Abstract
The objective of this study was to understand and optimize the formation of microalgae biofilms in specific culture conditions. Firstly, the adhesion of six freshwater algae species was compared. Chlorococcum sp. was selected because of the high adhesion biomass productivity (ABP) and adhesion rate achieved. Secondly, the adhesion of Chlorococcum sp. was compared with nine commonly used supporting materials, and glass fiber-reinforced plastic proved to be the optimal substrata. Thirdly, based on response surface methodology experiments, a second-order polynomial model was developed to examine the effect of culture period, initial total nitrogen concentration (ITNC) in manure wastewater, pH and culture volume of the growth chamber on the adhesion of Chlorococcum sp. using glass fiber-reinforced plastic. The experimental and modeling results showed that ITNC, pH and culture volume as well as the interactions between culture period and ITNC, culture period and culture volume were significant on ABP. Optimum culture conditions were predicted at a culture period of 11 days, ITNC of 70 mg L−1, pH of 8 and culture volume of 340 mL, under which the predicted maximum ABP was 4.26 g m−2 day−1. The prediction was close to validation experimental results, indicating that the model could be used to guide and optimize the attached culture of Chlorococcum sp. using glass fiber-reinforced plastic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are capable of producing high-valued products, such as food supplement docosahexaenoic acid, lutein, β-carotene, astaxanthin, pharmacy products and biofuels [1]. The advantages of high productivity potential, less competition with food production and less negative impact on the environment when compared with other biomass feedstock options make microalgae one of the most promising sources for the potential products, even for biofuels [2–4]. However, the conventional culture systems, such as open-pond and tubular photobioreactors containing over 99 % water and less than 1 % solids, make microalgae mass culture for biofuels neither economically viable nor sustainable from both energy and water supply standpoints [5–7].
Since many microorganisms have a natural tendency to attach to surfaces and grow on them [8], attached microalgal culture was developed. The principle is to form a thin layer of ‘algal film’ on the substrata with a small volume of culture medium, which is supplied to the supporting matrix materials to provide the nutrients and moisture [9]. The attached culture system could easily reduce the cost in microalgal harvesting and potentially increase the biomass productivity [9, 10].
The algal-biofilm formation characteristics depend on many factors, including microalgae species; culture conditions, such as the nitrogen concentration, CO2 supply, pH level, light intensity, culture density and culture age; interfacial and physiological factors [9, 11–13]. A few algae species (e.g., Scenedesmus dimorphus, Nitzschia amphibia, Chlorella vulgaris, Chroococcus minutus, Botrycoccus braunii and Nannochloropsis oculata) and various supporting materials (e.g., polyurethane, polystyrene, polyethylene, stainless steel, titanium and glass wool) have been investigated in attached microalgal culture [9, 11–15]. For example, Sekar et al. [11] studied the adhesion of C. vulgaris, N. amphibia and C. minutus to hydrophobic (perspex, titanium and stainless steel 316-L), hydrophilic (glass) and toxic (copper, aluminum brass and admiralty brass) substrata. All three organisms attached more on titanium and stainless steel and less on copper and its alloys. The results also indicated that the attachment was higher on rough surfaces when compared with smooth surfaces [11]. Johnson and Wen investigated the use of Chlorella sp. biofilms to produce biofuels. The algae were cultivated on polystyrene foam immersed in dairy manure wastewater and agitated with a rocking shaker. The authors reported biomass yield as large as 25.65 g m−2 and biomass productivity of 2.57 g m−2 day−1 [13].
The objective of this study was to understand and optimize the formation of microalgae biofilms. The adhesion of six freshwater algae species was compared to select the optimal algal species for attached culture. And then, the selected alga Chlorococcum sp. was cultivated using nine different substrata to compare the adhesion biomass productivity (ABP) and adhesion rate achieved. Response surface methodology (RSM) is a general linear or quadratic model in which attention is focused on the characteristics of the fit response function, in particular, where optimum estimate response values occur. In this study, second-order polynomial models were developed using RSM to investigate the influence and interaction of culture period, initial total nitrogen concentration (ITNC), pH and culture volume on ABP of the attached culture of Chlorococcum sp.
