Abstract
The present study aimed to evaluate the growth parameters and biological activities of two cyanobacterial and a chlorphyta species cultivated on BG110 and BG11 (as control media) respectively, as well as a combination of culture media (BG) with treated sewage wastewater (TSW) at various concentrations (100, 75, and 50% TSW) which were selected from six algal species (three cyanobacterial and three chlorophyta species) according to the concentration of auxins and cytokinins analyzed by HPLC. The experiments were conducted in triplicates and cultures were incubated at 25 ± 1 °C under continuous aeration and light intensity of 40µE/m2/s for 30 days. The Biological activities include antimicrobial (using agar well diffusion assay) and antioxidant (using DPPH radical methods) of absolute methanol extracts were evaluated. The obtained results revealed that maximum growth of the studied algal species on their specific culture media (BG11 and BG110) showed variable growth which was significantly enhanced when cultivated in combined media (BG11 or BG110 with TSW). HPLC analysis of auxin (IAA, indol acetic acid) and cytokinin (BA, benzyl adenin) in the investigated algal species grown under different media composition showed large variations depending on algal species and the type of culture media used. Maximum IAA concentration was recorded in Chlorella vulgaris and Nostoc muscorum cultivated in 100% TSW media. Concerning BA (cytokinin), Chlorella vulgaris and Nostoc muscorum showed the highest relative percentages on cultivation in 100% TSW. The antioxidant activity results of the algal species Nostoc muscorum cultured on BG110 showed higher activity with IC50 45.46 ug/ml when compared with Anabaena oryzae and Chlorella vulgaris (47.71 and 46.13 ug/ml), while IAA, BA, BHT (butylated hydroxytoluene), and ascorbic acid showed (26.97, 28.08, 13.19, and 13.34 ug/ml). The cultivation of Chlorella vulgaris on 100% TSW showed the highest antioxidant activity with IC50 33.99 ug/ml, while Anabaena oryzae showed 35.33 ug/ml on 50% TSW and Nostoc muscorum 39.30 ug/ml on 75% TSW. Concerning the cultivation of Chlorella vulgaris under nitrogen stress conditions, the highest antioxidant activity (IC50) was recorded under 0 × N2 and 0.5 × N2 (30.91 and 33.77 ug/ml). The antimicrobial activity showed that IAA and BA exhibited antibacterial activity against both Gram -ve and Gram + ve bacteria in addition to antifungal activity for Candida albicans only.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Microalgae are phylogenetically heterogeneous group of mostly photoautotrophic uni- and multicellular organisms presumably numbering between 200 and 800 thousand of species. Many species of microalgae are among the most promising and popular objects of biotechnology. Their cells are rich in vitamins, proteins, carbohydrates, fatty acids, enzymes, pigments, macro- and microelements, biologically active compounds with valuable medicinal properties [21] and [10].
These microalgae directly helps in nitrogen-fixation process, phosphate solubilization, or modulating plant hormone levels and indirectly helps in minimizing the effects of phytopathogens on plant growth and development by acting as biocontrol agents [26].
Growing algae in wastewater could be potentially useful to reduce wastes and turn it into a beneficial product. A variety of algae species and algae isolated from wastewater treatment plants such as Chlorella vulgaris, Scenedesmus sp., and Chlamydomonas are investigated with regard to their capability of treating municipal and artificial wastewater as well as their growth under various environmental conditions [27].
The biomass has many potential uses, which include bio-fuel, fish feed, and ethanol production. The algae can help in the elimination of harmful chemicals out of the wastewater and produce clean (drinking) water. This system could use the wastewater instead of being pumped into the ocean every day. Microalgae cultures offer an interesting alternative for wastewater treatment (urban, industrial, or agricultural effluents) because they provide a tertiary bio treatment coupled with the production of potentially valuable biomass, which can be used for various purposes [24] and [14].
Cultivation of high-lipid microalgae Chlorella pyrenoidosa using municipal sewage can both treat polluted water and gain biodiesel Wang et al. [36].
Several bioactive metabolites produced by algae have been discovered by screening programs, employing target organisms quite unrelated to those for which the metabolites evolved. Many of these chemicals have a diverse range of biological activities and chemical structures, which affect many biochemical processes within the cell. Such chemicals are related to the succession and regulation of algal and bacterial populations. These chemicals are expected to be synthesized under stress conditions and low growth rates and released at concentration large enough to be effective [13].
Phytohormones (plant growth regulators, PGRs) play a key role in the regulation of growth, development, and sustainability of the plants. Phytohormones of microalgae are considered as exogenous growth regulators, affecting the tolerance to factors of abiotic and biotic stress, as well as endogenous components of microalgae affecting the processes of the biosynthesis of pigments and lipids [32].
El semary and Mabrouk [9] found that the extracts from the microalgae (Poterioochromonas malhamensis and Synechocystis salina) showed considerable antimicrobial bioactivity against several multidrug-resistant isolates.
Natural antioxidants are highly desired for application in food industries as safer replacement to the synthetic antioxidants. The ultrasonic-assisted extraction were successfully employed for extracting antioxidants and quercetin from Ulva lactuca. This extract of algae can be considered a novel source of natural antioxidants [18].
This work aimed to evaluate the laboratory cultivation of six algal species (3 cyanobacteria and 3 chlorophyta) on synthetic culture media (BG110 & BG11) and combinations with secondary treated sewage wastewater (TSW) for biomass production, antimicrobial, and antioxidant activities.
