Abstract
A quick fed-batch saccharification (QFBs) strategy of pretreated wheat straw at high solid loadings (HSL) was developed to achieve high sugar concentrations and increase lignocellulosic ethanol productivities in the fermentation stage. Pretreatment time, number of feedings, and the time between them were evaluated using a Box-Behnken design. A surface response model was employed to maximize sugars released at 20% (w/w) HSL. Total sugar concentrations after 60 h with 20% (w/w), 25% (w/w), and 30% (w/w) HSL were 128.5 g/l, 142.4 g/l, and 163.5 g/l, respectively. These were 14.6–42% higher than their batch saccharification counterparts. The QFBs was implemented as part of sequential (SHF) and simultaneous saccharification-fermentation (SSF) strategies as well as the presaccharification stage of a semi-simultaneous saccharification-fermentation (SSSF) strategy. In the latter strategy, the highest ethanol concentration achieved was 31.7 g/l. The ethanol volumetric productivity was 2.2- and 3-fold their SHF and SSF counterparts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biochemical conversion of lignocellulosic biomass to biofuels and bioproducts is gaining importance as a circular economy alternative to phase out fossil materials. Stages such as pretreatment, enzymatic saccharification, fermentation, and purification are essential in biochemical-platform biorefineries [1]. The enzymatic saccharification, in particular, provides the monomeric sugars to the fermentation stage required to produce either bioproducts or biofuels. The efficiency and effectiveness of enzymatic saccharification are strongly dependent on the biomass type, pretreatment technology, and hydrolysis conditions (e.g., solid and enzyme loadings, temperature, pH) [2]. High sugar yields in saccharification are usually attained with lignocellulosic biomass low solid loading (4%, w/w). However, to obtain sugar concentrations that achieve cost-competitive conditions in the separation stages (e.g., >8% w/w for lignocellulosic ethanol at the separation input stream), the saccharification stage requires initial high solid loadings (HSL) (≥ 20% w/w) [3]. The use of HSL in lignocellulosic biomass processing also reduces water and time consumption, thus improving the economics and reducing the environmental impact of biorefining processes [4, 5].
The commercial-scale deployment of enzymatic saccharification at HSL exhibits challenging issues such as poor mass and energy transfer efficiencies, high concentration of inhibitors, and poor flowability in transport and mixing [6, 7]. These phenomena may cause lower sugar yields than at laboratory scale. Available free water at HSL is scarce due to the biomass hydrophilic properties generating localized enzyme accumulation ending up in inhibition [8] and promoting unproductive binding of cellulases to lignin [9]. Fed-batch strategies for lignocellulosic have been successfully tested [10], improving free water availability during the incremental loading of biomass and enzymes, speeding up the substrate liquefaction. Consequently, biomass flowability increases, ensuring a fast distribution of enzymes and products, thus achieving higher final sugar concentrations than those obtained in batch saccharification. Factors such as the pretreatment method to which the biomasses is submitted, prewashing, number of feedings (NF), the time between feedings (FT), initial SL (6–20%, w/w), and lignocellulosic biomass concentration increments (3–10%, w/w) have been considered in previous studies [11].
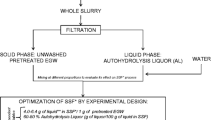
This work proposes a quick fed-batch saccharification (QFBs) strategy without prewash using wheat straw (WS). The QFBs strategy achieves high monomeric sugar concentrations at 20% (w/w) HSL, thus increasing lignocellulosic ethanol productivity. Factors such as NF, short FT, and pretreatment residence time (RT) were taken into consideration. The pretreatment employed in this work is carried out in a pilot-scale continuous tubular reactor (CTR) submitting the WS to autohydrolysis followed by a steam explosion (Ah-Se) stage, with lower production of inhibitors (furfural, acetic acid, 2,3-hydroxymethylfurfural (HMF)) than other technologies [12]. The pretreated WS exhibits high cellulose digestibility and hemicellulose solubilization [13, 14]. The proposed QFBs performance is compared against its batch counterpart. Higher solid loadings (25-30%, w/w) were also tested taking into consideration the increase in inhibitor concentrations due to larger HSL. This QFBs was tested as part of different saccharification-glucose-fermentation strategies such as SHF, SSF, and SSSF, achieving significant improvements in ethanol production and productivity.
