborne and bio-based polyurethanes. Moreover, this review outlines different approaches for applying organic coating. Finally, protection mechanisms of organic coatings are summarized.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lapses due to corrosion have severe consequences from both a human life and economic perspective, including the safety risks and plants outages. Corrosion inhibition for metal structures is an important step in extending the life and dependability of metal components in service [1, 2]. In recent years, corrosion experts have focused their efforts on surface functionalization of steel structures [3,4,5,6]. It is concerned with the inclusion of additional characteristics to certain materials in order to meet precise criteria. There are a variety of ways to protect metallic assets, but one of the most successful is to employ anticorrosion coatings [7, 8]. Corrosion inhibition coatings are typically used as functional barriers in a variety of settings, including prolonged contact with water, burying in soils, being exposed to ultraviolet radiation in industrial regions and air pollution [9]. Corrosion protection coatings can be classified as inorganic [10,11,12], organic [13,14,15,16], and hybrid [17,18,19,20] coatings (Fig. 1).
The coating a reactive metal’s outer layer with an organic coatings is a smart strategy to avoid corrosion while also obtaining other surface qualities without compromising mechanical characteristics. They are the most extensively used way for protecting metallic surfaces from corrosion, and they are especially important in transportation and infrastructure [21]. They provide protection by preventing the entry of hostile substances, such as water and oxygen, from reaching the coating/support interface, and hence are the first barrier coatings. They are made up of a complex blend of pigments, polymers, corrosion inhibitors, binders, fluid carriers and other additives. Pigments play a variety of roles in coating performance. The polymeric binder plays a crucial role in the coating's adherence to the substrate [21].
Emissions of volatile organic coatings are a serious health danger for workers as well as a long-term pollution threat to the environment. Ozone-depleting emissions like chlorinated fluorocarbons are particularly noticeable [22]. Over the years, chromate-based coatings such as primers and pigments have shown to be the most efficient corrosion protection solution. They must, however, be replaced with more ecologically friendly technology due to environmental and human health issues. As a result, there is more support and development of high-performance smart anticorrosive coatings for a wide range of industries [23, 24]. In the last few decades, extensive research has been conducted in order to develop new smart and green corrosion protection systems which has resulted in a massive search for water-based substitutes [25,26,27]. Volatile organic solvent emissions have been reduced through the use of exhaust air engineering controls, reduced paint usage through high transfer efficiency application methods, and the creation of novel coating formulas. The use of novel solvent less resins, the emergence of high-performance aqueous coatings, and heat-cured electrostatic powder coating are also examples of innovative formulations [28,29,30]. Examples of smart coatings include self-cleaning and super hydrophobic, antimicrobial, corrosion sensing, self-healing, and antifouling coatings [12]. The applications of smart organic coatings in various fields are depicted in Fig. 2 and are discussed in the following section.
The smart coat category is a well-known inhibitory product in the coatings business. These smart coatings, which contain inhibitors as well as other additives, reflect improvements in the corrosion inhibition characteristics as well as its multifunctional and environmentally beneficial features. When they come into contact with a severe environment, they have auto-responsive characteristics.
This review discusses self-healing, corrosion sensing, anti-fouling, self-cleaning and anti-microbial organic coatings. It also discusses bio-based and water-borne epoxy resins, hyper-branched polyesters, and waterborne and bio-based polyurethanes, among other smart anticorrosive organic coatings. Furthermore, this review discusses various methods for applying organic coatings. Finally, the organic coating’s protective mechanisms are summarised.
2 Types of anticorrosive organic coating
Organic coatings are used for a variety of reasons, including protection, aesthetics and practical functions including anti-fouling. They help constructions last longer by providing resistance to humidity, weather, abrasion, and chemicals. They have toughness and aesthetic appearance. The efficiency of organic coatings is affected by the mechanical characteristics of the smart coating technique, the kind and quantity of suspended inhibitors, pre-treatment of the surface of a metal, adherence of the coating towards the bottom of metal base, and the other additives that limit substrates corrosion[31]. The major characteristics of organic coatings are shown in Fig. 3. They are classified into three broad categories namely architectural coatings, product coatings and special purpose coatings. Paints and varnishes used in houses and buildings are called architectural coatings. These paints are less costly. Latex-based coatings (dispersions of polymer particles in a suspending medium, usually water) account for the majority of architectural coatings. Industrial/product coatings are applied to a broad range of objects, including appliances, automobiles, magnet wires, metal cans, furniture, chewing gum wrappers, and so on. Due to the different nature of product needs and hence types of coatings required, there is much more research and development in this market segment. Coatings for marine applications, aircraft, and maintenance coatings for bridges, chemical plants, storage tanks and other structures are examples of special purpose coatings. Major types of organic coatings are discussed in the following section Fig4.
2.1 Self-healing organic coatings
In recent years, the capacity of coatings to self-heal is a desirable aspect of corrosion inhibitor compounds. They are made by incorporating active inhibitory chemicals into polymer coatings through a technique that entails the progressive discharge of inhibitors from torn coating [32]. To retain mechanical qualities and prevent corrosion, the polymer matrix is intelligently healed once a damage happens. The chemical composition of the self-healing coatings determines its functionality. Inhibitors containing groups like as free radicals, aromatics, − OH, -SH, -C = C, -C = O-, − NH2, -COOH, -S–S-, -Si–O are used as active agents in micro and nanoforms. To incorporate self-healing qualities, two ways are available: intrinsic and extrinsic procedures. Additives are incorporated in the polymer coating after a trigger is applied in extrinsic strategy [33,34,35]. Additives such as corrosion inhibitors, capsules, hollow fibres, and vascular networks can offer extrinsic regeneration. As a result of the mechanical impact, the capsules shatter, releasing the healing agents, subsequently polymerize to create a protective film and restore its barrier property. The sol–gel procedure was used to create environmentally benign, self-healing, and highly effective anticorrosive coatings from poly(methyl methacrylate) and cerium oxide nanoparticles(NPs). The nanoscale dispersion of cerium oxide NPs into a poly(methyl methacrylate) matrix provided excellent anticorrosive efficiency and durability [36].
Because of their potential to store active anions, conductive polymers like poly pyrrole and poly aniline have sparked considerable interest in the field of protective coatings. The healing process is accomplished when conductive polymers are reduced by the released anions. The capacity of conductive polymers to self-heal and their environmental friendliness make them potential candidates to for replacing conventional hexavalent chromium coatings. Polyaniline, when combined with molybdate ions, hinders iron dissolution. Polyalinine has oxidising characteristics that cause steel passivation, and when combined with tetraoxomolybdate ions, self-healing can be obtained. The polyaniline-molybdate ion composite is an oxidizer that protects the substrate against anodic galvanic corrosion [37]. Fluorine resin coating made of a copolymer of vinyl ether and polychloro-trifluoroethylene has self-healing abilities. Its self-healing ability can be further improved by adding titanium or zinc powder. The added metal powder enables faster the manufacturing of corrosion resistant coatings [38]. By incorporating 8-hydroxyquinoline into poly(ethyleneimine) and poly(styrene sulphonate)coating, the protective qualities can be improved [39].
The intrinsic technique makes use of the polymer resins’ ability to heal on their own, without the need for external assistance when there is a crack on the surface of coating, is utilized. Dynamic reversible bonds, including reversible covalent and non-covalent bonds, are used in the intrinsic self-healing mechanism. The intrinsic chemical linkages and physical conformations of the polymer networks in the coating matrices are recovered to heal these coatings. In contrast to inhibitor-based systems, their healing effectiveness is in principle independent of the metal substrate. The Diels–Alder process was used to create an intrinsic mendable epoxy system with bifunctional adducts, which has shown to be particularly promising in corrosion resistant coatings area [40].
Natural ingredients like healing agents can be used to fill these capsules, which are considered an excellent green manufacturing method. Henna leaf extract has strong corrosion resistance. Henna leaves extract with acrylic coating was said to provide effective protection [41]. As greener corrosion inhibitors, several researchers employed oils such as linseed oils [42], tungoil [43], sunflower oil [44], and neem oil [45]. With the help of ambient oxygen, these natural oils oxidase, forming a film of polymerized dried oils that protects the coated surface even more.
Microcapsules made of GO can be used as strengthening healing capsules. Scratches on hot-dip galvanised steel surfaces were healed by microcapsules containing GO-linseed oil in waterborne PU coatings [46]. Epoxy coatings over mild steel incorporating GO/polysterene capsules with 8-HQ inhibitors can be employed as cheap self-healing anticorrosion compounds [47]. Metal parts used in undersea vehicles could be effectively protected by a graphene oxide(GO) incorporated mesoporous silicon dioxide layer nanosphere structure modified with tannic acid [48].
The use of renewable resources to create self-healing UV-curable coatings is crucial for environmental conservation and long-term growth. UV-curable coatings of biological origin with significant reparability solely with the help of heating be a substantial step forward in the long-term development of organic coatings. UV-curing coating films based on 2-furoic acid-glycidyl methacrylate and itaconic acid-glycidyl methacrylate showed outstanding self-healing, easily repaired, and remodelable properties [27].
2.2 Corrosion sensing organic coatings
They are typically pH-responsive coatings that must be exposed to oxygen reduction after an oxidative corrosion response. The commencement of corrosion on the alloys and metals covered with it is accompanied by an increase in pH at the location. Because of oxidation at high pH levels, some colour changing dyes or chemicals in the film matrix of these coatings fluoresce or change colour. The color shift or fluorescence is observable when the corroding species reacts with the matrices, which are usually transparent [49]. Anticorrosive species and additives are included in microcapsules within the colour dyes. They are also pigments designed to release anticorrosive species in the event of damage or when they detect the presence of corroding species without causing visible colour changes. For this purpose, hydroxyquinolines [50], schiff bases [51], phenolphthalein [52], fluorescein [53], 7-amino-4-methylcoumarin [32], bromothymol blue [54], and 7-diethylamino-4-methylcoumarin [55] have all been combined with the base coatings and inhibitory additives. This corrosion detecting indicator is best positioned near to the substrate in the primer layer and covered with a transparent topcoat. Nanotechnology has recently been applied in the development of corrosion sensing coatings and paints, which are designed to reduce the expense of maintaining specific buildings and structures made of metallic materials. Nanocapsules containing colour dyes, pigments and pH-sensitive additives preferably with a transparent top layer, have also been reported [56].
