Abstract
Biomass is a renewable source and potentially sustainable fossil fuel replacement due to its availability, lower processing cost, high conversion, and lower life cycle carbon emissions. Pyrolysis can be used to convert biomass into bio-oil, but the quality of bio-oil is usually poor exhibiting high viscosity, thermal instability, and corrosiveness. This review article is focused on the application of catalytic pyrolysis towards obtaining high-quality bio-oil and advanced techniques for bio-oil characterisation. Structural arrangement (i.e., mesoporous, microporous), number of acid sites (Lewis and Brønsted acid sites), and amount of metal loading play a key role on deoxygenation reactions and selective production of aromatic hydrocarbons. Hierarchical zeolites doped with noble metals favour hydrogenation of C▬O or C〓O and reduce coke deposition in the production of polycyclic aromatics. Overall reaction mechanisms, aromatic yield and selectivity, the effect of Si/Al ratio, and process challenges of metal loaded zeolites are summarized. The advantages and disadvantages of different types of advanced analytical techniques for bio-oil characterisation are also discussed. The results showed that two-dimensional gas chromatography (2D GC) technique can identify 70% of chromatograph from bio-oil analysis. However, there is need to combine analytical techniques to accurately quantify bio-oil components.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biomass is widely used as a renewable source for substitution of fossil fuel and a precursor for the production of chemicals [1]. For example, the conversion of lignocellulosic biomass (i.e., sugarcane, corn) into bioethanol has been extensively investigated [2, 3]. Biomass is a carbon-rich biological material widely used due to its availability, lower processing cost, and higher conversion [4]. In the course of biomass conversion, there is no overall increase in carbon footprint, making biomass a potential sustainable renewable energy source, and having a critical role in environmental mitigation and energy supply. Biomass can be grouped into four subgroups: (1) agricultural and forestry residues, (2) municipal and industrial solid waste, (3) herbaceous crops: Napier grass and weeds, and (4) aquatic and marine biomass [5]. Biomass is converted into bioenergy and chemicals via biological and thermochemical processes [6]. The thermochemical processes are carried out at high temperatures, between 300 and 1400°C [7, 8]. Amongst the thermochemical processes, pyrolysis is widely used, with biomass conversion by high heat energy (207–434 kJ/kg) in the absence of oxygen [9, 10].
Pyrolysis enhances the energy density of biomass with the flexibility to be carried out at a small scale or remote location setups [11]. Pyrolysis is a flexible and attractive process to converting biomass into bio-oil, chemicals, and heating energy. Slow pyrolysis is usually performed in batch mode for long periods of residence time (5–30 min) at low temperatures and heating rates [12]. The decomposition of biomass gives rise to three main products: biogas, bio-oil, and biochar. Bio-oil is the main product of pyrolysis, with a higher heating value than the raw material, which can be converted into different chemicals [13]. Catalysts have been used to improve the efficiency of pyrolysis process and to upgrade the bio-oil quality [14].
Catalytic pyrolysis operates in in-situ and ex-situ modes [15]. In the in-situ catalytic pyrolysis process, the biomass and catalyst are mixed before the pyrolysis process. In the case of the ex-situ catalytic pyrolysis process, the biomass is separated from the catalyst, and pyrolytic vapour from the pyrolysis process reacts with the catalyst in a secondary reactor [15]. Nevertheless, there are not many comparative studies in the literature to understand the catalytic mechanisms and kinetic pathways of in-situ and ex-situ catalytic pyrolysis. The most important catalyst groups used in catalytic pyrolysis are zeolites, mesoporous catalysts, and biomass-derived catalysts.
Zeolites with distinctive pore structure and acidity (Lewis and Brønsted acid sites), are used in bio-oil upgrading [16, 17]. The zeolites predominantly used in pyrolysis are ZSM-5, Beta-zeolites, and Y-zeolites [18,19,20]. ZSM-5 has demonstrated excellent efficiency in deoxygenation reactions for aromatic compounds, producing bio-oil with low oxygen content and high calorific value [21]. Metals have been loaded into zeolites to enhance bio-oil quality due to their high resistance to coke deposition and high acidity [22]. However, mass transfer limitations, catalyst deactivation over coke deposition, and sintering should be optimized for zeolite catalytic pyrolysis. Mesoporous catalysts, with a pore size range of 20–30 Å, are used in catalytic pyrolysis due to their unique porosity and high surface area (900–1100 m2/g) [23, 24]. Mesoporous silica catalysts, such as SBA-15, MCM-41, and MUS-S, are widely used in catalytic pyrolysis of biomass due to their supramolecular structure, and their propensity to synthesize different crystalline structures [25]. Biochar produced during biomass pyrolysis is also utilized as biomass-derived catalyst for bio-oil upgrading [26].
In this review article, the impact of biomass composition on the quality and yield of bio-oil produced via pyrolysis has been discussed. Different types of pyrolysis, product distribution, and key factors on the process performance are also discussed in the subsequent sections. In the review article more stress has being laid on bio-oil physicochemical properties and its upgrading by catalytic pyrolysis. The reaction mechanisms and application of heterogeneous catalysts to produce high quality bio-oil are explained in detail. In the last section, advanced analytical techniques used for bio-oil characterisation are also reviewed. The main objectives of this review article are to (1) summarise the basic features of catalytic pyrolysis to produce high quality bio-oil, (2) recommend different types of catalysts for specific products/chemicals production, and (3) summarise bio-oil advanced characterisation techniques.
2 Biomass composition
Biomass comprises hemicellulose, cellulose, lignin, and a small number of other extractives [27]. Agricultural and forestry residues have a high energy content, which mainly consists of cellulose [28]. However, herbaceous plants are generally continuous, with loosely bonded fibres, which contains a small lignin percentage that connects the cellulose fibres [29]. Lignin has a higher resistance to heat and chemical degradation than cellulose and hemicellulose [27].
As shown in Fig. 1, different biomass feedstocks comprise different amounts of hemicellulose, cellulose, and lignin. The total amount of lignin and cellulose is one of the determinant factors for subsequent energy and chemical conversion processing. Numerous herbaceous crop families, such as elephant grass, Bermuda grass, esparto grass, alfalfa-full flower, contain 10–25% hemicellulose, 20–40% cellulose and 10–30% lignin [29, 37, 38]. Typical switch grasses contain 32% cellulose, 19.2% hemicellulose, and 15–30% lignin [30]. Generally, biomass with lower lignin content and higher cellulose/hemicellulose content is desired for activated carbon production [39]. High lignin content gives the lowest aromatic yield and the highest coke yield while high hemicellulose content contributes to low coke yield and high noncondensable gas production [40].
The elemental composition of different biomass groups, pyrolysis conditions, bio-oil yield and composition obtained by conventional pyrolysis are summarized in Table 1. Herbaceous crop biomass has an overall elemental composition of 41–49% carbon, 44–47% oxygen, with bio-yield in the range of 44–60%. The energy per unit mass increases with decreasing oxygen content in the feedstock. For example, agricultural residues have a high oxygen percentage (38–47%), which reduces the calorific value of the bio-oil [61]. Municipal and industrial wastes are primary sources of nitrogenous compounds, having a nitrogen content of 3–8% [62].
The quality and yield of bio-oil produced by thermochemical conversion is strongly affected by the elemental composition of biomass. The bio-oil constituents typically depend on the carbon and hydrogen content of biomass. As shown in Table 1, spruce wood contains a high carbon and hydrogen content, 49.11% and 6.14 %, respectively, which enhance the bio-oil phenolic content [47]. The hydrogen amount seems to increase the heating value and aromatic compounds of bio-oil, but slightly varies over biomass types. Amongst the agricultural residues, oak showed the highest aromatic content due to its high hydrogen content (7.16%).
Similarly, municipal and industrial wastes have a high conversion into bio-oil with a yield in the range of 30.1–65%. The coffee husk showed higher conversion into phenolic and aromatic compounds due to high carbon to hydrogen ratio (7.33) [57]. The average C, O, and H percentages of the aquatic and marine biomass are 41.62, 5.90, and 44.26%, respectively. The variation in the elemental composition of biomass results in a high variation in the bio-oil yield and composition. The high carbon to hydrogen ratio (8.2) of P. indicus results in a high aromatic yield in the bio-oil [58]. In contrast, Nannochloropsis showed a lower aromatic yield due to lower carbon to hydrogen ratio of 6.3, but the pyrolysis conditions were different which makes this comparison difficult. Understanding the degree of biomass composition variation helps to design an effective thermochemical process for biomass conversion [63]. The bio-oil properties such as viscosity, pH, and chemical composition depend on the feedstock biomass type, pyrolysis conditions and reactor design. The effect of pyrolysis process parameters on bio-oil quality is discussed in detail in Section 3.2.2.
3 Biomass processing methods
3.1 General overview
Biomass consists of various precursors to produce green chemicals and fuels [64]. In general, biomass conversion is undertaken by two types of processes: biological and thermochemical processes [65]. Thermochemical methods are preferred over the biological processes due to the short reaction times and high degradation efficiency [66,67,68,69].
The product distribution and bio-oil quality from thermochemical processes depend on the residence time, heating rate, temperature, degree of oxidation, the feedstock particle size, and moisture content. The thermochemical processes can be classified into three primary processes: pyrolysis, gasification, and liquefaction [65]. The main products of pyrolysis and gasification are biogas, bio-oil, and biochar, whereas bio-crude and sugars are the main products of the liquefaction process. The intermediate products obtained from thermochemical processes such as sugars, bio-crude, and biogas can be further converted into bioenergy and chemicals via catalytic pyrolysis, steam reforming, fermentation, water–gas shift reaction, and hydro-processing [70, 71].
Liquefaction is an alternative thermochemical process, primarily designed for producing liquid fuel from biomass [72]. The process is carried out in an aqueous medium at a pressure of between 5 and 20 MPa, and temperatures between 250 and 370°C. These are subcritical conditions in which complex biomass structures decompose by hydrolysis and repolymerize into smaller molecules such as levoglucosan, hydroxyacetaldehyde, hydroxyacetone, pyruvic aldehyde, glyceraldehyde, and furfural [73].
Gasification is a thermochemical process to converting biomass into gaseous fuel with the presence of a gasifying agent [74]. The gasification process is carried out at high temperatures, between 500 and 1400°C, and at a range of pressures, from atmospheric pressure to 33 bar [7]. The gaseous products during gasification are CO2, H2, CH4, CO, and N2 [71]. Biomass moisture content varies between 30 and 60% while gasification process requires biomass with a moisture content between 10 and 15%. Therefore, drying biomass is a fundamental pretreatment process to meet the moisture content criteria for gasification, which significantly increases the overall processing costs [75]. Complex operation, high energy costs because of low moisture content requirement for the biomass and relatively high processing temperature make gasification process unsuitable for biomass conversion [76]. On the other hand, pyrolysis is a versatile process to efficiently convert biomass into bio-oil, which is suitable for all types of biomass [77].
