Abstract
Attached cultivation system has emerged as a cost-effective approach for the cultivation of microalgae. However, few microalgae species are still restricted to grow as biofilm due to the lack of adhesive element known as extracellular polymeric substance (EPS). The main objective of our study is to develop a novel attached cultivation strategy for EPS-lacking microalgae, Scenedesmus sp. DDVG I. We also aimed to identify cost-effective substratum showing good performance for biofilm growth. The approach was experimentally studied by conditioning a variety of substrata with EPS derived on varying days from cyanobacteria, Limnothrix sp. DDVG II. The EPS-coated substrata were used for the attached cultivation system of Scenedesmus sp. Additionally, we compared the biochemical properties of the EPS matrix derived on varying days using different extraction methods, their performance on adhesion strength, and microalgal growth. As a result, rough substrata were highly prone to the colonization of the EPS matrix than the normal one. The EPS derived from 12-day-old Limnothrix sp. culture (EPS-12) showed the highest potential on adhesion strength. Rough substrata coated with EPS-12 showed the maximum growth of Scenedesmus sp. DDVG I as a biofilm. Among the rough substrata, rough polylactic acid (rPLA) sheet coated with EPS-12 was found to be the best substratum showing the highest adhesion capability of 94.60 ± 4.2% and the maximum aerial biomass productivity of 31.6 ± 1.20 g/m2/day. The results indicate that EPS-lacking Scenedesmus sp. can be cultured with the attached cultivation technique to improve its biomass productivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Microalgae are emerging as the most versatile biomass for numerous applications such as biofuel production [1,2,3] wastewater treatment [4, 5], animal feed [6], and pharmaceutical and cosmetic industries [7]. There are still limitations for commercial production of microalgal biomass among which harvesting is a challenging task involving high capital cost and extra operational cost [8]. Generally, microalgae are cultivated in either open pond or photobioreactors in which the cells are in a suspended condition. At the time of biomass recovery, the conventional harvesting techniques viz. centrifugation, filtration, flocculation, and floatation are used [9]. These techniques are very expensive, and 30% of the total capital cost is still spent on harvesting [10]. To address this issue, the attached cultivation system has recently emerged as an alternative approach for biomass harvesting as well as wastewater treatment. In these systems, microalgal biomass is grown as a thin biofilm on supporting substrata wherein the biomass is more concentrated to 18% (g/g) [9] as compared to 0.4% (g/g) [11] for the suspended condition. The development of microalgal biofilm depends on several factors such as extracellular polymeric substance (EPS) matrix, substrata including polyethylene [9], cardboard and glass [10], loofah sponge, and polyurethane foam [11], and types of microalgal species depending on the presence and absence of EPS. According to Flemming et al. [12], the substratum is initially conditioned with adhesive mucilage called EPS. EPS matrix is a biopolymer which is predominantly comprised of various proteins, polysaccharides, lipids, uronic acids, and ionizable functional groups like carboxylic, phosphoric, amino, sulfate, and hydroxyl groups [13, 14]. Cyanobacteria, fungi, and some microalgal species are the major EPS producer [15, 16]. The components of EPS favor the site for colonization and multiplication of microalgal cell as a biofilm. For instance, the ethyl cellulosic backbone and cationic groups of polysaccharides present in the EPS matrix promote its adherence to the substratum and accelerates microalgal growth [12]. EPS production can be influenced by age of the culture that can significantly affect the adhesion strength and biofilm growth. Besides, suitable and effective substrata or supporting materials can induce good adhesion strength for EPS [17]. Yuan et al. [18] suggested that the physicochemical properties (surface texture, hydrophobicity, and surface free energy) of the substratum may influence the EPS-substratum interaction. Zhang et al. [19] demonstrated that the cracks and crevices created on rough substrata could provide a greater area for attachment and thus resulting in higher cell attachment and greater biomass production. Roostaei et al. [20] concluded hydrophobic substrata to be more favorable for cell adhesion than a hydrophilic one. The probable reason may be that the EPSs are mostly hydrophobic in nature which have greater affinity towards hydrophobic substrata to reduce their contact with water [21]. While Ozkan and Berberoglu [22] reported a hydrophilic substratum is more susceptible to adhesion than a hydrophobic substratum, however, Genin et al. [23] found no correlation between adhesion and surface hydrophobicity. Thus, the selection of effective materials is still a challenging task to enrich the research on the attached cultivation system.
Johnson and Wen [24] cultivated Chlorella sp. on polystyrene foam as attached cultivation, resulting a significant biomass accumulation (25.65 g m−2day−1) with efficient removal of 79% total nitrogen and 93% total phosphorus from dairy manure wastewater. A pilot scale rotating algal biofilm reactor using cotton duct as attaching material produced 8–14 g m−2day−1 biomass of Chlorella vulgaris [25]. Christenson and Sims [9] found the cotton cord as an effective substratum for the growth of algal-bacterial consortium, resulting in biomass productivity of 20–31 g m−2 day−1. Recently, Zhang et al. [26] found non-woven fabric to be a good substratum for the cultivation of filamentous algae, Tribonema minus, and the average biomass productivity obtained was 9.73 ± 2.19 g m−2 day−1. Lignocellulosic material such as pine sawdust could also support the algal attachment with the maximum biofilm productivity of 10.9 g m−2 day−1 [27]. Despite all these extensive efforts, some microalgal species which are high-value feedstock for biodiesel production have been restricted from growing as a biofilm due to the lack of EPS production. For example, according to the finding of Gaignard et al. [16], microalgae such as Nannochloris sp. (21–23% lipid content) [28], Tetraselmis striata (30–31% lipid content) [29], and certain strains of Scenedesmus sp. (18–20% lipid content) [30] were identified as non-EPS producer. To address this gap, our study was primarily focused on the attached cultivation of EPS-lacking microalgal species, Scenedesmus sp. with an aid of EPS derived from cyanobacterium, Limnothrix sp. Selection of the microalgae strain was based on its fast growth rate, high biomass, and oil production [3, 30], while the cyanobacterium was chosen because of its high EPS production [16]. We also aimed to identify cost-effective substrata showing good performance for the biofilm growth. The approach was experimentally studied by conditioning a variety of substrata with cyanobacterial EPS produced on varying days. Additionally, we compared the biochemical properties of those EPS using different extraction methods and their performance on adhesion strength and the microalgal growth.