Materials and methods
Algal strain and subculture
The algae Scenedesmus dimorphus (UTEX 417) and Chlorella protothecoides (UTEX 1806) were obtained from the University of Texas at Austin Culture Collection of Algae (Austin, TX). The algae C. vulgaris (FACHB-31), Scenedesmus obliqnus (FACHB-416) and S. dimorphus (FACHB-496) were obtained from the Freshwater Algae Culture Collection of the Institute of Hydrobiology (Wuhan, China). Chlorococcum sp. was donated by Dr. Wenqiao Yuan from North Carolina State University (Raleigh, NC). The six freshwater algae species were maintained in Modified Basal medium, which contained 1,000 mg L−1 urea, 1,250 mg L−1 KH2PO4, 1,000 mg L−1 MgSO4·7H2O, 500 mg L−1 EDTA, 114.2 mg L−1 H3BO3, 111 mg L−1 CaCl2·2H2O, 49.8 mg L−1 FeSO4·7H2O, 88.2 mg L−1 ZnSO4·7H2O, 14.2 mg L−1 MnCl2·4H2O, 15.7 mg L−1 CuSO4·5H2O and 4.9 mg L−1 Co(NO3)2·6H2O (1,000 mg L−1 Urea was used instead of 1,250 mg L−1KNO3, and MoO3 and ethylenediaminetetraacetic acid were not included in the Modified Basal medium) [16]. The medium was adjusted to pH 7 and autoclaved at 121 °C for 15 min. The inocula were grown in 250-mL Erlenmeyer flasks, each containing 120 mL of medium, and incubated at 26 ± 2 °C in an orbital shaker set to 125 rpm. The illumination was provided by 18-W cool white fluorescent light at 60–80 μmol photons m−2 s−1 measured with a Victor 1010A. The inoculation culture was used for experiments once algal cell concentration reached 106 cells mL−1.
Comparison of the adhesion of six microalgae species
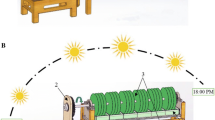
The experiments were carried out in three steps. In the first step of the experiments, the adhesion of six freshwater algae was compared using stainless steel sheet under the attached culture system as shown in Fig. 1. The supporting material was cut into a 10 × 19 cm piece and fixed at the bottom of a growth chamber. The chamber was incubated with 10 % (v/v) sub-cultured cells. Modified Basal medium was used. The growth chamber was placed on a rocking shaker and continuously illuminated with cool white fluorescent light at 100 μmol photons m−2 s−1. The rocking shaker provided a smooth gentle rocking motion at 12° from the horizontal plane at approximately eight revolutions per minute. The surface of the supporting material was alternatively submerged into the culture medium that provided nutrients for algal growth and exposed to illumination that provided light for algal photosynthesis. The culture temperature was 26 ± 2 °C. The culture lasted for 15 days, and distilled water was added once every day to compensate the evaporation.
Selection of the optimal supporting material
To choose the appropriate supporting material for attached microalgal culture, nine materials were compared in the second step of the experiments, including glass fiber-reinforced plastic (1-mm thick, Anping Chunsheng Hardware Mesh Co., Ltd.), plastic film (1-mm thick, Shandong Taikang Biodegradable Packing Materials Co., Ltd.), silicone film (1-mm thick, Guangzhou Clean and Simple Cleaning Products Co., Ltd.), Maifan stone sheet (2-mm thick, Yantai Jiaze International Trading Co., Ltd.), polyethylene foam (3-mm thick, Ningbo Mylon Rubber and Plastic Co., Ltd.), frosted glass (2-mm thick, Qingdao AEON Glass Co., Ltd.), polyurethane sheet (1-mm thick, Zibo Saitong Polyurethane Co., Ltd.), stainless steel sheet (1-mm thick, Tianjin Xinao Hongye Steel Sales Co., Ltd.) and polycarbonate sheet (1-mm thick, Guangdong Guoweixing Plastic Technology Co., Ltd.). All the materials are easily obtained from the local market and can also be purchased through vendors. The materials selected basically followed three rules: (1) hydrophobic property—although not universal, a general trend is that a higher adhesion density is observed over a hydrophobic surface [17–19]; (2) easy collection—avoiding materials with porous surface, such as loofah sponge, polyurethane foam and nylon sponge. Substantial algae may grow inside the pores, which makes harvesting of the biomass from these materials very difficult [13]; (3) durability—it is important to maintain the rigid structure during the growth and harvest cycle. The experiments were duplicated and carried out with the same conditions as in the first step of the experiments.