2 Materials and methods
2.1 Wheat straw characterization
Wheat straw (WS) was provided by Champiñones el Encinal S.A. de C.V. (Jalisco, Mexico) from the 2016 harvest of La Barca region (Jalisco, Mexico). This biomass was milled with an agricultural 2-blade and 6-hammer mill (Molinos Azteca, Mexico) and sieved with a 1.27-cm mesh. The WS sugar composition was measured using NREL “Analytical Procedures for the Determination of Structural Carbohydrates in Biomass” [15], resulting in 41.7 ± 2.2 (%) and 25.2 ± 2.3 (%) of cellulose and hemicellulose (dry basis), respectively. Water content was measured with an MS-70 moisture analyzer (A&D, Japan). Water content in WS was 62.58 ± 0.6 (16 min of RT), 55.6 ± 0.9 (34 min of RT), and 61.8 ± 2.1 (54 min of RT) after pretreatment. Carbohydrates, inhibitory compounds, and ethanol produced were measured by a Waters Alliance e2695 high-performance liquid chromatograph (HPLC) (Waters Corp., USA), equipped with UV and IR detectors and an Aminex HPX-87H column (Bio-Rad, USA) using H2SO4 (5 mM) as mobile phase with 0.6 ml/min flow rate and an oven temperature of 60 °C.
2.2 Thermochemical pretreatment and inhibitors production
Milled WS was hydrated using tap water with a 3-kg water/kg WS ratio for 4 h before pretreatment in a semi-pilot continuous tubular reactor (CTR) with sequential Ah-Se stages at 1034 kPa and 180 °C [16]. WS was pretreated using three residence times (RT) (16, 34, and 52 min) determined in previous studies guaranteeing high biomass digestibility in the saccharification stage [12, 13]. Liquors and pretreated WS were collected after pretreatment and stored (−4 °C) at sterile conditions.
2.3 Box-Behnken design of QFBs at HSL with WS
A Box-Behnken design (BBD), shown in Table 1, was used for evaluating three factors with central point triplicates: RT (16, 34, and 54 min pretreatment time), FT (2, 3, and 4 h) and NF with proportional dry mass increments throughout feedings (2 [15%, 20% (w/w)], 4 [11.2%, 15%, 17.8%, 20% (w/w)], and 6 [9.7%, 12.6%, 15%, 17%, 18.6%, 20% (w/w)]). During the fed-batch mode, the WS was added according to NF and FT configuration. The chosen response variables were Glc yield, Xyl yield, and total sugar concentration. These were measured 36 h after the start of saccharification when the total solid content was reached for all configurations.
2.4 Quick fed-batch and batch saccharifications at HSL
Commercial enzyme cocktails Htec2 and Ctec2 (Novozymes®, Denmark) were used in saccharification experiments. The enzyme load is expressed in filter paper units (FPU) per gram of glucan available in the raw WS (FPU/gGlu) and measured according to NREL “Analytical Procedures for Measurement of Cellulase Activity” [17]. The enzyme cocktail mixture of Htec2-Ctec2 was dossed at 40 FPU/gGlu (10 FPUHtec2 + 30 FPUCtec2).
Saccharification experiments were carried out in 250-ml serological flasks with 100 g of total reaction mass incubated in a Minitron® incubator (Infors HT, Switzerland) at 50 °C and 250 rpm. All QFBs began at 6% (w/w) solid loading. Citrate buffer (50 mM, pH 5) was used as reaction media. The enzyme cocktail mixture (Htec2-Ctec2) was added with each WS addition to keep the concentration at 40 FPU/gGlu (i.e., 16.7 FPU/gWS) on the fed-batch mode. Glucose (Glc) and xylose (Xyl) concentrations were determined in aliquots by HPLC analysis. All reactions were performed in triplicate.
2.5 Saccharification-fermentation strategies at HSL
The commercial yeast Saccharomyces cerevisiae strain Ethanol-Red® (Lesaffre, France) was used in the glucose fermentation experiments. Dried yeast was activated by incubation for 2 h in a minimal fermentation broth (3 g/l KH2PO4, 9.3 g/l (NH4)2SO4, 2.25 g/l MgSO4.7H2O, 20 g/l glucose) at 35 °C and 200 rpm. Cells were counted and inoculated at 9 × 106 cell/ml into the WS saccharified slurries.