3.2 Pyrolysis
Pyrolysis is a thermochemical degradation of biomass by high heat energy (207–434 kJ/kg) in the absence of oxygen [9, 10]. The pyrolysis process is ascribed as the sum of three main routes: char formation, depolymerization, and fragmentation [78]. The char formation pathway results in producing solid residue with a high amount of polycyclic aromatic hydrocarbons [79]. The primary steps in this path are the production and incorporation of benzene rings in a polycyclic structure [80, 81]. Depolymerization consists of degradation of polymeric structures and at low temperatures the degraded monomers condense into a liquid fraction [82]. Fragmentation results in incondensable gas formation and a variety of organic compounds that are condensable at ambient temperature [80, 83].
3.2.1 Types of pyrolysis processes
Based on processing pyrolysis parameters, the conversion of biomass is divided into three classes: slow, fast, and flash. Operation conditions for various types of pyrolysis are summarised in Table 2. Slow pyrolysis is mainly applicable for charcoal production and chemicals like acetic acid, furfural, and phenols. Most slow pyrolysis literature is primarily focused on biochar production and its applications [89, 90]. Of the slow processes, carbonization, with a low heating rate of 0.1–0.4°C/s, is a widely applicable process carried out without condensation of the pyrolysis products [91]. Carbonization is a biomass conversion technique for charcoal production and performed when biochar is the desired product. The ideal feedstock moisture content for slow pyrolysis is between 15 and 20% [92].
Flash pyrolysis produces high bio-oil yield (70%) and low gas and tar amounts in comparison with slow pyrolysis [93]. Flash pyrolysis takes place at high temperatures (650–1000°C) and requires short residence time (max. 2 s). During the process, the feedstock is heated rapidly to be vaporized and then condensed into bio-oil [94, 95]. On the other hand, fast pyrolysis takes place at moderately low temperature (500–800°C) and short residence vapour time (5 s) [96]. During fast pyrolysis, approximately 60–75% of the biomass is converted into bio-oil [97]. However, fast pyrolysis temperatures higher than 650°C favour biogas production [65]. Fast pyrolysis is a flexible and desired process to transform biomass into a liquid that is easily stored and transported for biofuel and chemical production [98, 99].
Vacuum pyrolysis is the decomposition of biomass in a pyrolysis reactor under vacuum to reduce vapours residence time [34]. Vacuum pyrolysis is characterised by a slow heating rate and takes place at temperature between 350 and 520°C resulting in low bio-oil yield (35–50 wt.%) [100]. Hydropyrolysis is the decomposition of biomass in the presence of hydrogen gas [101]. During hydropyrolysis hydrogen gas is reduced to form hydrogen radical, which reacts with pyrolytic vapour [99, 102]. The amount of aromatic hydrocarbons produced via catalytic pyrolysis is much lower than via catalytic hydropyrolysis [88].
3.2.2 Pyrolysis process parameters
The pyrolysis processing parameters affect the composition and yield of the desirable products. The main processing parameters include the heating rate, temperature, gas flow rate, reactor design, and particle size [103]. Any of the three pyrolysis products, such as bio-oil, biogas, or biochar can be improved by optimizing pyrolysis conditions [104,105,106,107,108]. The impact of operational conditions on the quality and yield of the pyrolysis products is summarized in the next paragraphs.
Temperature plays a predominant role in the degradation of high molecular weight components of biomass into smaller molecular fragments. Partial degradation of the biomass structure at the molecular level occurs at a temperature below 300°C that produces heavy residual tar. In contrast, large molecular weight biomass degradation occurs at a temperature higher than 550°C, enhancing the composition of bio-oil [109]. Some studies suggest that the temperature to achieve the highest bio-oil yield is 450–550°C. However, optimum processing temperature to maximise bio-oil yield depends on biomass composition and pyrolysis conditions such as heating rate and gas flow rate [105, 110, 111]. Ji-lu et al. [112] conducted rice husk pyrolysis in a fluidized bed at a temperature between 420 and 540°C, and obtained a maximum bio-oil yield of 56 wt.% at 465°C. This study demonstrated that a further increase of the pyrolysis temperature decreased bio-oil yield to 45 wt.%.
Biomass particle size affects mass and heat transfer rates during pyrolysis, which have an impact on bio-oil yield. The bio-oil yield is usually higher for biomass particles with a size lower than 2 mm [113]. Small biomass particles enhance the biomass decomposition rate due to a better and faster mass and heat transfer rates [114].The fast decomposition of small particles favours high bio-oil yields, however the larger particles cause slow decomposition and favour the production of char [115]. Biomass particles lower than 0.6 mm reduce bio-oil yield due to quick decomposition and participation in secondary reactions leading to an increase in biogas yield [116]. The mass and heat transfer rates are also depending on the types of reactors used in the pyrolysis process.
Many reactor types such as, fixed bed, fluidized bed, rotative, vacuum, plasma, and microwave have been used for pyrolysis. Fluidized bed reactor (bubbling) is frequently used to achieve high heating transfer rates (uniform temperature distribution) resulting in a high bio-oil yield of 70–75% [84]. However, it requires small biomass particles and is difficult to remove the biochar. Microwave reactor is another option that is mainly used due to high heating rates, high temperatures and short residence times resulting in a bio-oil yield of 60–70% [117]. However, the high processing costs, high power consumption and the need to use microwave absorbers limit its application [118]. Several researchers used plasma reactor for biomass pyrolysis, but despite of its high operating costs, high energy and small biomass particles requirements, the bio-oil yield was still low between 30 and 40% [119].
Pyrolysis process produces a significant amount of vapour during biomass conversion, which can promote side reactions, giving rise to thermal cracking, repolymerization, and recondensation into biochar, resulting in a reduction of bio-oil production [95, 103]. Nitrogen gas is preferably used to remove vapours from the pyrolysis reactor because is chemically stable, inexpensive, and abundant. Increasing the nitrogen gas flow rate reduces the residence time of vapour in the pyrolysis reactor [115]. Choi.et.al. [120] showed that an increase in nitrogen flow rate increased the noncondensable gas percentage, from 22.2 to 31.9%. The increased nitrogen flow rate enhanced the vigorous bubbling motion and improved both mixing and heat transfer rates. For example, Mohamed. A.R. et al. [121] showed that an increase of nitrogen gas flow rate from 150 ml/min to 500 ml/min, in empty fruit bunch pyrolysis fluidized the bed reactor decreased bio-oil yield from 45.7 to 37.8%. However, the noncondensable gas percentage increased from 28.4 to 35.1%.
Heating rate is also another key pyrolysis variable that influences the extent of degradation during pyrolysis. The abundance of volatile matter during the degradation process increases with an increase of the heating rate due to the endothermic decomposition of feedstock [122]. An increase of the heating rate also impacts on the optimal pyrolysis temperature for bio-oil production. For example, Debdoubi et al. [123] conducted pyrolysis of esparto by varying the pyrolysis temperatures from 400 to 700°C and using different heating rates of 50°C/min, 150°C/min, and 250°C/min. The researchers found the optimum heating rate for 57% of bio-oil yield was 150°C/min at 500°C. However, higher bio-oil yield was achieved for a heating rate of 250°C/min at 550°C. In general, a comprehensive ANOVA analysis of all parameters is necessary to optimize the pyrolysis process to obtain high bio-oil quality and yield.
3.3 Bio-oil from pyrolysis: composition and properties
Bio-oil is a dark brown colour liquid that can be used for power generation or extraction of various chemicals. Huber et al. [124] stated that typical pyrolysis bio-oil contains acids (propionic and acetic), alcohols (ethanol, methanol and ethylene glycol), phenols, aldehydes (acetaldehyde, formaldehyde and ethanediol), ketones, aromatics and furans, regardless of the type of feedstock. Table 1 presents the major chemical groups present in bio-oil for different biomass feedstock processed via pyrolysis.
Biomass with higher lignin content gives a higher bio-oil yield. Coffee husk pyrolysis at higher temperatures produces bio-oil with low molecular weight compounds of ketones, acids, and aromatic hydrocarbons [125]. Bio-oil obtained from herbaceous crops, for example Para grass and Arundo donax, contains a high amount of phenolic and high molecular weight aromatic compounds, making this feedstock desirable for phenol extraction. However, bio-oil produced from the pyrolysis process exhibits high viscosity, is corrosive, and thermally unstable. These properties make bio-oil undesirable for the synthesis of fuel and chemicals [126].
Different physicochemical properties of bio-oil produced via biomass pyrolysis and typical crude oil properties are summarized in Table 3. The concentration of elemental oxygen and moisture content in bio-oil are much higher than in crude oil and explain the low heating value of bio-oil. Several studies have reported that the quality of bio-oil is affected by physicochemical properties, such as pH, elemental composition, oxygen content, char, suspended solid, and ash content [123, 131, 132].
Kinematic viscosity of bio-oil ranges from 35 to 100 cP, which depends on the types of biomass and pyrolysis processing parameters. Bio-oil viscosity tends to increase over time during storage due to further chemical reactions between the bio-oil components [29]. Boucher et al. [133] reported the effect of adding a stabilizing agent (alcohol) on the viscosity of bio-oil. When bio-oil was stored in 10% methanol, the viscosity only increased from 20 to 22 cP over four months at 20°C. Similarly, 20% of ethanol showed a marginal increment, from 13 cP to 15 cP, on the viscosity of bio-oil at 40°C [134]. High viscosity of bio-oil causes incomplete combustion and poor atomisation during applications; however, adding organic solvents could enhance physicochemical properties and storage stability of bio-oil [135].
The presence of acetic and formic acids in the bio-oil increases acidity (pH <3). Reactive oxygenated compounds in bio-oil causes a change in viscosity, which alters thermal and storage stability [136]. Thereby these acids make the bio-oil corrosive and unsuitable for handling storage vessels and equipment [137]. Ash content in bio-oil arises from the different inorganic compounds such as sodium, magnesium, and potassium (predominantly) in the feedstock. Thangalazhy-Gopakumar et al. [138] reported that bio-oil synthesis from wood biomass showed 0.09 to 0.2% ash content. Moisture content in bio-oil results from dehydration reactions during pyrolysis and moisture in the feedstock [55]. In general, bio-oil can have 15–30% moisture content depending on the type of biomass [139]. Heo et al. [55] reported bio-oil with moisture content ranging from 40 to 60% obtained by pyrolysis of sawdust with 9.1% moisture content The rise in bio-oil moisture content is due to esterification reactions taking place between bio-oil constituents.
The complexity of bio-oil composition limits its application as an alternative energy source. The separation of bio-oil fractions has been employed to improve the calorific value and recover valuable chemicals from bio-oil. Several methods such as solvent extraction, distillation, centrifugation, and column chromatography have been employed to recover and separate of bio-oil fractions [140, 141]. Amongst the bio-oil fractions, phenolic compounds are most suitable for various applications including pharmaceuticals, resin manufacturing, fine chemicals, and food processing [142]. Solvent extraction of phenolic compounds from bio-oil is mainly performed by hexane, chloroform dichloromethane, and toluene [141]. However, the requirement of large volumes of solvent makes the solvent extraction undesirable.