2 Materials and methods
2.1 Chemical and solvents
Diphenyl-2-picrylhydrazyl (DPPH), sodium carbonate, Folin-Ciocalteu’s phenol reagent, gallic acid, aluminium chloride hexahydrate (AlCl3. 6 H2O), gallic acid, quercetin, penicillin G potassium + streptomycin, trypan blue, neutral red, glacial acetic acid, diphenyl, and dimethyl poly siloxane were obtained from Sigma-Aldrich (Sigma-Aldrich, Milan, Italy). Phosphate buffer saline, calcium and magnesium free, trypsin–EDTA Mueller–Hinton agar Gibco were obtained from Thermo Fisher Scientific, USA. Complete media of DMEM supplemented with fetal bovine serum (SeraLab-Bio-Connect B.V., Begonialaan, the Netherlands). Sodium nitrite, gentamycin, nystatin, ethanol HPLC grade, and sodium hydroxide were supplied by Roth company (Overland Park, KS, United States).
2.2 Algal species
Cyanobacterial species (Anabaena oryzae, Nostoc linckia, and Nostoc muscorum) were obtained from the Microbiology Department, Soils, Water and Environment Res. Inst. (SWERI), Agric. Res., Center (ARC), and Chlorophyta species (Chlorella vulgaris, Dichtyochloropsis splendida, and Muriella sp.) were obtained from the culture collection of Dr. Sanaa Shanab, Professor of Phycology, Department of Botany and Microbiology, Faculty of Science, Cairo University, Giza, Egypt.
2.3 Bacterial strains
Gram–ve bacteria [Escherichia coli (ATCC:9637),and Klebsiella pneumoniae (ATCC:10,031)] and Gram + ve bacteria [Staphylococcus aureus (ATCC:6538) and Streptococcus mutans (ATCC:25,175)] were used as tested strains for antibacterial activities.
2.4 Fungal strains
Candida albicans (ATCC:10,231) and Aspergillus niger (ATCC:32,856) were used as tested strains for antifungal activities.
2.5 Antifungal agents
Nystatin (Oxoid and Remel microbiology products); Streptoquin containing streptomycin (Medical Union pharmaceuticals, Egypt); Pencitard containing benzathine benzylpenicillin (North China pharmaceutical Co., Ltd.)
2.6 Antibacterial agents
Gentamycin containing gentamycin sulfate (Schering-Plough Co., USA), Ampicillin (Oxoid and Remel microbiology products).
2.7 Culture and maintenance conditions
The investigated algae were cultured and maintained on liquid BG110 free nitrogen [19] for cyanobacterial species and BG11 media for green algal species. Cultures were incubated in a growth chamber under continuous aeration (1.25 l/min), 16: 8 h light and dark cycle and light intensity 40 µE/m2/s at 25 ± 1 °C for 30 days. Bacterial strains were streaked and cultured for 48 h in dark at 28 ˚C on sterile Petri-dishes containing sterilized solidified nutrient agar medium. Each liter of the medium contained: 5 g peptone,2 g yeast extract; 1 g beef extract; 5 g NaCl and solidified with 15 g agar. The pH of the medium was adjusted to 7.0 before autoclaving [8]. Fungi were cultured and maintained on sterile slants of sterilized solidified Czapek’s medium in an incubator at 28 ± 1˚C in the dark. The Czapek’s medium was prepared according to Raper and Fennell [17]. Each liter contained: 2 g NaN03,1 g K2HPO4; 0.5 g MgSO4; 0.5 g KCl; 0.01 g FeSO4.7H2O; 5 g yeast extract; 30 g sucrose and solidified with 15 g agar.
2.8 Preparation of algal axenic culture (free from bacteria)
Purification of green algal and cyanobacterial species from bacteria is necessary for this study concerning secondary metabolite production to avoid interference of bacteria mixed with the algae under investigation. Antibiotics, penicilliumG, dihydrostreptomycin sulfate, and gentamycin sulfate at different concentrations were applied separatly according to the method described by Andersen and Robert [2]. The axenic cultures of the alga were used in all experimental work.
2.9 Wastewater sources
Sewage wastewater (SWW) was obtained from Zenain station, Giza, Egypt.
2.10 Treatment with sewage wastewater
This medium was investigated separately and in combination with standard medium, BG11 and BG110, (100%, 75%, and 50% sterilized wastewater) Wang et al. [35]. The SWW was sterilized using glass microfiber filter (0.22 µm) to remove large particles and indigenous bacteria for the experiment,this was signed as treated sewage wastewater (TSW). TSW was applied separately and in combination with BG110 to study their effects on cyanobacterial species. Similarly, TSW combinations were investigated with BG11 on green algae. BG11 and BG110 media were used as control media representing the standard synthetic media. The algal species were grown in 500 ml Erlenmeyer flasks. Ten percent algal inoculum was added to each flask (50 ml). The experiment was conducted in triplicates and cultures were incubated in an illuminated controlled culture condition at 25 ± 1 ºC, continuous aeration and light intensity of 40µE/m2/s for 30 days. In case of Chlorella vulgaris, BG11 with different nitrogen concentrations (N stress, ox, 0.5x, 2x, 4x) were investigated, and growth was compared with those recorded in combination media (BG11 + TSW).
2.11 PGRs extraction
The algal cells were harvested in each experiment by centrifugation at 5000 rpm for 10 min. The harvested cells were dried in an oven at 50 °C for 24 h till constant weight was achieved.The powdered cells was extracted overnight in 96% methanol containing 10 mg/L butylated hydroxytoluene (BHT) as standard antioxidant at 4 °C Sun et al. [30]. Then, the methanolic fraction was filtrated, and the residual pellets were re-extracted 3 times with 40% (10 ml) cold methanol. The combined methanol extracts were evaporated in the dark at room temperature. The aqueous solution was adjusted to pH 2.6–2.8 and then extracted 3 times by absolute ethyl acetate (50 ml/each extract). In addition, the ethyl acetate fraction was separated, dried over anhydrous MgSO4. Finally, the residue was dissolved in 4 ml of absolute methanol.