2 Materials and methods
2.1 Strains and medium
Wild-type microalgae strains, Scenedesmus sp. DDVG I (accession no. MN630585) and cyanobacterium Limnothrix sp. DDVG II (accession no. MN630310), were isolated from the local waterlogged area of the Indian Institute of Technology Guwahati, Assam, India (26° 11′ N, 91° 41′ E). The strains were cultivated in a modified BG11 medium (pH = 7.4) [31] consisted of the following ingredients (g/ L): NaNO3, 1.5; MgSO4.7H2O, 0.075; K2HPO4, 0.04; CaCl2, 0.036; citric acid, 0.006; ammonium ferric citrate, 0.006; Na2EDTA, 0.001; Na2CO3, 0.02; ZnSO4.7H2O, 0.22; MnCl2.4H2O, 1.81; CuSO4.5H2O, 0.08; Co (NO3)2.6H2O, 0.05; H3BO3, 2.86. The cultures were maintained using Erlenmeyer flasks at 25 °C in an orbital shaker (150 rpm) under a light intensity of 40.5 μmol m−2 s−1.
2.2 Fabrication of substratum
In this study, four different substrata were tested: glass slide (GS), polylactic acid (PLA) sheet, millipore filter membrane (FM), fiber-reinforced plastic (FRP). The substrata were modified with sandpaper Grit-100 to represent rough surfaces, i.e., rough glass slide (rGS), rough polylactic acid (rPLA) sheet, rough filter membrane (rFM), and rough fiber-reinforced plastic (rFRP). The dimension of the substrata was length × breadth = 7 × 2 cm.
2.3 Experimental setup and procedure
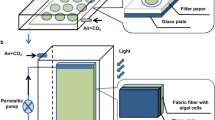
All the cultivations were conducted at 27 ± 1 °C under a light intensity of 40.5 μmol m−2 s−1 with 12 h:12 h (light:dark) under white fluorescent lamps. Scenedesmus sp. DDVG I and Limnothrix sp. DDVG II (initial inoculum of 0.2 g/L in both conditions) were cultivated in 500-mL Erlenmeyer flasks containing 300-mL BG11 media. EPSs produced by both the species were begun to be analyzed periodically after 4 days of the culture growth. Here, the types of EPS produced were named according to days of culture. For instance, EPSs produced on days 4, 8, 12, 16, and 20 were represented as EPS-4, EPS-8, EPS-12, EPS-16, and EPS-20, respectively. For the attached cultivation system, the reactor was set up in jam bottles (total volume = 500 mL) with 300 mL of BG11 medium in different batches. Briefly, the substrata were immersed vertically inside the jam bottles by an initial inoculation of Limnothrix sp. culture. These substrata coated with EPS were removed from the jam bottles periodically after 4 days. Routine analysis of substrata was done to investigate their performance for EPS adhesion. Subsequently, these substrata coated with EPS-4, EPS-8, EPS-12, EPS-16, and EPS-20 were transferred into other jam bottles by an initial inoculation of Scenedesmus sp. culture. The systems were operated for at least 20 days and harvested after reached a steady-state. Scenedesmus sp. biofilm was harvested from the substrata through scrapping. All experiments were performed in triplicates for each system. The overall experimentation is also illustrated in the schematic diagram (Fig. 1)
Schematic diagram of experimentation conducted under three-phases using 500-mL jam bottles at 40.5 μmol m−2 s−1 light intensity with 300-mL BG 11. a Substratum (length × breadth = 7 × 2 cm) is immersed vertically inside the jam bottle with initial inoculation of Limnothrix sp. DDVG II (red dashed arrows represent adhesion of EPS from Limnothrix cells on substratum. b EPS-coated substratum is used for the attached cultivation of microalgae, Scenedesmus sp. DDVG I (black dashed arrow represents an accumulation of cells as biofilm). c Microalgae cultivation in suspension condition
2.4 Analytical method
2.4.1 EPS extraction
Since there is no particular standard protocol for EPS extraction, EPS yield could not have relied on a single extraction method. Hence, two chemical methods i.e. Methods A and B were adopted according to conditions referred by Kawaguchi and Decho [13] and Klock et al. [32] with slight modifications. Method C was also carried out as a control. The EPS extraction procedures are summarized in Fig. 2. Briefly, the cell suspension was treated with different reagents: (Method A) 0.05-mM EDTA for 30 min at 40 °C, (Method B) 4-mM EDTA with 10% NaCl for 15 min at 40 °C. In Method C (control condition), the cell suspension was treated only with distilled water for 20 min at 50 °C. In the separation step, the EPS was separated by ultracentrifugation at 4 °C at different speeds: (Method A) 12,000 rpm for 15 min and followed by precipitation with ice-cold ethanol, (Method B) 10,000 rpm for 20 min and followed by precipitation with ice-cold ethanol, (Method C) 10,000 rpm for 20 min. Furthermore, the extracted EPS was purified using a dialysis membrane (Sigma-Aldrich, USA). Finally, the EPS was lyophilized and stored at − 20 °C for further characterization. The quantification of EPS was done by measuring the initial and final weight after drying in an oven at 70 °C overnight. The EPS with the highest concentration was further qualitatively analyzed for the biochemical composition.