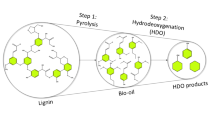
Direct application of bio-oil without upgrading is giving undesired results due to high oxygen content, high viscosity, thermal instability, and low calorific value. Bio-oil can be upgraded via different techniques such as hydrotreating, steam reforming, emulsification, and catalytic pyrolysis [143, 144]. Amongst bio-oil upgrading techniques, catalytic pyrolysis can lower decomposition temperature and requires low energy and hydrogen cracking throughout [145, 146]. Catalytic pyrolysis enhances the quality of bio-oil by removing oxygenated compounds in the form of CO, CO2, and H2O [147]. Catalytic pyrolysis is a potential process for high quality bio-oil production.
4 Biomass catalytic pyrolysis and reaction mechanism
4.1 Catalytic pyrolysis
Catalysts play a critical role in promoting process efficiency, targeting specific reactions and reducing processing temperature and time. Catalysts affect chemical composition and distribution of pyrolysis products. Catalytic pyrolysis has shown potential for converting oxygenated compounds in bio-oil mixture and consequently enhancing bio-oil quality. Catalysts have been used in the bio-oil upgrading process through various approaches [148, 149]. The process configuration of catalytic pyrolysis are grouped into in-situ and ex-situ modes, based on how pyrolytic vapour contacts with catalyst [150].
In-situ catalytic pyrolysis consists of mixing catalyst with biomass directly in the pyrolysis reactor [151]. For ex-situ, catalytic reaction occurs in a secondary independent reactor instead of the pyrolysis reactor [152]. Nevertheless, it is possible to convert oxygenated compounds effectively into hydrocarbons by either mode. However, during the in-situ process, the pyrolytic vapours could not react with substantial quantities of catalyst, which requires a higher biomass to catalyst ratio (i.e., 2:1) for adequate reaction [153]. Also, the optimum pyrolysis temperature is insufficient for in-situ upgrading, requiring a separate ex-situ reactor. Char formed during the in-situ catalytic pyrolysis can also lead to deactivation of the catalyst due to pores blockage [154]. The secondary reactor in ex-situ mode gives an advantage over in-situ mode such as, easy recovery of biochar without catalyst contamination and versatile temperature controls [155].
Synthesis of catalyst can be tailored to the final product requirements. Understanding the reaction mechanisms in the catalytic pyrolysis of bio-oil upgrading is fundamental. The mechanisms of catalyst pyrolysis depend on the reaction pathways of the catalytic system and specific compositions of biomass. The complexity of biomass matrix, inadequate mass transfer phenomena, and immobilisation of catalysts challenge the understanding of the catalyst pyrolysis mechanisms [156].
The major reaction pathways during catalytic pyrolysis are deoxygenation, ketonization, cracking, aldol condensation, and aromatization (Eqs. 1–5) [157, 158]. Hydrodeoxygenation (HDO) is a promising route to enhance the quality of bio-oil by removing oxygenated compounds in the form of CO, CO2, and H2O in the presence of H2 and catalyst [147]. The primary renewable fuel products from HDO include gasoline and diesel hydrocarbons. Various catalysts have been used during HDO including, noble metals, metal oxides, microporous (zeolites), and mesoporous.
HDO has significant benefits, such as high effectiveness on removing oxygen atoms, low reaction temperatures, and preserves the number of carbons in the products [159]. Various types of reactions are taking place during the hydrodeoxygenation process, including hydrogenation, decarboxylation, hydrogenolysis, dehydration, and hydrocracking [160]. Apart from phenolic molecules, aromatic compounds like guaiacols and syringol are also hydrogenated into a wide range of products, including cycloketones, cycloalcohols, arenes, methanol, and cycloalkanes [161, 162].
Conversion of phenols via HDO, as shown in Fig. 2, can be carried out through three different reaction paths: the first is the removal of oxygen by the cleavage of the C=O bond from the aromatic compound. Then cyclohexane and cyclohexene are formed after forming benzene in the presence of hydrogen. The second path is hydrogenation of phenol into cyclohexanol, followed by the removal of oxygen to produce cyclohexene and cyclohexane. The third path is the combination of both hydrodeoxygenation and hydrogenation to convert phenol compounds into cyclohexanone, which is immediately followed by hydrogenation to form cyclohexene, cyclohexanol, and cyclohexane [164, 165]. Eventually, all three paths lead to cyclohexane formation, which can also isomerize into methyl cyclopentane. The selection of one of the three paths to convert phenol into methyl cyclopentane depends on different parameters of catalysts such as metal composition, surface properties, reaction temperature, and required intermediate products.
Reaction mechanisms for phenols, adapted from [163]
4.2 Bio-oil quality: catalytic reactions and mechanisms
Zeolite catalysts have received much attention due to its relatively low cost, availability, and its potential to yield high quality bio-oil. Amongst zeolite catalysts, ZSM-5 (exhibiting high acidity and pore size) demonstrated excellent efficiency for bio-oil upgrading, producing less viscous, less acid, and high energy value bio-oil [166]. ZSM-5 also increased the concentration of aromatic hydrocarbons, organics, and gaseous compounds in bio-oil caused by aromatization, decarbonization, and cracking reactions [167, 168]. Zhang et al. [169] utilized ZSM-5 for ex-situ mode catalytic pyrolysis of corncobs using a fluidized bed reactor. The bio-oil obtained from the reactor showed a reduction of oxygenated compounds by 25% with a high heating value (HHV) of 34.6 MJ/kg, which is similar to heavy fuel oil and diesel values.
Many transition metals, such as cobalt, nickel, iron, cerium, and gallium, have been used to fine-tune ZSM-5 acidity to enhance bio-oil yields and decrease coke formation on catalysts [168, 170, 171]. Zeolite supports are frequently used to support metal-based catalysts because of the need to have metals and acidic sites to support the H2 and O-containing compounds activations. Zeolite supports with high Lewis and Brønsted acid site density favour high dehydration reaction. Kumar et.al. [172] prepared metal-based catalysts over zeolite support catalysts (Cu/zeolite, Ni/zeolite, and Cu–Ni/zeolite) to investigate their synergy effect on the deoxygenation reaction of pinewood. The authors found Cu–Ni/zeolite catalyst produced 34% of aliphatic hydrocarbons; however, monometallic combination favoured the production of aromatic hydrocarbons, Cu/zeolite: Ni/zeolite (1:1) generated 18.87% of aromatic hydrocarbons. Also, Cu/zeolite: Ni/zeolite (1:3) significantly reduced in comparison to noncatalytic pyrolysis, with 1.81% of acids, 6.42% of phenols, and 0.4% of ketones in the oxygenated compounds of bio-oil. Table 4 describes the key findings from modification of zeolites using transition metals and pyrolysis conditions. Selectivity and yield of catalysts depend on the catalyst to feedstock ratio, types, and percentage of metals. For example, incorporation of metals in the zeolite framework increases the production and composition of polycyclic aromatics while decreases bio-oil yield.
Metal catalyst activates hydrogenation of C▬O or C〓O to produce polycyclic aromatic hydrocarbons. Noble metal catalysts show better hydrogenation performance due to its stability and selectivity [176]. Metal electronic configuration and band structure also contribute to high hydrogenation performance. The binding capacity of substrate to metal catalyst surface depends on the availability of d orbital in spd hybrid bonding orbit. Therefore the higher d orbital percentage of noble metal the stronger interaction between substrate and catalyst [177]. The overall reaction pathways, advantages, and disadvantages of the use of metal-based catalysts in bio-oil production are summarized in Table 5.
Metal oxides are also viable for deoxygenation of the pyrolysis vapour to form aromatic hydrocarbons. Metal oxides are widely used in biomass pyrolysis because of their higher degree of active sites during reaction [186]. Additionally, they are highly temperature-stable and resistant against relatively nonpolar compounds under different pH conditions [112, 187]. Kaewpengkrow et al. [188] upgraded fast pyrolysis vapours from Jatropha curcas waste residue produced at 600°C using metal oxide/activated carbon catalysts prepared by wet impregnation. These metal oxide/activated carbon catalysts promoted aromatics formation and produced 86.56% hydrocarbon yield, considerably higher than 11.32% yield without catalysts.
The small size of micropores in the zeolite structure hinders the mass transfer of reactant and formation of polycyclic aromatic hydrocarbons [189]. Therefore to overcome this problem, hierarchically structured zeolites have been developed [190]. Hierarchical zeolites are vastly utilized in biomass catalysis because of their high surface area, better mass transfer, high selectivity, and yield [157]. As shown in Table 6 hierarchically structured zeolites can be achieved by creating zeolite materials with multiple porosity levels, i.e., mesoporous and microporous structures. Mesoporosity on zeolite materials is obtained by alkaline treatment (to remove Si atom) and acid treatment (to remove Al atom). The dealumination process increases the Si/Al ratio and enhances the formation of mesoporosity in the zeolite framework [196, 197]. Desilication of zeolites provides a well-controlled mesoporous formation with an optimal Si/Al ratio between 25 and 50 [195].
A wide variety of mesoporous silica has also been used for bio-oil upgrading such as MCM-41 (Mesoporous molecular sieve) and SBA (Santa Barbara Amorphous). MCM-14 exhibits a high surface area (1000 m2/g), narrow pore size distribution (20–30 Å), and a hexagonal arrangement [198]. However, due to weak acidity compared to aluminosilicate, MCM-14 is only applicable to a narrow range of processes. Acidic properties of mesoporous silica were enhanced by loading metals into the silica structure [23, 24]. Aluminium is the principal metal-doped into the structure of mesoporous silica to enhance catalytic cracking [199]. For instance, by optimizing the Al/Si ratio, the new mesoporous alumina–silica catalyst is created with high acid properties and high surface area. Similarly, different metals including Co, Sn, and Zr are used to prepare high-performing mesoporous silica catalysts [200].
Jeon et.al. [201] studied the application of mesoporous Pt and Al within SBA-15 support catalysts for catalytic pyrolysis of cellulose, hemicellulose, and lignin. AlSBA-15 and Pt/AlSBA-15 showed better catalytic performance than SBA-15 and Pt/SBA-15. In particular, Pt/AlSBA-15 showed a high yield (65 wt.%) for aromatics and furans. The presence of both acid sites and Pt are responsible for the conversion of levoglucosan into aromatics and furans during catalytic upgrading. Pd/SBA-15 revealed a better selectivity for the production of phenol from lignin-derived oligomers [202]. As shown in Fig. 3, the lignin depolymerized into monomeric phenols that were further converted to phenols without the side chain and unsaturated C▬C bond [203, 204]. The incorporation of acidity or alkalinity in the structure of mesoporous silica is likewise a promising methodology to duplicate its applications in catalysis [205]. The pore volume of mesoporous silica gives sufficient space to accommodate these species [206]. Table 7 presents a concise conclusion for the advantages and disadvantages of using mesoporous catalysts for bio-oil synthesis.
