Abstract
The present paper describes the use of an easily accessible polymer-grafted sulfonated carbon composite catalyst for the synthesis of α-hydroxy ethers as bio-lubricants from waste vegetable oil via chemical modification involving esterification, epoxidation, and ring-opening reaction. A variety of α-hydroxy ethers were synthesized using different fatty alcohols for the evaluation of physicochemical properties. The bio-lubricants synthesized from 2-ethyl hexanol and dodecanol were found to be most promising as they exhibited lower pour point (< − 27 °C) and higher viscosity index (VI) 97 and 143, respectively. High VI and a lower pour point indicate ROP-3 and ROP-5 to be the most promising candidates as bio-lubricant for various applications.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The growing concern for the environment leads to the use of renewable feedstocks such as vegetable oils and their derivatives for various applications including as fuels, lubricants, emulsifiers, plasticizers, surfactants, plastics, and solvents [1]. Owing to their easy availability, biodegradability, and environmentally friendly behavior, vegetable oils and derivatives have been considered as promising base stocks/formulation(s) for eco-friendly lubricants [2]. Their unique properties such as better lubricity, low volatility, high viscosity index, and high flash point make them as suitable solvents for fluid additives. However, the presence of unsaturation leads to poor oxidative and thermal stability [3]. Several attempts have been made to improve their oxidative stability [4,5,6] such as transesterification of rapeseed oil methyl ester with trimethylolpropane [7], selective hydrogenation [8, 9], and epoxidation [10,11,12]. In particular, epoxidation has found considerable interest owing to the higher reactivity of the oxirane ring for the further modification possibilities [13, 14]. Oxirane ring due to the ring strain can readily undergo to ring-opening reactions with nucleophiles to produce various valuable chemicals such as mono-alcohols, diols, alkoxy alcohols, hydroxy esters, N-hydroxyalkyl amides, mercapto alcohols, amino alcohols, and hydroxy nitrile [15, 16]. Ring-opening reactions are generally carried out in the presence of the acid catalyst, such as sulfuric acid, acetic acid, and p-toluene sulfonic acid [17]. However, corrosivity and tedious recovery of these homogeneous acidic catalysts make them unacceptable for practical applications. Subsequently, heterogeneous catalysts such as acidic ion exchange resins like Amberlyst 15 (dry) and Amberlite122R have been used, which offer several advantages such as low cost, easy availability, facile recovery, and recyclability. However, poor thermal stability and lower physical strength at higher temperature make their applicability limited.
In recent years, sulfonated carbon composites obtained from readily available bio-derived sources such as natural sugars have gained considerable interest as solid acid catalysts for acid-catalyzed reactions [18,19,20,21]. These sulfonated carbon composites have hitherto been known for esterification [22, 23] and alkylation reactions [24]; however, no report is known for the oxirane ring opening of lubricant range esters.
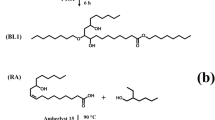
We report herein an efficient ring opening of oxiranes by fatty alcohols for the synthesis of a series of α-hydroxy ethers to be used as bio-lubricants in the presence of polymer-grafted sulfonated carbon composite (P–C–SO3H) as a heterogeneous acid catalyst. The reaction mainly consists of esterification, epoxidation, followed by oxirane ring opening (Scheme 1).
2 Experimental section
2.1 Materials and methods
All the substrates and reagents used were of analytical grade and used as received. Fatty alcohols (99%), oleic acid (~ 85%), formic acid (98–100%), and H2O2 (30 wt% in water) were procured from Merck, India. Acid oil was procured from a local vendor, Dehradun, India. The polymer-grafted sulfonated carbon composite (P–C–SO3H) was synthesized by following the reported procedure [22, 23]. Sulfur content of polymer impregnated sulfonated carbon composite P–C–SO3H was found to be 2.44 mmol H+ per g as determined by elemental analysis assuming that all the sulfur atoms are presented in –SO3H form. Further, acid site densities as calculated by using standard acid-base back titration method were found to be 2.54 mmol H+ per g of catalyst. The slightly higher value in comparison with the estimated values from elemental sulfur analysis might be due to the formation of phenolic-OH and –COOH groups resulting from the incomplete carbonization of glucose during the catalyst synthesis. The detailed synthetic methodology and characterization of the synthesized P–C–SO3H catalyst are given in the supporting information (Figs. S1–S3).