MOFs (Metal–Organic Framework) can produce strongly cross-linked epoxy/MOF composite coatings due to their high affinity interactions with inorganic and organic chemicals, which can modify the anti-corrosion properties of the epoxy coatings. Epoxy coatings containing modest levels of certain stimuli-responsive MOF nanocontainers also offer a barrier layer on the metal against corrosive media, and thereby protect the metal from further corrosion by managing the amount of released corrosion inhibitors [57]. It was discovered that acrylic polymer modified with phenanthroline can change colour after forming a complex with ferrous ions and can be utilised to detect corrosion at the metal-coating interface in neutral or near neutral pH circumstances [58].
The dispersion of nano sensors based on mesoporous silica nanocapsules in organic coatings introduced to metal substrates permits a highly delicate fluorescence recognition of the onset of metal dissolution, near to substrate flaws. As a result, this fascinating approach opens up new possibilities [59]. Corrosion inhibition of aluminium alloy substrate is achieved using anion-exchanging hydrotalcite chemicals distributed in organic resins. This component also gives the coating the ability to detect environmental changes that are a precursor to substrate corrosion [60]. Another corrosion-sensing method comprises an indicator interacting with metal ions freed during the corrosion process or low pH level at the anodic site of corrosion, where the metal dissolution happens, causing a change in the probe’s fluorescence.
By adding corrosion indicator chemicals into coating formulations, several authors have investigated techniques for corrosion prevention in aluminium and steel. Li and Calle have developed a microcapsule-carrying paint that exposes colour changes at triggered corrosion sites, leading to initial detection [61]. Meilun as examined the potential of smart anti-corrosive coatings for aluminium substrates. Smart anti-corrosive coatings are a relatively new technology that is critical in environments that prioritise safety, operability, and reliability [62].
2.3 Anti-fouling organic coatings
Marine biofouling costs the maritime industry a lot of money and generates a lot of issues. As a result, there is a high need for eco-friendly antifouling technology. Antifouling coating is a classic method of preventing marine biomass or creatures from adhering to surfaces. Antifouling is caused by a chemical reaction of responding fluid with the component surface. This type of coating is particularly important for the microbial driven corrosions of alloys and metals in aquatic environments. It promotes biological growth, which results in increased coating weight, decline of hydrodynamic characteristics and machine utilization, reduced speed and manoeuvrability, increased energy consumption, bio-entity cross contamination, and subsequent harm to coatings on ships. Organic coatings’ corrosion resistance and antifouling efficacy in maritime conditions have been greatly improved because to extensive study. An antifouling coating can also be applied to the top to prevent the attachment or formation of a biofilm or some fouling.
Fouling-resistant coatings can be made in two ways [63]. One is Chemically active or antifouling coatings, which work through preventing micro- and macro-fouling on the coating surface by restricting or lowering the settlement of the marine organisms employing the chemically active substances called biocides. Traditional biocides like Cu2O, CuO, and ZnO are still in use. To eliminate the biofoulings; the biocide incorporated in the coating is slowly exposed and released. Meanwhile, the biocide-depleted surface layer of the coating is polished/removed to reveal a new coating surface with enough biocide. To boost their anti-biofouling effectiveness, they were converted into NPs [64]. Many previously used biocides have been banned or will be outlawed as a result of rising environmental concerns, spurring the development of new biocide-releasing coatings. Antibiotics, inorganic ions, compounds and quaternary ammonium compounds are among the new ecologically friendly additives or biocides [65, 66].To avoid potential pollution of the environment, it is critical that the additives be extremely effective and fouling-selective. It only takes a very modest addition to make a significant difference. Some organic biocides that are harmless have been proposed. They are predicted to hasten the decomposition of biofoulant protein molecules, preventing or slowing the creation of the pre-conditioning fissure [67]. Serine protease can be encapsulated and utilized in a sol–gel coating and applied to a stainless steel surface for up to 9 months to keep the enzyme active against biofilm formation [68].
Foul-release coatings, on the other hand, are developed like that fouling microorganisms have only a weak connection to the surface, allowing them to be released by the small hydrodynamic forces produced by a flowing vessel. Fouling-releasing coatings are environmentally acceptable biocide-free antifouling option. Many research efforts have significantly shifted towards biocide-free, foul-releasing coatings which don't leach toxic compounds in the aquatic environments. Silicone-based polymers, for example, can be used to create a fouling-releasing coating that easily releases attached bio-fouling from the coated surface. The self-cleaning and antifouling properties of a composite poly (dimethysiloxane) covering containing SiO2-ZnO NPs have been described. The coatings, on the other hand, are expensive and ineffective in practise[69].
Another option is to use a brush made of hydrophilic polymer. Due to entropic repulsion, a hydrophilic polymer “brush” can hinder protein adhesion, preventing the creation of a biofilm for biofouling attachment. One of the most often utilised hydrophilic polymers is polyethylene glycol. Using atom transfer radical polymerization to graft polyethylene glycol brushes on a substrate surface, polyethylene glycol -based coatings can be made from self-assembled monolayers. As a result, the surface’s resistance to protein attachment increases as the density of the grafted polyethylene glycol and its chain length grows [70]. Some antifouling coating researchers are interested in the biomimetic attachment of polyethylene glycol to diverse substrates. Polyethylene glycol conjugated to a trimeric catecholate surface has been produced and immobilised on TiO2 and stainless steel to prevent the adsorption of human blood and germs [71]. Poly-cationic antibacterial compounds made by quaternary tertiary amine groups with alkyl halides also have antifouling properties, as the extremely positively charged polymer chains can harm bacteria's negatively charged cell membranes. They can be used to generate antibacterial surfaces by incorporating them into polymers, dendrimers and particles on a variety of substrates [72]. Yuan et al. used the ring opening reaction of a star-like poly (glycidylmehacrylate) to create hydroxyl-rich cationic derivatives, which they used to create an antibacterial and antifouling coating [73].
Antifouling biocides can be made from natural compounds derived from marine microbes [74], aquatic plants [75], seaweeds [76], marine animals [77], terrestrial sources [78]. They have the benefit of being more specific than heavy metals and are compatible with biological systems. Xu et al. developed 5-octylfuran-2(5H)-one (butenolide), an environmentally friendly antifoulant generated from Streptomyces spp. and showed solid antifouling action by limiting the larval settling of important fouling sort bryozoans and barnacles [79].
2.4 Self-cleaning organic coatings
Hydrophilic, hydrophobic, and super hydrophobic are the three types of self-cleaning coatings. A hydrophilic coating causes water to spread over the surfaces, carrying dirt and other impurities away. The surface contact angle between the liquid drop and the solid material is what determines the self-cleaning activity. Water’s behavior on surfaces, the preventing of flaws or imperfections on surface which could cause the coating to peel off as water enters, are all important considerations for effectively applying these coatings. The wettability and water contact angle of a solid coated surface are influenced by its geometric structure, chemical composition, roughness, and energy.
Hydrophilic coatings require polymers with superior film forming capabilities, as well as flexibility, toughness, and other features, so that a minor amount is required to give coatings with better mechanical attributes like toughness and optical transparency. Because of its favourable physical and chemical properties, TiO2 is a well-established key component of hydrophilic coatings. The contact angle shift of water droplet with the dirt on the substrate has been attributed to TiO2's photocatalytic and hydrophilic properties in a self-cleaning coat [80]. Anti-UV, self-cleaning and anti-bacterial paints are made with nano TiO2. Water droplets bead off of a fully healed surface, collecting up dirt and other surface impurities along the way [81].
The intrinsic chemical characteristics and surface microstructures of a material surface determine its hydrophobicity. The hydrophobic qualities of hydrophobic coatings result in water droplets and other surface contaminants spinning off healed surface, leaving the surface dry and clean. Surface-treated Al2O3 NPs help in the increase of hydrophobicity and scratch resistance [82].
Super hydrophobic coatings have sparked much interest in both basic research and industrial applications. Surfaces possess rough topology with small surface energy will have super hydrophobic wetting qualities in general. In recent investigations on metal corrosion protection, super hydrophobic surfaces have been identified as a significant technical accomplishment. Super hydrophobic surfaces can reduce the interaction between metal substrates and aqueous corrosive species by minimising water contact area and time or generating extra air barrier coatings, resulting in enhanced anticorrosive performance. Using modified silica particles in polystyrene, Power et al. demonstrated a viable technique for developing super hydrophobic coatings with self-cleaning and anticorrosion properties [83]. Vinyl triethoxysilane-based aqueous coatings are super hydrophobic, self-cleaning, and self-repairing [84]. Selim et al. developed an anti-fouling and self-cleaning silicone/β-MnO2 nanorod composite with a super hydrophobic coating [85].
Self-cleaning coatings with biocidal qualities are created by mixing nano-Ag and nano-TiO2 into the painting layer. Biocidal coatings incorporating biocides into NPs were created, and they are designed to deliver biocides once they are required. As a result, their biocidal efficacy lasts longer. Polymer based low-surface energy coatings have also been touted as self-cleaning coatings suitable for glass exteriors. NPs were employed to make transparent coatings that did not interfere with light passing through the glass [86].
2.5 Anti-microbial organic coatings
To prevent implant-related infection, antibacterial coatings are applied to device surfaces. Antibacterial agents, such as antibiotics, bioactive compounds, and inorganic antimicrobial agents, are released by active coatings into the environment. Among the available coating techniques and technologies for surface modification and treatment, it is desirable to include biocidal materials as an effective and flexible strategy for regulating microbial adherence, colonisation, and biofilm formation on surfaces. Protective polymeric coatings made of vegetable oil and Ag NPs can provide a strong barrier against moisture, corrosive acids and aggressive chloride ions. Silver, copper oxide and zinc oxide were previously employed to generate antimicrobial nanocomposite coatings. Patil et al. created antibacterial and anticorrosive poly urethane coatings using silver doped chicken egg-shell hydroxyapatite NPs. It was cost-effective, environmentally friendly, and long-lasting, with good anticorrosion properties and bacterial resistance against a variety of microorganisms[87].The research and development of non-toxic corrosion inhibitors is critical. The use of organic and inorganic fillers has been shown to increase the mechanical and barrier properties of coatings. El Fattah et al. found that adding chitosan to an epoxy coating increased corrosion resistance and antibacterial activity. Chitosan also improved the hardness, adherence, and impact strength of epoxy coatings while lowering their abrasion resistance. The addition of chitosan to epoxy coatings improved the epoxy coating's alkali, acid, and solvent resistance [88]. Epoxies, epoxy-polyurethane and epoxy-polyols coatings made from linseed and Pongamiaglabra seed oils are antimicrobial and corrosion-resistant [89].
