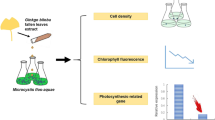
Abstract
Microalgae in genus Chlorella and Scenedesmus are common in aquatic ecosystems and are widely used for various studies on algal growth and applications. Macroalgae may play an important role for control of microalgal growth, attributable to their rich content of bioactive compounds. In this study, the brown seaweed Ascophyllum nodosum was extracted with 70% acetone and the extract was used to treat the green microalgae, Chlorella vulgaris and Scenedesmus sp. Cell density and chlorophyll a concentration were used as growth indexes to evaluate the effects of A. nodosum extract (ANE) on the microalgae. The ANE with concentrations > 1% exhibited significant capability of inhibition of the growth of microalgae by over 80%. On the contrary, 1% ANE caused varying degrees of acceleration of cell proliferation and chlorophyll a synthesis in C. vulgaris and Scenedesmus sp., respectively. Analysis of antioxidant activities of the enzymes superoxide dismutase (SOD) and catalase (CAT) revealed the impact of ANE on the antioxidant defense system of the microalgae. The SOD and CAT activities were significantly depressed by high concentrations (> 2%) ANE, while a slight increase of the enzyme activities was observed with 1% ANE at the early period, which could be correlated to the growth response. Therefore, the mechanism of microalgae control could be related to the interaction between the ANE and the antioxidant defense systems. Phlorotannins are proposed as the principal algistatic components in the ANE which could be utilized in controlling microalgae growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The microalgae Chlorella and Scenedesmus have been widely used as indicators for environmental monitoring and studies on the relationship between the algal growth and environmental stress (Gao et al. 2017). They are dominant in algal blooms (Auer et al. 1996) arising from water eutrophication, having detrimental influences on biological environment, public health, and economies (Anderson et al. 2002; Davidson et al. 2012). On the other hand, the economic value of both microalgae has been realized in the fields of biofuel, health care products, pigments, and aquaculture. These applications of the microalgae are attributable to the abundance of lipids, chlorophyll, proteins, and other nutrients (Rosenberg et al. 2014; Eustance et al. 2015;). More recently, heavy metal adsorption, and biogas and biofuel production by these microalgae have also been investigated (Mirghaffari et al. 2015; Jia et al. 2016; Mahdy et al. 2016; Senturk and Yildiz 2016).
Methods to control the growth of these microalgae have been widely studied. Physical (Anderson 2004) and chemical (Paerl 2008) methods have long been used to control the growth of microalgae. Low-dose-rate gamma irradiation has been reported to induce the enhancement of biomass and lipid content of marine microalgae for biofuel production (Jeong et al. 2017). Sun et al. (2014) also found that lower Se concentrations positively promoted the growth of Chlorella vulgaris, but the effect was opposite in the treatments with higher Se concentrations. Biological approaches feature higher efficiency, lower cost, and lower toxicity than physical and chemical methods (Oh et al. 2010; Wang et al. 2017). Aquatic plants (Marshall and Orr 1948) and algicidal bacteria (Salomon and Imai 2006) can directly attack the harmful algae or release algicidal compounds into water (Anderson 2009). Allelochemicals excreted by or isolated from aquatic plants has drawn much attention for algae-bloom control (Della Greca et al. 1998; Gross 2003; Hong et al. 2008a, b). A negative correlation of the abundance between microalgae and macroalgae has been reported (Crawford 1979; Lee and Olsen 1985; Fong et al. 1993). In view of the rapid effect, inhibitory compounds excreted by macroalgae instead of nutrient or sunlight competition have been proposed to influence the growth of microalgae (Fletcher 1975; Borowitzka 2016). Macroalgae of the Chlorophyta and Rhodophyta can affect the growth of microalgae and this has been attributed to compounds such as terpenoids (Konig et al. 1999), bromoperoxidase (Ohsawa et al. 2001), and polyunsaturated fatty acids (Chiang et al. 2004; Alamsjah et al. 2008; Oh et al. 2010).