Response surface methodology (RSM) and validation experiments
RSM is widely used in the optimization design of multi-parameter multi-level experiments [20, 21]. In the third step of the experiments, the central composite design (CCD) of RSM was introduced to analyze the effect of culture period, initial total nitrogen concentration (ITNC), pH and culture volume on adhesion biomass productivity (ABP) of Chlorococcum sp. using glass fiber-reinforced plastic. Pre-treated manure wastewater was used as culture medium. The manure wastewater was obtained from a piggery farm close to Fuzhou University (Fuzhou, Fujian, China). The wastewater was centrifuged to remove any large solid particles and then autoclaved at 121 °C for 15 min to avoid any contaminations from live microorganisms. The total nitrogen and phosphorus concentration of the autoclaved wastewater was around 70 ± 0.2 and 4.7 ± 0.1 mg L−1, respectively. To investigate the optimal ITNC level for the best adhesion of microalgae, diluted wastewater was carried out by adding adequate distilled water into the autoclaved wastewater. The same batch of wastewater was used throughout the experiments. The wastewater was stored at −20 °C and immediately used for algal culture after thawing and warming to room temperature.
Table 1 shows the small-scale CCD that has 21 experiments, including eight fractional factorial designs (24−1), eight star points (2 × 4) and five replicates at center points. Based on the experience regarding algal growth [22], the culture period was chosen from 7 (early exponential growth period) to 19 days (stationary growth period) with a center point of 13 days (exponential growth period). ITNC ranged from 30 to 70 mg L−1 with a center point of 50 mg L−1, which was in accordance with the optimal total nitrogen concentration of manure wastewater for algae culture in most of the researches [22–24]. pH was selected from 6 to 9 with a center point of 7.5, because weakly alkaline media were preferred by most of the microalgae species for either growth or flocculation [25, 26]. Since the light contact area of each chamber was the same, culture volume was chosen from 200 mL (33 %) to 500 mL (100 %) with a center point of 350 mL (50 %) based on the submerging level of the substrata in the maximum dip angle. According to spherical design of the response surface, the parameter of α was chosen to be 2. The RSM and validation experiments were duplicated under the same conditions.
Analysis
Biomass dry weight (DW) of suspended algae (DW1) was measured by filtering aliquot algal sample on pre-weighed glass fiber-filter paper with pore size of 0.45 μm (Q/IEFJ01-1997, Xingya purification material factory, Shanghai, China). The filters were then dried at 105 °C in an oven for 4 h. Algal biomass DW was determined by the difference between the two weights. The adhesion biomass was collected by scraping the attached cells from the supporting material surface and then re-suspending with distilled water to determine the biomass DW (DW2). The calculations of ABP and adhesion rate are presented in Eqs. 1 and 2.
where DW2 and V 2 are the biomass dry weight (g L−1) and volume (l) of the re-suspended algal solution from the attached algae; A is the area (0.019 m2) of the supporting material; DW1 and V 1 are the biomass DW (g L−1) and volume (l) of the suspended algae.
pH was measured using pH paper (Q/GHSC 1544-2009, Shanghai SSS reagent CO., LTD.), once every day and adjusted with 0.5 M HCl or NaOH solution to maintain the objective pH value. The total nitrogen and phosphorus concentrations were measured using the alkaline potassium persulfate digestion–UV spectrophotometric method and ammonium molybdate spectrophotometric method, respectively [27, 28]. Both methods were carried out with a Mi-parameter meter (5H-3BA, Lian-hua Tech. Co., Ltd, China).