For bacteria and corrosion prevention, Xu et al. used aqueous epoxy coatings containing poly m-aminophenol—GO on metal surfaces. The study make use of antibacterial and corrosion inhibiting properties of poly m-aminophenol. The composite has been shown to have antibacterial and anticorrosion properties [90]. Super hydrophobic coatings based on basalt salt and epoxy resin were created by Zheng et al. for use in severe marine environments. The developed coating has outstanding antibacterial and corrosion resistant properties [91].
3 Selected groups of smart anticorrosive organic coatings
3.1 Bio-based and water-borne epoxy resins
Epoxy coatings are organic polymers made by combining epoxy resins with co-reactants/hardeners/curatives which can include a wide range of compounds such as Lewis acids or bases, polyamines, acid anhydrides or in a chemical reaction. They have been applied in a variety of excellent characteristics of coatings due to their heat and water repellent, chemical stability, and great metal adhesion [92]. Epoxy resins provide higher surface coverage and anticorrosive activity than basic organic corrosion inhibitors due to their macromolecular composition. During metal-inhibitor interactions, the polar functional groups on the periphery of epoxy resins serve as adsorption centres [93]. Several epoxy resins have been employed as anti-corrosive coating materials in both pure and cured forms, particularly for carbon steel in acidic and saline environments. In the offshore industry, epoxy coating techniques are commonly employed as anti-corrosion solutions. Coats with excellent abrasion, chemical resistance and good barrier property are made with epoxy-based paints. It is usually applied as part of a multilayer system that includes a primer, two or three intermediate layers, and a topcoat. The durability,viscosity, adherence, solvent resistance, flexibility, and substrate wetting of an epoxy coating are all determined by the molecular weight of the epoxy resin [15].
The types of epoxy and curing agent used have a big impact on the qualities of the cured resins. The curing agents that are now in use are derived from petroleum. Furthermore, several popular curing agents, such as polyamides, isophorone diamine, other polyamines and anhydrides are toxic before curing, posing additional environmental and health risks [16].
Extensive research has been conducted around the world to develop safe and secure epoxy resins and curing agents’ derived sustainable bio based sources [94]. Plant oils have piqued attention as polymer building blocks due to their low cost, environmental friendliness, and ease of epoxidation, resulting in bio-based epoxy resins [95]. Catechins, saccharides, cardanols, tannins, lignin, terpenes, and rosins are important among the renewable resources [96]. Plant oils are commonly used in thermoset resins due to their high amount of carbon–carbon double bonds, which provide excellent polymerization handles. Starting with vegetable oils like soybean oil and castor oil, the manufacturing of bio-based epoxy resins is attracting a lot of interest. Epoxy resins based on linseed oil has become prominent in thermoset uses as a consequence of global push to develop bio-based epoxy resins for coatings applications [97]. Epoxy resins of saccharide have also been extensively studied in coating applications [96].
Liquid epoxy resin or solid epoxy dispersion are used in water-borne epoxy coating technology. It takes advantage of water as a low-cost catalytic solvent for delivering greater molecular weight epoxy polymers with low viscosities. They have good permeability and are frequently utilised in industrial applications as coatings and adhesives. They have been widely used to protect metals against corrosion. Using aqueous dispersions to deliver these resins draws attention to extending the paint pot life. Additionally, when water is utilised as the solvent, paints can be applied more easily [98]. The key benefits of this method are the minimal organic solvent content and great adherence to the intermediate coat and substrates. They also have superior abrasion resistance, better dry performance, are simple to mix, and have a minimal odour. Hydrophobic epoxy resin and hydrophilic amine based curing agent make up the majority of recently developed aqueous epoxy coating systems. The epoxy resin in these waterborne epoxy systems is in the form of well distributed hydrophobic phase in water [99]. Galgoci et al. created an aqueous epoxy resin system that included a non-ionic stabilised solid-type resin dispersion and an amine curing agent. The system has minimal volatile organic components, dries faster, hardens faster, and resists chemicals and aquatic environments well [100]. Fluorinated graphene modified aqueous epoxy resin has been shown to have better barrier characteristics, which adds to improved corrosion resistance [101]. Modification of waterborne epoxy resins with dopamine grafted metal- organic framework results in the enhancement of corrosion resistance and water resistance characteristics of epoxy resins [102]. Wang et al. developed a 3D network filler from stacking fly ash, GO, and multi-walled carbon nanotubes with the use of a silane coupling agents to improve the anti-corrosion performance and wear resistance of aqueous epoxy coatings. The modification enhances multi-dimensional cross-linking reactions between filler and filler, resin and filler, and resin and resin, which improves the cross linking ability of the water-based resin [103]. The corrosion resistance of waterborne epoxy resins doped with hexagonal boron nitride and strontium zinc phosphate was improved. This is owing to the synergetic effect of hexagonal boron nitride's physical barrier role and strontium zinc phosphate's inhibition [104].
3.2 Hyper branched polyesters
Hyper branched polyesters have sparked a lot of interest in recent years because of the relatively new and low-cost technology. They are frequently employed as promising resins for lowvolatile organic coatings formulation and protection against severe corrosive conditions. Hyper branched polyester is a three-dimensional dendritic polyester with a significant branched structure that can be employed as a surface modification to manage the inorganic–organic interface between the filler and the polymer matrix [105, 106]. Multiple active terminal functional groups of hyper branched polyester can make intimate contact with the polymer matrix, acting as a bridge between the two interfaces, enhancing filler dispersibility and interaction bonding and therefore coating performance [107]. They have a high density of functional end capping groups, which could be used as cross-linking sites for curing agents to create desirable crosslinking. The mechanical toughness of such strongly cross-linked coatings is predicted to be exceptional. They have numerous important qualities, including an internal cavity, strong reactivity, reduced melting, low solution viscosity, and good solubility, attributable to their adjustable size and non-entangled globular structures. These features, in general coating practise, denote effective flow paired with low solvent content to produce products that are chemically resistant, durable, and easy to clean [108].
In the field of coatings, the most commonly investigated highly branching polymers are primarily two: UV curable coatings based on hyper branched aliphatic polyesters and hyper branched polyester-amides. The catalysed esterification of the hydroxyl end-groups with acid chloride or anhydride is a simple way to convert a hyperbranched hydroxyl-functional polyester to a thermoset resin structure [109].
For corrosion protection, aqueous organosilane–polyester coatings were developed utilising methyltrimethoxysilane, polyester resin and 3-glycidoxytrimethoxysilane.The addition of organosilane into the polyester boosted the coatings' electric resistance, toughness, and hydrophobicity, resulting in improved corrosion resistance [110]. To make low-volatile organic coatings and recycle PET materials, Ikladious et al. developed alkyd resins based on glycolyzed PET waste and different aliphatic hyper branched polyesters with fatty acid [111]. A Sacha inchi oil-based alkyd resin was created by Hadzich et al. The combination of penterythritol and Sacha inchi oil improved anticorrosion behaviour and adhesion qualities in extreme environments, according to the findings [112]. Bat et al. developed a hyper branched fatty acid-based resin with excellent metal adhesion, adaptability and wear resistance [113]. Ashish et al. created an aqueous anti-corrosive coating made of a hyper-branched polyester polymer with excellent adhesion, strong cross-link density, and corrosion resistance [114].
3.3 Waterborne and bio-based polyurethanes
Polyurethanes (PU) have a wide range of applications due to their unique characteristics, including coatings, adhesives, and sealants, and recent research has focused on understanding the chemistry and physics of PUs. They are organic polymers that are formed by combining a monoglyceride, a polyol, and a diisocyanate to make urethane oil or urethane alkyd. When compared to typical alkyd resins, PU-based combinations provide a superior anticorrosive coating [115]. They are applied toimprove the appearance, lifetime, scratch resistance, and corrosion resistance of items. PU adhesives and sealants offer strong bonding and tight sealing. These adhesives have the benefit of developing “green strength” quickly, which means the material offers an initial bond before it fully cures. The rising need for high-performance PU coatings at a rational price has opened up a whole new world of possibilities for studying and constructing PU backbones with various architectures [116]. For low-volatile organic, weather-resistant coatings, Naik et al. developed a hyper branched urethane alkyd polymer. In the field of low-pollution weather-resistance coatings, these moisture-cured resins can be employed as a binder ingredient [117].
The development of waterborne PU is the initial step toward creating non-volatile solvent-free, non-polluting, and sustainable coating systems. In addition, aqueous PU has good mechanical, physical, and anticorrosion characteristics. They adhere to a variety of surfaces, including glass and polymeric fibres, with ease. Polyurethanes, in general, are hydrophobic and water insoluble. As a result, they must be changed in order to disperse in water, such as by adding ionic groups and/or non-ionic hydrophilic regions to the polymer structure [118]. Solvent-free approach was used to make waterborne polyurethane dispersions from various polycarbonate diols [119], m-di-(2-isocyanato propyl) benzene and carboxylic diols [120]. The production of well-dispersed graphene reinforced aqueous PU composite coatings was reported by Li et al. The ability of graphene-reinforced PU coatings to build a network in the PU matrix that prevents foreign molecules from permeating can be attributed to their better anticorrosive performance [121].