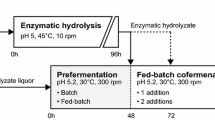
Fermentation stages were conducted in 250-ml serological flasks incubated at 35 °C and 200 rpm in a Minitron® incubator (Infors HT, Switzerland) and supplemented with salts (3 g/l KH2PO4, 9.3 g/l (NH4)2SO4, 2.25 g/l MgSO4.7H2O). SHF was carried out with 72 h saccharified WS slurry adjusted to pH (5) with NaOH (1 M). SSF was initiated with yeast inoculation and 6% (w/w) of pretreated WS, enriched with citrate buffer (50 mM, pH 5). Pretreated WS was loaded under QBFs conditions to achieve 20% (w/w) HSL. For the SSSF strategy, a 24 h prehydrolysis was carried out first according to the QFBs proposed in this work, followed by the slurry supplementation as described above. All experiments were performed in duplicate.
2.6 Calculation of saccharification and fermentation yields and productivities
The total sugar concentration (g/l) comes from the sum of Glc and Xyl concentration quantified for HPLC analysis. Yields were calculated based on the available sugars in the raw WS. The ethanol yield (YE) was calculated using Eq. 1. Ethanol productivity in the saccharification and fermentation stage was calculated by Eq. 2.
where [EtOH]t is ethanol concentration (g/l), [Glc]T is total glucose (g/l) concentration based on raw WS composition, and tT is the total processing time considering the saccharification and fermentation stage. Values 0.9 and 0.51 are mass correction factors of cellulose depolymerization and fermentation stoichiometry, respectively [18, 19].
2.7 Statistical analysis and surface response model calculations
All experimental results are expressed as standard error means (SEM). Statistical significance was calculated by a variance analysis (ANOVA) followed by a Dunnett comparison test using Prism (GraphPad Software, Inc.®, La Jolla, CA, USA). Values with p < 0.05 were considered significant. Response surface models were calculated with Minitab 18 software (Minitab® LLC, USA). A Fisher’s F-test was run for experiment and model significance. Response surfaces were drawn with Matlab® R2018b software (Mathworks, Inc. USA).
3 Results and discussion
3.1 Evaluation of the QFBs at 20% (w/w) HSL
Experimental results of saccharification at 20% (w/w) HSL of pretreated WS with the proposed QFBs strategy are compiled in Table 1. In the BBD, 36 h time mark was chosen to analyze the sugars released with the QFBs. Results at 48 and 72 h were also analyzed (data not shown). However, small contributions to the total sugar production from NF and FT in BBD at 72 h suggest that a kinetic effect of QFBs reduced at large saccharification times. Experiments R-3, R-5, R-7, and R-14 achieved concentrations above 100 g/l in sugars after 36 h of total reaction time, with R7 being the highest (115.9 g/l). In contrast, the lowest total sugar concentration was 82.9 g/l (R-2), 28.5% lower than the highest concentration achieved (R-7). Short RT (16 min), 4 feedings (NF), and 3 h FT factors were overrepresented in the experiments with high total sugar concentration. Regarding glucose yield, experiments R-3, R-4, R-5, and R-7 achieved values higher than 61% of the theoretical yield based on the glucose provided by the raw WS. Regarding xylose yields, experiments R-1, R-3, R-5, and R-7 reached values higher than 64% of the theoretical yield. In these experiments, 16 min RT was the only overrepresented value. These results indicate that 16 min RT improves sugar concentrations in saccharification. In contrast, large RT values resulted in lower total sugar concentrations [12, 13]. Previous works demonstrated that Ah-Se pretreatment removes hemicellulose fraction, releasing xylose, and xylooligosaccharides as a function of RT in CTR [13, 14]. This hemicellulose solubilization could make the Htec2 cocktail unnecessary, as previous reports indicate [20, 21]. However, better results in total sugars released by QFBs were obtained if high xylose content did not appear before saccharification (data not shown). The cellulose fraction in WS is usually slightly changed by AH-Se pretreatment. Small component modifications were reported at very large RT (54 min), conducting to low glucose production in saccharification at very low solid loading (1%, w/w) [13].
The experimental results produced second-order polynomial models, shown in Eqs. 3 to 5, correlating the response variables (i.e., Glc yield, Xyl yield, and total sugar concentration) with the experimental factors. Table 2 shows the regression coefficients (R2) values and results obtained from the ANOVA. Good R2 values were obtained for the three response variable models (0.93, 0.96, and 0.92 for Glc yield, Xyl yield, and total sugar concentration models, respectively).
Factors values producing the maximal response (i.e., glucose, xylose yields and total sugar concentration) values are shown in row “Maximization conditions” of Table 2. Response surfaces corresponding to Eqs. 1 to 5 are shown in Fig. 1, confirming that the QFBs produced the highest concentration of total sugars as well as glucose and xylose yields using WS pretreated with 16 min RT and NF equal or less than 5. Experimental points and calculated values of factors for maximal sugar concentrations and yields are shown as red dots and blue dots, respectively. They also illustrate the importance of RT and NF factors and their quadratic counterparts on maximizing total sugar release achieved by the QFBs. The FT factor showed a marginal contribution to both sugars (glucose and xylose) production.