Phlorotannins isolated from the brown alga Ecklonia kurome significantly decreased the swimming cell density of three red tide microalgae species within 30 min of application (Nagayama et al. 2003). Phlorotannins are also well known to have diverse effects on biological systems, such as anti-viral (Ahn et al. 2004), anti-cancer (Parys et al. 2010), antibacterial (Lopes et al. 2012), and antioxidant activity (Abu et al. 2013; Queguineur et al. 2013). They are usually extracted from brown algae using various solvents (Gall et al. 2015), e.g., 70% acetone is the most effective solvent for phlorotannin extraction from Fucus vesiculosus (Koivikko et al. 2005). The brown alga Ascophyllum nodosum is rich in phlorotannins (Zubia et al. 2009) with approximately 6.5% of the dry weight (Breton et al. 2011; Queguineur et al. 2013). However, A. nodosum extract (ANE) containing phlorotannins has not been studied for controlling freshwater microalgal growth. Moreover, the effect of phlorotannins on cell physiology of microalgae is not well understood.
Environmental stresses such as herbicides (Qian et al. 2008) and allelopathic effect of macrophytes (He et al. 2008) cause oxidative damage in microalgae by triggering the increase in reactive oxygen species (ROS) such as superoxide radical (O2•−), hydrogen peroxide (H2O2) and the hydroxyl radical (•OH) (Mittler 2002). For protecting cells against the potential damage by ROS, the activity of antioxidant enzymes is generally stimulated (Gao et al. 2017). These enzyme activities have been used to assess the toxicity to microalgae (Huang et al. 2012; Pereira et al. 2014; Sun et al. 2014). In this study, C. vulgaris and Scenedesmus sp. were selected for investigation of the effects of and A. nodosum extract (ANE) containing phlorotannins on the growth and antioxidant defense systems of the freshwater microalgae.
Material and methods
Freshwater microalgae
The unialgal inoculants of Chlorella vulgaris (PKU AC176) and Scenedesmus sp. (PKU AC158) were obtained from Peking University Algal Collection (PKU AC) and cultured in sterilized BG11 medium (Rippka et al. 1979). The pH value was adjusted to 7.1 using 1 M NaOH or HCl. The stationary microalgal cultivation was under an irradiance of 40–60 mmol photons m−2 s−1 and a photoperiod of 12 h light: 12 h dark at 24 °C. Microalgal cells in the logarithmic phase were used throughout the experiments.
Preparation of ANE
Dry powder of A. nodosum was purchased from Starwest Botanicals Inc., Canada. Portions (3 g) of milled powder (100 mesh) were extracted using 100 mL of 70% acetone. Three-hour ultrasonic extraction (ultrasonic time: 3 s; rest time: 9 s) was done at 400 W by an ultrasonic cell disintegrator (JY92-IIN, Ningbo Xinzhi Instruments, Inc., China) in an ice-water bath. The supernatant after centrifugation (12,857×g for 10 min at 4 °C) was concentrated at 30 °C under reduced pressure and then freeze-dried to obtain the crude extract (green-brown powder).
Microalgal assays with ANE
The crude extract (about 0.6 g) was re-suspended in 20 mL deionized water, and added to the suspensions of C. vulgaris and Scenedesmus sp. in BG11 medium (100 mL for each culture). Three separate cultures were prepared for each experiment. The concentration of extract in the suspensions varied from 1 to 4% (v/v). Before and after the cultivation for 1 to 7 days under the conditions stated in Section 2.1, cells densities were measured with Countstar Automated Cell Counter (Inno-Alliance Biotech, Inc., USA). The percent inhibition was calculated using the following formula: \( \mathrm{Inhibition}\ \left(\%\right)=100-\frac{\mathrm{cell}\ \mathrm{densities}\ \mathrm{in}\ \mathrm{treatment}\mathrm{s}\ \mathrm{with}\ \mathrm{extract}}{\mathrm{cell}\ \mathrm{densities}\ \mathrm{in}\ \mathrm{control}\ \mathrm{treatment}}\times 100. \) The concentration of chlorophyll a was determined every other day by hot-ethanol extraction method (Jespersen and Christoffersen 1987) with ultrasonic cell disintegration. The effective concentration of the ANE that inhibits 50% of the microalgae population (EC50) during the growth period was calculated using Probit analysis of transformed chemical concentration by plotting the natural logarithm values versus percentage of inhibition in MINITAB software (Release 15.1., Minitab Inc., 2007).