2.2 Esterification of oleic acid and acid oil
The esterification was carried out by using oleic acid (20 mmol), 2-ethylhexanol (20 mmol), and tetramethylguanidine hydrogen sulfate (TMG.HSO4) catalyst (5 wt% of total weight) [25] into a round-bottomed flask, and the resulting mixture were heated at 150 °C for 6 h under stirring. The reaction progress was monitored by thin-layer chromatography (TLC) using silica gel (SiO2). After completion of the reaction, the organic layer was washed with petroleum ether, dried over anhydrous MgSO4, and concentrated under reduced pressure to give the corresponding ester (2-ethylhexyl oleate). Conversion of acid to ester was calculated by employing the acid value (AV). The acid value of the sample was determined by the acid-base titration method. The FFA conversion (reduction in acid value) was calculated using the following equation:
where ai is the initial acidity and at is the acidity at time t (6 h). Further, the crude product was purified by percolation through basic alumina. After purification, the product was analyzed by 1H and 13C NMR.
Further, to generalize the reaction, waste refinery acid oil was esterified under the described reaction conditions. The conversion was estimated by acid-base titration technique. Further, the ester (EAE) was purified by percolation through basic alumina and analyzed by FTIR, 1H, and 13C NMR spectroscopic methods.
2.2.1 Epoxidation of 2-ethylhexyl oleate
A mixture containing 2-ethylhexyl oleate (5.6 g, 20 mmol) and formic acid (1.5 ml, 40 mmol) at 4 °C (ice bath) was added to an aqueous solution of 30 wt% H2O2 (6.25 ml, 80 mmol) dropwise, and the reaction was continued under vigorous stirring at room temperature for 5 h. After completion, the reaction mixture was washed with saturated NaHCO3 solution and brine solution until neutralization was achieved. Furthermore, the organic layer was subjected to usual workup and concentrated under reduced pressure to give 2-ethylhexyl 9, 10-epoxy stearate (EPO) as a white product (4.8 g). The product was further confirmed by comparing its FTIR, 1H, and 13C NMR spectral data with authentic samples.
Under similar conditions, the 2-ethylhexyl ester of acid oil was epoxidized to the corresponding epoxide as a brownish product, EPA (9.0 g). The product was further confirmed by FTIR, 1H, and 13C NMR spectral analyses.
2.2.2 Synthesis of α-hydroxy ethers by oxirane ring opening
Oxirane ring opening was carried out by taking the epoxide of 2-ethylhexyl ester of acid oil (10 mmol) and octanol (10 mmol) as representative substrates under variable conditions, i.e., catalyst (0.5–5%), temperature 50–150 °C, and reaction time 1–6 h. After the reaction, the catalyst was recovered by simple filtration, and the residue obtained was washed with diethyl ether. The organic layer was dried and concentrated under reduced pressure. The product was further analyzed by 1H NMR.
Furthermore, epoxide of 2-ethylhexyl fatty ester of acid oil was subjected to ring opening with different alcohols such as hexanol, decanol, 2-ethylhexanol, dodecanol, and glycerol under optimized experimental conditions. All the products were obtained in 85.5–96.5% yields.
2.2.3 Characterization of the products
Characterization of esters
Fatty acid composition of the acid oil was found to be major component, C18:1, and minor components, C18:2, C18:0, C16:0, as determined by gas chromatography. FTIR, 1H, and 13C NMR data of 2-ethylhexyl oleate and 2-ethylhexyl ester of acid oil are summarized in Table 1. In FTIR, the appearance of carbonyl band –C=O of ester at 1739 cm−1, a multiplet at 1.2–1.3, 1.6 ppm related to the –CH2, –CO–CH2–CH2 protons in 1H NMR and peaks at 64, 173 ppm for –O–CH2–, –COO in 13C NMR, confirmed the successful formation of respective esters.
Characterization of epoxides
The corresponding epoxides of 2-ethylhexyl oleate (EPO) and ester of 2-ethylhexyl acid oil (EPA) were confirmed by the disappearance of the signals corresponding to H–C= and –C=C– at 3008 cm−1and 1514 cm−1, respectively. Further, the appearance of a band at 1033 cm−1 due to oxirane ring formation confirmed the successful synthesis of corresponding epoxides. Also, in 1H NMR spectra, the absence of peaks between 5.3 and 5.5 ppm due to the protons of –C=C– and new multiplet in between of 2.5 and 3.5 ppm due to the epoxide ring protons confirmed the formation of epoxide successfully.