Biomass waste (sawdust) was also used to produce a highly efficient magnetic solid-acid catalyst through a fast pyrolysis–sulphonation process [213]. First, the Fe3+ ions were adsorbed into the biomass waste to achieve Fe-loaded biomass, then pyrolysis to produce biochar. Finally, solid-acid magnetic porous catalyst was prepared via sulfonation method from the biochar. The fast pyrolysis method induced reduction of Fe3+ to Fe3O4 and incorporated magnetism into the material, which was kept after sulfonation. The catalyst exhibits a surface area of 296.4 m2/g and acidity of 2.57 mmol/g. The catalyst had notable catalytic activity, including dehydration, esterification, and hydrolysis for distinct acid catalytic reactions. A furfural yield of 6% in dimethyl sulfoxide (DMSO) was obtained at 150°C with a xylose conversion of 96%. The sulfonated catalyst was less active, producing only 45% furfural under the same conditions, due to its lower acidity of 1.26 mmol/g than the iron catalyst. The catalyst was also extremely efficient in producing 94% glucose and fructose [213].
5 Advanced analytical techniques for bio-oil characterisation
Evaluating bio-oil chemical and physical characteristics is a significant process to decide future applications as well as upgrading techniques to improve the composition. Bio-oil physical characteristics, such as viscosity, pH, ash content, moisture content, cetane index, refractive index, heating values, and elemental composition, can be performed accurately by the existing standards procedure. However, qualitative and quantitative analysis of chemical properties remains challenging. The complexity and number of compounds in bio-oil require multiple analytical methods for its chemical characterisation. Therefore, the spectroscopic and chromatographic techniques are implemented and interpreted as complementary. Nuclear magnetic resonance (NMR), thermogravimetric analysis (TGA), gel permeation chromatography (GPC), gas chromatography (GC), and Fourier-transform infrared spectroscopy (FTIR) have been used to elucidate the chemical characteristics of bio-oils at distinct levels [214]. Amongst the analytical technique, NMR spectroscopy and GC are the most comprehensive techniques to characterise bio-oil components. The next section will discuss the application of NMR, GC, and TGA for bio-oil chemical analysis.
5.1 Gas chromatography
Gas chromatography (GC) is a widely used separation method to identify thermally stable volatile compounds. The flame ionisation detector (FID) and thermal conductivity detector (TCD) have been used for GC detector because of highly sensitive, rapid response, and a wide range of linear dynamics. GC-FID can be used for bio-oil composition characterisation such as phenols, aldehydes, alcohols organic acids, sugars, and ketones [215]. Additionally, GC-FID has been used to estimate the concentrations of compounds in biochar from the noncondensate stream during pyrolysis [216]. A conventional 1-D GC is usually used to separate bio-oil in a nonpolar or a weak-polar column depending on the boiling point or vapour pressure [217,218,219].
5.1.1 Conventional gas chromatography (1-D GC)
Conventional 1-D GC techniques employed in bio-oil characterisation are based on bio-oil compounds boiling point and volatility [220, 221]. Also, the type of detector, polarity difference between the molecules and their interaction with column material are an important consideration. Types of solvent used during sample preparation should not affect the early eluting of bio-oil fractions. However, most solvents except acetone hinder separation, such as chloroform, tetrahydrofuran, and ethyl acetate elute along with low molecular weight fraction of bio-oil [222]. Co-elutions of solvent and bio-oil fractions hinder absolute quantification while using FID and TCD. Therefore, combining GC with mass spectrometry (GC-MS) will help to accurately identify peaks, which are not detected by FID such as alkanes, C5–C15 hydrocarbon, 2-methoxy and phenols [223].
1-D GC mostly uses nonpolar or slightly polar column for boil-oil characterisation, which oversight polar fraction [218, 219]. Therefore, it requires additional GC columns to quantify both nonpolar and polar compounds. 1-D GC identifies a small portion of high molecular weight or nonvolatile polar fractions of bio-oil due to the low volatility nature of the compounds [224, 225]. Derivatization enhances the detectability of nonvolatile polar fractions by converting into volatile low polarity derivatives using derivatization reagents [226]. Silylation and N-methyl N-(trimethylsilyl) trifluoroacetamide are amongst the most used derivation agents for quantification of hydroxyl and carboxyl groups compounds [227, 228].
5.1.2 Two-dimensional gas chromatography (2-D GC)
2-D GC (GC × GC) analytical technique employs two independent columns with different polarity for GC separation, superior peak detection and resolution [229]. Bio-oil analysis conducted by 2-D GC identified 70% of chromatograph; however, 1-D GC only identified about 47% [222]. Quantification of bio-oil components with 2-D GC depends on the type of columns and the modulator. Typically, the first column is nonpolar or slightly polar, while the second column is polar [230]. Bio-oil fractions are separated by their volatility in the first column, while the second column separates via hydrogen-bonding, π–π interactions, and steric effects [231]. The chromatographic resolution of 2-D GC technique can be improved by increasing resolution in the first column and optimizing split-flow [232]. Analysing all bio-oil fractions using a single analytical is almost impossible, therefore there is a need to combine GC with other technique such as NMR to better understand the chemical and molecular weight properties of bio-oil samples. Gas chromatography is an effective technique to analysis volatile components in bio-oil; however, analysing higher molecular weight molecules of bio-oil is still a challenge.
5.2 Nuclear magnetic resonance (NMR) spectroscopy
NMR spectroscopy provides structural information of high molecular weight compounds in bio-oil. NMR is a powerful technique to analyse bio-oil functional groups such as aromatic, carbonyl, olefin, aliphatic, and methoxy/hydroxyl from the integration of appropriate chemical shift regions [233, 234]. The advantages of NMR over other spectroscopy are its simplicity, short analysis time, and ability to acquire information about the bio-oil composition from a single spectrum [235]. Hydrogen (1H) and carbon (13C) NMR techniques are widely used to analyse the hydrogen-carbon framework of bio-oil. Accuracy and repeatability of NMR analysis depend on solvent, baseline compensation, selection of chemical-shift regions, and longitudinal relaxation [236]. Polar solvents are mainly used for analysis of bio-oil components such as furan, ketones, phenols, and organic acids [235]. During sample preparation dried bio-oil is dissolved in polar deuterated solvents such as dimethylsulfoxide (DMSO-d6), deuterated dichloromethane (DCM-d2), deuterium oxide (D2O), and ethanol-d2. The hydrogen bond strength of polar solvent affects NMR analysis of bio-oil, solvents such as ethanol, dichloromethane, and water, exhibit strong hydrogen bonding, but DMSO-d6 exhibits much less hydrogen proton shifts. Also, solvent signals such as CDCl3 (13C NMR 77.00 ppm and 1H NMR 7.25 ppm) overlap with aromatic group chemical shift region and interfere with quantification of bio-oil fractions. Therefore, the use of DMSO-d6 as solvent allows the collection of chemical structure information from both 13C and 1H NMR [218]. In the following section, the application of 1H and 13C NMR for bio-oil analysis with chemical shift assignments will be discussed.
5.2.1 1H NMR
1H NMR is the most extensively and convenient spectrometric technique used to quantify the oxygenated compounds in bio-oil [237]. The abundance of hydrogen atoms (major isotope 1H) in an organic compound makes 1H NMR spectroscopy analysis sensitive to identifying bio-oil constituents. This technique is characterised by fast analysis and high sensitivity [238]. Table 8 summarizes the major chemical shifts of bio-oil components and the hydrogen percentage of bio-oil obtained from noncatalytic and ZSM-5 catalytic pyrolysis of pinewood. Bio-oil produced with ZSM-5 catalyst contained more hydrogen from ethers (3.0–4.2 ppm) than the noncatalyst pyrolysis bio-oil. The chemical shift range of 9.5–11.0 ppm is assigned to aldehydes and phenols while carboxylic acid proton is assigned to the range of 11.0–12.5 ppm. The spectral overlap of aldehydes and phenols in the region from 9.5 to 11.0 ppm made the quantification of phenols difficult because of low resolution and chemical shift overlaps of 1H NMR. Therefore, it is required to use several characterisation techniques simultaneously to obtain a full insight into bio-oil composition. However, the chemical shift of the hydrogen atom on alkanes and aromatic groups shows clear signals making 1H NMR spectroscopy suitable for the analysis of aromatic ring rich bio-oil.
1H NMR spectroscopy can also explain the effect of biomass types in the overall chemical composition of bio-oil. Mullen et al. [234] used 1H NMR to characterise bio-oil from different energy crops and categorised bio-oil composition based on the hydrogen atom percentage. 1H NMR is an essential and sensitive technique for determining hydrogen distributions in bio-oil; however, chemical shift ranges are not well-known because of several overlaps. Therefore, to obtain distinguished chemical shift range, the 1H NMR spectrum should complement additional NMR techniques such as 13C NMR spectroscopy.
5.2.2 13C NMR
13C NMR provides a quantitative analysis of carbon atoms in the different functional groups, which can be used as complementary information for bio-oil characterisation [240]. The low natural abundance of 13C atom makes 13C NMR spectroscopy less sensitive, therefore it provides a better signal to noise ratio by accumulating large numbers of transient [241]. The 13C NMR chemical shift of carbon atom from various compounds in bio-oil is summarized in Table 9. The region between 1 and 60 ppm corresponds to alkyl hydrocarbons, which enhance the energy content of bio-oil [232]. The region between 50 and 65 ppm provides information about hydroxyl or methoxy functional groups in bio-oil while the region from 65 to 105 ppm explains carbohydrate (levoglucosan) in bio-oil. 13C NMR spectra between 150 and 215 ppm resonates with the presence of acid, ketones, esters, and aldehydes. 13C NMR spectroscopy techniques provide valuable qualitative analysis; however, spectra overlap occurs due to bio-oil complexity, limiting its application. Therefore, it is required to correlate both 13C NMR and 1H NMR spectra information to obtain a better insight into the overlapping regions.
5.3 Thermal analysis
Thermal properties of bio-oil are studied using thermogravimetry (TGA) and its derivatives (DTG). TGA measures weight losses based on the volatility of molar fractions against the temperature or time at a specific heating rate. Thermal degradation takes place in three stages: the first stage corresponds to carbon dioxide, carbon monoxide, and water removal from the feedstock at a temperature less than 200°C; the primary degradation occurs in the second stage at a temperature between 460°C and 680°C; and the final stage of decomposition occurs at slow reaction rate at a temperature higher than 680°C [243].
TGA analysis helps on the characterisation of evaporation, combustion, and thermal degradation of bio-oil. Also, TGA analysis of biomass generates information about carbon, water, ash, cellulose, hemicellulose, and lignin content that can be used to enhance the quality and composition of bio-oil. The percentage of weight loss in a region during the thermal analysis of biomass provides information about the reactivity; for example, biomass containing high lignin content showed low reactivity resulting in high biochar production. TGA data is also used to optimize bio-oil yield by analysing the ash content in different biomass, where higher ash content corresponds to lower bio-oil yield [244]. TGA has further been used to determine the amount of coke deposited in porous catalysts such as zeolites. The formation of coke on internal and external surfaces of catalysts causes catalyst deactivation and reduces catalyst activity for bio-oil upgrading [245].