2.4.2 Characterization of the chemical composition of EPS
Total protein content was estimated by the Lowry method [33] using bovine serum albumin (Sigma-Aldrich, USA) as the standard. Polysaccharides were determined by the phenol-sulfuric method using D-glucose (Sigma-Aldrich, USA) as the standard [34]. The monosaccharide composition of the EPS was further analyzed according to the method of Comte et al. [35]. The lyophilized EPS was methanolized in 0.4-mL 2-M HCl in methanol (14 h at 85 °C). It was subsequently hydrolyzed with 2-M trifluoroacetic acid (TFA) in a sealed glass test tube (1 h at 121 °C). Before analysis, the sample was filtered through a 0.22-μm Nylon syringe filter (Axiva, India). The monosaccharide compositions of the hydrolysate were determined by HPLC (Perkin Elmer, Series 200, USA) equipped with an RI detector using a carbohydrate analysis column (Agilent Hi-Plex series, USA) and eluted with the solvent system 0.0008-N H2SO4 at a flow rate of 0.5 mL/min.
The lyophilized EPS was further ground to a powder using a mortar-pestle to identify its functional groups. One milligram of EPS powder was mixed with 180 mg of KBr and compacted to form a pellet. The transmission spectra from 4000 to 500 cm−1 were acquired using an FTIR spectrometer (Shimadzu IRAffinity-1, Japan). Powdered EPS samples were analyzed directly on marked glass slides through a 100× water immersion objective with an exposure time of 100 s.
2.4.3 Physiochemical characterization of substratum
Substrata were screened for their hydrophobicity through contact angle measurements. It was performed using Drop Shape Analyzer (KRUSS DSA25, Germany). The probe liquids used were Milli-Q water and diiodomethane (non-polar solvent). FTIR spectroscopy was performed using an ATR spectrophotometer (Shimadzu IRAffinity-1, Japan). The texture and topography of the surfaces were investigated by atomic force microscope (Agilent 5500 series, Model: Cypher, Oxford) on a quartz substrate in acoustic mode (non-contact) using a silicon cantilever with a force constant of 33 N/m and a resonance frequency of 304 kHz. The roughness of the substrata was analyzed by using WSxM 5.0 Develop software.
2.4.4 Microalgae cell harvest
Biomass of Scenedesmus sp. DDVG I in the biofilm was determined by calculating the difference between the total dry weights of the biomass scrapped off from the substrata and the weight of the EPS. The percentage of adhesion capacity is the amount of microalgal cell attached to the substrata over the total algae biomass produced in suspension which is demonstrated in Eq. (1). Confocal laser scanning microscopy (CLSM) was used to analyze the pattern of Scenedesmus cells developed within the EPS matrix. Images at discrete focal planes (z-stack) were taken using the microscope (JSM-7610F Jeol, USA). Chlorophyll within the cells was excited using an argon laser with a wavelength of 488 nm and emitted fluorescence was observed using a 505-nm long-pass filter and a 20 Kx magnification objective lens.
where Bb is the microalgal biomass accumulated as a biofilm on substratum and Bs is the total biomass produced in the suspension culture condition.
2.5 Statistical analysis
All the quantitative data were represented as mean and standard deviation. The difference in the means of two groups was analyzed by Welch’s two-sample t test. Multiple groups were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s HSD. All the statistical analyses were performed using the R-statistical package. In all the statistical tests, p < 0.05 was considered significant.
3 Results and discussion
3.1 Growth and EPS production
The result in Fig. 3a shows the biomass growth curve of Limnothrix sp. DDVG II over cultivation period of 24 days. Limnothrix sp. achieved its exponential state after day 4 where the biomass concentration increased from 0.32 ± 0.037 to 0.69 ± 0.05 g/L. The maximum biomass concentration reached up to 1.82 ± 0.06 g/L in 10 days. The culture did not show further increase in biomass concentration after 10 days which marked the beginning of the stationary state. Meanwhile, the production of EPS and the yield were determined periodically using three different extraction methods. Results in Fig. 3b show that during the initial 4 days of Limnothrix sp. growth, EPS-4 concentration was very low with the yield of 1.5 ± 0.05 mg/L (for Method A), 0.9 ± 0.02 mg/L (Method B), and 0.72 ± 0.00 mg/L (Method C). The EPS concentration was significantly increased (p < 0.001) over 12 days of growth where the culture Limnothrix sp. reached an early stationary state. The yields of EPS-12 extracted using Methods A, B, and C were 21.5 ± 1.5 mg/L, 21.0 ± 1.1 mg/L, and 18.1 ± 1.0 mg/L, respectively. After day 12, there was an insignificant increase in EPS concentration. Moreno et al. [36] demonstrated that Anabaena sp. could produce 17 mg/L of EPS during the stationary phase. Likewise, Trabelsi et al. [37] also reported that the maximum EPS yield (120 mg/L) from Arthrospira platensis was obtained during the stationary phase. A study by De Philippis and Vincenzini [38] reported that cyanobacterial EPS are the specialized metabolites released by the organism when its growth is limited by different factors such as temperature, nitrogen concentration, or irradiance. Mainly during the late exponential or stationary phase, EPS provides a protective shield for the physiological adaptation to external changes [38]. On the other hand, when we quantitatively analyzed the EPS produced from Scenedesmus sp. DDVG I, the yield was almost negligible.