3-D response surface plots showing the effect of factors RT, NF, and FT on total sugar concentration (a) and glucose (b) and xylose (c) yields employing the proposed QFBs at 20% (w/w) HSL with WS. Red dots indicate the experimental results. Light-gray dots and green dots indicate the adjusted maximal experimental condition
The ANOVA results shown in Table 2 showed that RT is the only statistically significant factor (p < 0.001) common to the three models, confirming the relevant role of the Ah-Se technology and its pretreatment conditions on the release of the hemicellulose fraction [14]. NF and its quadratic factor are also statistically significant in total sugar concentration and glucose yield models. Neither FT nor its quadratic factors were statistically significant in any model.
Since FT was not statistically significant, further experiments were carried out to corroborate that shorter FT could render similar values in the response variables to the maximal values calculated by the models. Feeding times periods of 2 h, 2.5 h, 4 h, and 6 h were tested together with 16 min and 5 feedings as RT and NF values, respectively (Fig. 2). Results at 36 h of saccharification indicate that glucose and xylose released were similar under the FT conditions tested. In comparison, at 72 h, the xylose content remains equivalent; meanwhile, glucose releases decrease on the FT increments. As being previously reported, not only large FT results in low sugar production from by-product inhibition, but also, other factors such as NF (solid increments) and enzyme dosage contribute to this behavior [11, 22, 23]. A large FT (6 h) showed the lowest glucose release at 72 h with WS. This result confirms that the inhibitory effect is stronger with large FT, replicating similar behavior to previous reports [22, 23]. The total sugar produced at 35 and 72 h of saccharification time with FT conditions tested was not statistically significant. Interestingly, 2.5 h in FT (130.6 g/l) outperformed total sugar production at 36 h (108.2 g/l) by 17.2%.
3.2 Comparison with batch mode
The QFBs with 16 min RT, 5 NF, and 2.5 h FT at 20% (w/w) HSL was compared with its batch counterpart in 60 h experiments. The QFBs reached its target HSL after 12.5 h, as shown in Fig. 3. After 0.5–1.25 h, WS flowability improved. Visual evidence is including as supplementary material using the aforementioned QFBs conditions in a 1.5-l bioreactor showing the change in flowability. In comparison, the corresponding batch saccharification required 16–18 h to achieve similar flowability conditions. The total sugar concentration after 60 h for the batch saccharification was 105.7 g/l, while QFBs reached 128.5 g/l. The sugar concentration in QFBs was 21.6% higher than its batch saccharification counterpart as shown in Fig. 3. This contribution to total sugar production comes from glucose release improvements. In contrast, a marginal contribution is originated from xylose release with the QFBs. This result confirms that RT pretreatment was the strongest factor tested contributing to hemicellulose depolymerization. Meanwhile, fed-batch conditions (NF and FT) were non-significant to xylose improvements. Future studies will be required to understand the enzyme dosage contribution and use of the Htec2 cocktail in QFBs.
3.3 Quick fed-batch saccharification with WS at higher solid loadings
The effect on sugar production by the QFBs with higher solid loadings (25% and 30% w/w) using WS was tested with 16 min RT, 2.5 h FT, and extending the NF as shown in Fig. 4. The total sugar concentrations attained at 25% and 30% (w/w) HSL were 142.4 g/l and 163.5 g/l at 48 h after the last feeding, respectively. For 25% (w/w) HSL, total sugar concentration was 15% higher than its counterpart saccharification in batch mode (123.8 g/l). The batch mode ES with WS at 30% (w/w) did not progress through 98 h of reaction time. This result emphasizes the benefit of water availability using QFBs.