Characterization of α-hydroxy ethers
The epoxide of 2-ethylhexyl ester of acid oil (EPA) was subjected to oxirane ring-opening with different alcohols (hexanol, octanol, 2-ethylhexanol, decanol, dodecanol, glycerol) to give corresponding α-hydroxy ethers (ROP-1, ROP-2, ROP-3, ROP-4, ROP-5, ROP-6) as shown in Scheme 1. The spectral data (FTIR, 1H, and 13C NMR) of the products are summarized in Table 1. In the FT-IR spectra of the synthesized products, the characteristic bands appeared at 2926 and 2854 (–CH2–); 1737 (C=O of ester); 1462 (–CH2–); 1376 (–CH3–); 1245, 1175, and 1096 cm−1 (ester C–O stretch); and additional peaks at 1145 (–C–OR–) and 3452 cm−1 (–OH). In 1H NMR disappearance of peaks at δ 2.5–3.5 ppm and the emergence of new characteristics peaks in between 3.1–3.9 ppm and at 1.4–1.5 ppm due to -CH(OR′)-; -CH(OH)-; -OH and (–CH2CH)OR′)–; –CH2CH(OH)– respectively confirmed the formation of α-hydroxy ethers. The resonating signal at δ 73 ppm was assigned to the branching site (–CH(OR′)– and 83 ppm due to branching hydroxy group (–CH(OH)–) in 13C NMR spectra.
3 Results and discussion
Acid oil, a waste residue achieved after the refining of the vegetable oil having FFA (149 mg KOH/g), has been used as a feedstock for the synthesis of lubricant range esters (α-hydroxy ethers) in the present study. The intended products were obtained by esterification, epoxidation, and finally ring opening of the oxirane ring. Initially, the reaction conditions were optimized by choosing oleic acid as a model substrate. Once the optimized reaction conditions were achieved, acid oil was treated under similar conditions. The esterification of oleic acid with 2-ethylhexanol was done using acidic 1,1,3,3-tetramethylguanidinium hydrogen sulfate (TMG.HSO4) as a catalyst. The best conversion was obtained using a 5 wt% catalyst at 150 °C in 6 h as described in the “Experimental section.” Further esterification of the acid oil was performed with 2-ethylhexanol under the optimized reaction conditions to yield corresponding 2-ethylhexyl ester of acid oil. Subsequently, 2-ethylhexyl ester of oleic acid and acid oil was subjected to epoxidation using performic acid (HCOOH/H2O2), and then oxirane ring was opened with different alcohols in the presence of P–C–SO3H acid catalyst to afford desired α-hydroxy ethers.
3.1 Effect of reaction parameters on oxirane ring opening
At first, the effect of catalyst loading, reaction temperature, and reaction time was evaluated on the epoxide of 2-ethylhexyl ester of acid oil with 1-octanol. The results obtained by varying the reaction temperature from 50 to 150 °C are summarized in Fig. S4. As shown, the best conversion (96.83%) was achieved at 100 °C. Further enhancement in temperature did not make a significant difference in the conversion. Next, the reaction was studied by varying the catalyst concentration from 0.5 to 5 wt% (P–C–SO3H) at 100 °C for 5 h (Fig. S5). The maximum 96% conversion was achieved when 2 wt% of the catalyst was utilized. Further, the reaction was found to be affected adversely with increasing the catalyst concentration beyond 2 wt%, which is most likely due to the possibility of the formation of by-products under highly acidic conditions.
Furthermore, the effect of reaction time was evaluated by withdrawing the samples after a definite period and measured by 1H NMR spectroscopy. The reaction was found to be very fast in the beginning and gave about 61% conversion in 1 h. However, as the reaction proceeds, a marginal increment in the reaction rate was observed, and the maximum 96% conversion was achieved in 5 h (Fig. S6). Next, the reaction was generalized by using different linear and branched alcohols such as hexanol, decanol, 2-ethylhexanol, dodecanol, and glycerol under optimized experimental conditions. The results of these experiments are summarized in Table 2. As can be seen from Table 2, all the epoxides were efficiently converted and afforded high to the excellent yield of the α-hydroxy ethers in 86–97%.