The first-order and second-order response surface models, which are commonly used in fitting RSM experimental data [20, 21], were developed to study the effects of culture period, ITNC, pH and culture volume and their interactions on ABP. Because ABPs fit the second-order model (R 2 = 0.95) much better than the first-order model, the first-order regression results were not reported. A second-order polynomial equation was developed to investigate the effect of independent variables in terms of linear, quadratic and interactions as in the following equation:
where Y is ABP (g m−2 day−1); X 0 stands for the model intercept; X 1, X 2, X 3 and X 4 are the levels of culture period, ITNC, pH and culture volume, respectively; a i …a ij are the regression coefficients. The P value of each term was determined to remove insignificant terms. The analysis of variance was carried out through the Design Expert software version 7.0 (Statease, Minneapolis, MN).
Results and discussion
Optimization of algae species
Table 2 shows the adhesion biomass productivity (ABP) and adhesion rate achieved with six microalgae species using a stainless steel sheet. Chlorococcum sp., the unicellular alga with spherical or slightly oblong cells of varied size around 10–12 μm, was selected as the optimal species for attached culture because of the maximum ABP of 0.53 ± 0.05 g m−2 day−1 and the highest adhesion rate of 53.3 ± 0.8 % achieved. The variation in attachment observed in the present study may be related to the attachment mechanism and their differential ability to produce exopolymeric substances (EPS). EPS are adhesive metabolites which provide a three-dimensional hydrated matrix that cements the growing cells, forming the biofilm [29]. EPS are not unique to bacteria. Some of the most abundant EPS producers are microalgae (in particular, diatoms). The green alga Penium margaritaceum has been shown to produce large amounts of EPS (predominantly polysaccharides) [30, 31]. Researches indicate that EPS are involved in the processes of flocculation, adhesion and biofilm formation [32].
Selection of the optimal supporting material
The adhesion of Chlorococcum sp. varied significantly with different supporting materials as shown in Fig. 2. Glass fiber-reinforced plastic, which was characterized by its micro-porous surface with high roughness and wave shape (shown in Fig. 1), was selected as the optimal substrata based on the maximum ABP of 1.47 ± 0.06 g m−2 day−1 and highest adhesion rate of 81.2 ± 2.0 % achieved. Most researches reveal that the characteristics of supporting materials are crucial to the attachment of microalgae [11–13]. Cao et al. [33] studied the effect of surface texture on algal adhesion for a mechanical–biological system that was intended to grow algae biofilms over a metal conveyor belt floating in open sea for biofuel production. They reported that microscale textures made by laser over stainless steel surface increased the attachment of green algal S. dimorphus [33].
Generally, the adhesion of microalgae can be grouped into two stages: (1) initial adhesion and (2) deliberate secondary adhesion. Initial adhesion of cells is preceded by adsorption of algal cells to the solid surface forming a conditioning film. This initial adhesion is often reversible. Adhesion of cells to a surface is followed by a committed secondary adhesion through the production of adhesive EPS resulting in an irreversible adhesion [34]. Microalgae formed a thick “mat” on the concave area of glass fiber-reinforced plastic as shown in Fig. 1. It is believed that the specific shape of the glass fiber-reinforced plastic may help to trap the algal cells in the concave area and increase the stability of the initial attachment. According to Morikawa’s research, the use of immobilized cells is an effective technique to increase cell mass concentration inside the reactor [35]. Due to the gravity forces, algal cells may easily be settled in the concave area of glass fiber-reinforced plastic to form the first layer biofilm, and then the biofilm could be possibly enlarged with a more stable adhesion force produced by EPS.