3.4 Comparison with other fed-batch strategies
Table 3 presents HSL fed-batch enzymatic saccharification strategies published in the scientific literature. The saccharification results are closely linked to the cellulose content in the biomass and the dose and type of enzymes used. However, multiple factors intervene in the efficiency of saccharification, such as pretreatment, water availability, type and origin of biomass, inhibition of enzymes, reaction scale, and type of reactor, among others. In the studies presented in the table, the enzyme dose ranged from 8.8 to 40 FPU/g of cellulose, showing that the enzyme dose is not a trivial parameter, and must be determinate according to the characteristics of the pretreated biomass and the operational conditions in the saccharification process. For example, twelve out of thirteen works included in the table employ a prewashing stage to remove chemicals, catalysts, and inhibitors [10, 22,23,24,25,26,27,28,29,30,31,33]. The prewash also removes glycans, thus modifying LB composition, which can improve the saccharification yield. Most fed-batch strategies reported periods over 18–48 h to achieve the target HSL. The QFBs R-16, in contrast, took shorter times to reach its target HSL, achieving the highest sugar productions for 20, 25 and 30% (w/w) HSL with WS. In conclusion, the different methodologies proposed to reach HSL present some advantages such as not carrying out a prewashing stage of the pretreated biomass, shorter saccharification times, reaching higher solid loads (more than 20%), or lower doses of enzymes. However, to determine if these advantages positively impact the cost of lignocellulosic ethanol production, it is necessary to validate the strategies at a pilot level and carry out sustainability analyses.
3.5 Semi-simultaneous saccharification-fermentation of WS at HSL
The uses of SSF and SSSF are efficient ways to produce lignocellulosic ethanol. These strategies reduce inhibition caused by mono- and oligosaccharide accumulation in the system, as well as yeast stress caused by high sugar concentration at HSL [34]. In particular, SSSF uses a prehydrolysis stage to initiate the saccharification-fermentation task, thus reducing the fermentation time. This prehydrolysis stage promotes the lignocellulosic biomass liquefaction improving mixing in the system [35]. As a result, ethanol productivity in the SSSF is usually higher than the SSF [36, 37].
Therefore, the proposed QFBs with R-16 maximizing factor values at 20% (w/w) HSL was included as the presaccharification stage in an SSSF. Other strategies such as SHF and SSF were also tested, using the QFBs in the corresponding saccharification stage. The fermentation was carried out as described in Sect. 2.5. Table 4 shows the results of the SSF, SHF, and SSSF experiments. The fermentation kinetics are shown in Fig. 5. High ethanol production and glucose depletion were achieved at 24 h in SHF (Fig. 5a). In contrast, the SSSF reached similar conditions in 18 h (Fig. 5b), depleting the carbon source in the fermentation broth. SHF and SSSF achieved similar results in ethanol titration (32.7 g/l and 31.7 g/l, respectively) and yield (%) (51.4 and 50.2, respectively). However, the SHF processing time (96 h) was more than twice of SSSF time (42 h), thus affecting the ethanol productivity (\( {Q}_{E_{SF}} \)). Table 4 shows that ethanol concentration, yield, and productivity are strongly dependant on the SL and fermentation strategy. The highest productivity (0.75 g/lh) was achieved in the SSSF strategy being 2.2- and 3-fold higher than those of SHF and SSF, respectively.
Inhibitor concentrations impact negatively in downstream bioprocessing. The Ah-Se pretreatment used in this work produces small amounts of inhibitors compared to other pretreatment technologies (see supplementary material). Nevertheless, inhibitor concentration (3.24 ± 0.03 g/l of acetic acid, 1.51 ± 0.58 g/l of furfural and 37.1–10.3 mg/l of HMF) at 20% (w/w) HSL delayed the fermentation onset for 4–6 h as can be seen in Fig. 5. The S. cerevisiae metabolism transforms furfural into furfuryl alcohol, affecting cell growth as a side effect [45]. The fermentative capacity of S. cerevisiae was restored once the furfural concentrations were reduced. The furfural consumption rate was higher than that of HMF.
Table 4 includes the results of those fed-batch strategies already published that were tested in saccharification-fermentation strategies. The QFBs strategy proposed in this work produced ethanol yields of around 50% below the best yields reported at equivalent solid loadings (79.1% in [41]). However, productivity was one of the highest reported in HSL conditions. Similar productivity (0.77 g/lh) was previously reported with delignified CS at low SL (8%, w/w) [38].
4 Conclusions
The QFBs strategy proposed in this work gives rise to high sugar concentrations with WS using saccharification fed-batch strategies. The fed-batch factors (NF and FT) promote free water availability (flowability) in the enzymatic saccharification, thus improving the glucose release. The use of QFBs as part of the SSSF strategy demonstrated a significant increase in ethanol productivity compared to SHF and SSF strategies without the need for a prewash stage.