Biomass growth of a Limnothrix sp. DDVVG II (presented as mean ± standard deviation of triplicate experiments). b EPS yield (mg/ L) extracted during specific cultivation period of Limnothrix sp. DVVG through the different extraction methods. Bars represent the average values over three replicates; error bars depict standard error of the mean (n = 3). Data were analyzed by ANOVA and Tukey’s HSD (***p < 0.001, **p < 0.01, *p < 0.05)
Overall, by comparing the EPS yield, we observed that the extraction methods, Methods A and B, were more efficient when compared with Method C (control condition). However, the yield obtained from Methods A and B did not differ significantly from each other. The reason for higher extraction yield through chemical methods may be influenced by the use of chemical solvents. The metal ions such as NaCl and EDTA from the reagents form strong interchain linkages and chelate divalent ions present within the EPS [35]. They form EPS complexes that loosen EPS from cell surfaces and also reduce the strength of the EPS matrix [39]. The motive of the addition of NaCl in Method B was that the sodium salts such as NaCl, NaOH, and CH3COONa would increase the pH of the system. Hence, the dissociation of acidic groups in EPS would take place and cause repulsion between the negatively charged moieties. Thus, the solubility of EPS might increase in the solvent and facilitate the extraction process [13]. Furthermore, ethanol precipitation and dialysis are common techniques for isolation and purification of the EPS against the solvent [40]. The present study also showed the effective isolation and purification of the polymeric fraction of EPS through ethanol precipitation in combination with dialysis.
3.2 Biochemical composition of EPS
Cyanobacterial EPS are mainly composed of heteropolysaccharides consisting of monomer units like neutral sugars (glucose, galactose, mannose, arabinose, and xylose) and at least one uronic acid [41]. Results from Table 1 clearly show the presence of polysaccharides (489.1–829.4 mg/g) and proteins (17.45–29.36 mg/g) abundantly in the purified EPS. While, the monomer units of the polysaccharides were rich in neutral sugars specifically glucose (15.2–29.74%), galactose (14.61–21.25%), xylose (12.85–24.6%), and uronic acid (0.6–2.5%) (Table 1). The variations in the compositions of polysaccharides, uronic acids, and proteins in the EPS are due to the influence of centrifugation, incubation time, and the solvents (NaCl, EDTA, acetone, ethanol, etc.) [32]. According to Higgins and Novack [42], varieties of protein moieties and hexose sugar present in the EPS matrix play a major role to signify the hydrophobic and hydrophilic behavior of EPS.
3.3 FTIR spectroscopy
The FTIR spectra of EPS are shown in Online Resource 1 and band assignments corresponding to the IR spectra are represented per literature (see Online Resource 2) [35, 43]. The spectral band at 3750–3747 cm−1 attributed to the vibrational stretching of OH– group of the polymeric compounds. The intense band at 3290–3288 cm−1 represents NH– stretching vibrations of amide and amine. The broad absorption peak at 2977–2950 cm−1 was assigned to asymmetrical stretching vibrations of C–H from an aliphatic CH2 group, which reveals the presence of lipids and proteins. Peak intensity at 2877–2870 cm−1 attributed to the asymmetrical stretching of CH3 from the lipids. The peak observed at 2380–2300 cm−1 could be due to the presence of –C=O– group and asymmetrical vibrational stretching of the C=N group. The asymmetrical absorption peak at 1760–1746 cm−1 represents the stretching vibration of C=O of esters. The intense broad peak at 1680–1670 cm−1 corresponds to the stretching vibration of the C–N and deformation vibration of N–H of the amide group. The peak at 1540–1523 cm−1 represents C–H bends and side-chain stretching of C–N of the amide group. The intense peak at 1377 cm−1 reveals the phosphorylated protein. The stretching of C–O–C and C–O at 1236 cm−1 corresponds to the presence of carbohydrates and the peak at 1125–1000 cm−1 confirms the presence of uronic acids (O-acetyl ester linkage bonds). Furthermore, several visible bands obtained at 1000–600 cm−1 correspond to the presence of phosphate and sulfur functional groups. Many studies documented that uronic acids and sulfated sugars in EPS have an affinity towards substratum and support in initial immobilization [34]. Domozych et al. [44] reported the presence of xylose, fucose, and glucuronic acid after analyzing different functional groups in EPS of Penium margaritaceum [44]. It was also confirmed that the presence of varieties of biochemical contents in EPS could aid in forming a cross-bridge between cells and the solid surface.
3.4 FESEM and EDX analysis
The morphology and the major elemental composition of EPS were analyzed through FESEM and EDX respectively. The EPS was observed as ribbon, cross-linking, and mesh-like structures because of the richness in polysaccharides (Online Resource 3 (a)). The observed morphology of EPS was comparable to the typical structure of EPS obtained from cyanobacteria and bacteria (such as Gluconacetobacter xylinus) [45, 46]. A high distribution of carbon (C) and oxygen (O) was detected in the EPS. A mixture of minerals such as calcium (Ca), phosphorus (P), sodium (Na), and sulfur (S) was detected in low quantities. Silicon (Si), magnesium (Mg), chloride (Cl), and other trace minerals namely sulfur (S) were also detected in the EPS (Online Resource 3 (b)). According to Rossi and De Philippis [47], the distribution of cations such as Na and Ca in the EPS enhances its binding capacity with the negative charges of the sulfate groups. The advantage of having elements in EPS is that EPS could provide a mini-environment by harboring these elements as mineral salt which is essential for the growth and development of microalgae [47].
3.5 Evaluation of the appropriate substratum
3.5.1 Surface hydrophobicity and texture
The physicochemical properties of the substrata including water and diiodomethane contact angle, the free surface energy of the substrata, and the textures are summarized in Table 2. The static water contact angle, θ of rPLA, PLA rGS, GS, FRP, and rFRP were measured as 53.0, 67.4, 20.7, 72, and 81.3, respectively, showing slightly hydrophilic, while of FM showed a higher θ of 122 being more hydrophobic. [48]. Ji et al. [49] observed that attachment of Pseudochlorococcum was stronger on the hydrophilic fiberglass than the hydrophobic surfaces. However, Sekar et al. [40] observed that hydrophobic surfaces favor higher attachment of bacterial EPS and microbial cells, which is mediated by the water exclusion mechanism. In this study, all the substrata were prone to the colonization of EPS, resulting in a significant accumulation of EPS (p < 0.001) (Fig. 4). Furthermore, the result in Table 2 shows the surface energy of the substrata to be in the range of 39.3–70.7 mJ/m2. Gross et al. [25] suggested that substrata like metals, plastics, rubbers, having surface energy in the range of 20–60 mJ/m2 provide a site for adhesion of microalgae or cyanobacterial EPS.