Furthermore, the recyclability and reusability of the catalyst were checked for the ring opening of epoxide of 2-ethylhexyl oleate with 1-octanol under described reaction conditions. The catalyst could easily be recovered by simple decantation, washed with methanol, and dried at 80 °C for 4–5 h before recycling run. The recovered catalyst was reused for the subsequent six runs using fresh substrates under optimized conditions. The results of these are mentioned in Fig. 1. As can be seen in results, the catalyst exhibited consistent activity, and no leaching of sulfonic acid groups was observed during reactions. In order to confirm the leach-proof nature of the catalyst, we measured the sulfur content of the used catalyst that was recovered after the sixth recycling run. The value of sulfur content in the recovered catalyst was found to be 2.43 mmol H+ per g as determined by elemental analysis assuming that all the sulfur atoms are presented in –SO3H form. This value is almost similar to the fresh one (2.44 mmol H+ per g), which evidently suggests that the catalyst is quite stable and truly heterogeneous in nature without showing any detectable leaching of –SO3H groups.
3.2 Evaluation of physicochemical properties of synthesized products
The reactants oleic acid and acid oil (1–2); their esters ESE and EAE (3–4); epoxides EPO and EPA (5–6); and their α-hydroxy ethers ROP-1, ROP-2, ROP-3, ROP-4, ROP-5, and ROP-6 (7–12) were evaluated for physicochemical properties such as viscosity at 40 and 100 °C, density, viscosity index, and pour point as shown in Table 3.
The pour point of the oleic acid and acid oil was improved after esterification in ESE and EAE (Table 3, entries 3–4) due to the addition of 2-ethyl hexyl ester moiety. The pour point of oleic acid and acid oil was determined as + 6.0 °C and − 9 °C, respectively. The chemical structure of the oleic acid is not so linear and has some steric hindrance due to an unsaturation between C-9 and C-10 cis conformation, which breaks the linearity of the molecule. But, it is not sufficient to overcome the intermolecular interaction forces due to hydrogen bonding between the molecules. After esterification of oleic acid and acid oil, the pour point value decreases to − 15 and − 30 °C, respectively. The addition of branched-chain alcohol (2-ethylhexanol) after esterification increases the effective steric hindrance of the molecule and considerably reduces the pour point as compared with their fatty acids. Thus, after the esterification, the reduction in intermolecular interactions due to the lack of hydrogen bonding reduces the flow behavior of the esters. The study is comparable with J.P.C. Marques et al. [26]. The pour point was further increased in epoxidized products (EPO and EPA). This is due to the intermolecular interactions after the oxirane ring formation, whereas a significant variation was observed in corresponding α-hydroxy ethers, as shown in Table 3, entries 7–12. As anticipated, the chain length of the alcohol improved the pour point of the product, which can be assumed due to the effective formation of highly disorder macrocrystalline structures with higher alkyl chain length at reduced temperatures. Among all the α-hydroxy ethers formed, ROP-3 and ROP-5 (< − 27 °C) exhibited best results which are probably due to the branched and long-chain structure of the corresponding alcohols (Table 3, entry 9 and 11).
Besides this, insertion of α-hydroxy ether moiety at the mid-chain in the structure not only influences the pour point but also increases their viscosity at 40 °C. After the oxirane ring-opening reaction, the formation of one free hydroxyl group provides intermolecular hydrogen bonding interactions between the molecules. As shown in Table 3, the viscosity of synthesized esters of oleic acid and acid oil (entries 3–4) became lower as compared with their corresponding acids (entry 1, 2); however, after epoxidation, a remarkable enhancement in the viscosity was observed (Table 3, entries 5–6). The viscosity of synthesized α-hydroxy ethers (ROP-1 to ROP-6) at 40 °C and 100 °C was found to be significantly increased than their epoxide counterparts. This enhancement in viscosity is presumed due to the higher molecular weight and stronger intermolecular interactions resulting from the branching at the middle of the product molecule [27]. A significant decrease in pour point (< − 27 °C) was observed in the case of ROP-5 due to enhancement of steric hindrance at mid-point by the addition of branched chain moiety. Furthermore, a dramatic improvement in viscosity, 140.90 at 40 °C, was observed (Table 3, entry 12) when glycerol, a highly viscous alcohol, was used for the ring opening. The presence of three hydroxyl group (one hydroxyl group (–OH) in the main chain and other two free hydroxyl group of glycerol moiety) provides strong intermolecular hydrogen bonding, which led to a very high viscosity [28]. Viscosity index (VI) is defined as the change of viscosity with variations in temperature. The viscosity index of esters ESE and EAE was found to be higher 168.02 and 184.29, respectively, as compared with their corresponding acids 153 and 161.21. The epoxidation of the esters lowers the VI. Further, VI decreases for α-hydroxy ether derivatives after oxirane ring opening due to the reduced linearity of the molecule by the branching in the mid-chain. In ROP-6, a significant decrease in VI, i.e., 48, was observed due to the insertion of the glycerol molecule.