References
Achinas S, Euverink GJW (2016) Consolidated briefing of biochemical ethanol production from lignocellulosic biomass. Electron J Biotechnol 23:44–53. https://doi.org/10.1016/j.ejbt.2016.07.006
Machineni L (2020) Lignocellulosic biofuel production: review of alternatives. Biomass Convers Biorefinery 10:779–791. https://doi.org/10.1007/s13399-019-00445-x
Larsen J, Øtergaard Petersen M, Thirup L et al (2008) The IBUS process - Lignocellulosic bioethanol close to a commercial reality. Chem Eng Technol 31:765–772. https://doi.org/10.1002/ceat.200800048
Xiros C, Janssen M, Byström R, Børresen BT, Cannella D, Jørgensen H, Koppram R, Larsson C, Olsson L, Tillman AM, Wännström S (2017) Toward a sustainable biorefinery using high-gravity technology. Biofuels, Bioprod Biorefining 11:15–27. https://doi.org/10.1002/bbb.1722
Saini JK, Agrawal R, Satlewal A, Saini R, Gupta R, Mathur A, Tuli D (2015) Second generation bioethanol production at high gravity of pilot-scale pretreated wheat straw employing newly isolated thermotolerant yeast Kluyveromyces Marxianus DBTIOC-35. RSC Adv 5:37485–37494. https://doi.org/10.1039/c5ra05792b
Koppram R, Tomás-Pejó E, Xiros C, Olsson L (2014) Lignocellulosic ethanol production at high-gravity: challenges and perspectives. Trends Biotechnol 32:46–53. https://doi.org/10.1016/j.tibtech.2013.10.003
Chen H, Fu X (2016) Industrial technologies for bioethanol production from lignocellulosic biomass. Renew Sustain Energy Rev 57:468–478. https://doi.org/10.1016/j.rser.2015.12.069
Viamajala S, McMillan JD, Schell DJ, Elander RT (2009) Rheology of corn stover slurries at high solids concentrations - effects of saccharification and particle size. Bioresour Technol 100:925–934. https://doi.org/10.1016/j.biortech.2008.06.070
Palonen H, Tjerneld F, Zacchi G, Tenkanen M (2004) Adsorption of Trichoderma reesei CBH I and EG II and their catalytic domains on steam pretreated softwood and isolated lignin. J Biotechnol 107:65–72. https://doi.org/10.1016/j.jbiotec.2003.09.011
Zhang Y, Liu Y-Y, Xu J et al (2012) High solid and low enzyme loading based saccharification of agricultural biomass. 7:345–353
Agrawal R, Bhadana B, Mathur AS, Kumar R, Gupta RP, Satlewal A (2018) Improved enzymatic hydrolysis of pilot scale pretreated rice straw at high total solids loading. Front Energy Res 6:115. https://doi.org/10.3389/fenrg.2018.00115
Pérez Pimienta JA, Papa G, Rodriguez A, Barcelos CA, Liang L, Stavila V, Sanchez A, Gladden JM, Simmons BA (2019) Pilot-scale hydrothermal pretreatment and optimized saccharification enables bisabolene production from multiple feedstocks. Green Chem 21:3152–3164. https://doi.org/10.1039/c9gc00323a
Pérez-Pimienta JA, Papa G, Gladden JM, Simmons BA, Sanchez A (2020) The effect of continuous tubular reactor technologies on the pretreatment of lignocellulosic biomass at pilot-scale for bioethanol production. RSC Adv 10:18147–18159. https://doi.org/10.1039/d0ra04031b
Rodríguez F, Sanchez A, Amaya-Delgado L (2018) Xyloligosaccharides production from lignocellulosic biomass using a pretreatment continuous tubular reactor. Modelling and experimental validation. Ind Crops Prod 134:62–70. https://doi.org/10.1016/j.indcrop.2019.03.058
Sluiter A, Hames B, Ruiz R, et al (2008) Determination of structural carbohydrates and lignin in biomass: laboratory analytical procedure (LAP). https://www.nrel.gov/docs/gen/fy11/42618.pdf
Jaramillo I, Sanchez A (2018) Mass flow dynamic modeling and residence time control of a continuous tubular reactor for biomass pretreatment. ACS Sustain Chem Eng 6:8570–8577. https://doi.org/10.1021/acssuschemeng.8b00882
Adney B, Baker J (2008) Measurement of cellulase activities laboratory. Tech Rep NREL/TP-510-42628 8
Luo P, Liu Z, Yang C, Wang G (2008) Study of simultaneous saccharification and fermentation for steam exploded wheat straw to ethanol. Front Chem Eng China 2:447–451. https://doi.org/10.1007/s11705-008-0069-1