TGA analysis has frequently been used to determine chemical kinetic parameters such as a preexponential factor (A) and activation energy (E) using different modelling methods [246]. Modelling of chemical kinetics uses TGA analysis conducted via nonisothermal and isothermal with multiple and single heating rates [247, 248]. However, more than one reaction pathway is considered to study kinetic parameters of thermal decomposition of biomass [249, 250].
6 Conclusions
Biomass is a renewable source and potential fossil fuel replacement due to its availability, lower processing cost, higher conversion, and lower carbon emissions. Pyrolysis is an attractive and flexible process of converting biomass into bio-oil, which can be utilized for the production of energy and chemicals. However, bio-oil obtained from biomass pyrolysis process is not suitable for fossil fuel substitution due to the high amount of oxygenate compounds (i.e., phenols, ketones, aromatic hydrocarbons, sugars, alcohols). Therefore, there is a need to upgrade bio-oil properties by converting the oxygenated compounds into aromatic hydrocarbons. Catalysts have been used to upgrade bio-oil properties, but not all the desired properties of a fuel have been achieved yet. According to our literature review, most catalytic upgrading of bio-oil has been carried out via a monocatalytic system (acid or base catalysts), which is unable to address all oxygenated compounds available in the bio-oil. Also, catalyst deactivation over coke deposition and sintering promote lower catalytic activity. Future research should focus on synthesising robust bifunctional catalysts to address both acidic and alkaline bio-oil fractions.
In addition, hierarchical zeolites have been used to enhance the bio-oil quality. The sequential dealumination–desilication process is used to create additional mesoporosity in the zeolite framework. However, the optimum amount of mesoporosity for high bio-oil quality is still unknown, so we recommend that future research should be focused on studying the effect of both mesoporosity and loading of metal oxides on bio-oil quality and yield. Analysing chemical composition of bio-oil is fundamental for the optimisation of the pyrolysis process and its application as alternative energy source. NMR spectroscopy and GC techniques are mainly used to obtain structural and molecular weight information. To obtain a comprehensive understanding of bio-oil molecular fractions, combining both analytical techniques is required. Further work should be carried out to better understand the impact of pyrolysis processing parameters on bio-oil composition using statistical techniques.
References
Bridgwater AV (2003) Renewable fuels and chemicals by thermal processing of biomass. Chem Eng J 91(2):87–102
Asadullah M et al (2013) Production and detailed characterization of bio-oil from fast pyrolysis of palm kernel shell. Biomass Bioenergy 59:316–324
Kang Q et al (2014) Bioethanol from lignocellulosic biomass: current findings determine research priorities. Sci World J 2014:298153
Kobayashi N, Fan L-S (2011) Biomass direct chemical looping process: a perspective. Biomass Bioenergy 35(3):1252–1262
Panwar NL, Kothari R, Tyagi VV (2012) Thermo chemical conversion of biomass—eco friendly energy routes. Renew Sust Energ Rev 16(4):1801–1816
Torres W, Pansare SS, Goodwin JG (2007) Hot gas removal of tars, ammonia, and hydrogen sulfide from biomass gasification gas. Catal Rev 49(4):407–456
Morrin S et al (2012) Two stage fluid bed-plasma gasification process for solid waste valorisation: technical review and preliminary thermodynamic modelling of sulphur emissions. Waste Manag 32(4):676–684
Antunes E et al (2017) Biochar produced from biosolids using a single-mode microwave: characterisation and its potential for phosphorus removal. J Environ Manag 196:119–126
Di Blasi C et al (2001) Pyrolytic behavior and products of some wood varieties. Combust Flame 124(1):165–177
Mythili R et al (2013) Characterization of bioresidues for biooil production through pyrolysis. Bioresour Technol 138:71–78
Laird DA et al (2009) Review of the pyrolysis platform for coproducing bio-oil and biochar. Biofuels Bioprod Biorefin 3(5):547–562
Chaiwong K et al (2013) Study of bio-oil and bio-char production from algae by slow pyrolysis. Biomass Bioenergy 56:600–606
Alvarez J et al (2014) Bio-oil production from rice husk fast pyrolysis in a conical spouted bed reactor. Fuel 128:162–169
Iliopoulou EF, Triantafyllidis KS, Lappas AA (2019) Overview of catalytic upgrading of biomass pyrolysis vapors toward the production of fuels and high-value chemicals. Energy Environ 8(1):e322
Wang K, Johnston PA, Brown RC (2014) Comparison of in-situ and ex-situ catalytic pyrolysis in a micro-reactor system. Bioresour Technol 173:124–131
Stephanidis S et al (2011) Catalytic upgrading of lignocellulosic biomass pyrolysis vapours: effect of hydrothermal pre-treatment of biomass. Catal Today 167(1):37–45
Stefanidis S et al (2011) In-situ upgrading of biomass pyrolysis vapors: catalyst screening on a fixed bed reactor. Bioresour Technol 102(17):8261–8267
van Donk S et al (2003) Generation, characterization, and impact of mesopores in zeolite catalysts. Catal Rev 45(2):297–319
Deng Y et al (2013) Large-pore ordered mesoporous materials templated from non-Pluronic amphiphilic block copolymers. Chem Soc Rev 42(9):4054–4070
Li W et al (2013) Ordered mesoporous materials based on interfacial assembly and engineering. Adv Mater 25(37):5129–5152
Guo X et al (2009) Analysis of coke precursor on catalyst and study on regeneration of catalyst in upgrading of bio-oil. Biomass Bioenergy 33(10):1469–1473
Shi Y et al (2017) Recent progress on upgrading of bio-oil to hydrocarbons over metal/zeolite bifunctional catalysts. Catal Sci Technol 7(12):2385–2415
Yang Y et al (2016) Ce-promoted Ni/SBA-15 catalysts for anisole hydrotreating under mild conditions. Appl Catal B Environ 197:206–213
Linares N et al (2011) Incorporation of chemical functionalities in the framework of mesoporous silica. Chem Commun 47(32):9024–9035
Nava R et al (2009) Upgrading of bio-liquids on different mesoporous silica-supported CoMo catalysts. Appl Catal B Environ 92(1):154–167
Han T et al (2019) Catalytic pyrolysis of lignin using low-cost materials with different acidities and textural properties as catalysts. Chem Eng J 373:846–856
Balat M (2011) Production of bioethanol from lignocellulosic materials via the biochemical pathway: a review. Energy Convers Manag 52(2):858–875
Tsai W, Lee M, Chang Y (2006) Fast pyrolysis of rice straw, sugarcane bagasse and coconut shell in an induction-heating reactor. J Anal Appl Pyrolysis 76(1-2):230–237
Dhyani V, Bhaskar T (2018) A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew Energy 129:695–716
Imam T, Capareda S (2012) Characterization of bio-oil, syn-gas and bio-char from switchgrass pyrolysis at various temperatures. J Anal Appl Pyrolysis 93:170–177
Chang S et al (2013) Effect of hydrothermal pretreatment on properties of bio-oil produced from fast pyrolysis of eucalyptus wood in a fluidized bed reactor. Bioresour Technol 138:321–328
Abdullah N, Gerhauser H (2008) Bio-oil derived from empty fruit bunches. Fuel 87(12):2606–2613
Piskorz J et al (1988) Liquid products from the fast pyrolysis of wood and cellulose. In: Research in thermochemical biomass conversion. Springer, p 557–571
Isahak WNRW et al (2012) A review on bio-oil production from biomass by using pyrolysis method. Renew Sust Energ Rev 16(8):5910–5923
Braga RM et al (2014) Characterization and comparative study of pyrolysis kinetics of the rice husk and the elephant grass. J Therm Anal Calorim 115(2):1915–1920
Shi X, Wang J (2014) A comparative investigation into the formation behaviors of char, liquids and gases during pyrolysis of pinewood and lignocellulosic components. Bioresour Technol 170:262–269
Mullen CA, Boateng AA (2008) Chemical composition of bio-oils produced by fast pyrolysis of two energy crops. Energy Fuel 22(3):2104–2109. https://doi.org/10.1021/ef700776w
Prasad S, Singh A, Joshi H (2007) Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resour Conserv Recycl 50(1):1–39
Stefanidis SD et al (2014) A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J Anal Appl Pyrolysis 105:143–150
Zhang L et al (2018) Catalytic pyrolysis of biomass and polymer wastes. Catalysts 8(12):659
Tsai W, Lee M, Chang Y (2007) Fast pyrolysis of rice husk: product yields and compositions. Bioresour Technol 98(1):22–28
Torri C et al (2010) Comparative analysis of pyrolysate from herbaceous and woody energy crops by Py-GC with atomic emission and mass spectrometric detection. J Anal Appl Pyrolysis 88(2):175–180
Zanzi R, Sjöström K, Björnbom E (2002) Rapid pyrolysis of agricultural residues at high temperature. Biomass Bioenergy 23(5):357–366
Chen Z et al (2015) Pyrolysis behaviors and kinetic studies on Eucalyptus residues using thermogravimetric analysis. Energy Convers Manag 105:251–259
Elliott DC et al (2009) Catalytic hydroprocessing of biomass fast pyrolysis bio-oil to produce hydrocarbon products. Environ Prog Sustain Energy 28(3):441–449
Şensöz S, Can M (2002) Pyrolysis of pine (Pinus brutia Ten.) chips: 1. Effect of pyrolysis temperature and heating rate on the product yields. Energy Sources 24(4):347–355
Lyu G, Wu S, Zhang H (2015) Estimation and comparison of bio-oil components from different pyrolysis conditions. Front Energy Res 3:28
Boateng AA et al (2007) Bench-scale fluidized-bed pyrolysis of switchgrass for bio-oil production. Ind Eng Chem Res 46(7):1891–1897
Bartoli M et al (2016) Production of bio-oils and bio-char from Arundo donax through microwave assisted pyrolysis in a multimode batch reactor. J Anal Appl Pyrolysis 122:479–489
Chen D, Zhou J, Zhang Q (2014) Effects of heating rate on slow pyrolysis behavior, kinetic parameters and products properties of moso bamboo. Bioresour Technol 169:313–319
Li LIN, Zhang H (2005) Production and characterization of pyrolysis oil from herbaceous biomass (Achnatherum Splendens). Energy Sources 27(4):319–326
Sahoo D et al (2019) Value-addition of water hyacinth and para grass through pyrolysis and hydrothermal liquefaction. Carbon Resour Conver 2(3):233–241