Second-order model analysis
Second-order polynomial Model A was developed to analyze the effect and interactions of independent variables (culture period, initial total nitrogen concentration (ITNC), pH and culture volume) on ABP. The equation in terms of coded factors (not actual factor) is shown below:
The significance of the regression model and individual variables were determined at 95 % confidence level. As shown in Table 3, the P value of Model A is lower than 0.05, which indicates that the model is statistically significant. Based on the P values, the variables of ITNC, pH and culture volume, as well as the interactions between culture period and ITNC, culture period and culture volume were significant on ABP.
Influence of ITNC
The influence of ITNC on ABP when culture period, pH and culture volume were constant at the center point is shown in Fig. 3. In the researched area, ABP was enhanced as the ITNC increased. Nutrients, especially nitrogen supplement, are important for algae biomass growth [36, 37] and also for the adhesion of microalgae [23]. By raising nitrogen concentration, the biomass productivity of most algae species increases [37, 38]. EPS, as an important factor on the adhesion of microalgae, is also affected by the nitrogen supplement [39, 40]. Hoa et al. [40] revealed that the EPS components, namely proteins and carbohydrates, had a more profound effect on adhesion compared to total EPS, with protein being more significant than carbohydrate. When nitrogen increased from 0 to 56 mg L−1, the protein component of EPS increased from 1.47 to 9.4 mg g−1; as the nitrogen concentration continued to go up, the protein component of EPS dropped down [40]. ABP was positively related to both biomass productivity and EPS quantity. When nitrogen concentration increased in the present study, the biomass productivity and protein component of EPS may get enhanced, leading to the increase of ABP.
Influence of pH
The influence of pH on ABP when culture period, ITNC and culture volume are constant at the center point is shown in Fig. 4. ABP was slightly decreased first as the pH increased from 6 to 7 and then enhanced when pH continued to go up to 9. It is well known that pH plays an important role in both microalgal growth and flocculation. It is possible for most of the algae species to achieve higher biomass productivity as the pH goes up from 6 to 9 [25, 26]. However, since microalgae membrane and EPS are mainly composed of polysaccharides, proteins and lipids, the components of extracellular metabolites could be varied with different pH [41]. At low pH, the dissociation of carboxyl groups is inhibited, while the dissociation of amine groups is enhanced, so the negative surface charge of algae is weakened [42], which may increase the adhesion of microalgae. The results were in accordance with those of the present study that slightly higher ABP was achieved at a low pH level of 6. On the other hand, according to Zhang et al.’s [42] research, when pH was above 9, microalgae cells may release EPS to protect themselves from the environment, which may cause the change in surface charges. The results were also in accordance with Sekar et al.’s [11] research showing that the attachment of N. amphibia was significantly higher at pH 9 when compared with pH 7 on glass substrata.
Influence of culture volume
The influence of culture volume on ABP when culture period, ITNC and pH were constant at the center point is shown in Fig. 5. ABP was enhanced first as the culture volume increased from 200 to 300 mL and then sharply decreased when culture volume continued to go up to 500 mL. Rising culture volume may increase the nutrient supplement for cell growth, but also decrease the cell concentration of the suspended solution. Sekar et al. [11] studied the effect of cell concentration on the adhesion of N. amphibia using both titanium and glass substrates. As the cell concentration increased from 2 × 102 to 2.3 × 105 cells mL−1, the attachment of N. amphibia on both substrates was significantly enhanced [11]. It is believed that the increase in cell concentration may increase the frequency of the cell–cell encounters and lead to the enhancement of the attachment of microalgae, and vice versa in general [25]. As a result, the culture volume provided should be balanced between sufficient nutrient supplement and high cell concentration.