The soluble protein and antioxidant enzyme activity assays
Assay kits for measuring total protein, superoxide dismutase (SOD), and catalase (CAT) activities were purchased from Nanjing Jiancheng Bioengineering Institute, China. On each day from day 0 to day 4, microalgal cells were collected by centrifugation (12,857×g) at 4 °C for 10 min. The cell pellets were washed by phosphate-buffered saline (PBS) solution (50 mM, pH 7.0) twice and re-suspended in 1 mL PBS solution. Cell homogenization was conducted by ultrasonic cell disintegration at 400 W for 4 cycles (ultrasonic time: 3 s; rest time: 9 s) in an ice bath. The supernatant was used for soluble protein content and antioxidant enzyme activity measurements. Total protein was measured by colorimetric method based on BCA (bicinchoninic acid) at 562 nm (Smith et al. 1985) using the Epoch Microplate Spectrophotometer (BioTek Instruments, Inc., USA). Bovine serum albumin was used as standard. SOD activity was measured by using WST-1 (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2, 4-disulfophenyl)-2H–tetrazolium, monosodium salt) to produce a water-soluble formazan with O2˙− and determining the inhibitory ability of SOD to the rate of the reduction of WST-1 with O2˙−. CAT activity was measured by the decrease of the absorbance of H2O2 at 240 nm, as CAT could catalytically decompose H2O2 (Aebi 1974).
Analysis of algistatic compositions in the ANE
The crude extract was re-suspended in acetone and further extracted for five times until the residue became whitish. The acetone-soluble fraction was concentrated, vacuum dried and re-suspended in 20 mL deionized water. This solid–iquor mixture (1 mL) was added to the cultures of C. vulgaris and Scenedesmus sp. in sterilized BG11 medium (30 mL for each culture with three replicates), in parallel with the crude extract for comparison and sterile water as control.
Total phenolic compounds (PC) in different fractions were analyzed by the Folin–Ciocalteu method adapted from Audibert et al. (2010). ANE, Folin–Ciocalteu reagent and sodium carbonate were mixed and incubated at room temperature for 1 h. The optical absorbance of the solution was measured at 765 nm. The content of PC shown as gram of gallic acid equivalents (GAE) per gram of ANE (dry weight) was calculated according to a calibration curve obtained with gallic acid.
Statistical analyses
The mean and standard deviation of triplicates were calculated for each treatment and the control at each sampling time of both microalgae. The statistical significance of differences between treatments and the control were tested by independent-samples t tests (SPSS Version 19.0. SPSS Inc., USA).
Results
The effects on the growth of microalgae
The ANE was successfully prepared with 70% acetone (ESM_1). Without the ANE treatment, the cell density of both species increased rapidly in 7 days (Fig. 1). The ANE (> 1%) significantly (p < 0.01) inhibited the cell proliferation in both species after day 2. The algistatic effect was enhanced with increasing concentration, and the inhibitory ratios for C. vulgaris and Scenedesmus sp. reached 84.3 and 83.6%, respectively. Obvious difference between the two species was observed for 1% ANE. It reduced the cell density of Scenedesmus sp., but surprisingly promoted the growth of C. vulgaris (p < 0.01) after day 3. Flocculation was visible in the cultivation of C. vulgaris with 1 and 2% extract (Fig. 2a(1, 2)). The EC50 values of C. vulgaris remained in the range of 2.49 to 2.86%, while the EC50 for Scenedesmus sp. decreased steadily from 3.35 to 1.28% in 7 days (Table 1). This implies the dose-dependent and time-dependent inhibitory effects of the ANE on C. vulgaris and Scenedesmus sp., respectively.
Effects on photosynthesis of microalgae
Chlorophyll a content was also influenced by the ANE in a concentration-dependent manner (Fig. 3). Total chlorophyll a concentrations of both species significantly decreased after treated with 3 and 4% ANE (p < 0.01). The decrease in chlorophyll a concentration turned to be less significant for lower concentrations of extract. The 1% extract could even increase the total content of chlorophyll a in Scenedesmus sp. By comparing the chlorophyll a concentration per cell, the 3 and 4% ANE hindered the synthesis of chlorophyll a in both species. The dilute (1 and 2%) ANE significantly enhanced the synthesis of chlorophyll a in cells of Scenedesmus sp. (p < 0.01), but the effects on C. vulgaris were negligible (Fig. 3c, d).