Aerial EPS productivity (mg/m2/day) developed on different substrata due to adhesion of EPS produced from Limnothrix sp. over 20 days of the culture growth. Bars represent the average values over three replicates; error bars depict standard error of the mean (n = 3). Data were analyzed by ANOVA and Tukey’s HSD (***p < 0.001, **p < 0.01, *p < 0.05)
Results in Fig. 5 demonstrate the formation of micropatterns over the substrata after modification with sandpaper grit-200 when compared to normal substrata. The surface of rPLA, PLA, and rFRP became more irregular that their roughness increased to 82 ± 25 nm, 79 ± 21 nm, and 72 ± 19 nm, respectively. However, the roughness of FM was unable to be analyzed as the surface roughness was beyond the detection limit. According to Characklis et al. [51], rough surfaces favor more attachment of EPS because of the increased convection associated with rough surfaces.
3.5.2 FTIR of substrata
FTIR spectra of the normal and modified substrata are represented in Online Resource 4 and the spectral bands have been assigned according to documented literature [52, 53]. FTIR spectra indicated the presence of carboxyl, hydroxyl, and carbonyl groups on the surface of the substrata spread from the 400 to 4000-cm−1 regions. We observed that there was no significant difference in peak distribution of normal and rough substrata. The intense peak at 905 cm−1 and 740–770 cm−1 was attributed to Si–OH and silicon oxides, respectively. In PLA, the peaks at 1747 cm−1, 1180 cm−1, and 1080 cm−1 confirmed the presence of C–O bond, C–O–C bond, and C–C–(O)–O bond, respectively. FRP also showed the presence of carbonyl group (C=O bond) at 1713 cm−1, methyl group at 1381 cm−1, and hydroxyl group (O–H bond) at 3518 cm−1. These functional groups (carboxylic, O-acetyl, O-methyl) have binding potential with the water and small ionic compounds (salts) present in the media and form a layer of ions known as the “conditioning layer.” Thereby, this layer helps in the initial adhesion of the EPS and microalgae [54].
3.6 Effect of substrata on EPS adhesion
The substrata immobilized with EPS derived on different culture periods represented as EPS-4, EPS-8, EPS-12, EPS-16, and EPS-20. The EPS-4 which was secreted during the initial four days of Limnothrix sp. growth was very less, thus the yields of EPS-4 accumulated on the substrata were not reported. The substratum was covered with a thin film of EPS-12 significantly (p < 0.001) over the 12 days. Among the substrata, the rough substrata showed higher adhesion for EPS-12 than the normal one. rPLA with EPS-12 showed the highest aerial EPS yield (2.25 ± 0.02 g/m2/day) followed by rFRP (2.24 ± 0.02 g/m2/day), rGS (2.08 ± 0.04 g/m2/day), and FM (2.01 ± 0.1 g/m2/day). Figure 6a also confirms the proper immobilization of EPS-12 on the rPLA with thickness ~ 2 μm. However, all the substrata showed an insignificant increase (p > 0.3) in coverage of aerial yield of EPS-14, EPS-16, and EPS-20 over 20 days of operation. Overall, the findings showed that rough substrata were ideal for adhesion. Gross et al. [50] found a rough surface to be more suitable for biofilm formation than normal substrata. Characklis et al. [43] found the increased biofilm formation on rough surfaces due to the increase in the surface area for the attachment. Moreover, we observed that EPS-12 produced during the early stationary phase of the culture showed good adhesion strength on substrata. We have discussed earlier in the previous section about the correlation between the biochemical composition of EPS and its adhesion properties. Characklis et al. [51] found deterioration in adhesion strength of EPS with an increase in culture age [51].
Confocal Z-stack images. a EPS colonization on rPLA with a thickness of ~2 μm. b Accumulation of Scenedesmus cells on rPLA with EPS-12. Red fluorescence is emitted from the chlorophyll content of algal biomass that is distributed over the rPLA with thickness of ~10 μm (excitation and emission wavelength of 488 and 505 nm, respectively)
3.7 Microalgal accumulation on substrata
Results in Table 3 show that rough substrata coated with EPS-12 exhibit a high adhesion rate of Scenedesmus cells. The accumulation of Scenedesmus cells on different substrata is also illustrated in Online Resource 5. Among the substrata, rPLA with EPS-12 showed the highest adhesion capacity (94.6 ± 4.2%) for microalgae accumulation with maximum biomass productivity of 31.6 ± 1.2 g/m2/day (p < 0.001). The pattern of biomass accumulated over rPLA coated with EPS-12 was observed through confocal microscopy. In Fig. 6b, we observe red fluorescence with a thickness of ~10 μm developed over the substratum. The red fluorescence was emitted by the chlorophyll content of the microalgal biomass, which showed proper accumulation of cells. EPS-12-coated rFRP, rGS, and FM showed the adhesion capacity of 87.6 ± 2.8%, 79.28 ± 2.8%, and 88.9 ± 2.2%, respectively, achieving algal biomass productivity of 30.9 ± 1.20 g/m2/day, 29.8 ± 1.10 g/m2/day, and 27.6 ± 0.90 g/m2/day, respectively. However, all the substrata without EPS showed a negligible amount of Scenedesmus sp. cell accumulation. The adhesion capacity and the biomass productivity for the substrata without EPS were significantly very low (p < 0.0001). Even the aerial biomass productivity accumulated over rPLA with EPS-4 was higher (1.20 ± 0.02 g/m2/day) significantly (p < 0.02) when compared with aerial biomass productivity (0.90 ± 0.00 g/m2/day) over rPLA without EPS (Table 3). This suggested that the presence of EPS as a film on substrata induced the microalgal attachment. It was also confirmed that Scenedesmus sp. DDVG I is a type of species that does not secrete its EPS but it somehow can grow on substrata with the aid of EPS derived from other sources. In suspension cultivation, Scenedesmus sp. cells did not show adhesion even on the jam bottles over 20 days of cultivation. The culture in the suspension medium took more than 36 h to settle down. Hence, the biomass was harvested through centrifugation, resulting in biomass productivity of 22 ± 1.01 g/L/day. According to documented literature, the adhesion strength of EPS is not dependent on the amount of EPS; rather, the age of EPS and its varieties of biochemical composition play a major role in adhesion (EPS [55]). In another study, Wagner et al. [56] reported that with increasing EPS age, the chemical diversity of EPS changes tremendously. The mesh-like inter-crossed structure of EPS provides the sites for entrapment of microalgae cells, and thereby the biomass accumulation occurs. In this study, 12-day-old EPS showed the highest potential of microalgae adhesion. However, some studies reported that the EPS content does not influence adhesion [12]. There is no good reason to believe that adhesive properties and the attraction of microbial cells are related. These conflicting results may be explained by the complexity of EPS and its structure [57]. Overall, rPLA would be ideal for attached cultivation and its performance is also compared with the documented literatures in Table 4.