The design of greener chemical methods to replace petroleum-based products such as diisocyanates and diols is a difficult task. As a result, starch, lignin, cellulose, vegetable oils and fats are being investigated as a means of generating polymers from sustainable natural materials. They have esters and carbon double bonds that can be modified to make polyols, resulting in bio-based PUs. As corrosion inhibitors, Marathe and colleagues blended natural polyol and polyester from neem oil with quinoline. Quinoline was encapsulated in a micro-reservoir, which allowed it to overcome the drawbacks of adding it directly to a coating layer. The study reported that addition an corrosion inhibitor to neem oil-based smart PU coatings improves their anticorrosive properties [122]. Functional soybean oil-based polyols are another green resource for PU coatings [123]. Cashew nut shell liquid was successfully used as a precursor in the production of bio-based PU coatings. Cardanol, a phenolic compound produced from cashew nut shell liquid, comprises a reactive phenolic group and an aliphatic double bond that could be tailored to create novel functional materials for coating applications [124].
Tensile strengths, hardness, flexibility, adherence, and water resistance are all advantages of terpene-based polyols (derived from turpentine) that can be cross linked with polyisocyanate to make PU coatings. PU coatings have also been developed using eucalyptus tar compounds and castor oil [125]. Siyanbola et al. developed an environmentally acceptable approach to synthesise carbon NPs from Eucalyptus globulus leaves to enhance a castor oil-based PU coating. The composite outperforms the neat PU coating in terms of mechanical, hydrophobicity, thermal and anticorrosion properties [126].
4 Approaches for applying organic coating
Painting, powder coating, electrophoretic coating, and sol–gel coating are different approaches for applying organic coatings. The advantages and disadvantages of different types of organic coatings are depicted in Table 1.
4.1 Painting
The first stage in painting is to choose an alkali-resistant primer, which could be polyvinyl butyral, vinyl epoxy, acrylic, baked phenolic or polyurethane. Pigments such as chromate and titanium dioxide have been used to improve corrosion resistance. This is the simplest and most customizable way of applying a coating. Its main drawbacks are the need for organic solvents and the multi-step process [127].
Standard methods used for painting to structural steelwork include application by brush, roller, traditional air spray and airless spray. Brush application, in its original form, creates extremely strong shearing forces between the liquid paint and the substrate. This substantially aids the intimate wetting of the steel surface, resulting in enhanced dry paint film adhesion. Roller application is significantly faster than brushing and is used for big flat surfaces, but it requires the paint to have the right rheological qualities. It is therefore not a recommended application method for coating awkward corners, bolt heads, and so on. In spray drying, the paint is atomized into fine droplets and sprayed onto the covered surface, where the droplets link together to form a continuous coating. Air spraying or airless spraying can be used to atomize the material. This method can be used to apply solvent-free compounds like two-pack commodities that can be blended right at the spray gun nozzle while being applied. In order to produce the best outcomes, costly equipment and highly experted labours are required [128].
4.2 Powder coating
Powder coating is a solids-based coating that is applied as a dry powder and then heated to form a film. It uses a solid binder and pigment application process. When heated, the solid binder melts, bonds the pigment, and when cooled, forms a pigment coating. Powder coatings have become increasingly popular in recent years, and the demands for functional powder coatings have gradually increased [129]. Electrostatic powder spraying, flame spraying of thermoplastic powders and fluidized bed spraying are the most common methods for powder coating. In most cases, the electrostatic spray procedure is the most flexible and versatile of these processes. Following heat fusion, the powder is usually attached to the substrate as a film [130]. Because the powder coating technique do not need solvents, it was appealing from an environmental standpoint; excess powder could theoretically be collected and recycled, allowing product usage to approach 100%. This technology also has high economic benefits, energy savings, and performance advantages, as well as the elimination of hazardous waste [131]. Powder coating, on the other hand, has several drawbacks. The powder must be completely dry, the coating should be thick, coating recessed portions is challenging, and an extreme heat is required, which may be incompatible with particular substrates [132].
4.3 Electrophoretic deposition
It is basically a two-step procedure. Under an electric field, charged particles suspended in a liquid migrate towards the electrode in the first step. The particles deposit on the electrode in the second step, creating a moderately dense homogeneous film. An appropriate heat-treatment such as firing or sintering is frequently required as a post- electrophoretic deposition processing step. Cathodic electrophoretic deposition and anodic electrophoretic deposition are the two types of electrophoretic deposition [133].
In a typical electrophoretic coating process, surface treatment is essential. It has a big impact on the coating’s quality and performance. In industry, wet silane and phosphating are the most commonly used pretreatments for electrophoretic coating [134, 135].
Electrophoretic deposition has the advantages of a quick formation time, a simple apparatus, a wide range of substrate shapes, and no need for binder breakdown because the green coatings includes few or no organics. In comparison to other progressive coatings procedures, the method is extremely flexible, allowing it to be simply customised for a given purpose. However, the procedure necessitates intricate electrical regulation [136, 137].
5 Sol–gel coating
In this process, a solid phase is created by the gelation of the colloidal suspension. It can be dried to form a dry gel state, which employed to eliminate unreacted organic byproducts, stabilise the gel into densify it, or add crystallinity. The following are some of the benefits of employing sol–gel coatings: The temperature of the processing is kept low, usually near room temperature. Because liquid precursors are employed, coatings can be formed on complex forms and thin films can be produced without the requirement for machining or melting. Green coating technologies are used to generate the sol–gel films. Compounds which does not introduce impurities to the ultimate product are used as starting components. There is no waste produced during the creation of sol–gels, and there is no need to wash them. Further advantages include the product’s broad range of formulations, excellent adhesive to the substrate, as well as chemical and thermal stability [138, 139]. However, mechanical deposition processes such as dipping, spin-coating, and spraying have a tiny thickness limit, which is a key limitation of sol–gel technology [140].
6 Protection mechanism of organic coatings
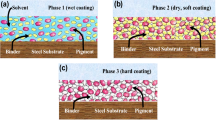
Anticorrosive coatings are classed based on the mechanisms that protect a metal against corrosion. Corrosion can take several forms, depending on the systems, design of materials and engineering, environments, and other factors. Anticorrosive coatings include three fundamental defensive processes. To a large extent, the processes of the three types of coatings are similar, but the primary difference is in the nature of the pigments and substrates, as shown in Fig. 4.
6.1 Barrier coatings or impermeable coatings
Barrier protection is regarded as the most common form of protection mechanism. It works by preventing hostile species like water, ions, gases, and electrons from entering the surface. This can be accomplished by adding pigments to the coating or using a chemical conversion layer. This sort of coating is commonly employed on immersed structures and can be utilised as a topcoat, primer or intermediate. When the binder is made up of macromolecules with a stiff structure, a significant cross linking, or crystalline chain segments, it acts as a physical barrier to diffusion. However, the solid structure and toughness of the coating may cause it to become brittle, resulting in a loss of mechanical capabilities. The additions of barrier pigments prolong the diffusion paths of the invading species. Barrier coatings, such as epoxy, are widely used and have been shown to efficiently prevent corrosion until coating faults occur. Pits or holes in the coating form as a result of mechanical shocks or age, and this is when the coating fails. Through flaws, the corrosive species assaults the underlying metal, increasing the exposed surface and thereby speeding up the corrosion process [11].
When graphene and its derivatives are integrated into organic coatings, they have the ability to act as an efficient barrier against the diffusion of corrosive materials including H2O, O2, and Cl [141]. In addition, the alignment of graphene and its derivatives in the polymer matrix contributes corrosion resistance. Organic–inorganic nanocomposites based on traditional epoxy, polyurethane, and acrylic components mixed with ceramic nanofillers including ceria, silica, and zirconia demonstrated excellent barrier properties, preserving steel and aluminium alloys for longer periods of time. The addition of inorganic nanofillers to the organic matrix results in a thick, homogeneous nanocomposite that acts as a diffusion barrier, limiting water absorption and ionic species diffusion to a very small amount. Epoxy resins with GO-ZrO2 attachment offer superior barrier protection. Because of their high aspect ratio, 2D sheet structure, and homogenous dispersion in the epoxy matrix, studies have shown that sheets like GO-ZrO2 hybrids can provide an additional barrier layer to impede electrolyte permeation and provide good barrier protection [142].
Organic–inorganic hybrids such as PMMA-SiO2 [143], epoxy-SiO2 [144], and polyurethane-SiO2 [145] coatings, all of which use silica as an inorganic phase, have recently been investigated for their structural and anticorrosive capabilities on metallic alloys. The scientific community and industry have paid close attention to their exceptional performance as a passive barrier with great corrosion resistance and durability. On a molecular or nanoscale, covalent conjugation of the organic and inorganic phases produces novel materials with distinct characteristics. The polymeric phase of this nanocomposite material serves to hermetically seal the structure, avoiding the passage of corrosive species like chloride ions, water, and oxygen, while also giving the coating a hydrophobic property. The ceramic phase, which leads to the formation of crosslinked silica nodes, compresses the polymeric chain segments and creates covalent Si–O-M connections at the film/metal contact, ensuring long-term adhesion [146].
6.2 Inhibitive coatings
Unlike impermeable based coatings, inhibitive coatings prevent corrosion by the interacting with environment to form a protective layer on the surface of the metal. The study of any chemical substance's inhibitory efficacy in corrosion inhibitor coatings is focused with the substance directly, which is usually employed as an inhibitor, rather than just as a paint addition. If the coating includes corrosion resistant pigments or chemical compounds that hinder chemical or electrochemical metal corrosion, this mechanism is activated. A leaching mechanism is used for active protection. This is a complicated process in which the inhibitor is liberated from the coating and migrates to corroding sites, passivating the substrate in the coating defect area [147]. Inhibitory pigments are mostly inorganic salts that are water soluble to a certain degree. Because pigments have a poor solubility, they can only be released slowly if the coating has flaws. They are mostly used in industrial sectors where there is a high risk of atmospheric corrosion and they are not meant to be submerged in water or buried in soil. This type of coating is usually used as a primer because it is only useful if soluble components may react with the metal. Calcium plumbate, lead cyanamide, dibasic lead phosphite, lead silicochromate, zinc chromate, zinc phosphate, and zinc tetraoxichromate are examples of inhibitive anticorrosive pigments. These anticorrosive pigments only protect in the presence of water, either through their water-soluble fractions or through water-soluble reaction products with certain binders, implying that their anticorrosive abilities are only formed in the presence of water. Anticorrosive pigments, with the exception of zinc phosphate and zinc dust, have a number of drawbacks, one of which is that they can be harmful to health. These pigments have been subjected to stringent requirements in terms of handling, application, storage, and disposal [11].