Effects on soluble protein and antioxidant enzyme activities
Before the cultivation, the initial concentrations of soluble protein were 8.47 μg mL−1 for C. vulgaris and 67.40 μg mL−1 for Scenedesmus sp. The total concentration of soluble protein in the microalgal cells cultured without ANE increased steadily over time (Fig. 4). Application of extracts above 1% significantly stimulated the synthesis of soluble protein in both microalgal species within 1 day. The highest concentrations of total protein in cells of C. vulgaris and Scenedesmus sp. cultured with 3% ANE were 47 and 17 fold that of the control, respectively. After 2 days, the stimulation became less significant.
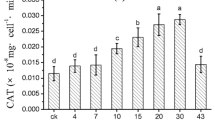
High activities of SOD and CAT in C. vulgaris were observed during the first day, which suggested antioxidant enzymes of C. vulgaris were more sensitive to the environmental changes. The 2 and 3% extracts severely inhibited the SOD activities in both microalgae for the entire study period (p < 0.05) (Fig. 5a, b). Instead of being inhibited, the SOD activity of both microalgae significantly increased (p < 0.05) by 24-h exposure to the 1% extract and then decreased to the control level 1 day later. The inhibitory effect on CAT activity of C. vulgaris was highly significant in the 3% ANE, and became less effective for lower concentrations, while for Scenedesmus sp., the inhibitory effect was clear for all treatments on day 1, but the CAT activities for all treatments were not significantly different from control thereafter (Fig. 5d).
Algistatic component analysis
The content of PC in the crude extract (about 0.6 g) was determined to be 24.33%, corresponding to 5.76% of the dry weight of A. nodosum. The acetone-soluble fraction was equivalent to 38.48% of the crude extract, but contained 73.86% of the total PC extracted from A. nodosum. The acetone-soluble fraction containing 46.7% PC demonstrated strong inhibition of both C. vulgaris and Scenedesmus sp. with percentage inhibition of 73.8 and 83.7%, respectively, which were comparable with the crude extract with equivalent contents of PC. This consistency implies that PC would have played the important role of algistatic effects. The PC content contributing to the 96 h EC50 of ANE for C. vulgaris and Scenedesmus sp. was determined to be 60.14 and 46.14 mg L−1, respectively.
Discussion
The cell density and chlorophyll a concentration are commonly reported for toxicity indexes of algicidal components (Zhang et al. 2015). With low concentration of the ANE, the stimulated cell division in C. vulgaris was similar to its general response to other slight stresses (Sun et al. 2014). For chlorophyll a content, the different trend between total and per cell concentration might be due to the inconsistent rate of cell division and chlorophyll a synthesis. The enhanced chlorophyll a synthesis in individual cells of Scenedesmus sp. was similar with the previous study on the toxicity of wastewater against Scenedesmus obliquus (Zhang et al. 2015), indicating that chlorophyll a synthesis in Scenedesmus sp. was more sensitive than biomass production. Intensive photosynthesis may be one of the steps in cellular recovery by increasing the rate of the light utilization and synthesizing more carbohydrates (Rym 2012). Therefore, low dosage of the ANE may be applied for acceleration of the microalgal cultivation.
Flocculation of C. vulgaris can be induced by environmental stresses such as high pH, Zn2+ and Cd2+ in the medium (Vandamme et al. 2012; Nguyen et al. 2014; Alam et al. 2015). Alam et al. (2015) indicated the flocculating microalgae were more tolerant to Zn2+ and Cd2+ stress in contrast with non-flocculating microalgae. Flocculation ability was also correlated to the inhibitor tolerance of other microorganisms such as yeast Saccharomyces cerevisiae (Westman et al. 2014). Therefore, flocculation may be one of the responses for C. vulgaris to improve the tolerance to the stress of A. nodosum extract.
The enhancement of protein synthesis could be one of the responses towards the stress induced by exposure to ANE. Protein can be synthesized by plants under stressful conditions, such as salinity stress, osmotic stress, extreme temperature, anaerobiosis, infection with pathogens, gaseous pollutants, UV radiation, and so on (Dubey 1999). Soluble protein synthesis was found to be enhanced or induced by UV radiation in the algae Chlamydomonas reinhardtii (Nicholson and Howe 1989). These stress-induced proteins have been reported to be osmosis pressure adjustors (Cheng et al. 2016), regulator of chlorophyll synthesis (Tzvetkova-Chevolleau et al. 2007) or enzymes with antioxidant activities. Nagayama et al. (2003) reported algicidal activity of phlorotannins due to the interaction between enzyme and the channel proteins in cell membranes. Moreover, acceleration of protein synthesis could damage the cells due to changes in osmosis pressure.