2.12 Wastewater analysis
Chemical and physical parameters of treated sewage wastewater were analyzed as reported by APHA [4] as shown in Table 1.
2.13 Determination of growth rate
-
a-
Determination of algal growth rates by optical density (O.D.)
Growth of all axenic algal cultures was determined by measuring the optical density at 550 nm (cyanobacteria) and 660 nm (chlorophyta) at 5-day interval through the incubation period of 30 days at the controlled culture conditions according to the method described by Wang et al. [35]. The algal harvest was at the beginning of the stationary phase [15].
-
b-
Determination of algal growth rate by dry weight (D.wt)
Algal dry biomasses were determined at 5-day interval during the incubation period. Twenty ml of algal suspension was filtrated, washed, and dried in oven at 105 °C for 24 h according to the method described by Talukddar [31].
2.14 Determination of phosphorus (P)
The total phosphorus in different algal treatments was extracted as reported by Soltanpour [29] and spectrophotometrically determined according to procedures of Watanabe and Olsen [37].
2.15 Determination of potassium (K)
The total potassium in tested samples was determined by Flame photometry according to APHA method [3].
2.16 Determination of total nitrogen (N)
The determination of total nitrogen in the samples was carried out according to Micro-Kjeldahel method [1].
2.17 HPLC conditions
The standard hormonal sample and the investigated algal methanol extracts were subjected successively to be analyzed by high performance liquid chromatography (HPLC). The quantitative analysis of phytohormones was performed with YL9100 HPLC system with C18 column, UV at 254 nm and 40 ºC with gradient mode of methanol and acetic acid. The amount of IAA and BA in the algae grown on BG110 or BG11 and 100% TSW were calculated from the dose growth curves and the HPLC analysis of algal hormones.
2.18 Antioxidant activity using DPPH method
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) test was carried out as described by Burits and Bucar [5]. One ml of algal crude methanol extracts at different concentration (50–500 μg/ml) was mixed with 1 ml DPPH reagent [0.002% (w/v)/methanol water solution]. After an incubation period (30 and 60 min), the absorbance was measured at 517 nm. The percentage and IC50 of antioxidant activity was calculated. As % antioxidant activity = Ac-At / Ac × 100 where: At was the absorbance of the algal extract samples or antioxidant standards (BHT and Ascorbic acid) or plant growth regulators (PGRs) substances (BA and IAA) and Ac the absorbance of methanolic DPPH solution.
2.19 Antimicrobial activities
The antimicrobial activity of synthesized hormonal compounds was determined using agar well diffusion method according to Scott [22].
All the algal extracts were tested in vitro at a concentration of 15 mg/ml for their antibacterial activity against Staphylococcus aureus and Streptococcus mutans (Gram + ve bacteria) and Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae (Gram − ve bacteria) using nutrient agar medium. Ampicillin was used as standard drug for Gram + ve, while Gentamicin was used for Gram –ve bacteria, respectively. DMSO was used as solvent and –ve control. Nystatin was tested against the fungal strains; Candida albicans (ATCC:10,231) and Aspergillus niger (ATCC:32,856).
Under aseptic condition, the sterilized media was poured onto the sterilized petri-dishes (20–25 ml, each petri dish) and allowed to solidify at room temperature. Bacterial suspension was prepared in sterilized saline equivalent to McFarland 0.5 standard solution (1.5 × 105 CFU/ml) and its turbidity was adjusted to O.D = 0.13 using spectrophotometer at 625 nm. Optimally, within 15 min after adjusting the turbidity of the inoculum suspension, a sterile cotton swab was dipped into the adjusted suspension and was flooded on the dried agar surface then allowed to dry for 15 min with lid in place. Wells of 6 mm diameter was made in the solidified media with the help of sterile cork borer tool. 100 μl of algal extract was added to each well. The plates were incubated at 37 °C for 24 h (for antibacterial activities) and 72 h (for antifungal activities). This experiment was carried out in triplicate, and zones of inhibition were measured in mm scale.
2.20 Statistical analysis
Data were subjected to an analysis of variance, and the means were compared using the least significant difference (LSD) test at the 0.05 levels (p ≤ 0.05), as recommended by Snedecor and Cochran [28].
3 Results and discussion
3.1 Growth parameters
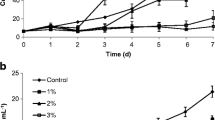
The growth rate has been estimated as dried mass (mg per 10 ml sample) every 5 days; then it has expressed as dry weight (gm/L) and optical density (OD) (Fig. 1 A and B). The growth rates of six algal species, three of them were chlorophyta (green algae) (Chlorella vulgaris, Muriella sp. and Dictyochloropsis splendida), which were cultivated on BG11 medium, and the Cyanobacteria (Anabaena oryzae, Nostoc muscurum, and Nostoc linkia), which were cultivated on BG110 medium appeared with short lag phase (day 5), different log phase lengths and short decline phase as recorded in Fig. 1 A and B. The growth rate of algae increased gradually from the first cultivation day until they reached the maximum biomass productivity then declined. Maximum growth rates of Chlorella vulgaris and Nostoc linkia was at day 25, while that of Dictyochloropsis splendida, Anabaena oryzae, and Nostoc muscurum was at day 20 and Muriella sp. maximum growth rate was at day 30, as shown in Fig. 1 A and B. The optical density method of green algae was recorded at 660 nm, while the cyanobacteria were at 550 nm.