The addition of lithium salts to epoxy coatings improves their corrosion resistance. Lithium salts were studied as a possible alternative for hexavalent chromium in organic coatings and as a leachable corrosion inhibitor. Active corrosion inhibition was demonstrated by the creation of a protective layer in a damaged area when coatings containing lithium carbonate or lithium oxalate were applied [148]. According to studies, lithium ions were incorporated into PMMA-silica nanocomposites, resulting in a thick, highly cross-linked hybrid network that acts as a diffusion barrier against corrosive species. A self-healing mechanism was proposed, which describes the development of a protective layer of redox reaction products induced by lithium ions, which blocks the corrosion process not only in localised defects (pits), but also in artificially damaged zones, extending the durability of the PMMA-silica coatings significantly. PMMA-silica hybrids modified by lithium constitute a novel class of costeffective functional coatings that have the potential to substitute the harmful hexavalent chromium-conversion procedure now employed to protect metallic structures against corrosion [149].
In the PMMA-cerium oxide have been shown to be particularly promising materials for protective coatings due to their superior resistance to corrosion, active self-healing potential, and environmental friendliness. The detailed studies showed that Ce ions act as self-healing agents, forming insoluble cerium oxide and hydroxide species in the scratch track as a result of reactions between leached cerium ions and hydroxyl groups, preventing the corrosion process from progressing and thus extending the coating lifetime [36].
6.3 Sacrificial coatings
For corrosion prevention, they depend on the galvanic corrosion principle. The substrate is covered with an alloy and metal that is electrochemically higher active than the substrate. For the corrosion protection of steel structures, coatings made with metallic zinc powder are widely used. Because they require an electrical contact between the substrate and the sacrificial metal to be effective, these coatings are only used as primers. The advantages of sacrificial metallic coatings include cathodic protection with barrier properties, onsite spraying and repair capabilities, quick handling after application, low cost, and great mechanical resistance[150].
7 Conclusion and future perspectives
Because of growing concern that fossil fuels are a serious environmental problem, all countries must adopt cleaner and more environmentally friendly industry and transportation technology. Corrosion is a major problem for most industries around the world because it results in disasters and substantial financial loss. Corrosion-inhibiting coatings are typically used as functional barriers in a variety of conditions, including constant water immersion, burying in soils, being exposed to ultraviolet radiation in commercial sectors, hot abrasive liquids, and air pollution. Over the last two decades, new expectations for significant technological innovation with greater performance, as well as environmental issues, have pushed the emergence of novel coating systems in the paint industry forward. Organic coating is a popular corrosion protection method because it offers an effective barrier to corrosive media. Thus the functionalization of organic coatings can lead to significant advancements in the field of anticorrosive coatings and modify their properties for specific applications. The industry's task is to keep or modify properties at a fair cost while also addressing the need for environmentally friendly coatings. Due to the depletion of non-renewable feedstock, the production of bio-based eco-friendly organic coatings from sustainable resources employing green solvents is a new topic of research. The development of more efficient coating system technologies has a promising future, because to recent technological breakthroughs in inspection methodologies and a greater understanding of corrosive conditions. An adequate interdisciplinary engineering approach is necessary to produce high-quality coatings with multi-functional characteristics.
Data availability
Not applicable.
References
Cui G, Bi Z, Wang S, Liu J, Xing X, Li Z, Wang B (2020) A comprehensive review on smart anti-corrosive coatings. Prog Org Coatings 148:105821. https://doi.org/10.1016/j.porgcoat.2020.105821
Honarvar Nazari M, Zhang Y, Mahmoodi A, Xu G, Yu J, Wu J, Shi X (2022) Nanocomposite organic coatings for corrosion protection of metals: A review of recent advances. Prog Org Coatings 162:106573. https://doi.org/10.1016/j.porgcoat.2021.106573
de Almeida Bino MC, Eurídice WA, Gelamo RV et al (2021) Structural and morphological characterization of Ti6Al4V alloy surface functionalization based on Nb2O5 thin film for biomedical applications. Appl Surf Sci 557:149739. https://doi.org/10.1016/j.apsusc.2021.149739
Zhang F, Liu W, Wang S et al (2021) Ke Pi: InvestigationSurface functionalization of Ti3C2Tx and its application in aqueous polymer nanocomposites for reinforcing corrosion protection. Compos Part B Eng 217:108900. https://doi.org/10.1016/j.compositesb.2021.108900
Gonzalez AS, Riego VV et al (2021) Functional antimicrobial surface coatings deposited onto nanostructured 316l food-grade stainless steel. Nanomaterials 11:1055. https://doi.org/10.3390/nano11041055
Baig N, Saleh TA (2021) A facile development of superhydrophobic and superoleophilic micro-textured functionalized mesh membrane for fast and efficient separation of oil from water. J Environ Chem Eng 9:105825. https://doi.org/10.1016/j.jece.2021.105825
Guo Q, Wang G, Batista ER et al (2021) Two-Dimensional Nanomaterials as Anticorrosion Surface Coatings for Uranium Metal: Physical Insights from First-Principles Theory. ACS Appl Nano Mater 4:5038–5046. https://doi.org/10.1021/acsanm.1c00525
Deyab MA, El Bali B, Mohsen Q, Essehli R (2021) Design new epoxy nanocomposite coatings based on metal vanadium oxy-phosphate M0.5VOPO4 for anti-corrosion applications. Sci Rep 11:1–8. https://doi.org/10.1038/s41598-021-87567-3
Olajire AA (2018) Recent advances on organic coating system technologies for corrosion protection of offshore metallic structures. J Mol Liq 269:572–606
Kao WH, Su YL, Horng JH, Wu WC (2021) Mechanical, tribological, anti-corrosion and anti-glass sticking properties of high-entropy TaNbSiZrCr carbide coatings prepared using radio-frequency magnetron sputtering. Mater Chem Phys 268:124741. https://doi.org/10.1016/J.MATCHEMPHYS.2021.124741
Chen W, Hu T, Wang C et al (2020) The effect of microstructure on corrosion behavior of a novel AlCrTiSiN ceramic coating. Ceram Int 46:12584–12592. https://doi.org/10.1016/J.CERAMINT.2020.02.022
Židov B, Lin Z, Stojanović I, Xu L (2021) Impact of inhibitor loaded mesoporous silica NPs on waterborne coating performance in various corrosive environments. J Appl Polym Sci 138:49614. https://doi.org/10.1002/APP.49614
Adibzadeh E, Mirabedini SM, Behzadnasab M, Farnood RR (2021) A novel two-component self-healing coating comprising vinyl ester resin-filled microcapsules with prolonged anticorrosion performance. Prog Org Coatings 154:106220. https://doi.org/10.1016/j.porgcoat.2021.106220
Ji S, Gui H, Guan G et al (2021) Molecular design and copolymerization to enhance the anti-corrosion performance of waterborne acrylic coatings. Prog Org Coatings 153:106140. https://doi.org/10.1016/j.porgcoat.2021.106140
Verma C, Olasunkanmi LO, Akpan ED, et al (2020) Epoxy resins as anticorrosive polymeric materials: A review. React Funct Polym 156:104741. https://doi.org/10.1016/j.reactfunctpolym.2020.104741
Akbarzadeh S, Ramezanzadeh M, Ramezanzadeh B, Bahlakeh G (2020) A green assisted route for the fabrication of a high-efficiency self-healing anti-corrosion coating through GO nanoplatform reduction by Tamarindus indiaca extract. J Hazard Mater 390:122147. https://doi.org/10.1016/j.jhazmat.2020.122147
Yu M, Lu Q, Cui Z et al (2020) Siloxane-epoxy composite coatings for enhanced resistance to large temperature variations. Prog Org Coatings 139:105457. https://doi.org/10.1016/J.PORGCOAT.2019.105457
Pourhashem S, Saba F, Duan J et al (2020) Polymer/Inorganic nanocomposite coatings with superior corrosion protection performance: A review. J Ind Eng Chem 88:29–57. https://doi.org/10.1016/J.JIEC.2020.04.029
Harb SV, Trentin A, Uvida MC, Hammer P (2020) Advanced organic nanocomposite coatings for effective corrosion protection. In: Corrosion protection at the nanoscale. Elsevier 315–343. https://doi.org/10.1016/B978-0-12-819359-4.00017-9
Jena G, Anandkumar B, Sofia S et al (2020) Fabrication of silanized GO hybrid coating on 316L SS with enhanced corrosion resistance and antibacterial properties for marine applications. Surf Coatings Technol 402:126295. https://doi.org/10.1016/J.SURFCOAT.2020.126295
Lyon SB, Bingham R, Mills DJ (2017) Advances in corrosion protection by organic coatings: What we know and what we would like to know. Prog Org Coatings 102:2–7
Burghardt TE, Pashkevich A (2018) Emissions of Volatile Organic Compounds from road marking paints. Atmos Environ 193:153–157. https://doi.org/10.1016/j.atmosenv.2018.08.065
Zafar F, Ghosal A, Sharmin E et al (2019) A review on cleaner production of polymeric and nanocomposite coatings based on waterborne polyurethane dispersions from seed oils. Prog Org Coatings 131:259–275
Ataei S, Khorasani SN, Neisiany RE (2019) Biofriendly vegetable oil healing agents used for developing self-healing coatings: A review. Prog Org Coatings 129:77–95. https://doi.org/10.1016/J.PORGCOAT.2019.01.012
Jing LC, Wang T, Cao WW et al (2020) Water-based polyurethane composite anticorrosive barrier coating via enhanced dispersion of functionalized GO in the presence of acidified multi-walled carbon nanotubes. Prog Org Coatings 146:105734. https://doi.org/10.1016/j.porgcoat.2020.105734