Dowe N, Mcmillan J (2008) SSF experimental protocols -- lignocellulosic biomass hydrolysis and fermentation. Tech Rep NREL/TP-510-42630
Sant’Ana da Silva A, Fernandes de Souza M, Ballesteros I, Manzanares P, Ballesteros M, P. S. Bon E (2016) High-solids content enzymatic hydrolysis of hydrothermally pretreated sugarcane bagasse using a laboratory-made enzyme blend and commercial preparations. Process Biochem 51:1561–1567. https://doi.org/10.1016/j.procbio.2016.07.018
Alvira P, Negro MJ, Ballesteros I, González A, Ballesteros M (2016) Steam explosion for wheat straw pretreatment for sugars production. Bioethanol 2:66–75. https://doi.org/10.1515/bioeth-2016-0003
Mukasekuru MR, Hu J, Zhao X, Sun FF, Pascal K, Ren H, Zhang J (2018) Enhanced high-solids fed-batch enzymatic hydrolysis of sugar cane bagasse with accessory enzymes and additives at low cellulase loading. ACS Sustain Chem Eng 6:12787–12796. https://doi.org/10.1021/acssuschemeng.8b01972
Gao Y, Xu J, Yuan Z, Zhang Y, Liu Y, Liang C (2014) Optimization of fed-batch enzymatic hydrolysis from alkali-pretreated sugarcane bagasse for high-concentration sugar production. Bioresour Technol 167:41–45. https://doi.org/10.1016/j.biortech.2014.05.034
Gladis A, Bondesson PM, Galbe M, Zacchi G (2015) Influence of different SSF conditions on ethanol production from corn stover at high solids loadings. Energy Sci Eng 3:481–489. https://doi.org/10.1002/ese3.83
Du J, Zhang F, Li Y et al (2014) Enzymatic liquefaction and saccharification of pretreated corn stover at high-solids concentrations in a horizontal rotating bioreactor. Bioprocess Biosyst Eng 37:173–181. https://doi.org/10.1007/s00449-013-0983-6
Geng W, Jin Y, Jameel H, Park S (2015) Strategies to achieve high-solids enzymatic hydrolysis of dilute-acid pretreated corn stover. Bioresour Technol 187:43–48. https://doi.org/10.1016/j.biortech.2015.03.067
Du J, Cao Y, Liu G et al (2017) Identifying and overcoming the effect of mass transfer limitation on decreased yield in enzymatic hydrolysis of lignocellulose at high solid concentrations. Bioresour Technol 229:88–95. https://doi.org/10.1016/j.biortech.2017.01.011
Liu Y, Xu J, Zhang Y, Yuan Z, Xie J (2015) Optimization of high solids fed-batch saccharification of sugarcane bagasse based on system viscosity changes. J Biotechnol 211:5–9. https://doi.org/10.1016/j.jbiotec.2015.06.422
Caspeta L, Caro-Bermúdez MA, Ponce-Noyola T, Martinez A (2014) Enzymatic hydrolysis at high-solids loadings for the conversion of agave bagasse to fuel ethanol. Appl Energy 113:277–286. https://doi.org/10.1016/j.apenergy.2013.07.036
Li K, Qin JC, Liu CG, Bai FW (2016) Optimization of pretreatment, enzymatic hydrolysis and fermentation for more efficient ethanol production by Jerusalem artichoke stalk. Bioresour Technol 221:188–194. https://doi.org/10.1016/j.biortech.2016.09.021
Gupta R, Kumar S, Gomes J, Kuhad RC (2012) Kinetic study of batch and fed-batch enzymatic saccharification of pretreated substrate and subsequent fermentation to ethanol. Biotechnol Biofuels 5:16. https://doi.org/10.1186/1754-6834-5-16
Hernández-Beltrán JU, Hernández-Escoto H (2018) Enzymatic hydrolysis of biomass at high-solids loadings through fed-batch operation. Biomass and Bioenergy 119:191–197. https://doi.org/10.1016/j.biombioe.2018.09.020
Sharma S, Kumar R, Gaur R, Agrawal R, Gupta RP, Tuli DK, Das B (2015) Pilot scale study on steam explosion and mass balance for higher sugar recovery from rice straw. Bioresour Technol 175:350–357. https://doi.org/10.1016/j.biortech.2014.10.112
Fockink DH, Urio MB, Chiarello LM, Sánchez JH, Ramos LP (2016) Principles and challenges involved in the enzymatic hydrolysis of cellulosic materials at high total solids. En: Green Fuels and Technology. pp 147–173 Springer, Cham. https://doi.org/10.1007/978-3-319-30205-8_7
Horan E, Zhang J, Viikari L et al (2011) Thermostable endoglucanases in the liquefaction of hydrothermally pretreated wheat straw. Biotechnol Biofuels 4:1–10. https://doi.org/10.1186/1754-6834-4-2