3.2 Growth rate of algal species
The growth rates of all Chlorella vulgaris treatments and control (Fig. 2 A and B) appeared with short lag phase (day 5), different log phase length, and short decline phase. The growth rate of control medium BG11 and TSW (treated sewage wastewater) treatments increased gradually from the first cultivation day until they reached the maximum biomass productivity then declined. The day by which the maximum productivity was reached differ by treatments. Maximum growth rates of C. vulgaris reached about 900 mg/L at day 25 of cultivation when treated with control medium BG11, which represent approximately about twofold of that recorded with 50% TSW at day 50 of cultivation. Treatment with 100% and 75% TSW expressed lower growth rates reaching 356 and 373 mg/L at day 50 of cultivation, which was lower than that recorded with control medium (900 mg/L) at day 25 of cultivation. Treating C. vulgaris with control medium BG11 showed high productivity compared to other treatments even in the decline phase which recorded 715 mg/L at day 30 of cultivation as shown in Fig. 2A. The optical density method shown in Fig. 2A also recorded that treatment with control medium BG11 expressed maximum productivity at day 25 and even at decline phase (day 30 of cultivation) the productivity remains high as compared to the values of other treatments.
Also, the growth rate of C. vulgaris with control medium BG11 and different concentrations of nitrogen treatments (0, 0.5, 2, 4 × of NaNO3 in control medium) recorded in Fig. 2B increased gradually from the first cultivation day until they reached the maximum biomass productivity then declined. The day by which the maximum productivity was reached differ by treatments. Maximum growth rates of C. vulgaris reached about 1000 mg/L at day 35 of cultivation when treated with 2 × conc (3 g/l) of NaNO3. Treatment with zero nitrogen conc. reached 92 mg/L at day 20, half nitrogen conc. expressed 166 mg/L at day 25 of cultivation, and 4 × conc. recorded 835 mg/L at day 35, which was lower than that recorded with control medium (900 mg/L) at day 25 of cultivation. Treating C. vulgaris with 2x conc. of nitrogen showed high productivity compared to other treatments even in the decline phase which recorded 949 mg/L at day 40 of cultivation as shown in Fig. 2B. The optical density method shown in Fig. 2B also recorded that treatment with 2 × nitrogen conc. expressed maximum productivity at day 35, and even at decline phase (day 40 of cultivation), the productivity remains high as compared to the values of other treatments. Increasing nitrogen conc than that in control medium (1.59 g/l) showed stimulatory effect which reached its maximum at 2x (3 g/l) and more evaluated conc 4x (4 g/l) were inhibitory.
As shown in Fig. 3A, the growth rates of all Anabaena oryzae treatments and control medium BG110 appeared with short lag phase (day 5), different log phase length, and short decline phase. The growth rate of control medium BG110 and treatments increased gradually from the first cultivation day until they reached the maximum biomass productivity then decline. It was clearly noticed that the day by which the maximum productivity was reached differ by treatments. Maximum growth rates of A. oryzae reached about 600 mg/L at day 25 of cultivation when treated with 100% TSW, which represent about 1.5-fold of that recorded with control medium BG110 at day 20 of cultivation. Treatment with 50% and 75% TSW expressed respectively low growth rates reaching 50 and 300 mg/L at day 15 of cultivation, which was lower than that recorded by control medium (400 mg/L) at day 20 of cultivation. Treating A. oryzae with 100% TSW showed high productivity compared to other treatments even in the decline phase which recorded 400 mg/L at day 30 of cultivation, which is similar to the maximum growth rate of control recorded at day 25. The optical density method shown in Fig. 3A also recorded that treatment with 100% TSW expressed maximum productivity at day 25, and even at decline phase (day 30 of cultivation), the productivity remains high as compared to the values of other treatments and control.
The growth rates of all Nostoc muscurum treatments and control BG110 (Fig. 3B) appeared with short lag phase (day 5), different log phase length, and short declined phase. The growth rate of control medium BG110 and treatments increased gradually from the first cultivation day until they reached the maximum biomass productivity then decline. The day by which the maximum productivity was reached differ by treatments. Maximum growth rates of Nostoc muscurum reached about 350 mg/L at day 20 of cultivation when treated with control medium BG110. Treatment with 100% TSW and 75%TSW represented about 273 mg/L at day 25 and 253 mg/L at day 30 of cultivation, respectively. Treatment with 50% expressed low growth rates reaching 109 at day 15 of cultivation, which was lower than that recorded with control medium (350 mg/L) at day 20 of cultivation. Treating N. muscurum with control medium BG110 showed high productivity compared to other treatments as shown in Fig. 1 A and B. The optical density method shown in Fig. 1A also showed the same trend and recorded that treatment with control medium BG110 expressed maximum productivity.
This means the maximum OD was more early reached on algal cultivation with combined media (BG11, BG110, and TSW) than the control one (BG11 or BG110) growth of cyanobacterial species (Anabaena oryza and Nostoc muscorum) at 100% TSW, while green Chlorella vulgaris at 50% TSW. These results may be due to the richness of TSW in anions, cations, some trace elements in addition to IAA and BA and NPK compared to the synthetic media used (BG11 and BG110).
The results were in an agreement with the results obtained by Wang et al. [34] showing that some microalgae such as Chlorella sp. could adapt well on different wastewaters (municipal wastewater treatment plant, MWTP) with no lag phase observed. Similar observation and results were recorded by Shalaby et al. [24] for Nostoc muscorum cultivated at different types of wastewaters (sewage, industrial, and agriculture).