4 Conclusions
The present study established an ideal technique for the cultivation of Scenedesmus sp. in a cost-effective way aiming at easy harvesting. This work demonstrated the possibilities of growing non-adhesive microalgal as a biofilm on substrata coated with EPS. The study reported an inclusive study for the extraction of EPS during different stages of the cyanobacterial growth and the effect of the physicochemical properties on the initial colonization. Chemical extraction was found to be a good method for quantitative and qualitative analysis of EPS. Rough substrata showed to be effective for the adhesion of EPS. Among them, rPLA coated with EPS-12 showed the maximum algal accumulation of 31.6 ± 1.2 g/m2/day. For scaling up the system, we should need to emphasize on the total cost. The total capital cost for all the substrata in this study did not exceed $30. They are easily available in the local market. Moreover, the substrata can be either reused several times after scrapping off the biofilm.
References
Mahesh R, Naira VR, Maiti SK (2019) Concomitant production of fatty acid methyl ester (biodiesel) and exopolysaccharides using efficient harvesting technology in flat panel photobioreactor with special sparging system via Scenedesmus abundans. Bioresour Technol 278:231–241. https://doi.org/10.1016/J.BIORTECH.2019.01.091
Parmar A, Singh NK, Pandey A, Gnansounou E, Madamwar D (2011) Cyanobacteria and microalgae: a positive prospect for biofuels. Bioresour Technol 102:10163–10172. https://doi.org/10.1016/j.biortech.2011.08.030
Tsavatopoulou VD, Aravantinou AF, Manariotis ID (2019) Biofuel conversion of Chlorococcum sp. and Scenedesmus sp. biomass by one- and two-step transesterification. Biomass Convers Biorefinery 1–9. https://doi.org/10.1007/s13399-019-00541-y
Azam R, Kothari R, Singh HM, Ahmad S, Ashokkumar V, Tyagi VV (2020) Production of algal biomass for its biochemical profile using slaughterhouse wastewater for treatment under axenic conditions. Bioresour Technol 306:123116. https://doi.org/10.1016/j.biortech.2020.123116
Kothari R, Ahmad S, Pathak V V et al (2019) Algal-based biofuel generation through flue gas and wastewater utilization: a sustainable prospective approach. Biomass Convers. Biorefinery 1–24
Kusmayadi A, Leong YK, Yen H-W, Huang CY, Chang JS (2021) Microalgae as sustainable food and feed sources for animals and humans – biotechnological and environmental aspects. Chemosphere 129800. https://doi.org/10.1016/j.chemosphere.2021.129800
Hu J, Nagarajan D, Zhang Q, Chang JS, Lee DJ (2018) Heterotrophic cultivation of microalgae for pigment production: a review. Biotechnol Adv 36:54–67
Rajendran A, Hu B (2016) Mycoalgae biofilm: development of a novel platform technology using algae and fungal cultures. Biotechnol Biofuels 9:112. https://doi.org/10.1186/s13068-016-0533-y
Christenson LB, Sims RC (2012) Rotating algal biofilm reactor and spool harvester for wastewater treatment with biofuels by-products. Biotechnol Bioeng 109:1674–1684. https://doi.org/10.1002/bit.24451
Ozkan A, Kinney K, Katz L, Berberoglu H (2012) Reduction of water and energy requirement of algae cultivation using an algae biofilm photobioreactor. Bioresour Technol 114:542–548. https://doi.org/10.1016/j.biortech.2012.03.055
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Flemming HC, Neu TR, Wozniak DJ (2007) The EPS matrix: the “House of Biofilm Cells”. J Bacteriol 189:7945–7947. https://doi.org/10.1128/JB.00858-07
Kawaguchi T, Decho AW (2000) Biochemical characterization of cyanobacterial extracellular polymers (EPS) from modern marine stromatolites (Bahamas). Prep Biochem Biotechnol 30:321–330. https://doi.org/10.1080/10826060008544971
Silipo A, Molinaro A, Molteni M, Rossetti C, Parrilli M, Lanzetta R (2010) Full structural characterization of an extracellular polysaccharide produced by the freshwater cyanobacterium oscillatoria planktothrix FP1. Eur J Org Chem 2010:5594–5600. https://doi.org/10.1002/ejoc.201000749
Berner F, Heimann K, Sheehan M (2015) Microalgal biofilms for biomass production. J Appl Phycol 27:1793–1804. https://doi.org/10.1007/s10811-014-0489-x
Gaignard C, Laroche C, Pierre G, Dubessay P, Delattre C, Gardarin C, Gourvil P, Probert I, Dubuffet A, Michaud P (2019) Screening of marine microalgae: investigation of new exopolysaccharide producers. Algal Res 44:101711. https://doi.org/10.1016/j.algal.2019.101711
Shen Y, Xu X, Zhao Y, Lin X (2014) Influence of algae species, substrata and culture conditions on attached microalgal culture. Bioprocess Biosyst Eng 37:441–450. https://doi.org/10.1007/s00449-013-1011-6
Yuan H, Zhang X, Jiang Z, Chen X, Zhang X (2019) Quantitative criterion to predict cell adhesion by identifying dominant interaction between microorganisms and abiotic surfaces. Langmuir 35:3524–3533. https://doi.org/10.1021/acs.langmuir.8b03465