Analysis of superoxide dismutase (SOD) and catalase (CAT) activities within the cells of these two microalgae provided an understanding of the different physiological response to the ANE. The cellular antioxidant defense system containing SOD and CAT for oxidative stress (Mager and Dekruijff 1995) is considered to be the first defense line against ROS damage with capability to convert O2•− to H2O2 and O2, and further convert H2O2 to H2O (Ken et al. 2005; Ballesteros et al. 2009). This system also plays an important role in radical scavenging and intracellular protective mechanism. The occurrence of non-inhibition on the cell division and the chlorophyll a synthesis by ANE of low concentration may be correlated with a rise in enzyme activity and the recovery ability in the microalgal cells. Increasing SOD activities of both microalgae in the early stage indicated that SOD contributed to the scavenging of O2•− produced as a result of the ANE. There was no significant difference of CAT activities in both microalgae between low-dose treatment and control. That suggests that CAT did not play a role in scavenging H2O2 in this study and other antioxidant enzymes may function as H2O2 scavengers produced from the dismutation of superoxide catalyzed by SOD or direct production via photorespiration. Both microalgae showed low concentration tolerance to the stress induced by ANE and the fast recovery in cell division and chlorophyll a synthesis, which is in accordance with the behavior by the exposure to nonylphenol (Gao et al. 2017). However, the exposure to the ANE at high concentration could depress the activity of antioxidant enzymes despite enhanced protein synthesis. Inactive SOD and CAT failed to remove more O2•− and H2O2 induced by high dosage of ANE, which might destroy the defensive system and cause the inhibition of microalgal growth.
In conclusion, the ANE at concentrations higher than 1% had a consistent inhibitory effect on the growth of C. vulgaris and Scenedesmus sp. with a maximum percentage inhibition of over 80%. ANE at 1% significantly stimulated the cell proliferation and chlorophyll a synthesis of C. vulgaris and Scenedesmus sp., respectively. The analysis of antioxidant enzyme activity implied that the inhibitory effects on the activities of SOD and CAT within the antioxidant defense system could be one of the algistatic mechanisms. Phlorotannins are proposed to be the key algistatic compounds with active concentrations at the ppm level. Due to the abundance of phlorotannins, the ANE has the potential for controlling microalgae growth.
References
Abu R, Jiang ZD, Ueno M, Okimura T, Yamaguchi K, Oda T (2013) In vitro antioxidant activities of sulfated polysaccharide ascophyllan isolated from Ascophyllum nodosum. Int J Biol Macromol 59:305–312
Aebi H (1974) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 673–684
Ahn MJ, Yoon KD, Min SY, Lee JS, Kim JH, Kim TG, Kim SH, Kim NG, Huh H, Kim J (2004) Inhibition of HIV-1 reverse transcriptase and protease by phlorotannins from the brown alga Ecklonia cava. Biol Pharm Bull 27:544–547
Alam MA, Wan C, Zhao XQ, Chen LJ, Chang JS, Bai FW (2015) Enhanced removal of Zn2+ or Cd2+ by the flocculating Chlorella vulgaris JSC-7. J Hazard Mater 289:38–45
Alamsjah MA, Hirao S, Ishibashi F, Oda T, Fujita Y (2008) Algicidal activity of polyunsaturated fatty acids derived from Ulva fasciata and U. pertusa (Ulvaceae, Chlorophyta) on phytoplankton. J Appl Phycol 20:713–720
Anderson DM (2004) Prevention, control and mitigation of harmful algal blooms: multiple approaches to HAB management. Harmful algae management and mitigation Asia-Pacific economic cooperation (Singapore): APEC Publication #204-MR-042, pp 123–130
Anderson DM (2009) Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coast Manage 52:342–347