Interactions between culture period and ITNC
The interactions between culture period and ITNC on ABP when pH and culture volume are constant at the center point are shown in Fig. 6. In a nitrogen-deficient situation (ITNC around 30–50 mg L−1), increasing culture period increases ABP to some extent. Generally, a smooth biomass growth curve is often found in nitrogen-deficient situations [22]. However, as culture period increases, the production of EPS is enhanced. Becker [36] investigated the EPS production, adhesion strength and density of Amphora coffeaformis over surfaces with different surface energy. They reported that EPS production and adhesion strength were increased with time [36]. Therefore, it is reasonable to achieve higher ABP as culture period increases in a nitrogen-deficient situation. On the other hand, in a nitrogen-sufficient situation (ITNC around 50–70 mg L−1), the maximum ABP resulted in low level of culture period and ABP decreased as culture period increased. In a nitrogen-sufficient situation, it is usual for algae cells to reach an exponential growth period around 7–10 days [22], achieving the maximum cell growth rate. The high cell concentration may increase the cell–cell encounters and lead to the maximum ABP. Generally, microalgal cells carry negative charges that prevent them from aggregation in suspension [25]. The adhesion of microalgae cells to substrata is often affected by the cell surface charges. Since culture volume and pH were constant, the cationic ions in the solution that could be used to neutralize or reduce the cell surface charges were constant. When cell concentration increased to some extent, there were limited cationic ions for neutralization and, therefore, the attachment of microalgae could be weakened, leading to the decrease of ABP.
Interactions between culture period and culture volume
The interactions between culture period and culture volume on ABP when ITNC and pH are constant at the center point is shown in Fig. 7. When culture volume was low, increasing culture period decreased the ABP, probably due to the deficient nutrient supplement for biomass growth. On the other hand, when culture volume was high, increasing culture period increased the ABP. As previously mentioned, rising culture volume decreased the cell concentration in the solution. In this case, prolonging culture period may be an effective way of increasing cell concentration to achieve a higher ABP.
Optimization and validation
Based on the second-order polynomial Model A presented in Eq. 4, the predicted maximum ABP was 4.26 g m−2 day−1 at culture period 11 days, ITNC 70 mg L−1, pH 8 and culture volume 340 mL. The predicted condition was validated by duplicate experiments, and the measured ABP was 3.96 ± 0.12 g m−2 day−1 with adhesion rate 90.8 ± 0.8 %. The prediction was close to validation of experimental results, indicating that the model can be used to guide and optimize the attached culture of Chlorococcum sp. using glass fiber-reinforced plastic.
ABP versus adhesion rate
Although the objective of this study was to optimize ABP, it does not mean that adhesion rate is not important to attached microalgal culture. Table 4 summarizes both adhesion rate and ABP obtained from RSM experiments. The adhesion rate varied from 48.0 ± 0.5 to 95.2 ± 0.8 % and averaged at 81.1 %. The results indicated that for attached culture of Chlorococcum sp. using glass fiber-reinforced plastic, adhesion rate was high even without optimization; however, ABPs were lower than 2.4 g m−2 day−1 on average. It is therefore more important to maximize ABP than adhesion rate for attached culture of Chlorococcum sp. However, because adhesion rate is dependent on both algal species and substrata, the same conclusion may not be true for other algae species or substrata.
Summary and conclusions
The formation of microalgae biofilms with various algae species, substrates and culture conditions was investigated. Among the six freshwater algae species and nine different substrates studied, Chlorococcum sp. and glass fiber-reinforced plastic proved to be the optimal algae species and substrata for attached culture based on the high adhesion biomass productivity (ABP) and adhesion rate achieved. Response surface methodology (RSM) experiments were carried out to analyze the influence and interactions of independent variables (culture period, initial total nitrogen concentration (ITNC), pH and culture volume) on ABP. A second-order polynomial model was also developed to optimize the attached culture conditions for maximum ABP. Results showed that increasing the culture period was conducive to enhance ABP in a nitrogen-deficient situation, but decreased ABP in a nitrogen-sufficient situation. Prolonging the culture period increased ABP when culture volume was high, but decreased ABP when culture volume was low. The predicted maximum ABP was 4.26 g m−2 day−1 at culture period 11 days, ITNC 70 mg L−1, pH 8 and culture volume 340 mL. The predicted ABP was validated by experiments with acceptable accuracy, indicating that the model could be used to guide and optimize the attached culture of Chlorococcum sp. using glass fiber-reinforced plastic.