Zhang Q, Yu Z, Jin S, Liu C, Li Y, Guo D, Hu M, Ruan R, Liu Y (2020) Role of surface roughness in the algal short-term cell adhesion and long-term biofilm cultivation under dynamic flow condition. Algal Res 46:101787. https://doi.org/10.1016/j.algal.2019.101787
Roostaei J, Zhang Y, Gopalakrishnan K, Ochocki AJ (2018) Mixotrophic microalgae biofilm: a novel algae cultivation strategy for improved productivity and cost-efficiency of biofuel feedstock production. Sci Rep 8. https://doi.org/10.1038/s41598-018-31016-1
Wang ZW, Liu Y, Tay JH (2005) Distribution of EPS and cell surface hydrophobicity in aerobic granules. Appl Microbiol Biotechnol 69:469–473. https://doi.org/10.1007/s00253-005-1991-5
Ozkan A, Berberoglu H (2013) Physico-chemical surface properties of microalgae. Colloids Surf B: Biointerfaces 112:287–293. https://doi.org/10.1016/j.colsurfb.2013.08.001
Genin SN, Stewart Aitchison J, Grant Allen D (2014) Design of algal film photobioreactors: material surface energy effects on algal film productivity, colonization and lipid content. Bioresour Technol 155:136–143. https://doi.org/10.1016/j.biortech.2013.12.060
Johnson MB, Wen Z (2010) Development of an attached microalgal growth system for biofuel production. Appl Microbiol Biotechnol 85:525–534. https://doi.org/10.1007/s00253-009-2133-2
Gross M, Henry W, Michael C, Wen Z (2013) Development of a rotating algal biofilm growth system for attached microalgae growth with in situ biomass harvest. Bioresour Technol 150:195–201. https://doi.org/10.1016/j.biortech.2013.10.016
Zhang Y, Ji C, Zhou W, Wang H, Wang J, Liu T (2020) Studies on the attached cultivation of filamentous oleaginous microalga Tribonema minus. J Ocean Univ China 19:691–699. https://doi.org/10.1007/s11802-020-4256-0
Zhang Q, Liu C, Li Y, Yu Z, Chen Z, Ye T, Wang X, Hu Z, Liu S, Xiao B, Jin S (2017) Cultivation of algal biofilm using different lignocellulosic materials as carriers. Biotechnol Biofuels 10:115. https://doi.org/10.1186/s13068-017-0799-8
Jazzar S, Quesada-Medina J, Olivares-Carrillo P, Marzouki MN, Acién-Fernández FG, Fernández-Sevilla JM, Molina-Grima E, Smaali I (2015) A whole biodiesel conversion process combining isolation, cultivation and in situ supercritical methanol transesterification of native microalgae. Bioresour Technol 190:281–288. https://doi.org/10.1016/j.biortech.2015.04.097
Patidar SK, Kim SH, Kim JH, Park J, Park BS, Han MS (2018) Pelagibaca bermudensis promotes biofuel competence of Tetraselmis striata in a broad range of abiotic stressors: dynamics of quorum-sensing precursors and strategic improvement in lipid productivity. Biotechnol Biofuels 11:102. https://doi.org/10.1186/s13068-018-1097-9
Difusa A, Talukdar J, Kalita MC, Mohanty K, Goud VV (2015) Effect of light intensity and pH condition on the growth, biomass and lipid content of microalgae Scenedesmus species. Biofuels 6:37–44. https://doi.org/10.1080/17597269.2015.1045274
Srivastava G, Nishchal, Goud VV (2017) Salinity induced lipid production in microalgae and cluster analysis (ICCB 16-BR_047). Bioresour Technol 242:244–252. https://doi.org/10.1016/j.biortech.2017.03.175
Klock JH, Wieland A, Seifert R, Michaelis W (2007) Extracellular polymeric substances (EPS) from cyanobacterial mats: characterisation and isolation method optimisation. Mar Biol 152:1077–1085. https://doi.org/10.1007/s00227-007-0754-5
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (2000) Total protein estimation by Lowry’s method. Exp BL 301:1–3
Dubois M, Gilles KA, Hamilton JK et al (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Comte S, Guibaud G, Baudu M (2006) Relations between extraction protocols for activated sludge extracellular polymeric substances (EPS) and EPS complexation properties: Part I. Comparison of the efficiency of eight EPS extraction methods. Enzym Microb Technol 38:237–245. https://doi.org/10.1016/j.enzmictec.2005.06.016
Moreno J, Vargas MA, Olivares H, Rivas J́, Guerrero MG (1998) Exopolysaccharide production by the cyanobacterium Anabaena sp. ATCC 33047 in batch and continuous culture. J Biotechnol 60:175–182. https://doi.org/10.1016/S0168-1656(98)00003-0
Trabelsi L, M’sakni NH, Ben OH et al (2009) Partial characterization of extracellular polysaccharides produced by cyanobacterium Arthrospira platensis. Biotechnol Bioprocess Eng 14:27–31. https://doi.org/10.1007/s12257-008-0102-8
De Philippis R, Vincenzini M (1998) Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol Rev 22:151–175. https://doi.org/10.1016/S0168-6445(98)00012-6
Liu H, Fang HHP (2002) Extraction of extracellular polymeric substances (EPS) of sludges. J Biotechnol 95:249–256. https://doi.org/10.1016/S0168-1656(02)00025-1
Underwood GJC, Paterson DM, Parkes RJ (1995) The measurement of microbial carbohydrate exopolymers from intertidal sediments. Limnol Oceanogr 40:1243–1253. https://doi.org/10.4319/lo.1995.40.7.1243
Parikh A, Madamwar D (2006) Partial characterization of extracellular polysaccharides from cyanobacteria. Bioresour Technol 97:1822–1827. https://doi.org/10.1016/j.biortech.2005.09.008