Anderson DM, Glibert PM, Burkholder JM (2002) Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries 25:704–726
Audibert L, Fauchon M, Blanc N, Hauchard D, Gall EA (2010) Phenolic compounds in the brown seaweed Ascophyllum nodosum: distribution and radical-scavenging activities. Phytochem Anal 21:399–405
Auer MT, Effler SW, Storey ML, Connors SD, Sze P, Siegfried CA, Auer NA, Madsen JD, Smart RM, Eichler LW, Boylen CW, Sutherland JW, Bloomfield JA, Wagner BA, Danehey R, Ringler NA, Gandino C, Hirethota P, Tango P, Arrigo MA, Morgan C, Millard C, Murphy M, Sloan RJ, Niehaus SL, Whitehead KA (1996) Biology. In: Effler SW (ed) Limnological and engineering analysis of polluted urban lake: prelude to environmental management of Onondaga Lake, New York. Springer, NY, pp 384–534
Ballesteros ML, Wunderlin DA, Bistoni MA (2009) Oxidative stress responses in different organs of Jenynsia multidentata exposed to endosulfan. Ecotox Environ Safe 72:199–205
Borowitzka MA (2016) Chemically-mediated interactions in microalgae. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer International Publishing, Cham, pp 321–357
Breton F, Cerantola S, Gall EA (2011) Distribution and radical scavenging activity of phenols in Ascophyllum nodosum (Phaeophyceae). J Exp Mar Biol Ecol 399:167–172
Cheng X, Deng G, Su Y, Liu JJ, Yang Y, Du GH CZY, Liu FH (2016) Protein mechanisms in response to NaCl-stress of salt-tolerant and salt-sensitive industrial hemp based on iTRAQ technology. Ind Crop Prod 83:444–452
Chiang IZ, Huang WY, Wu JT (2004) Allelochemicals of Botryococcus braunii (Chlorophyceae). J Phycol 40:474–480
Crawford SA (1979) Farm pond restoration using Chara vulgaris vegetation. Hydrobiologia 62:17–31
Davidson K, Gowen RJ, Tett P, Bresnan E, Harrison PJ, McKinney A, Milligan S, Mills DK, Silke J, Crooks AM (2012) Harmful algal blooms: how strong is the evidence that nutrient ratios and forms influence their occurrence? Estuar Coast Shelf Sci 115:399–413
Della Greca M, Ferrara M, Fiorentino A, Monaco P, Previtera L (1998) Antialgal compounds from Zantedeschia aethiopica. Phytochemistry 49:1299–1304
Dubey RS (1999) Protein synthesis by plants under stressful conditions. In: Handbook of plant and crop stress, second edition. CRC Press, Boca Raton pp 365–397
Eustance E, Wray JT, Badvipour S, Sommerfeld MR (2015) The effects of limiting nighttime aeration on productivity and lipid accumulation in Scenedesmus dimorphous. Algal Res 10:33–40
Fletcher RL (1975) Heteroantagonism observed in mixed algal cultures. Nature 253:534–535
Fong P, Donohoe RM, Zedler JB (1993) Competition with macroalgae and benthic cyanobacterial mats limits phytoplankton abundance in experimental microcosms. Mar Ecol Prog Ser 100:97–102
Gall EA, Lelchat F, Hupel M, Jegou C, Stiger-Pouvreau V (2015) Extraction and purification of phlorotannins from brown algae. Methods Mol Biol 1308:131–143
Gao QT, Wong YS, Tam NFY (2017) Antioxidant responses of different microalgal species to nonylphenol-induced oxidative stress. J Appl Phycol 29:1317–1329
Gross EM (2003) Allelopathy of aquatic autotrophs. Crit Rev Plant Sci 22:313–339
He F, Deng P, Wu XH, Cheng SP, Gao YN, Wu ZB (2008) Allelopathic effects on Scenedesmus obliquus by two submerged macrophytes Najas minor and Potamogeton malaianus. Fresen Environ Bull 17:92–97
Hong Y, Hu HY, Li FM (2008a) Physiological and biochemical effects of allelochemical ethyl 2-methyl acetoacetate (EMA) on cyanobacterium Microcystis aeruginosa. Ecotox Environ Safe 71:527–534
Hong Y, Hu HY, Xie X, Li FM (2008b) Responses of enzymatic antioxidants and non-enzymatic antioxidants in the cyanobacterium Microcystis aeruginosa to the allelochemical ethyl 2-methyl acetoacetate (EMA) isolated from reed (Phragmites communis). J Plant Physiol 165:1264–1273