To inspect why TSW supported higher growth rates of algae in the present study referred to the physical and chemical analyses of both TSW and BG11 media (Table 1). TSW appeared to be slightly acidic (pH 6.48) while BG11 medium was neutral (pH 7). Both TSW and BG11 media have nearly similar amount of potassium (17.9 and 17.5 mg/L, respectively). TSW showed richness in anions compared with those found in BG medium. The data recorded in Table 1 revealed that the total nitrogen in BG11 media was more than 8 folds the nitrogen content of treated sewage water (248 and 28.5 mg/L respectively). Concerning trace elements, elevated amount of copper, zinc, and cobalt were noticed in TSW, while the concentrations of boron, phosphate, iron, and manganese were much more in BG medium.
3.3 HPLC results
Tables 2 and 3 showed the HPLC analysis of different plant hormones (ABA, GA3, IAA, and BA) in all the investigated cyanobacterial and green algal species. IAA conc. ranges 0.003, 0.005, and 0.010 mg/g and BA ranges (0.001, 0.002, and 0.004 mg/g) in green algae. While in cyanobacterial species, IAA conc. ranges 0.0002, 0.002, and 0.003 mg/g and BA ranges 0.005, 0.013, and 0.014 mg/g.
Analysis of plant growth regulators especially IAA and BA in different algal extracts of Anabaena oryzae cultivated in TSW and BG110 media was performed using HPLC (as shown in Table 4). The analyses revealed that both IAA and BA amounts were increased in alga cultivated in BG110 and TSW combination media (50, 75, and 100%) and when compared with BG110 medium. Moreover, TSW 100% recorded highest concentration of IAA (0.051 mg/g) when compared with BG110 medium (0.003 mg/g). However, algae cultivated in 50% TSW recorded the highest concentration of BA (0.044 mg/g) when compared with BG110 medium (0.013 mg/g). These results may be due to the abiotic stress occurred due to algal cultivation of A. oryzae in sewage wastewater which was rich with macro and micro nutrients as shown in Table 1 stress led to secretion of different secondary metabolites from algal cells as type of self-defense.
Analysis of plant growth regulators especially IAA and BA in different extracts of Nostoc muscurum cultivated in TSW and BG110 media was performed using HPLC (as shown in Table 4). The obtained results revealed that IAA concentration was increased in alga cultivated in 50, 75 and 100% treated media (BG110 and TSW combination by 0.050, 0.025, and 0.053 mg/g respectively, when compared with 100% BG110 medium (0.002 mg/g), while BA amount was increased in 100% and 50% treated media by 0.120 and 0.017 mg/g respectively and decreased in 75% TSW by 0.004 mg/g compared with 100% BG110 medium by 0.002 mg/g. treated alga with TSW 100% recorded the highest concentration of IAA and BA when compared with BG110 medium.
Analysis of plant growth regulators especially IAA and BA of Chlorella vulgaris cultivated in TSW (50, 75, and 100%), BG11 and nitrogen stress conditions (0x, 0.5x, 2 × and 4x) media was performed using HPLC as shown in Table 5. The obtained results revealed that IAA amount was increased in alga cultivated in 50 and 100% of TSW and BG11 combination by 0.014 and 0.032 mg/g respectively, when compared with BG11 medium by 0.01 mg/g, while, BA amount was increased in 100, 75, and 50% of TSW and BG11 combination by 0.695, 0.005, and 0.033 mg/g respectively, also in nitrogen stress conditions (0x, 0.5x, and 4x) by 0.005, 0.006, and 0.005 mg/g respectively, when compared with BG11 medium by 0.004 mg/g. TSW 100% recorded highest concentration of IAA and BA when compared with BG11 medium.
These results were in agreement with the results obtained by Rodríguez-Meizoso et al. [20] who reported that microalgae live in complex habitats and are subjected to stress and/or extreme natural environmental conditions, such as changes in salinity, temperature, and nutrients. Thus, these microorganisms must rapidly adapt to new environmental conditions to survive and thus produce a great variety of biologically active secondary metabolites that are not found in similar organisms lived in normal conditions.
3.4 N, P, and K results
Table 6 showed the percentage of NPK in Anabaena oryzae cultivated in various media conditions (BG110 and TSW). The results indicated that algal cell (cultivated on control medium, BG110) had relatively high amount of nitrogen (11.82%). In contrast, alga contained a moderate amount of potassium and phosphorus (1.46 and 1.11%, respectively). The percentage of these nutrients decreased in algal cells cultivated in mixed media (BG110 and TSW) with increasing the percentage of TSW media as shown in Table 6. The nitrogen decrease from 11.82% in control media (BG110) to 7.1% in 50% TSW followed by 6.88% in 75% TSW then 5.22% in 100% TSW, and the same trend was observed in cases of phosphorus and potassium.
Table 6 also showed the percentage of NPK in Nostoc muscurum cultivated in various media conditions (BG110 and TSW). The results indicated that algal cell (cultivated on 75% TSW) had relatively high amount of nitrogen (16.55%). In contrast, alga contained a low amount of potassium (0.030%) and a moderate amount of phosphorus (0.61%). The percentage of these nutrients decreased in algal cells cultivated in media (BG110 combined with 50% TSW and 100%) as shown in Table 6. The nitrogen decrease from 16.55% in 75% TSW medium to 15.08% in control medium(BG110) followed by 8.02% in 100% TSW then 6.29% in 50% TSW. However control medium (BG110) was observed increase in cases of phosphorus and potassium.