Khanjani J, Hanifpour A, Pazokifard S, Zohuriaan-Mehr MJ (2020) Waterborne acrylic-styrene/PDMS coatings formulated by different particle sizes of PDMS emulsions for outdoor applications. Prog Org Coatings 141:105267. https://doi.org/10.1016/j.porgcoat.2019.105267
Wang S, Wu Y, Dai J et al (2020) Making organic coatings greener: Renewable resource, solvent-free synthesis, UV curing and repairability. Eur Polym J 123:109439. https://doi.org/10.1016/j.eurpolymj.2019.109439
Faccini M, Bautista L, Soldi L et al (2021) Environmentally friendly anticorrosive polymeric coatings. Appl Sci 11:3446
Li J, Ding Y, Gao Q et al (2020) Ultrathin and flexible biomass-derived C@CoFe nanocomposite films for efficient electromagnetic interference shielding. Compos Part B Eng 190:107935. https://doi.org/10.1016/j.compositesb.2020.107935
Thomas J, Singh V, Jain R (2020) Synthesis and characterization of solvent free acrylic copolymer for polyurethane coatings. Prog Org Coatings 145:105677. https://doi.org/10.1016/j.porgcoat.2020.105677
de Wit JHW, van der Weijde DH, Ferrari G (2011) Organic coatings. In: Corrosion mechanisms in theory and practice: Third Edition. Elsevier 863–906. https://doi.org/10.1201/b11020
Ulaeto SB, Rajan R, Pancrecious JK et al (2017) Developments in smart anticorrosive coatings with multifunctional characteristics. Prog Org Coatings 111:294–314
Stankiewicz A, Szczygieł I, Szczygieł B (2013) Self-healing coatings in anti-corrosion applications. J Mater Sci 48:8041–8051
Sauvant-Moynot V, Gonzalez S, Kittel J (2008) Self-healing coatings: An alternative route for anticorrosion protection. Prog Org Coatings 63:307–315. https://doi.org/10.1016/J.PORGCOAT.2008.03.004
Nardeli JV, Fugivara CS, Taryba M et al (2020) Self-healing ability based on hydrogen bonds in organic coatings for corrosion protection of AA1200. Corros Sci 177:108984. https://doi.org/10.1016/j.corsci.2020.108984
Harb SV, Trentin A, de Souza TAC, et al (2020) Effective corrosion protection by eco-friendly self-healing PMMA-cerium oxide coatings. Chem Eng J 383:1-11. https://doi.org/10.1016/j.cej.2019.123219
Karpakam V, Kamaraj K, Sathiyanarayanan S et al (2011) Electrosynthesis of polyaniline-molybdate coating on steel and its corrosion protection performance. Electrochim Acta 56:2165–2173. https://doi.org/10.1016/j.electacta.2010.11.099
Yabuki A, Yamagami H, Noishiki K (2007) Barrier and self-healing abilities of corrosion protective polymer coatings and metal powders for aluminum alloys. Mater Corros 58:497–501. https://doi.org/10.1002/maco.200604041
Andreeva DV, Fix D, Möhwald H, Shchukin DG (2008) Buffering polyelectrolyte multilayers for active corrosion protection. J Mater Chem 18:1738–1740. https://doi.org/10.1039/b801314d
Dello Iacono S, Martone A, Amendola E (2018) Corrosion-resistant self-healing coatings. In: AIP Conference Proceedings. 1990:020010. https://doi.org/10.1063/1.5047764
Zulkifli F, Ali N, Yusof MSM et al (2017) Henna leaves extract as a corrosion inhibitor in acrylic resin coating. Prog Org Coatings 105:310–319. https://doi.org/10.1016/j.porgcoat.2017.01.017
Wang H, Zhou Q (2018) Evaluation and failure analysis of linseed oil encapsulated self-healing anticorrosive coating. Prog Org Coatings 118:108–115. https://doi.org/10.1016/j.porgcoat.2018.01.024
Samadzadeh M, Boura SH, Peikari M et al (2011) Tung oil: An autonomous repairing agent for self-healing epoxy coatings. Prog Org Coatings 70:383–387. https://doi.org/10.1016/j.porgcoat.2010.08.017
Baharom Z, Baba NB, Ramli R, et al (2019) Microencapsulation of natural self-healing agent as corrosion coating. In: AIP conference proceedings. American Institute of Physics Inc 2068(1):020103. https://doi.org/10.1063/1.5089402
Bagale UD, Sonawane SH, Bhanvase BA et al (2018) Green synthesis of nanocapsules for self-healing anticorrosion coating using ultrasound-assisted approach. Green Process Synth 7:147–159. https://doi.org/10.1515/gps-2016-0160
Li J, Feng Q, Cui J et al (2017) Self-assembled GO microcapsules in Pickering emulsions for self-healing waterborne polyurethane coatings. Compos Sci Technol 151:282–290. https://doi.org/10.1016/j.compscitech.2017.07.031
Daradmare S, Pradhan M, Raja VS, Parida S (2016) Encapsulating 8-hydroxyquinoline in GO-stabilized polystyrene containers and its anticorrosion performance. J Mater Sci 51:10262–10277. https://doi.org/10.1007/s10853-016-0254-4
Wang W, Wang H, Zhao J et al (2019) Self-healing performance and corrosion resistance of GO–mesoporous silicon layer–nanosphere structure coating under marine alternating hydrostatic pressure. Chem Eng J 361:792–804. https://doi.org/10.1016/j.cej.2018.12.124
Bryant DE, Greenfield D (2006) The use of fluorescent probes for the detection of under-film corrosion. Prog Org Coatings 57:416–420. https://doi.org/10.1016/j.porgcoat.2006.09.027
Tian Z, Shi H, Liu F et al (2015) Inhibiting effect of 8-hydroxyquinoline on the corrosion of silane-based sol-gel coatings on AA 2024–T3. Prog Org Coatings 82:81–90. https://doi.org/10.1016/j.porgcoat.2015.01.018
Langer E, Waśkiewicz S, Kuczyńska H (2019) Application of new modified Schiff base epoxy resins as organic coatings. J Coatings Technol Res 16:1109–1120. https://doi.org/10.1007/s11998-019-00185-7
Maia F, Tedim J, Bastos AC, et al (2013) Nanocontainer-based corrosion sensing coating. Nanotechnology 24 https://doi.org/10.1088/0957-4484/24/41/415502
Dhole GS, Gunasekaran G, Naik R et al (2020) Fluorescence based corrosion detecting epoxy coating. Prog Org Coatings 138:105425. https://doi.org/10.1016/j.porgcoat.2019.105425
Zhang J, Frankel GS (1999) Investigation of the corrosion-sensing behavior of an acrylic-based coating sys. Corrosion 55:957
Li Y (2020) Temperature and humidity sensors based on luminescent metal-organic frameworks. Polyhedron 179:114413. https://doi.org/10.1016/j.poly.2020.114413
Salaluk S, Jiang S, Viyanit E et al (2021) Design of Nanostructured Protective Coatings with a Sensing Function. ACS Appl Mater Interfaces 13:53046–53054. https://doi.org/10.1021/ACSAMI.1C14110/SUPPL_FILE/AM1C14110_SI_001.PDF
Zhang M, Ma L, Wang L et al (2018) Insights into the Use of Metal-Organic Framework As High-Performance Anticorrosion Coatings. ACS Appl Mater Interfaces 10:2259–2263. https://doi.org/10.1021/acsami.7b18713
Dhole GS, Gunasekaran G, Ghorpade T, Vinjamur M (2017) Smart acrylic coatings for corrosion detection. Prog Org Coatings 110:140–149. https://doi.org/10.1016/j.porgcoat.2017.04.048
Exbrayat L, Salaluk S, Uebel M et al (2019) Nanosensors for Monitoring Early Stages of Metallic Corrosion. ACS Appl Nano Mater 2:812–818. https://doi.org/10.1021/acsanm.8b02045
Buchheit RG, Guan H, Mahajanam S, Wong F (2003) Active corrosion protection and corrosion sensing in chromate-free organic coatings. In: Progress in Organic Coatings. 174–182. https://doi.org/10.1016/j.porgcoat.2003.08.003
Li W, Calle LM (2007) Controlled release microcapsules for smart coatings. In: NACE - International Corrosion Conference Series. OnePetro 072281–0722811
Mathiazhagan A, Joseph R (2011) Nanotechnology-a new prospective in organic coating-review. Int J Chem Eng Appl 225–237. https://doi.org/10.7763/ijcea.2011.v2.108
Basu S, Hanh BM, Ismail MH et al (2020) Laboratory and Field Testing Assessment of Next Generation Biocide-Free, Fouling-Resistant Slippery Coatings. ACS Appl Polym Mater 2:5147–5162. https://doi.org/10.1021/ACSAPM.0C00916/SUPPL_FILE/AP0C00916_SI_013.AVI
Han X, Wu J, Zhang X et al (2021) Special issue on advanced corrosion-resistance materials and emerging applications. The progress on antifouling organic coating: From biocide to biomimetic surface. J Mater Sci Technol 61:46–62. https://doi.org/10.1016/j.jmst.2020.07.002
Mohanan S, Maruthamuthu S, Kalaiselvi N et al (2005) Role of quaternary ammonium compounds and ATMP on biocidal effect and corrosion inhibition of mild steel and copper. Corros Rev 23:425–444. https://doi.org/10.1515/corrrev.2005.23.4-5-6.425
Guo J, Yuan S, Jiang W et al (2018) Polymers for combating biocorrosion Front Mater 5:10
Jin H, Wang J, Tian L et al (2022) Recent advances in emerging integrated antifouling and anticorrosion coatings. Mater Des 213:110307. https://doi.org/10.1016/j.matdes.2021.110307
Regina VR, Søhoel H, Lokanathan AR et al (2012) Entrapment of subtilisin in ceramic sol-gel coating for antifouling applications. ACS Appl Mater Interfaces 4:5915–5921. https://doi.org/10.1021/am301554m
Selim MS, Shenashen MA, Elmarakbi A et al (2017) Synthesis of ultrahydrophobic and thermally stable inorganic–organic nanocomposites for self-cleaning foul release coatings. Chem Eng J 320:653–666. https://doi.org/10.1016/j.cej.2017.03.067
Szleifer I (1997) Protein adsorption on surfaces with grafted polymers: A theoretical approach. Biophys J 72:595–612. https://doi.org/10.1016/S0006-3495(97)78698-3
Khalil F, Franzmann E, Ramcke J et al (2014) Biomimetic PEG-catecholates for stabile antifouling coatings on metal surfaces: Applications on TiO2 and stainless steel. Colloids Surfaces B Biointerfaces 117:185–192. https://doi.org/10.1016/j.colsurfb.2014.02.022
Carpenter AW, Worley BV, Slomberg DL, Schoenfisch MH (2012) Dual action antimicrobials: Nitric oxide release from quaternary ammonium-functionalized silica NPs. Biomacromol 13:3334–3342. https://doi.org/10.1021/bm301108x
Yuan H, Yu B, Fan L-H et al (2016) Multiple types of hydroxyl-rich cationic derivatives of PGMA for broad-spectrum antibacterial and antifouling coatings. Polym Chem 7:5709–5718. https://doi.org/10.1039/C6PY01242F
Josphine JS, Manjusha WA, (2021) Evaluation of antifouling potential of staphylococcus sp. isolated from marine sea water. Ann Rom 25:6650–6661. https://www.annalsofrscb.ro.