Huang LD, Lu DH, Zhang P, Diao JL, Zhou ZQ (2012) Enantioselective toxic effects of hexaconazole enantiomers against Scenedesmus obliquus. Chirality 24:610–614
Jeong DH, Jeong MH, Jeong SK, Yang K, Jo WS (2017) Effect of continuous exposure to low-dose-rate gamma irradiation on cell growth and lipid accumulation of marine microalgae. Aquacult Int 25:589–601
Jespersen AM, Christoffersen K (1987) Measurements of chlorophyll-a from phytoplankton using ethanol as extraction solvent. Arch Hydrobiol 109:445–454
Jia Q, Xiang W, Yang F, Hu Q, Tang M, Chen C, Wang G, Dai S, Wu H, Wu H (2016) Low-cost cultivation of Scenedesmus sp. with filtered anaerobically digested piggery wastewater: biofuel production and pollutant remediation. J Appl Phycol 28:727–736
Ken CF, Hsiung TM, Huang ZX, Juang RH, Lin CT (2005) Characterization of Fe/Mn—superoxide dismutase from diatom Thallassiosira weissflogii: Cloning, expression, and property. J Agr Food Chem 53:1470–1474
Koivikko R, Loponen J, Honkanen T, Jormalainen V (2005) Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J Chem Ecol 31:195–212
Konig GM, Wright AD, Linden A (1999) Plocamium hamatum and its monoterpenes: chemical and biological investigations of the tropical marine red alga. Phytochemistry 52:1047–1053
Lee V, Olsen S (1985) Eutrophication and management initiatives for the control of nutrient inputs to Rhode Island coastal lagoons. Estuaries 8:191–202
Lopes G, Sousa C, Silva LR, Pinto E, Andrade PB, Bernardo J, Mouga T, Valentao P (2012) Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS One 7:e31145
Mager WH, Dekruijff AJJ (1995) Stress-induced transcriptional activation. Microbiol Rev 59:506
Mahdy A, Mendez L, Tomas-Pejo E, del Mar MM, Ballesteros M, Gonzalez-Fernandez C (2016) Influence of enzymatic hydrolysis on the biochemical methane potential of Chlorella vulgaris and Scenedesmus sp. J Chem Technol Biotechnol 91:1299–1305
Marshall SM, Orr AP (1948) Further experiments on the fertilization of a sea loch (Loch Craiglin)—the effect of different plant nutrients on the phytoplankton. J Mar Biol Assoc UK 27:360–379
Mirghaffari N, Moeini E, Farhadian O (2015) Biosorption of Cd and Pb ions from aqueous solutions by biomass of the green microalga, Scenedesmus quadricauda. J Appl Phycol 27:311–320
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Nagayama K, Shibata T, Fujimoto K, Honjo T, Nakamura T (2003) Algicidal effect of phlorotannins from the brown alga Ecklonia kurome on red tide microalgae. Aquaculture 218:601–611
Nguyen TD, Frappart M, Jaouen P, Pruvost J, Bourseau P (2014) Harvesting Chlorella vulgaris by natural increase in pH: effect of medium composition. Environ Technol 35:1378–1388
Nicholson P, Howe CJ (1989) Stress-induced protein synthesis in Chlamydomonas reinhardtii. FEMS Microbiol Lett 51:283–287
Oh MY, Lee SB, Jin DH, Hong YK, Jin HJ (2010) Isolation of algicidal compounds from the red alga Corallina pilulifera against red tide microalgae. J Appl Phycol 22:453–458
Ohsawa N, Ogata Y, Okada N, Itoh N (2001) Physiological function of bromoperoxidase in the red marine alga, Corallina pilulifera: production of bromoform as an allelochemical and the simultaneous elimination of hydrogen peroxide. Phytochemistry 58:683–692
Paerl H (2008) Chapter 10: Nutrient and other environmental controls of harmful cyanobacterial blooms along the freshwater-marine continuum. Adv Exp Med Biol 619:217–237
Parys S, Kehraus S, Krick A, Glombitza KW, Carmeli S, Klimo K, Gerhauser C, Konig GM (2010) In vitro chemopreventive potential of fucophlorethols from the brown alga Fucus vesiculosus L. by anti-oxidant activity and inhibition of selected cytochrome P450 enzymes. Phytochemistry 71:221–229