The percentage of NPK was showed in Table 7 of Chlorella vulgaris cultivated in various media conditions (BG11 and TSW). The results indicated that algal cells (cultivated on control medium, BG11) had relatively high amount of nitrogen (8.64%). In contrast, alga contained a moderate amount of potassium and phosphorus (1.28% and 0.51%, respectively). The percentage of these nutrients decreased in algal cells cultivated in mixed media (BG11 and TSW). The nitrogen decrease from 8.64% in control media (BG11) to 7.09% in 50% TSW followed by 7.045% in 75% TSW then 4.31% in 100% TSW, and the same trend was observed in cases of phosphorus and potassium. Also, the percentage of NPK was showed in Table 7 of C. vulgaris cultivated in various media conditions (BG11 and nitrogen different conc. 0.0 N, 0.75 g/l, 3 g/l, and 6 g/l). Under nitrogen stress conditions, the nitrogen content decrease from 8.64% in control media (BG11) to 3.9% in 4 × N followed by 2 × N (3.51%) then 0.5 × and 0 N media by 0.375% and 0.365% respectively, while phosphorus decrease from 1.28 in control media (BG11) to 4 × (0.20%) followed by 0.5 × (0.047%) then 0 and 2 × media by 0.041 and 0.039% respectively. In the case of potassium, 2× medium recorded the heighest result with 0.71% followed by 0.5× (0.55%).
These results may be due to the composition of media (BG11 and TSW) from these nutrients (N, P, and K) as recorded in Table 1. It is clear from the data that the N, P, and K contents in treated sewage wastewater were less than those in the control media (BG11 and BG110), and this may be correlated with the percentage of these nutrients in algal cells as shown in Tables 6 and 7.
3.5 Antioxidant activity
The obtained results using the antioxidant bioassays of methanol extracts of three selected algal species revealed that the antioxidant activity was concentration and time-dependent against DPPH radical assay. The antioxidant activity reached to IC50 47.71 µg/mL for Anabaena oryzae cultivated on medium BG110; this activity increased in algal cells cultivated in mixed media (50% BG110 with 50% TSW and 25% BG110 with 75% TSW) and recorded in Fig. 4 with IC50 35.33 and 43.33 µg/ml, respectively. However, this activity was decreased in alga cultivated in 100% TSW media with IC50 54.49 µg/ml when compared with IC50 values of natural and synthetic antioxidant standards (Ascorbic acid and BHT) (13.34 and 13.59 µg/ml). It is the same trend of Nostoc muscurum that the antioxidant activity reached to IC50 45.46 µg/mL Fig. 4 cultivated on medium BG110; this activity increased in algal cells cultivated in mixed media (50% BG110 with 50% TSW and 25% BG110 with 75% TSW) with IC50 34.42 and 39.30 µg/ml, respectively). However, this activity was decreased in alga cultivated in 100% TSW media with IC50 48.86 µg/ml when compared with IC50 values of natural and synthetic antioxidant standards (13.34 and 13.59 µg/ml). Moreover, plant growth regulators standards such as IAA and BA recorded high antioxidant activity with IC50 26.97 and 28.08 µg/ml by about twofold more than those recorded by ascorbic acid (vit. C) and BHT as shown in Fig. 4.
The antioxidant activity reached to IC50 46.13 µg/mL for Chlorella vulgaris cultivated on medium BG11; this activity increased in algal cells cultivated in TSW and nitrogen different conc. (50% TSW, 75% TSW, and 100% TSW) with IC50 34.72, 34.87, and 33.99 µg/ml, respectively) and (0x, 0.5x, 2x, 4x) with IC50 30.91, 33.77, 37.84, and 36.63 µg/mL as shown in Figs. 4 and 5.
So, it was clear now that the cultivation of samples under stress (TSW and nitrogen different conc.) increased its ability of antioxidant activity when compared with alga cultivated in synthetic medium (BG110 and BG11). This activity may be due to the active ingredient formation during the cultivation of samples under a biotic stress. These active ingredients include plant growth regulators (especially IAA and BA), phenolic compounds, and Phycobiliprotein. The obtained results were in agreement with the results reported by Shalaby [23], Shanab et al. [25], Mtaki et al. [16], and Shalaby et al. [24]. The antioxidant activity of IAA and BA may be due to the chemical structure and the atoms such as nitrogen in both the chemical structures of IAA and BA. This structure helps to scavenge the free radicals and increased the antioxidant values, which were in agreement with the results obtained by Chitra et al. [6].
3.6 Antimicrobial activities
The antimicrobial activity of selected algal extracts grown on control culture media (BG11 and BG110) were investigated against Gram –ve bacterial strains (E. coli and K. pneumonia) and Gram + ve (S. aureus and S. mutans) as well as against fungal species (A. niger and C. albicans) using well diffusion method and compared to the activity exhibited by IAA, BA, and standard bacterial and fungal drugs.
The obtained results recorded in Table 8 showed that IAA and BA exhibited antimicrobial activity against both Gram –ve and Gram + ve bacteria. Benzyladenine (BA) demonstrated higher activity than IAA for both types of bacteria except to K. pneumonia (Table 9), IAA activity higher than BA as shown in Table 10, and showed moderate activity against C. albicans (anticandidal activity), but no antifungal activity was observed. The IAA and BA exhibited MIC ranges of 125–250 ppm.
Chlorella vulgaris and Nostoc muscorum grew on control media showed no activity against all the tested microorganisms, while Anabaena oryzae extract exhibited low to moderate antibacterial activity against E. coli (17.6 ± 0.5 mm) and S. aureus (13.6 ± 0.5 mm) as shown in Table 8. The MIC for A. oryzae was 250 ppm compared to the standard drugs used (Table 10).