Kyei SK, Darko G, Akaranta O (2020) Chemistry and application of emerging ecofriendly antifouling paints: a review. J Coatings Technol Res 17:315–332
Pinteus S, Lemos MFL, Alves C et al (2021) The marine invasive seaweeds Asparagopsis armata and Sargassum muticum as targets for greener antifouling solutions. Sci Total Environ 750:141372. https://doi.org/10.1016/j.scitotenv.2020.141372
Jin H, Tian L, Bing W et al (2022) Bioinspired marine antifouling coatings: Status, prospects, and future. Prog Mater Sci 124:100889. https://doi.org/10.1016/j.pmatsci.2021.100889
Chen L, Duan Y, Cui M, et al (2021) Biomimetic surface coatings for marine antifouling: Natural antifoulants, synthetic polymers and surface microtopography. Sci Total Environ 766:144469. https://doi.org/10.1016/j.scitotenv.2020.144469
Xu Y, He H, Schulz S et al (2010) Potent antifouling compounds produced by marine Streptomyces. Bioresour Technol 101:1331–1336. https://doi.org/10.1016/j.biortech.2009.09.046
Parkin IP, Palgrave RG (2005) Self-cleaning coatings. J Mater Chem 15:1689–1695
Wang S, Wang Y, Zou Y et al (2020) A self-adjusting PTFE/TiO2 hydrophobic double-layer coating for corrosion resistance and electrical insulation. Chem Eng J 402:126116. https://doi.org/10.1016/j.cej.2020.126116
Dhoke SK, Mangal Sinha TJ, Khanna AS (2009) Effect of nano-Al2O3 particles on the corrosion behavior of alkyd based waterborne coatings. J Coatings Technol Res 6:353–368. https://doi.org/10.1007/s11998-008-9127-3
Pawar PG, Xing R, Kambale RC et al (2017) Polystyrene assisted superhydrophobic silica coatings with surface protection and self-cleaning approach. Prog Org Coatings 105:235–244. https://doi.org/10.1016/j.porgcoat.2017.01.016
Wu X, Yang F, Gan J et al (2021) A flower-like waterborne coating with self-cleaning, self-repairing properties for superhydrophobic applications. J Mater Res Technol 14:1820–1829. https://doi.org/10.1016/j.jmrt.2021.07.096
Selim MS, Yang H, El-Safty SA et al (2019) Superhydrophobic coating of silicone/β–MnO 2 nanorod composite for marine antifouling. Colloids Surfaces A Physicochem Eng Asp 570:518–530. https://doi.org/10.1016/j.colsurfa.2019.03.026
Ganesh VA, Raut HK, Nair AS, Ramakrishna S (2011) A review on self-cleaning coatings. J Mater Chem 21:16304–16322
Patil CK, Jirimali HD, Paradeshi JS et al (2019) Functional antimicrobial and anticorrosive polyurethane composite coatings from algae oil and silver doped egg shell hydroxyapatite for sustainable development. Prog Org Coatings 128:127–136. https://doi.org/10.1016/j.porgcoat.2018.11.002
El-Fattah MA, El Saeed AM, Azzam AM et al (2016) Improvement of corrosion resistance, antimicrobial activity, mechanical and chemical properties of epoxy coating by loading chitosan as a natural renewable resource. Prog Org Coatings 101:288–296. https://doi.org/10.1016/j.porgcoat.2016.09.002
Sharmin E, Ashraf SM, Ahmad S (2007) Synthesis, characterization, antibacterial and corrosion protective properties of epoxies, epoxy-polyols and epoxy-polyurethane coatings from linseed and Pongamia glabra seed oils. Int J Biol Macromol 40:407–422. https://doi.org/10.1016/j.ijbiomac.2006.10.002
Xu R, Li J, Xiong Z et al (2020) Antibacterial waterborne epoxy coatings containing poly m-aminophenol-deposited GO. Prog Org Coatings 147:105802. https://doi.org/10.1016/j.porgcoat.2020.105802
Zheng H, Li Z, Liu L et al (2021) Superhydrophobic composite coatings in bacterial culture media: Durable antibacterial activity and enhanced corrosion resistance. Compos Commun 27:100857. https://doi.org/10.1016/j.coco.2021.100857
Kausar A (2020) Performance of corrosion protective epoxy blend-based nanocomposite coatings: a review. Polym Technol Mater 59:658–673
Tator KB (2018) Epoxy resins and curatives. In: Protective organic coatings. ASM International, 63–79
Marotta A, Faggio N, Ambrogi V et al (2021) Biobased furan-based epoxy/TiO2 nanocomposites for the preparation of coatings with improved chemical resistance. Chem Eng J 406:127107. https://doi.org/10.1016/j.cej.2020.127107
Pouladi J, Mirabedini SM, Eivaz Mohammadloo H, Rad NG (2021) Synthesis of novel plant oil-based isocyanate-free urethane coatings and study of their anti-corrosion properties. Eur Polym J 153:110502. https://doi.org/10.1016/j.eurpolymj.2021.110502
Baroncini EA, Kumar Yadav S, Palmese GR, Stanzione JF (2016) Recent advances in bio-based epoxy resins and bio-based epoxy curing agents. J Appl Polym Sci 133: 4103. https://doi.org/10.1002/app.44103
Alam M, Akram D, Sharmin E et al (2014) Vegetable oil based eco-friendly coating materials: A review article. Arab J Chem 7:469–479. https://doi.org/10.1016/J.ARABJC.2013.12.023
Dehan V, Bourgeat-Lami E, D’Agosto F et al (2017) High-performance water-based barrier coatings for the corrosion protection of structural steel. Steel Constr 10:254–259. https://doi.org/10.1002/stco.201710034
Elmore JD, Kincaid DS, Komar PC, Nielsen JE (2002) Waterborne epoxy protective coatings for metal. J Coatings Technol 74:63–72. https://doi.org/10.1007/bf02697969
Galgoci EC, Komar PC, Elmore JD (1999) High performance waterborne coatings based on dispersions of a solid epoxy resin and an amine-functional curing agent. J Coatings Technol 71:45–52. https://doi.org/10.1007/bf02697895
Dou B, Xiao H, Lin X et al (2021) Investigation of the anti-corrosion properties of fluorinated graphene-modified waterborne epoxy coatings for carbon steel. Coatings 11:1–16. https://doi.org/10.3390/coatings11020254
Wang N, Zhang Y, Chen J et al (2017) Dopamine modified metal-organic frameworks on anti-corrosion properties of waterborne epoxy coatings. Prog Org Coatings 109:126–134. https://doi.org/10.1016/j.porgcoat.2017.04.024
Wang C, Wang Z, Liu S et al (2021) Anti-corrosion and wear-resistant coating of waterborne epoxy resin by concrete- like three-dimensional functionalized framework fillers. Chem Eng Sci 242:116748. https://doi.org/10.1016/j.ces.2021.116748
Wu Y, Yu J, Zhao W et al (2019) Investigating the anti-corrosion behaviors of the waterborne epoxy composite coatings with barrier and inhibition roles on mild steel. Prog Org Coatings 133:8–18. https://doi.org/10.1016/j.porgcoat.2019.04.028
Kausar A (2020) High performance epoxy/polyester-based nanocomposite coatings for multipurpose applications: A review. J Plast Film Sheeting 36:391–408. https://doi.org/10.1177/8756087920910481
Guo T, Li H, Ma X et al (2020) Hyperbranched polyester modified GO on anti-corrosion performance of epoxy composite coatings for electric power system. Plast Rubber Compos 49:245–253. https://doi.org/10.1080/14658011.2020.1735180
Patil AM, Jirimali HD, Jagtap RN (2020) Study of coating performance of bio-based hyperbranched polyester polyol/GO composites in PU-coating. J Macromol Sci Part A Pure Appl Chem 58:81–89. https://doi.org/10.1080/10601325.2020.1826330
Patil AM, Gite VV, Jirimali HD, Jagtap RN (2021) Fully Biobased Nanocomposites of Hyperbranched-Polyol and Hydroxyapatite in Coating Applications. J Polym Environ 29:799–810. https://doi.org/10.1007/s10924-020-01903-8
Gurunathan T, Mohanty S, Nayak SK (2016) Hyperbranched Polymers for Coating Applications: A Review. Polym - Plast Technol Eng 55:92–117. https://doi.org/10.1080/03602559.2015.1021482
Pathak SS, Khanna AS (2009) Investigation of anti-corrosion behavior of waterborne organosilane-polyester coatings for AA6011 aluminum alloy. Prog Org Coatings 65:288–294. https://doi.org/10.1016/j.porgcoat.2008.12.006
Ikladious NE, Asaad JN, Emira HS, Mansour SH (2017) Alkyd resins based on hyperbranched polyesters and PET waste for coating applications. Prog Org Coatings 102:217–224. https://doi.org/10.1016/j.porgcoat.2016.10.015
Hadzich A, Gross GA, Leimbach M, et al (2020) Effect of polyalcohols on the anticorrosive behaviour of alkyd coatings prepared with drying oils. Prog Org Coatings 145 https://doi.org/10.1016/j.porgcoat.2020.105671
Bat E, Gündüz G, Kisakürek D, Akhmedov IM (2006) Synthesis and characterization of hyperbranched and air drying fatty acid based resins. Prog Org Coatings 55:330–336. https://doi.org/10.1016/j.porgcoat.2006.01.005
Singh AP, Suryanarayana C, Baloji Naik R, Gunasekaran G (2016) Development of hyperbranched polyester polyol-based waterborne anticorrosive coating. J Coatings Technol Res 13:41–51. https://doi.org/10.1007/s11998-015-9720-1