Kojima Y et al (2015) Pyrolysis characteristic of kenaf studied with separated tissues, alkali pulp, and alkali lignin. Biofuel Res J 8:317–323
Cao J-P et al (2011) Preparation and characterization of bio-oils from internally circulating fluidized-bed pyrolyses of municipal, livestock, and wood waste. Bioresour Technol 102(2):2009–2015
Heo HS et al (2010) Bio-oil production from fast pyrolysis of waste furniture sawdust in a fluidized bed. Bioresour Technol 101(1):S91–S96
Weldekidan H et al (2019) Energy conversion efficiency of pyrolysis of chicken litter and rice husk biomass. Energy Fuel 33(7):6509–6514
Setter C et al (2020) Energy quality of pellets produced from coffee residue: characterization of the products obtained via slow pyrolysis. Ind Crop Prod 154:112731
Luo Z et al (2004) Research on biomass fast pyrolysis for liquid fuel. Biomass Bioenergy 26(5):455–462
Adamczyk M, Sajdak M (2018) Pyrolysis behaviours of microalgae Nannochloropsis gaditana. Waste Biomass Valoriz 9(11):2221–2235
Yang W et al (2014) Direct hydrothermal liquefaction of undried macroalgae Enteromorpha prolifera using acid catalysts. Energy Convers Manag 87:938–945
Alper K, Tekin K, Karagöz S (2015) Pyrolysis of agricultural residues for bio-oil production. Clean Techn Environ Policy 17(1):211–223
Hameed Z et al (2021) Gasification of municipal solid waste blends with biomass for energy production and resources recovery: current status, hybrid technologies and innovative prospects. Renew Sust Energ Rev 136:110375
Zhang Q, Yang Z, Wu W (2008) Role of crop residue management in sustainable agricultural development in the North China Plain. J Sustain Agric 32(1):137–148
Torres-Mayanga PC et al (2019) Production of biofuel precursors and value-added chemicals from hydrolysates resulting from hydrothermal processing of biomass: a review. Biomass Bioenergy 130:105397
Zhang L, Xu C, Champagne P (2010) Overview of recent advances in thermo-chemical conversion of biomass. Energy Convers Manag 51(5):969–982
Bridgwater A (2001) Thermal conversion of biomass and waste: the status. Bio-Energy Research Group, Aston University, Birmingham
Effendi A, Gerhauser H, Bridgwater AV (2008) Production of renewable phenolic resins by thermochemical conversion of biomass: a review. Renew Sust Energ Rev 12(8):2092–2116
Amen-Chen C, Pakdel H, Roy C (2001) Production of monomeric phenols by thermochemical conversion of biomass: a review. Bioresour Technol 79(3):277–299
Jenkins B et al (1998) Combustion properties of biomass. Fuel Process Technol 54(1-3):17–46
Sikarwar VS et al (2017) Progress in biofuel production from gasification. Prog Energy Combust Sci 61:189–248
Damartzis T, Zabaniotou A (2011) Thermochemical conversion of biomass to second generation biofuels through integrated process design—a review. Renew Sust Energ Rev 15(1):366–378
Gopirajan PV, Gopinath KP, Sivaranjani G, Arun J (2021) Optimization of hydrothermal liquefaction process through machine learning approach: process conditions and oil yield. Biomass Conv Bioref 1–10. https://doi.org/10.1007/s13399-020-01233-8
Barreiro DL et al (2013) Hydrothermal liquefaction (HTL) of microalgae for biofuel production: state of the art review and future prospects. Biomass Bioenergy 53:113–127
Ruiz JA et al (2013) Biomass gasification for electricity generation: review of current technology barriers. Renew Sust Energ Rev 18:174–183
Franco C et al (2003) The study of reactions influencing the biomass steam gasification process☆. Fuel 82(7):835–842
Chan YH et al (2019) An overview of biomass thermochemical conversion technologies in Malaysia. Sci Total Environ 680:105–123
Jiang X et al (2014) Investigation into advantage of pyrolysis over combustion of sewage sludge in PCDD/Fs control. Fresenius Environ Bull 23(2a):550–557
Collard F-X, Blin J (2014) A review on pyrolysis of biomass constituents: mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew Sust Energ Rev 38:594–608
McGrath TE, Chan WG, Hajaligol MR (2003) Low temperature mechanism for the formation of polycyclic aromatic hydrocarbons from the pyrolysis of cellulose. J Anal Appl Pyrolysis 66(1-2):51–70
Van de Velden M et al (2010) Fundamentals, kinetics and endothermicity of the biomass pyrolysis reaction. Renew Energy 35(1):232–242
Collard F-X et al (2012) Influence of impregnated metal on the pyrolysis conversion of biomass constituents. J Anal Appl Pyrolysis 95:213–226
Garcia-Perez M et al (2007) Characterization of bio-oils in chemical families. Biomass Bioenergy 31(4):222–242
López MB et al (2002) Composition of gases released during olive stones pyrolysis. J Anal Appl Pyrolysis 65(2):313–322
Basu P (2010) Biomass gasification and pyrolysis: practical design and theory. Academic press, Cambridge
Guedes RE, Luna AS, Torres AR (2018) Operating parameters for bio-oil production in biomass pyrolysis: A review. J Anal Appl Pyrolysis 129:134–149
Goyal H, Seal D, Saxena R (2008) Bio-fuels from thermochemical conversion of renewable resources: a review. Renew Sust Energ Rev 12(2):504–517
Zhang Q, Wang T, Wu V, Ma L, Xu Y (2010) Fractioned preparation of bio-oil by biomass vacuum pyrolysis. Int J Green Energy 7(3):263–272. https://doi.org/10.1080/15435071003795972
Resende FLP (2016) Recent advances on fast hydropyrolysis of biomass. Catal Today 269:148–155
Stamatov V, Honnery D, Soria J (2006) Combustion properties of slow pyrolysis bio-oil produced from indigenous Australian species. Renew Energy 31(13):2108–2121
Hagner M et al (2020) Performance of liquids from slow pyrolysis and hydrothermal carbonization in plant protection. Waste Biomass Valoriz 11(3):1005–1016
Antal MJ, Grønli M (2003) The art, science, and technology of charcoal production. Ind Eng Chem Res 42(8):1619–1640
Eke J, Onwudili JA, Bridgwater AV (2019) Influence of moisture contents on the fast pyrolysis of trommel fines in a bubbling fluidized bed reactor. Waste Biomass Valoriz 11(2):1–12
Balat M et al (2009) Main routes for the thermo-conversion of biomass into fuels and chemicals. Part 1: Pyrolysis systems. Energy Convers Manag 50(12):3147–3157
Bridgwater AV, Peacocke GVC (2000) Fast pyrolysis processes for biomass. Renew Sust Energ Rev 4(1):1–73
Heo HS et al (2010) Fast pyrolysis of rice husk under different reaction conditions. J Ind Eng Chem 16(1):27–31
Wei L et al (2006) Characteristics of fast pyrolysis of biomass in a free fall reactor. Fuel Process Technol 87(10):863–871
Czernik S, Bridgwater A (2004) Overview of applications of biomass fast pyrolysis oil. Energy Fuel 18(2):590–598
Venderbosch R, Prins W (2010) Fast pyrolysis technology development. Biofuels Bioprod Biorefin 4(2):178–208
Li Y, Khanal SK (2016) Bioenergy: principles and applications. John Wiley & Sons, Hoboken
Garcìa-Pérez M et al (2007) Vacuum pyrolysis of softwood and hardwood biomass: comparison between product yields and bio-oil properties. J Anal Appl Pyrolysis 78(1):104–116
Singh NR et al (2010) Estimation of liquid fuel yields from biomass. Environ Sci Technol 44(13):5298–5305
Galiasso R, González Y, Lucena M (2014) New inverted cyclone reactor for flash hydropyrolysis. Catal Today 220:186–197
Akhtar J, Saidina Amin N (2012) A review on operating parameters for optimum liquid oil yield in biomass pyrolysis. Renew Sust Energ Rev 16(7):5101–5109
Beis S, Onay Ö, Koçkar Ö (2002) Fixed-bed pyrolysis of safflower seed: influence of pyrolysis parameters on product yields and compositions. Renew Energy 26(1):21–32
Angın D (2013) Effect of pyrolysis temperature and heating rate on biochar obtained from pyrolysis of safflower seed press cake. Bioresour Technol 128:593–597
Fu P et al (2011) Effect of temperature on gas composition and char structural features of pyrolyzed agricultural residues. Bioresour Technol 102(17):8211–8219
Açıkalın K, Karaca F, Bolat E (2012) Pyrolysis of pistachio shell: effects of pyrolysis conditions and analysis of products. Fuel 95:169–177
Sricharoenchaikul V, Atong D (2009) Thermal decomposition study on Jatropha curcas L. waste using TGA and fixed bed reactor. J Anal Appl Pyrolysis 85(1):155–162
Lam SS et al (2012) Microwave-heated pyrolysis of waste automotive engine oil: influence of operation parameters on the yield, composition, and fuel properties of pyrolysis oil. Fuel 92(1):327–339
Pütün AE, Apaydın E, Pütün E (2004) Rice straw as a bio-oil source via pyrolysis and steam pyrolysis. Energy 29(12-15):2171–2180
Paenpong C, Pattiya A (2016) Effect of pyrolysis and moving-bed granular filter temperatures on the yield and properties of bio-oil from fast pyrolysis of biomass. J Anal Appl Pyrolysis 119:40–51
Ji-lu Z (2007) Bio-oil from fast pyrolysis of rice husk: yields and related properties and improvement of the pyrolysis system. J Anal Appl Pyrolysis 80(1):30–35
Bridgewater AV (2004) Biomass fast pyrolysis. Therm Sci 8(2):21–50
Ateş F, Pütün E, Pütün A (2004) Fast pyrolysis of sesame stalk: yields and structural analysis of bio-oil. J Anal Appl Pyrolysis 71(2):779–790
Tripathi M, Sahu JN, Ganesan P (2016) Effect of process parameters on production of biochar from biomass waste through pyrolysis: a review. Renew Sust Energ Rev 55:467–481
Varma AK, Mondal P (2017) Pyrolysis of sugarcane bagasse in semi batch reactor: effects of process parameters on product yields and characterization of products. Ind Crop Prod 95:704–717
Bhoi PR et al (2020) Recent advances on catalysts for improving hydrocarbon compounds in bio-oil of biomass catalytic pyrolysis. Renew Sust Energ Rev 121:109676
Antunes E et al (2018) Microwave pyrolysis of sewage biosolids: dielectric properties, microwave susceptor role and its impact on biochar properties. J Anal Appl Pyrolysis 129:93–100
Jahirul MI et al (2012) Biofuels production through biomass pyrolysis—a technological review. Energies 5(12):4952–5001
Choi HS, Choi YS, Park HC (2012) Fast pyrolysis characteristics of lignocellulosic biomass with varying reaction conditions. Renew Energy 42:131–135
Mohamed AR et al (2013) The effects of holding time and the sweeping nitrogen gas flowrates on the pyrolysis of EFB using a fixed-bed reactor. Proc Eng 53:185–191
Strezov V, Moghtaderi B, Lucas J (2003) Thermal study of decomposition of selected biomass samples. J Therm Anal Calorim 72(3):1041–1048