Cultivation of the selected algal species on 100% TSW showed neither antibacterial nor antifungal activities concerning extracts of C. vulgaris and A. oryzae, while N. muscorum showed activity against E. coli (19.6 ± 0.5 mm) and S. aureus (15.6 ± 0.5 mm) as recorded in Table 10, with MIC ranges 250 ppm.
Cultivation of C. vulgaris in different nitrogen conc. conditions (N-stress) showed higher antibacterial activity against S. aureus (20.3 ± 0.5 mm) which was comparable to that shown by the standard drug Ampicillin (22 ± 0.5 mm), with MIC ranges 125 ppm which is considered high compared to the range of MIC of standard drugs( 1.95–62.5) (Table 10).
Cultivation of the selected algal species on control media or on stressed 100% TSW conditions mostly do not permit the algae to manufacture antimicrobial compounds except in rare cases. Even under nitrogen stress conditions, C. vulgaris exhibited antibacterial activity against only S. aureus with high MIC value. These results may be algal species specific in case of C. vulgaris or due to metabolic alterations under stressed culture conditions (as in case of 100% TSW of N. muscorum and N-stressed C. vulgaris).
The obtained results in this study were in agreement with those obtained by Vehapi et al. [33] who reported that Chlorella vulgaris and Chlorella minutissima were cultivated on bold basal medium and municipal wastewater (ISKi) and Iroko tree extract water had strong antifungal activity against Aspergillus niger and Fusarium oxysporum using fumigation bioassay.
Microalgae grown under stress conditions (temperature, pH, salinity, light intensity, and medium components) effect the synthesis of bioactive compounds with antifungl, antibacterial, antiviral, antioxidant, anticoagulant, anticancer, and antiinflammatory activities [7].
The microalgal crude extracts of N. oculata, T. suecica, and Chlorella sp. in co-application with silver nanoparticles (AgNPs) had enhanced antimicrobial activity with the potential to overcome the global problem of microbial antibiotic resistance Hussein et al. [11].
Also, Iasimone et al. [12] reported that combined yeast and microalgal cultivation in a pilot-scale raceway pond for urban wastewater treatment and potential biomass production. Oleaginous yeast Lipomyces starkeyi and the wasrewater native microalgae (mostly Chlorella sp. and Scenedesmus sp.) was used to enhance lipid and biomass production from urban wastewaters.
Microalgal photosynthesis induced high pH and dissolved oxygen values resulted in matural bactericidal and antifungal activity; these results were concomitant with our results.
4 Conclusion
Growth of algal species was enhanced when cultured on combined media (control media + TSW conc.). Hormonal content (IAA/BA) showed variations depending on algal species and culture media. NPK contents in algae cultivated in control media were gradually decreased in combined media (50, 75, and 100% TSW). Elevation in antioxidant activity was recorded in algal cultivation on combined medium 50% TSW as well as under nitrogen stress conditions (in case of C. vulgaris). IAA and BA exhibited antimicrobial activity, while algae grown on control media showed no activity. Most of the selected algae cultured on media of 100% TSW or under N-stress conc. demonstrated no activity neither against bacteria (Gram –ve, Gram + ve) nor against fungi (C. albicans, A. niger) except in rare cases where antibacterial activity with high MIC values (125–250 ppm) was recorded.
Data availability
The data used and analysed in this study are available from the corresponding author on reasonable request.
References
A.O.A.C. (1995) Method of analysis, Association of Official Agriculture Chemists, 16th edn. Washington
Andersen RA, Robert A (eds.) (2005) Algal culturing techniques: a book for all phycologists. J Phycol 41: 906–908
APHA (1992) Standard methods for the examination of water and wastewater, 18th edn. American Public Health Association ,Washington, ISBN: 0–8–553060–5
APHA (1998) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, Washington, ISBN: 0–8–553060–5 Article ID 4910621, 11 pages
Burits M, Bucar F (2000) Antioxidant activity of nigella sativa essential Oil. Phytother Res 14:323–328
Chitra G, Selvi MS, Franklin DS, Sudarsan S, Sakthivel M, Guhanathan S (2019) pH-sensitive biopolymeric hydrogel-based on indole-3-acetic acid for wound healing and anti-cancer applications. SN Appl Sci 1(12). https://doi.org/10.1007/s42452-019-1339-x
De Morais MG, da Silva CK, Henrard AA, Costa JAV (2015) Carbon dioxide mitigation by microalga in a vertical tubular reactor with recycling of the culture medium. Afr J Microbiol Res 9(33):1935–1940
Downes, Ito (ed.) (2001) Compendium of methods for the microbiological examination of foods, 4th ed. American Public Health Association, Washington
El Semary AN, Mabrou M (2013) Molecular characterization of two microalgal strains in Egypt and investigation of the antimicrobial activity of their extracts. Biotechnol Agron Soc Environ 17(2):312–320
Gonçalves AL (2021) The use of microalgae and cyanobacteria in the improvement of agricultural practices: a review on their biofertilising, biostimulating and biopesticide roles. Appl Sci 11:871. https://doi.org/10.3390/app11020871
Hussein AH, Desy FS, Siti AMR, Julius Y, Fu S, Nor AMZ, Abdullah MA (2020) Phytochemical screening, metabolite profiling and enhanced antimicrobial activities of microalgal crude extracts in co-application with silver nanoparticle. Bioresour Bioprocess 7:39