Higgins MJ, Novak JT (1997) Characterization of exocellular protein and its role in bioflocculation. J Environ Eng 123:479–485. https://doi.org/10.1061/(ASCE)0733-9372(1997)123:5(479)
Allen MS, Welch KT, Prebyl BS, Baker DC, Meyers AJ, Sayler GS (2004) Analysis and glycosyl composition of the exopolysaccharide isolated from the floc-forming wastewater bacterium Thauera sp. MZ1T. Environ Microbiol 6:780–790. https://doi.org/10.1111/j.1462-2920.2004.00615.x
Domozych DS, Kort S, Benton S, Yu T (2005) The extracellular polymeric substance of the green alga Penium margaritaceum and its role in biofilm formation. Biofilms 2:129–144. https://doi.org/10.1017/S147905050500181X
Coutinho ML, Miller AZ, Gutierrez-Patricio S, Hernandez-Marine M, Gomez-Bolea A, Rogerio-Candelera MA, Philips AJL, Jurado V, Saiz-Jimenez C, Macedo MF (2013) Microbial communities on deteriorated artistic tiles from Pena National Palace (Sintra, Portugal). Int Biodeterior Biodegrad 84:322–332. https://doi.org/10.1016/j.ibiod.2012.05.028
Fang L, Catchmark JM (2014) Characterization of water-soluble exopolysaccharides from Gluconacetobacter xylinus and their impacts on bacterial cellulose crystallization and ribbon assembly. Cellulose 21:3965–3978. https://doi.org/10.1007/s10570-014-0443-8
Rossi F, De Philippis R (2015) Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life 5:1218–1238. https://doi.org/10.3390/life5021218
Arima Y, Iwata H (2007) Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 28:3074–3082. https://doi.org/10.1016/j.biomaterials.2007.03.013
Ji B, Zhang W, Zhang N, Wang J, Lutzu GA, Liu T (2014) Biofilm cultivation of the oleaginous microalgae Pseudochlorococcum sp. Bioprocess Biosyst Eng 37:1369–1375. https://doi.org/10.1007/s00449-013-1109-x
Gross M, Zhao X, Mascarenhas V, Wen Z (2016) Effects of the surface physico-chemical properties and the surface textures on the initial colonization and the attached growth in algal biofilm. Biotechnol Biofuels 9:38. https://doi.org/10.1186/s13068-016-0451-z
Characklis W, McFeters G, Marshall K (1990) Physiological ecology in biofilm systems. In: Characklis WG, Marshall KC, editors. Biofilms. Biofilms 341–394
Hwang T, Park SJ, Oh YK, Rashid N, Han JI (2013) Harvesting of Chlorella sp. KR-1 using a cross-flow membrane filtration system equipped with an anti-fouling membrane. Bioresour Technol 139:379–382. https://doi.org/10.1016/j.biortech.2013.03.149
Yeom CK, Lee KH (1996) Pervaporation separation of water-acetic acid mixtures through poly(vinyl alcohol) membranes crosslinked with glutaraldehyde. J Membr Sci 109:257–265. https://doi.org/10.1016/0376-7388(95)00196-4
Khan MF, Luong N, Kurian J, Brook MA (2017) Superwetting comonomers reduce adhesion of E. coli BL21. Chem Commun 53:3050–3053. https://doi.org/10.1039/c6cc09984j
Ohashi A, Harada H (1996) A novel concept for evaluation of biofilm adhesion strength by applying tensile force and shear force. Water Sci Technol 34:201–211. https://doi.org/10.1016/0273-1223(96)00647-6
Wagner M, Ivleva NP, Haisch C, Niessner R, Horn H (2009) Combined use of confocal laser scanning microscopy (CLSM) and Raman microscopy (RM): investigations on EPS - Matrix. Water Res 43:63–76. https://doi.org/10.1016/j.watres.2008.10.034
Ahimou F, Semmens MJ, Haugstad G, Novak PJ (2007) Effect of protein, polysaccharide, and oxygen concentration profiles on biofilm cohesiveness. Appl Environ Microbiol 73:2905–2910. https://doi.org/10.1128/AEM.02420-06
Acknowledgements
The authors would like to thank the Centre for Energy, Indian Institute of Technology Guwahati for providing research laboratory facilities. The authors are also grateful to the Department of Chemical Engineering, CoE-SuSPOL, Department of Chemistry and Department of Bioscience and Bioengineering, and Indian Institute of Technology Guwahati for providing analytical facilities. The authors would also like to thank the Ministry of Education, Government of India for the financial assistantship.
Author information
Authors and Affiliations
Contributions
Nongmaithem Debeni Devi: investigation, methodology, formal analysis, resources, data curation, writing - original draft, writing - review and editing, visualization. Rahul Tiwari: methodology, formal analysis, investigation, writing - original draft. Vaibhav V. Goud: supervision, project administration, funding acquisition, conceptualization, investigation, data curation, writing - review and editing, validation. All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 5785 kb)
Rights and permissions
About this article
Cite this article
Devi, N.D., Tiwari, R. & Goud, V.V. Cultivating Scenedesmus sp. on substrata coated with cyanobacterial-derived extracellular polymeric substances for enhanced biomass productivity: a novel harvesting approach. Biomass Conv. Bioref. 13, 2971–2983 (2023). https://doi.org/10.1007/s13399-021-01432-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01432-x