Koppram R, Nielsen F, Albers E et al (2015) Simultaneous saccharification and co-fermentation for bioethanol production using corncobs at lab, PDU and demo scales. Fuel Prod from Non-Food Biomass Corn Stover:155–179. https://doi.org/10.1201/b18437
Gonçalves FA, Ruiz HA, Nogueira CDC et al (2014) Comparison of delignified coconuts waste and cactus for fuel-ethanol production by the simultaneous and semi-simultaneous saccharification and fermentation strategies. Fuel 131:66–76. https://doi.org/10.1016/j.fuel.2014.04.021
Santos JRA, Lucena MS, Gusmão NB, Gouveia ER (2012) Optimization of ethanol production by Saccharomyces cerevisiae UFPEDA 1238 in simultaneous saccharification and fermentation of delignified sugarcane bagasse. Ind Crops Prod 36:584–588. https://doi.org/10.1016/j.indcrop.2011.10.002
Tomás-Pejó E, Oliva JM, Ballesteros M, Olsson L (2008) Comparison of SHF and SSF processes from steam-exploded wheat straw for ethanol production by xylose-fermenting and robust glucose-fermenting Saccharomyces cerevisiae strains. Biotechnol Bioeng 100:1122–1131. https://doi.org/10.1002/bit.21849
Li X, Lu J, Zhao J, Qu Y (2014) Characteristics of corn stover pretreated with liquid hot water and fed-batch semi-simultaneous saccharification and fermentation for bioethanol production. PLoS Oneuiy87:9. https://doi.org/10.1201/b18437
Lu J, Li X, Yang R, Yang L, Zhao J, Liu Y, Qu Y (2013) Fed-batch semi-simultaneous saccharification and fermentation of reed pretreated with liquid hot water for bio-ethanol production using Saccharomyces cerevisiae. Bioresour Technol 144:539–547. https://doi.org/10.1016/j.biortech.2013.07.007
Hiyama RYO, Harada A, Seiki G, Orihashi KEN (2016) Ethanol production from unpretreated waste medium of shiitake mushroom (Lentinula edodes) by semi-simultaneous saccharification and fermentation under high substrate concentration conditions. Cellul Chem Technol 50:771–780
de Oliveira RA, Gottschalk LMF, Freitas SP, Bon EP d S (2018) One-vessel saccharification and fermentation of pretreated sugarcane bagasse using a helical impeller bioreactor. Biomass Conv Bioref 8:1–10. https://doi.org/10.1007/s13399-017-0272-8
Shahsavarani H, Hasegawa D, Yokota D, Sugiyama M, Kaneko Y, Boonchird C, Harashima S (2013) Enhanced bio-ethanol production from cellulosic materials by semi-simultaneous saccharification and fermentation using high temperature resistant Saccharomyces cerevisiae TJ14. Seibutsu-kogaku Kaishi 115:20–23. https://doi.org/10.1016/j.jbiosc.2012.07.018
Sárvári Horváth I, Franzén CJ, Taherzadeh MJ et al (2003) Effects of furfural on the respiratory metabolism of Saccharomyces cerevisiae in glucose-limited chemostats. Appl Environ Microbiol 69:4076–4086. https://doi.org/10.1128/AEM.69.7.4076
Acknowledgements
The authors also acknowledge Dr. J. A. Perez-Pimienta and Dr. F. Rodriguez-Palomera for their assistance in the pretreatment of WS using the CR at the Bioenergy Futures Laboratory.
Funding
Financial support was received from the Energy Sustainability Fund 2014 (CONACYT-SENER, Mexico) Grants 245750 and 249564 (Mexican Bioenergy Innovation Centre, Bio-alcohols Cluster). JG received financial support from CONACYT in the form of Ph.D. scholarships (455101 and 291250).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. The conceptualization of the work was established by Jorge Gonzalez, Arturo Sanchez, and Lorena Amaya. Material preparation, data collection, and analysis were performed by Jorge Gonzalez and Alexander Valle. The first draft of the manuscript was written by all authors who commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gonzalez-Rios, J.A., Valle-Pérez, A.U., Amaya-Delgado, L. et al. A quick fed-batch saccharification strategy of wheat straw at high solid loadings improving lignocellulosic ethanol productivity. Biomass Conv. Bioref. 13, 4991–5003 (2023). https://doi.org/10.1007/s13399-021-01580-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01580-0