Iasimone F, Zuccaro G, D’Oriano V, Franci G, Galdiero M, Pirozzi D, De Felice V, Pirozzi F (2018) Combined yeast and microalgal cultivation in a pilot-scale raceway pond for urban wastewater treatment and potential biodiesel production. Water Sci Technol 77(3-4):1062–1071. https://doi.org/10.2166/wst.2017.620
Kumar J, Dangariya M, Nakum AK, Agarwal P, Panda A, Parida AK, Gangapur DR, Meena R, Agarwal PK (2020) Sargassum seaweed extract enhances macrophomina phaseolina resistance in tomato by regulating phytohormones and antioxidative activity. J Appl Phycol 32:4373–4384
Koutra E, Tsafrakidou P, Sakarika M, Kornaros M (2020) Chapter 11-Microalgal biorefinery”. In: Yousuf A (ed) Microalgae Cultivationfor Biofuels Production. Academic Press, Cambridge, pp 163–185
Mackinney G (1941) Absorption of light by chlorophyll solutions 140:315–322
Mtaki K, Kyewalyanga MS, Mtolera MSP (2020) Assessment of antioxidant contents and free radical-scavenging capacity of Chlorella vulgaris cultivated in Low Cost Media. Appl Sci 10:8611
Raper KB, Fennell DI (1965) The genus Aspergillus. Science 150(3697):736–737
Rashed S, El-Chaghaby G, Lima EC, Simoes dos reis G (2021) Optimizing the ultrasonic-assisted extraction of antioxidants from Ulva lactuca algal biomass using factorial design. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01516-8
Rippka R, Deruelles J, John J, Waterbury B, Herdman M, Roger Y (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Rodríguez-Meizoso I, Jaime L, Santoyo S, Señoráns FJ, Cifuentes A, Ibáñez E (2010) Subcritical water extraction and characterization of bioactive compounds from Haematococcus pluvialis microalga. J Pharm Biomed Anal 51(2):456–463
Romanenko V, Kosakovskaya IV, Romanenko PA (2015) Phytohormones of microalgae: biological role and involvement in the regulation of physiological processes. Pt I. Auxins, Abscisic Acid, Ethylene. Int J Algae 17(3):275–289
Scott AC (1989) Laboratory control of antimicrobial therapy. In: Collee JG et al (eds) Practical Medical Microbiology, 13th edn. Churchill Livingstone, Edinburgh, p 161
Shalaby E (2011) Algae as promising organisms for environment and health. Plant Signal Behav 9:1338–1350
Shalaby EA, Atta MB, Sleem IA, Mohamed MA, El-Shemy HA (2018) Cytotoxicity, antioxidant and antiviral potential of aqueous extract from Nostoc muscorum cultivated in various inexpensive media. Waste Biomass Valorization 8:1–13
Shanab SM, Mostafa SS, Shalaby EA, Mahmoud GI (2012) Aqueous extracts of microalgae exhibit antioxidant and anticancer activities. Asian Pac J Trop Med 2:608
Shunmugam S, Muralitharan G, Thajuddin N (2015) Cyanobacteria and algae potential sources of biological control agents used against phytopathogenic bacteria. In book: Sustainable Approaches to Controlling Plant Pathogenic Bacteria, CRC Press, pp 241–254. https://doi.org/10.1201/b18892-13
Singh SP, Singh P (2015) Effect of temperature and light on the growth of algae species: A review. Renewable and Sustainable Energy Reviews, Elsevier 50(C):431–444
Snedecor G, Cochran W (1982) Statistical methods. Iowa state University Press, Iowa, p 511
Soltanpour PN (1985) Use of ammonium bicarbonate DTPA soil test to evaluate elemental availability and toxicity. Commun Soil Sci Plant Anal 16:323–338
Sun FuJ, Wang X, Chu J, Yan C (2011) Progress in quantitative analysis of plant hormones. Chin Sci Bull 56:355–366
Talukddar J (2012) Influences of dissolved inorganic carbon and nitrogen sources on growth, total lipid content and calorific value of freshwater oleaginous Microalga Akistrodesmus falcatus (corda) Ralfs. Environ Eng Manag J 61:14–25
Thi Cam VD Tran DT, Le TG, Nguyen QT (2020) Characterization of endogenous auxins and gibberellinsproduced by Chlorella sorokiniana TH01 under phototrophicand mixtrophic cultivation modes toward applications in microalgal biorefinery and crop research. J Chem :2020. https://doi.org/10.1155/2020/4910621
Vehapi M, Yilmaz A, Ozcimeu D (2018) Antifungal activities of Chloralla vulgaris and Chlorella minutissima microalgae cultivated in bold basal medium, wastewater and tree extract water against Aspergillus niger and Fusarium oxysporium. Rom Biotechnol Lett :2018. https://doi.org/10.26327/RBL2018.228
Wang BG, Zhang WW, And DXJ, Li XM (2009) In vitro antioxidative activaties of extracts and semi-purified fractions of the marine red alga, Rhosomela confervoides (Rhodomelaceae). Food Chem 113:1101–1105
Wang L, Min M, Li Y, Chen P, Chen Y, Liu Y, Wang Y, Ruan R (2010) Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl Biochem Biotechnol 162(4):1174–1186
Wang Q, Jin W, Zhou X, Zhang C, Shuhong Gao S, Chen Y (2021) Enhancement of biodiesel-promising microalgae Chlorella pyrenoidosa growth using stimulants in municipal sewage. Biomass Conversion Biorefinery https://doi.org/10.1007/s13399-020-01204-z
Watanabe FS, Olsen SR (1965) Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from the soil. Soil Sci Soc Am J 29:677–678
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elakbawy, W.M., Shanab, S.M.M. & Shalaby, E.A. Biological activities and plant growth regulators producing from some microalgae biomass cultivated in different wastewater concentrations. Biomass Conv. Bioref. 13, 8075–8088 (2023). https://doi.org/10.1007/s13399-021-01610-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01610-x