References
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65:635–648
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(2):294–306
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54(4):621–639
Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science 329:796–799
Stephens E, Ross IL, King Z, Mussgnug JH, Kruse O, Posten C, Borowitzka MA, Hankamer B (2010) An economic and technical evaluation of microalgal biofuels. Nat Biotechnol 28:126–128
Moheimani NR, Borowitzka MA (2006) The long-term culture of the coccolithophore Pleurochrysis carterae (Haptophyta) in outdoor raceway ponds. J Appl Phycol 18:703–712
Carlozzi P (2003) Dilution of solar radiation through “culture lamination” in photobioreactor rows facing south north: a way to improve the efficiency of light utilization. Biotechnol Bioeng 81:305–315
Tredici MR (2010) Photobiology of microalgae mass cultures: understanding the tools for the next green revolution. Biofuels 1:143–162
Liu TZ, Wang JF, Hu Q, Cheng PF, Ji B, Liu JL, Chen Y, Zhang W, Chen XL, Chen L, Gao LL, Ji CL, Wang H (2012) Attached cultivation technology of microalgae for efficient biomass feedstock production. Bioresour Technol 127:216–222
Mulbry W, Kangas P, Kondrad S (2010) Toward scrubbing the bay: nutrient removal using small algal turf scrubbers on Chesapeake Bay tributaries. Ecol Eng 36(4):536–541
Sekar R, Venugopalan VP, Satpathy KK, Nair KVK, Rao VNR (2004) Laboratory studies on adhesion of microalgae to hard substrates. Biomed Life Sci 173(2):109–116
Yuan WQ, Cui Y, and Pei ZJ (2009) Algal cell–surface interaction: an overview and preliminary test. ASME International Manufacturing Science and Engineering Conference Vol.1 MSEC2009-84222
Johnson MB, Wen ZY (2010) Development of an attached microalgal growth system for biofuel production. Appl Microbiol Biotechnol 85(3):525–534
Moreno-Garrido I (2008) Microalgae immobilization: current techniques and uses. Bioresour Technol 99(10):3949–3964
Irving TE, Allen DG (2011) Species and material considerations in the formation and development of microalgal biofilms. Appl Microbiol Biotechnol 92(2):283–294
Sorokin C, Krauss RW (1958) The effect of light intensity on the growth rates of green algae. Plant Physiol 33:109–113
Ozkan A, and Berberoglu H (2011) Adhesion of Chlorella vulgaris on hydrophilic and hydrophobic surfaces. In: Proceedings of the ASME 2011 international mechanical engineering congress and exposition, IMECE2011-64133, Denver, Colorado, USA, 11–17 November 2011
Li Y, Gao Y, Li X, Yang J, Que G (2010) Influence of surface free energy on the adhesion of marine benthic diatom Nitzschia closterium MMDL533. Colloids Surf B Biointerfaces 75:550–556
Stanley M, Callow J (2007) Whole cell adhesion strength of morphotypes and isolates of Phaeodactylum tricornutum (Bacillariophyceae). Eur J Phycol 42:191–197
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Optimization of Nannochloropsis oculata growth using the response surface method. J Chem Technol Biotechnol 81:1049–1056
Azma M, Mohamed MS, Mohamad R, Rahim RA, Ariff AB (2011) Improvement of medium composition for heterotrophic cultivation of green microalgae, Tetraselmis suecica, using response surface methodology. Biochem Eng J 53(2):187–195
Shen Y, Yuan W, Pei Z, Mao E (2008) Culture of microalga Botryococcus in livestock wastewater. Trans ASABE 54(4):1395–1400
Urrutia I, Serra JL, Llama MJ (1995) Nitrate removal from water by Scenedesmus obliquus immobilized in polymeric foams. Enzyme Microb Technol 17(3):200–205
Kebede-Westhead E, Pizarro C, Mulbry W (2006) Treatment of swine manure effluent using freshwater algae: production, nutrient recovery, and elemental composition of algal biomass at four effluent loading rates. J Appl Phycol 18:41–46