Debdoubi A et al (2006) The effect of heating rate on yields and compositions of oil products from esparto pyrolysis. Int J Energy Res 30(15):1243–1250
Huber GW, Iborra S, Corma A (2006) Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem Rev 106(9):4044–4098
Vardon DR et al (2013) Complete utilization of spent coffee grounds to produce biodiesel, bio-oil, and biochar. ACS Sustain Chem Eng 1(10):1286–1294
Jacobson K, Maheria KC, Kumar Dalai A (2013) Bio-oil valorization: a review. Renew Sust Energ Rev 23:91–106
Oasmaa A, Czernik S (1999) Fuel oil quality of biomass pyrolysis oils state of the art for the end users. Energy Fuel 13(4):914–921
Dickerson T, Soria J (2013) Catalytic fast pyrolysis: a review. Energies 6(1):514–538
Kumar R, Strezov V (2021) Thermochemical production of bio-oil: a review of downstream processing technologies for bio-oil upgrading, production of hydrogen and high value-added products. Renew Sust Energ Rev 135:110152
Clauser NM et al (2021) Biomass waste as sustainable raw material for energy and fuels. Sustainability 13(2):794
Demirbas A (2007) The influence of temperature on the yields of compounds existing in bio-oils obtained from biomass samples via pyrolysis. Fuel Process Technol 88(6):591–597
Onay O (2007) Influence of pyrolysis temperature and heating rate on the production of bio-oil and char from safflower seed by pyrolysis, using a well-swept fixed-bed reactor. Fuel Process Technol 88(5):523–531
Boucher M, Chaala A, Roy C (2000) Bio-oils obtained by vacuum pyrolysis of softwood bark as a liquid fuel for gas turbines. Part I: properties of bio-oil and its blends with methanol and a pyrolytic aqueous phase. Biomass Bioenergy 19(5):337–350
Diebold JP (1999) A review of the chemical and physical mechanisms of the storage stability of fast pyrolysis bio-oils. National Renewable Energy Lab, Golden
Cai J et al (2016) Viscosity of aged bio-oils from fast pyrolysis of beech wood and Miscanthus: shear rate and temperature dependence. Energy Fuel 30(6):4999–5004
Meng J et al (2015) Thermal and storage stability of bio-oil from pyrolysis of torrefied wood. Energy Fuel 29(8):5117–5126
Zhang Q et al (2007) Review of biomass pyrolysis oil properties and upgrading research. Energy Convers Manag 48(1):87–92
Thangalazhy-Gopakumar S et al (2010) Physiochemical properties of bio-oil produced at various temperatures from pine wood using an auger reactor. Bioresour Technol 101(21):8389–8395
Park Y-K et al (2012) Wild reed of Suncheon Bay: potential bio-energy source. Renew Energy 42:168–172
Kim J-S (2015) Production, separation and applications of phenolic-rich bio-oil–a review. Bioresour Technol 178:90–98
Wei Y et al (2014) Liquid–liquid extraction of biomass pyrolysis bio-oil. Energy Fuel 28(2):1207–1212
Fini EH et al (2011) Chemical characterization of biobinder from swine manure: sustainable modifier for asphalt binder. J Mater Civ Eng 23(11):1506–1513
Zhang S et al (2019) Liquefaction of biomass and upgrading of bio-oil: a review. Molecules 24(12):2250
Mathimani T et al (2019) Review on cultivation and thermochemical conversion of microalgae to fuels and chemicals: process evaluation and knowledge gaps. J Clean Prod 208:1053–1064
Shan Ahamed T et al (2021) Upgrading of bio-oil from thermochemical conversion of various biomass—mechanism, challenges and opportunities. Fuel 287:119329
Gollakota ARK et al (2016) A review on the upgradation techniques of pyrolysis oil. Renew Sust Energ Rev 58:1543–1568
Wang S et al (2017) Lignocellulosic biomass pyrolysis mechanism: a state-of-the-art review. Prog Energy Combust Sci 62:33–86
Aho A et al (2010) Catalytic upgrading of woody biomass derived pyrolysis vapours over iron modified zeolites in a dual-fluidized bed reactor. Fuel 89(8):1992–2000
Foster AJ et al (2012) Optimizing the aromatic yield and distribution from catalytic fast pyrolysis of biomass over ZSM-5. Appl Catal A Gen 423-424:154–161
Ruddy DA et al (2014) Recent advances in heterogeneous catalysts for bio-oil upgrading via “ex situ catalytic fast pyrolysis”: catalyst development through the study of model compounds. Green Chem 16(2):454–490
Luo G, Resende FL (2016) In-situ and ex-situ upgrading of pyrolysis vapors from beetle-killed trees. Fuel 166:367–375
Wan S, Wang Y (2014) A review on ex situ catalytic fast pyrolysis of biomass. Front Chem Sci Eng 8(3):280–294
Gamliel DP et al (2015) Investigation of in situ and ex situ catalytic pyrolysis of miscanthus × giganteus using a PyGC–MS microsystem and comparison with a bench-scale spouted-bed reactor. Bioresour Technol 191:187–196
Stefanidis SD et al (2016) Catalyst hydrothermal deactivation and metal contamination during the in situ catalytic pyrolysis of biomass. Catal Sci Technol 6(8):2807–2819
Shirazi Y, Viamajala S, Varanasi S (2020) In situ and ex situ catalytic pyrolysis of microalgae and integration with pyrolytic fractionation. Front Chem 8:786
Hemberger P et al (2017) Understanding the mechanism of catalytic fast pyrolysis by unveiling reactive intermediates in heterogeneous catalysis. Nat Commun 8:15946
Rahman MM, Liu R, Cai J (2018) Catalytic fast pyrolysis of biomass over zeolites for high quality bio-oil—a review. Fuel Process Technol 180:32–46
Adjaye J, Bakhshi N (1995) Production of hydrocarbons by catalytic upgrading of a fast pyrolysis bio-oil. Part II: Comparative catalyst performance and reaction pathways. Fuel Process Technol 45(3):185–202
Nie L et al (2014) Selective conversion of m-cresol to toluene over bimetallic Ni–Fe catalysts. J Mol Catal A Chem 388:47–55
Shafaghat H, Rezaei PS, Daud WMAW (2016) Catalytic hydrodeoxygenation of simulated phenolic bio-oil to cycloalkanes and aromatic hydrocarbons over bifunctional metal/acid catalysts of Ni/HBeta, Fe/HBeta and NiFe/HBeta. J Ind Eng Chem 35:268–276
Yuan G, Keane MA (2007) Aqueous-phase hydrodechlorination of 2,4-dichlorophenol over Pd/Al2O3: reaction under controlled pH. Ind Eng Chem Res 46(3):705–715
Mahata N, Vishwanathan V (2000) Influence of palladium precursors on structural properties and phenol hydrogenation characteristics of supported palladium catalysts. J Catal 196(2):262–270
Patel M, Kumar A (2016) Production of renewable diesel through the hydroprocessing of lignocellulosic biomass-derived bio-oil: a review. Renew Sust Energ Rev 58:1293–1307
Zhao C et al (2011) Aqueous-phase hydrodeoxygenation of bio-derived phenols to cycloalkanes. J Catal 280(1):8–16
Echeandia S et al (2010) Synergy effect in the HDO of phenol over Ni–W catalysts supported on active carbon: effect of tungsten precursors. Appl Catal B Environ 101(1):1–12
Nishu et al (2020) A review on the catalytic pyrolysis of biomass for the bio-oil production with ZSM-5: focus on structure. Fuel Process Technol 199:106301
Mihalcik DJ, Mullen CA, Boateng AA (2011) Screening acidic zeolites for catalytic fast pyrolysis of biomass and its components. J Anal Appl Pyrolysis 92(1):224–232
French R, Czernik S (2010) Catalytic pyrolysis of biomass for biofuels production. Fuel Process Technol 91(1):25–32
Zhang H et al (2009) Comparison of non-catalytic and catalytic fast pyrolysis of corncob in a fluidized bed reactor. Bioresour Technol 100(3):1428–1434
Valle B et al (2010) Selective production of aromatics by crude bio-oil valorization with a nickel-modified HZSM-5 zeolite catalyst. Energy Fuel 24(3):2060–2070
Iliopoulou EF et al (2012) Catalytic upgrading of biomass pyrolysis vapors using transition metal-modified ZSM-5 zeolite. Appl Catal B Environ 127:281–290
Kumar R et al (2019) Enhanced bio-oil deoxygenation activity by Cu/zeolite and Ni/zeolite catalysts in combined in-situ and ex-situ biomass pyrolysis. J Anal Appl Pyrolysis 140:148–160
Sun L et al (2016) Comparision of catalytic fast pyrolysis of biomass to aromatic hydrocarbons over ZSM-5 and Fe/ZSM-5 catalysts. J Anal Appl Pyrolysis 121:342–346
Zheng Y et al (2017) Study on aromatics production via the catalytic pyrolysis vapor upgrading of biomass using metal-loaded modified H-ZSM-5. J Anal Appl Pyrolysis 126:169–179
Razzaq M et al (2019) Investigating use of metal-modified HZSM-5 catalyst to upgrade liquid yield in co-pyrolysis of wheat straw and polystyrene. Fuel 257:116119
Taylor MJ et al (2016) Highly selective hydrogenation of furfural over supported Pt nanoparticles under mild conditions. Appl Catal B Environ 180:580–585
Li X et al (2019) Recent advances in aqueous-phase catalytic conversions of biomass platform chemicals over heterogeneous catalysts. Front Chem 7:948
Hellinger M et al (2015) Catalytic hydrodeoxygenation of guaiacol over platinum supported on metal oxides and zeolites. Appl Catal A Gen 490:181–192
Jin X et al (2013) Lattice-matched bimetallic CuPd-graphene nanocatalysts for facile conversion of biomass-derived polyols to chemicals. ACS Nano 7(2):1309–1316
Lup ANK et al (2017) A review on reactivity and stability of heterogeneous metal catalysts for deoxygenation of bio-oil model compounds. J Ind Eng Chem 56:1–34
Bulushev DA, Ross JR (2011) Catalysis for conversion of biomass to fuels via pyrolysis and gasification: a review. Catal Today 171(1):1–13
Nilsen MH et al (2007) Investigation of the effect of metal sites in Me–Al-MCM-41 (Me=Fe, Cu or Zn) on the catalytic behavior during the pyrolysis of wooden based biomass. Microporous Mesoporous Mater 105(1):189–203
Shen Y et al (2014) In-situ catalytic conversion of tar using rice husk char-supported nickel-iron catalysts for biomass pyrolysis/gasification. Appl Catal B Environ 152:140–151
Guan G et al (2012) Catalytic steam reforming of biomass tar over iron- or nickel-based catalyst supported on calcined scallop shell. Appl Catal B Environ 115-116:159–168
Hensley AJ et al (2014) Enhanced Fe2O3 reducibility via surface modification with Pd: characterizing the synergy within Pd/Fe catalysts for hydrodeoxygenation reactions. ACS Catal 4(10):3381–3392
Zarnegar S (2018) A review on catalytic-pyrolysis of coal and biomass for value-added fuel and chemicals. Energy Sources Part A 40(12):1427–1433
Busetto L et al (2011) Application of the Shvo catalyst in homogeneous hydrogenation of bio-oil obtained from pyrolysis of white poplar: New mild upgrading conditions. Fuel 90(3):1197–1207