Pereira MM, Mouton L, Yepremian C, Coute A, Lo J, Marconcini JM, Ladeira LO, Raposo NRB, Brandao HM, Brayner R (2014) Ecotoxicological effects of carbon nanotubes and cellulose nanofibers in Chlorella vulgaris. J Nanobiotech 12:15. https://doi.org/10.1186/1477-3155-12-15
Qian HF, Chen W, Sheng GD, Xu XY, Liu WP, Fu ZW (2008) Effects of glufosinate on antioxidant enzymes, subcellular structure, and gene expression in the unicellular green alga Chlorella vulgaris. Aquat Toxicol 88:301–307
Queguineur B, Goya L, Ramos S, Martin MA, Mateos R, Guiry MD, Bravo L (2013) Effect of phlorotannin-rich extracts of Ascophyllum nodosum and Himanthalia elongata (Phaeophyceae) on cellular oxidative markers in human HepG2 cells. J Appl Phycol 25:1–11
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61
Rosenberg JN, Kobayashi N, Barnes A, Noel EA, Betenbaugh MJ, Oyler GA (2014) Comparative analyses of three Chlorella species in response to light and sugar reveal distinctive lipid accumulation patterns in the microalga C. sorokiniana. PLoS One 9:e92460
Rym BD (2012) Photosynthetic behavior of microalgae in response to environmental factors. In: Najafpour MM (ed) Applied photosynthesis. InTech, Riejeka, pp 22–46
Salomon PS, Imai I (2006) Pathogens of harmful microalgae. In: Granéli E, Turner JT (eds) Ecology of harmful algae. Springer, Berlin, pp 271–282
Senturk T, Yildiz S (2016) Adsorbent effect of Chlorella vulgaris and Scenedesmus sp (Chlorophyta) for the removal of some heavy metals and nutrients. Turk J Biochem 41:87–95
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Sun X, Zhong Y, Huang Z, Yang YF (2014) Selenium accumulation in unicellular green alga Chlorella vulgaris and its effects on antioxidant enzymes and content of photosynthetic pigments. PLoS One 9:e112270
Tzvetkova-Chevolleau T, Franck F, Alawady AE, Dall'Osto L, Carriere F, Bassi R, Grimm B, Nussaume L, Havaux M (2007) The light stress-induced protein ELIP2 is a regulator of chlorophyll synthesis in Arabidopsis thaliana. Plant J 50:795–809
Vandamme D, Foubert I, Fraeye I, Meesschaert B, Muylaert K (2012) Flocculation of Chlorella vulgaris induced by high pH: role of magnesium and calcium and practical implications. Bioresour Technol 105:114–119
Wang L, He F, Sun J, Hu Y, Huang T, Zhang Y, Wu Z (2017) Effects of three biological control approaches and their combination on the restoration of eutrophicated waterbodies. Limnology 18:301–313
Westman JO, Mapelli V, Taherzadeh MJ, Franzen CJ (2014) Flocculation causes inhibitor tolerance in Saccharomyces cerevisiae for second-generation bioethanol production. Appl Environ Microbiol 80:6908–6918
Zhang Y, Sun Q, Zhou J, Masunaga S, Ma F (2015) Reduction in toxicity of wastewater from three wastewater treatment plants to alga (Scenedesmus obliquus) in northeast China. Ecotoxicol Environ Saf 119:132–139
Zubia M, Fabre MS, Kerjean V, Le Lann K, Stiger-Pouvreau V, Fauchon M, Deslandes E (2009) Antioxidant and antitumoural activities of some Phaeophyta from Brittany coasts. Food Chem 116:693–701
Acknowledgements
The authors appreciate the invaluable discussion of Professor Li Fong Yau Sam from the National University of Singapore and the assistance on extraction work by Yushui Li and Tingting Li.
Funding
This work is financially supported by the Natural Science Foundation of China (No. 21405008), Shenzhen Research Grant (No. JCYJ20140903101648708 and KC2015ZDYF0014A), and by Shenzhen Municipal Government subsidies for postdoctoral research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, P., Geng, S., Feng, T. et al. Effects of Ascophyllum nodosum extract on growth and antioxidant defense systems of two freshwater microalgae. J Appl Phycol 30, 851–859 (2018). https://doi.org/10.1007/s10811-017-1287-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1287-z