Haghdadeh P, Ghaffari M, Ramezanzadeh B et al (2018) The role of functionalized GO on the mechanical and anti-corrosion properties of polyurethane coating. J Taiwan Inst Chem Eng 86:199–212. https://doi.org/10.1016/j.jtice.2018.02.009
Tsai PY, Chen TE, Lee YL (2018) Development and characterization of anticorrosion and antifriction properties for high performance polyurethane/graphene composite coatings. Coatings 8:250. https://doi.org/10.3390/coatings8070250
Naik RB, Malvankar NG, Mahato TK et al (2014) Novel moisture-cured hyperbranched urethane alkyd resin for coating application. J Coatings Technol Res 11:575–586. https://doi.org/10.1007/s11998-013-9561-8
Li J, Cui J, Yang J et al (2016) Reinforcement of graphene and its derivatives on the anticorrosive properties of waterborne polyurethane coatings. Compos Sci Technol 129:30–37. https://doi.org/10.1016/j.compscitech.2016.04.017
Xiao L, Shi J, Wu K, Lu M (2020) Self-healing supramolecular waterborne polyurethane based on host–guest interactions and multiple hydrogen bonds. React Funct Polym 148:104482. https://doi.org/10.1016/j.reactfunctpolym.2020.104482
Lee DK, Tsai HB, Da YZ, Tsai RS (2012) Polyurethane dispersions derived from polycarbonatediols by a solvent-free process. J Appl Polym Sci 126:E275–E282. https://doi.org/10.1002/app.36812
Li Y, Yang Z, Qiu H et al (2014) Self-aligned graphene as anticorrosive barrier in waterborne polyurethane composite coatings. J Mater Chem A 2:14139–14145. https://doi.org/10.1039/c4ta02262a
Marathe R, Tatiya P, Chaudhari A et al (2015) Neem acetylated polyester polyol-Renewable source based smart PU coatings containing quinoline (corrosion inhibitor) encapsulated polyurea microcapsules for enhance anticorrosive property. Ind Crops Prod 77:239–250. https://doi.org/10.1016/j.indcrop.2015.08.054
Ismail EA, Motawie AM, Sadek EM (2011) Synthesis and characterization of polyurethane coatings based on soybean oil–polyester polyols. Egypt J Pet 20:1–8. https://doi.org/10.1016/j.ejpe.2011.06.009
Kathalewar M, Sabnis A, D’Melo D (2014) Polyurethane coatings prepared from CNSL based polyols: Synthesis, characterization and properties. Prog Org Coatings 77:616–626. https://doi.org/10.1016/j.porgcoat.2013.11.028
Araújo RCS, Pasa VMD (2004) New Eucalyptus tar-derived polyurethane coatings. Prog Org Coatings 51:6–14. https://doi.org/10.1016/j.porgcoat.2004.04.002
Siyanbola TO, Neelambaram P, Mohanty S et al (2019) The effects of carbonized Eucalyptus globulus leaves on castor seed oil based urethane coating system. Prog Org Coatings 131:42–48. https://doi.org/10.1016/j.porgcoat.2019.02.018
Edavan RP, Kopinski R (2009) Corrosion resistance of painted zinc alloy coated steels. Corros Sci 51:2429–2442. https://doi.org/10.1016/j.corsci.2009.06.028
Bayliss D, Deacon D (2002) Steelwork corrosion control. Spon. 2nd Edition, Kindle Edition, pp 432
Dumain ED, Agawa T, Goel S et al (1999) Cure behavior of polyester-acrylate hybrid powder coatings. J Coatings Technol 71:69–75. https://doi.org/10.1007/bf02697908
Lafabrier A, Fahs A, Louarn G et al (2014) Experimental evidence of the interface/interphase formation between powder coating and composite material. Prog Org Coatings 77:1137–1144. https://doi.org/10.1016/j.porgcoat.2014.03.021
Iwamura G, Agawa T, Maruyama K, Takeda H (2000) A novel acrylic/polyester system for powder coatings. JOCCA - Surf Coatings Int 83:285–288. https://doi.org/10.1007/BF02692728
Farshchi N, Gedan-Smolka M (2020) Polyurethane powder coatings: A review of composition and characterization. Ind Eng Chem Res 59:15121–15132. https://doi.org/10.1021/acs.iecr.0c02320
Boccaccini AR, Zhitomirsky I (2002) Application of electrophoretic and electrolytic deposition techniques in ceramics processing. Curr Opin Solid State Mater Sci 6:251–260. https://doi.org/10.1016/S1359-0286(02)00080-3
Zhang J, Wu C (2009) Corrosion protection behavior of AZ31 magnesium alloy with cathodic electrophoretic coating pretreated by silane. Prog Org Coatings 66:387–392. https://doi.org/10.1016/j.porgcoat.2009.09.001
Li GY, Lian JS, Niu LY et al (2007) Effect of zinc-phosphate-molybdate conversion precoating on performance of cathode epoxy electrocoat on AZ91D alloy. Surf Eng 23:56–61. https://doi.org/10.1179/174329407X161663
Park JH, Park JM (2014) Electrophoretic deposition of GO on mild carbon steel for anti-corrosion application. Surf Coatings Technol 254:167–174. https://doi.org/10.1016/j.surfcoat.2014.06.007
Singh S, Singh G, Bala N (2019) Corrosion behavior and characterization of HA/Fe3O4/CS composite coatings on AZ91 Mg alloy by electrophoretic deposition. Mater Chem Phys 237:121884. https://doi.org/10.1016/j.matchemphys.2019.121884
Zheludkevich ML, Salvado IM, Ferreira MGS (2005) Sol-gel coatings for corrosion protection of metals. J Mater Chem 15:5099–5111. https://doi.org/10.1039/b419153f
Barranco V, Carmona N, Galván JC et al (2010) Electrochemical study of tailored sol-gel thin films as pre-treatment prior to organic coating for AZ91 magnesium alloy. Prog Org Coatings 68:347–355. https://doi.org/10.1016/j.porgcoat.2010.02.009
Liu L, Mandler D (2015) Sol-gel coatings by electrochemical deposition. In: The sol-gel handbook. John Wiley & Sons Ltd 373–414. https://doi.org/10.1002/9783527670819.ch12
Qi K, Sun Y, Duan H, Guo X (2015) A corrosion-protective coating based on a solution-processable polymer-grafted GO nanocomposite. Corros Sci 98:500–506. https://doi.org/10.1016/j.corsci.2015.05.056
Di H, Yu Z, Ma Y et al (2016) Corrosion-resistant hybrid coatings based on GO–zirconia dioxide/epoxy system. J Taiwan Inst Chem Eng 67:511–520. https://doi.org/10.1016/j.jtice.2016.08.008
Dos Santos FC, Harb SV, Menu MJ et al (2015) On the structure of high performance anticorrosive PMMA-siloxane-silica hybrid coatings. RSC Adv 5:106754–106763. https://doi.org/10.1039/c5ra20885h
Torrico RFAO, Harb SV, Trentin A et al (2018) Structure and properties of epoxy-siloxane-silica nanocomposite coatings for corrosion protection. J Colloid Interface Sci 513:617–628. https://doi.org/10.1016/j.jcis.2017.11.069
Maya-Visuet E, Gao T, Soucek M, Castaneda H (2015) The effect of TiO2 as a pigment in a polyurethane/polysiloxane hybrid coating/aluminum interface based on damage evolution. Prog Org Coatings 83:36–46. https://doi.org/10.1016/j.porgcoat.2015.02.001
Brusciotti F, Snihirova DV, Xue H et al (2013) Hybrid epoxy–silane coatings for improved corrosion protection of Mg alloy. Corros Sci 67:82–90. https://doi.org/10.1016/J.CORSCI.2012.10.013
Sellaiyan S, Hughes AE, Smith SV et al (2014) Leaching properties of chromate-containing epoxy films using radiotracers, PALS and SEM. Prog Org Coatings 77:257–267. https://doi.org/10.1016/j.porgcoat.2013.09.014
Visser P, Lutz A, Mol JMC, Terryn H (2016) Study of the formation of a protective layer in a defect from lithium-leaching organic coatings. Prog Org Coatings 99:80–90. https://doi.org/10.1016/j.porgcoat.2016.04.028
Trentin A, Harb SV, Uvida MC et al (2019) Dual Role of Lithium on the Structure and Self-Healing Ability of PMMA-Silica Coatings on AA7075 Alloy. ACS Appl Mater Interfaces 11:40629–40641. https://doi.org/10.1021/acsami.9b13839
Syrek-Gerstenkorn B, Paul S, Davenport AJ (2020) Sacrificial thermally sprayed aluminium coatings for marine environments: A review. Coatings 10:1–19. https://doi.org/10.3390/coatings10030267
Acknowledgements
Reshmy R (SR/WOS-B/587/2016) acknowledges Department of Science and Technology for sanctioning projects under DST WOS-B scheme.
Funding
Reshmy R (SR/WOS-B/587/2016) acknowledges Department of Science and Technology for sanctioning projects under DST WOS-B scheme.
Author information
Authors and Affiliations
Contributions
Deepa Thomas, Eapen Philip: Collecting articles and Writing original draft.
Reshmy R, Mukesh Kumar Awasthi: Project Administration, Conceptualization and Visualization.
Raveendran Sindhu: Reviewing and Editing.
Sarah B. Ulaeto: Reviewing and Editing.
Arivalagan Pugazhendhi: Supervision, Reviewing and Editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thomas, D., R, R., Philip, E. et al. Developments in smart organic coatings for anticorrosion applications: a review. Biomass Conv. Bioref. 12, 4683–4699 (2022). https://doi.org/10.1007/s13399-022-02363-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02363-x