Kaewpengkrow P, Atong D, Sricharoenchaikul V (2017) Selective catalytic fast pyrolysis of Jatropha curcas residue with metal oxide impregnated activated carbon for upgrading bio-oil. Int J Hydrog Energy 42(29):18397–18409
Yarulina I et al (2018) Recent trends and fundamental insights in the methanol-to-hydrocarbons process. Nat Catal 1(6):398–411
Wang Z et al (2015) Direct, single-step synthesis of hierarchical zeolites without secondary templating. J Mater Chem A 3(3):1298–1305
Li J et al (2014) Catalytic fast pyrolysis of biomass with mesoporous ZSM-5 zeolites prepared by desilication with NaOH solutions. Appl Catal A Gen 470:115–122
Ding K et al (2017) Effects of alkali-treated hierarchical HZSM-5 zeolites on the production of aromatic hydrocarbons from catalytic fast pyrolysis of waste cardboard. J Anal Appl Pyrolysis 125:153–161
Puértolas B et al (2015) Porosity–acidity interplay in hierarchical ZSM-5 zeolites for pyrolysis oil valorization to aromatics. ChemSusChem 8(19):3283–3293
Asadieraghi M, Wan Daud WMA (2015) In-situ catalytic upgrading of biomass pyrolysis vapor: Using a cascade system of various catalysts in a multi-zone fixed bed reactor. Energy Convers Manag 101:151–163
Kantarelis E et al (2019) Engineering the catalytic properties of HZSM5 by cobalt modification and post-synthetic hierarchical porosity development. Top Catal 62(7):773–785
Yue Y et al (2014) From natural aluminosilicate minerals to hierarchical ZSM-5 zeolites: A nanoscale depolymerization–reorganization approach. J Catal 319:200–210
Cho K et al (2012) Zeolite synthesis using hierarchical structure-directing surfactants: retaining porous structure of initial synthesis gel and precursors. Chem Mater 24(14):2733–2738
Antonakou E et al (2006) Evaluation of various types of Al-MCM-41 materials as catalysts in biomass pyrolysis for the production of bio-fuels and chemicals. Fuel 85(14):2202–2212
Twaiq FA, Mohamed AR, Bhatia S (2003) Liquid hydrocarbon fuels from palm oil by catalytic cracking over aluminosilicate mesoporous catalysts with various Si/Al ratios. Microporous Mesoporous Mater 64(1):95–107
Liang J et al (2017) Heterogeneous catalysis in zeolites, mesoporous silica, and metal–organic frameworks. Adv Mater 29(30):1701139
Jeon M-J et al (2013) Catalytic pyrolysis of biomass components over mesoporous catalysts using Py-GC/MS. Catal Today 204:170–178
Lu Q et al (2010) Catalytic upgrading of biomass fast pyrolysis vapors with Pd/SBA-15 catalysts. Ind Eng Chem Res 49(6):2573–2580
Du L et al (2013) A comparison of monomeric phenols produced from lignin by fast pyrolysis and hydrothermal conversions. Int J Chem React Eng 11(1):135–145
Wang C et al (2021) Integrated harvest of phenolic monomers and hydrogen through catalytic pyrolysis of biomass over nanocellulose derived biochar catalyst. Bioresour Technol 320:124352
Wang D et al (2010) Comparison of catalytic pyrolysis of biomass with MCM-41 and CaO catalysts by using TGA–FTIR analysis. J Anal Appl Pyrolysis 89(2):171–177
Brunelli NA, Venkatasubbaiah K, Jones CW (2012) Cooperative catalysis with acid–base bifunctional mesoporous silica: impact of grafting and co-condensation synthesis methods on material structure and catalytic properties. Chem Mater 24(13):2433–2442
Qiang L et al (2009) Analytical pyrolysis–gas chromatography/mass spectrometry (Py–GC/MS) of sawdust with Al/SBA-15 catalysts. J Anal Appl Pyrolysis 84(2):131–138
Tang Y et al (2011) Enhancement of Pt catalytic activity in the hydrogenation of aldehydes. Appl Catal A Gen 406(1):81–88
Yin Y et al (2017) Modification of as synthesized SBA-15 with Pt nanoparticles: nanoconfinement effects give a boost for hydrogen storage at room temperature. Sci Rep 7(1):1–10
Adam J et al (2005) Pyrolysis of biomass in the presence of Al-MCM-41 type catalysts. Fuel 84(12-13):1494–1502
Kim H et al (2016) Catalytic copyrolysis of particle board and polypropylene over Al-MCM-48. Mater Res Bull 82:61–66
Triantafyllidis KS et al (2007) Hydrothermally stable mesoporous aluminosilicates (MSU-S) assembled from zeolite seeds as catalysts for biomass pyrolysis. Microporous Mesoporous Mater 99(1-2):132–139
Liu W-J et al (2013) Facile synthesis of highly efficient and recyclable magnetic solid acid from biomass waste. Sci Rep 3:2419
Tessarolo NS et al (2016) Characterization of thermal and catalytic pyrolysis bio-oils by high-resolution techniques: 1H NMR, GC×GC-TOFMS and FT-ICR MS. J Anal Appl Pyrolysis 117:257–267
Staš M et al (2014) Overview of analytical methods used for chemical characterization of pyrolysis bio-oil. Energy Fuel 28(1):385–402
Eschenbacher A et al (2020) Insights into the scalability of catalytic upgrading of biomass pyrolysis vapors using micro and bench-scale reactors. Sustainable Energy Fuels 4(7):3780–3796
Naik S et al (2010) Supercritical CO2 fractionation of bio-oil produced from wheat–hemlock biomass. Bioresour Technol 101(19):7605–7613
Ingram L et al (2008) Pyrolysis of wood and bark in an auger reactor: physical properties and chemical analysis of the produced bio-oils. Energy Fuel 22(1):614–625
Ren S et al (2016) Analysis of switchgrass-derived bio-oil and associated aqueous phase generated in a semi-pilot scale auger pyrolyzer. J Anal Appl Pyrolysis 119:97–103
Bertero M, de la Puente G, Sedran U (2012) Fuels from bio-oils: bio-oil production from different residual sources, characterization and thermal conditioning. Fuel 95:263–271
Barnés MC et al (2015) A new approach for bio-oil characterization based on gel permeation chromatography preparative fractionation. J Anal Appl Pyrolysis 113:444–453
Sfetsas T et al (2011) Qualitative and quantitative analysis of pyrolysis oil by gas chromatography with flame ionization detection and comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry. J Chromatogr A 1218(21):3317–3325
Auersvald M et al (2019) Quantitative study of straw bio-oil hydrodeoxygenation over a sulfided NiMo catalyst. ACS Sustain Chem Eng 7(7):7080–7093
Lewis AJ et al (2015) Hydrogen production from switchgrass via an integrated pyrolysis–microbial electrolysis process. Bioresour Technol 195:231–241
Hui-Peng L et al (2009) Effects of phenols on the stability of FCC diesel fuel. Pet Sci Technol 27(5):486–497
Gellerstedt G et al (2008) Chemical structures present in biofuel obtained from lignin. Energy Fuel 22(6):4240–4244
Joseph J et al (2016) Compositional changes to low water content bio-oils during aging: an NMR, GC/MS, and LC/MS study. Energy Fuel 30(6):4825–4840
Tammekivi E et al (2019) Comparison of derivatization methods for the quantitative gas chromatographic analysis of oils. Anal Methods 11(28):3514–3522
Moraes MSA et al (2012) Analysis of products from pyrolysis of Brazilian sugar cane straw. Fuel Process Technol 101:35–43
Venkatramani C, Xu J, Phillips JB (1996) Separation orthogonality in temperature-programmed comprehensive two-dimensional gas chromatography. Anal Chem 68(9):1486–1492
Murray JA (2012) Qualitative and quantitative approaches in comprehensive two-dimensional gas chromatography. J Chromatogr A 1261:58–68
Negahdar L et al (2016) Characterization and comparison of fast pyrolysis bio-oils from pinewood, rapeseed cake, and wheat straw using 13C NMR and comprehensive GC × GC. ACS Sustain Chem Eng 4(9):4974–4985
Mattsson C et al (2016) Using 2D NMR to characterize the structure of the low and high molecular weight fractions of bio-oil obtained from LignoBoost™ kraft lignin depolymerized in subcritical water. Biomass Bioenergy 95:364–377
Mullen CA, Strahan GD, Boateng AA (2009) Characterization of various fast-pyrolysis bio-oils by NMR spectroscopy. Energy Fuel 23(5):2707–2718
Hao N et al (2016) Review of NMR characterization of pyrolysis oils. Energy Fuel 30(9):6863–6880
Bharti SK, Roy R (2012) Quantitative 1H NMR spectroscopy. TrAC Trends Anal Chem 35:5–26
Kanaujia PK et al (2014) Review of analytical strategies in the production and upgrading of bio-oils derived from lignocellulosic biomass. J Anal Appl Pyrolysis 105:55–74
David K et al (2010) 31P-NMR analysis of bio-oils obtained from the pyrolysis of biomass. Biofuels 1(6):839–845
Joseph J et al (2010) Chemical shifts and lifetimes for nuclear magnetic resonance (NMR) analysis of biofuels. Energy Fuel 24(9):5153–5162
Christensen ED et al (2011) Analysis of oxygenated compounds in hydrotreated biomass fast pyrolysis oil distillate fractions. Energy Fuel 25(11):5462–5471
Wang Y et al (2020) Analytical strategies for chemical characterization of bio-oil. J Sep Sci 43(1):360–371
Schnitzer MI et al (2007) The conversion of chicken manure to biooil by fast pyrolysis I. Analyses of chicken manure, biooils and char by 13C and 1H NMR and FTIR spectrophotometry. J Environ Sci Health B 42(1):71–77
Jiang X, Ellis N, Zhong Z (2011) Fuel properties of bio-oil/bio-diesel mixture characterized by TG, FTIR and 1H NMR. Korean J Chem Eng 28(1):133–137
Kanaujia PK et al (2013) Analytical approaches to characterizing pyrolysis oil from biomass. TrAC Trends Anal Chem 42:125–136
Hu X et al (2020) Coke Formation during thermal treatment of bio-oil. Energy Fuel 34(7):7863–7914
El-Sayed SA, Mostafa ME (2020) Thermal pyrolysis and kinetic parameter determination of mango leaves using common and new proposed parallel kinetic models. RSC Adv 10(31):18160–18179
Mureddu M et al (2018) Air-and oxygen-blown characterization of coal and biomass by thermogravimetric analysis. Fuel 212:626–637
Hu J et al (2019) Combustion behaviors of three bamboo residues: gas emission, kinetic, reaction mechanism and optimization patterns. J Clean Prod 235:549–561
Müller-Hagedorn M, Bockhorn H (2007) Pyrolytic behaviour of different biomasses (angiosperms)(maize plants, straws, and wood) in low temperature pyrolysis. J Anal Appl Pyrolysis 79(1-2):136–146
Branca C, Albano A, Di Blasi C (2005) Critical evaluation of global mechanisms of wood devolatilization. Thermochim Acta 429(2):133–141
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dada, T.K., Sheehan, M., Murugavelh, S. et al. A review on catalytic pyrolysis for high-quality bio-oil production from biomass. Biomass Conv. Bioref. 13, 2595–2614 (2023). https://doi.org/10.1007/s13399-021-01391-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01391-3