Molina Grima EM, Belarbi EH, Fernandez FGA, Medina AR, Chisti Y (2003) Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv 20:491–515
Valdes FJ, Hernandez MDR, Gomez A, Marcilla A, Chapuli E (2008) Study of the efficiency of different flocculants for effective microalgae harvesting. http://rua.ua.es/dspace/bitstream/10045/8536/1/Poster%20expoquimia%20floculacion%20(2008).pdf
GB 11894–89 (1989) Water quality—determination of total nitrogen–Alkaline potassium persulfate digestion-UV spectrophotometric method. Standards Press of China, Shanghai
GB 11893–93 (1989) Water quality—determination of total phosphorus–Ammonium molybdate spectrophotometric method. Standards Press of China, Shanghai
Bragadeeswaran S, Jeevapriya R, Prabhu K, Sophia Rani S, Priyadharsini S, Balasubramanian T (2011) Expolysaccharide production by Bacillus cereus GU812900, a fouling marine bacterium. Afr J Microbiol Res 5(24):4124–4132
Domozych DS, Kort S, Benton S, Yu T (2005) The extracellular polymeric substance of the green alga Penium margaritaceum and its role in biofilm formation. Biofilms 2(2):129–144
Domozych DS (2007) Exopolymer production by the green alga Penium margaritaceum: implications for biofilm residency. Int J Plant Sci 168(6):763–774
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8(9):623–633
Cao J, Yuan WQ, Pei ZJ, Tiffany D, Yan C, Michael B (2009) A preliminary study of the effect of surface texture on algae cell attachment for a mechanical–biological energy manufacturing system. J Manuf Sci E 131(6):064505
Bos R, van der Mei H, Busscher H (1999) Physico-chemistry of initial microbial adhesive interactions—its mechanisms and methods for study. FEMS Microbiol Rev 23:179–230
Morikawa M (2006) Review: beneficial biofilm formation by industrial bacteria Bacillus subtilis and related species. J Biosci Bioeng 101(1):1–8
Becker EW (1994) Microalgae: biotechnology and microbiology, vol. 18. Cambridge University, Cambridge, p 24
Shen Y, Yuan W, Pei Z, Mao E (2009) Heterotrophic culture of Chlorella protothecoides in various nitrogen sources for lipid production. Appl Biochem Biotechnol 160(6):1674–1684
Schenk PM, Thomas-Hall S, Stephens E, Marx UC, Mussgnug JH, Posten C, Kruse O, Hankamer B (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenergy Res 1(3):20–43
Vargas-Garcia MC, Lopez MJ, Elorrieta MA, Suarez F, Moreno J (2001) Influence of nutritional and environmental factors on polysaccharide production by Azotobacter vinelandii cultured on 4-hydroxybenzoic acid. J Ind Microbiol Biotechnol 27:5–10
Hoa PT, Nair L, Visvanathan C (2003) The effect of nutrients on extracellular polymeric substance production and its influence on sludge properties. Water SA 29:437–442
Wyatt NB, Gloe LM, Brady PV, Hewson JC, Grillet AM, Hankins MG, Pohl PI (2012) Critical conditions for ferric chloride-induced flocculation of freshwater algae. Biotechnol Bioeng 109(2):493–501
Zhang H, Kuang Y, Zhe L, Liu C (2011) Influence on surface characteristics of microalgae cell by solution chemistry. Adv Mater Res 287–290:1938–1942
Acknowledgments
This research was financially supported by the Natural Science Foundation of China (Award 51108085), “863” Project (No. 2012AA021704), the Natural Science Foundation of Fujian Province (Award No. 2011J05125) and the Program of the Education Department of Fujian Province (Award JA11030).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, Y., Xu, X., Zhao, Y. et al. Influence of algae species, substrata and culture conditions on attached microalgal culture. Bioprocess Biosyst Eng 37, 441–450 (2014). https://doi.org/10.1007/s00449-013-1011-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-013-1011-6