4 Conclusion
We have described the synthesis of various bio-lubricant esters from epoxide of 2-ethylhexyl ester of acid oil by ring opening with different alcohols using polymer-grafted sulfonated carbon composite (P–C–SO3H) as a heterogeneous acid catalyst. The use of heterogeneous catalyst offers several advantages such as secure handling, higher thermal stability, and efficient recovery and recycling as compared with the commonly used catalysts such as p-toluene sulfonic acid. A series of α-hydroxy ethers were synthesized using different alcohols by following esterification and epoxidation steps. A systematic study of the variation in pour point, viscosity, and viscosity index with different modifications introduced due to the various alcohols used for ring opening was performed. The difference of the polarities and symmetry of the synthesized samples were responsible for the significant differences in the physicochemical properties. Among the all, two products ROP-3 and ROP-5 exhibited best results as < − 27 °C pour point (PP) and viscosity index (VI) 97 and 143, respectively. The insertion of chain at the mid-point creates the steric hindrance in the molecules, which generate sufficient repulsion to better overcome the higher interaction forces. High VI and a lower pour point indicate ROP-3 and ROP-5 to be the most promising as bio-lubricant, thus enabling applications at lower temperatures.
References
Erhan SZ (ed) (2005) Industrial uses of vegetable oils. AOCS Press, Champaign
Erhan SZ, Sharma BK, Liu Z, Adhvaryu A (2008) Lubricant base stock potential of chemically modified vegetable oils. J Agric Food Chem 56(19):8919–8925
Becker R, Knorr A (1996) An evaluation of antioxidants for vegetable oils at elevated temperatures. Lubr Sci 8:95–117
Moser BR, Haas MJ, Winkler JK, Jackson MA, Erhan SZ, List GR (2007) Evaluation of partially hydrogenated methyl esters of soybean oil as biodiesel. Eur J Lipid Sci Technol 109:17–24
Dunn RO (2005) Effect of antioxidants on the oxidative stability of methyl soyate (biodiesel). Fuel Process Technol 86:1071–1085
Karavalakis G, Stournas S, Karonis D (2010) Evaluation of the oxidation stability of diesel/biodiesel blends. Fuel 89:2483–2489
Uosukainen E, Linko YY, Lamasa M, Tervakangas T, Linko P (1998) Transesterification of trimethylolpropane and rapeseed oil methyl ester to environmentally acceptable lubricants. J Am Oil Chem Soc 75:1557–1563
Johansson LE, Lundin ST (1979) Copper catalysts in the selective hydrogenation of soybean and rapeseed oils: I. The activity of the copper chromite catalyst. J Am Oil Chem Soc 56:974–980
Karl RK, Daniel KS, Medlin JW (2013) Selective hydrogenation of polyunsaturated fatty acids using alkanethiol self-assembled monolayer-coated Pd/Al2O3 catalysts. ACS Catal 3(9):2041–2044
Moser BR, Erhan SZ (2007) Preparation and evaluation of a series of α-hydroxy ethers from 9,10-epoxystearates. Eur J Lipid Sci Technol 109:206–213
Salimon J, Nadia SN (2010) Chemical modification of oleic acid oil for biolubricant industrial applications. Aust J Basic Appl Sci 4(7):1999–2003
Lathi PS, Mattiasson B (2007) Green approach for the preparation of biodegradable lubricant base stock from epoxidized vegetable oil. Appl Catal B Environ 69:207–212
Padwa A, Murphree SS (2006) Epoxides and aziridines-a mini review. Arkivoc 3:6–33
Suman SK, Dhawaria M, Porwal J, Aila M, Karanwal N, Behera B, Kaul S, Ghosh S, Jain SL (2015) Biocatalytic green approach for epoxidation of fatty compounds derived from soyadeodistillate under acid-free conditions. RSC Adv 5:53708
Rios LA, Weckes PP, Schuster H, Hoelderich WF (2005) Resin catalyzed alcoholysis of epoxidized fatty esters: effect of the alcohol and the resin structures. Appl Catal A Gen 284:155–161
Baumann H, Buhler H, Fochem H, Hirsinger F, Zobelein H, Falde J (1988) Natural fats and oils-renewable raw materials for the chemical industry. Angew Chem 27:41–62
Salimon J, Abdullah BM, Yusop RM, Salih N, Emad Y (2013) Synthesis and optimization ring opening of monoepoxide linoleic acid using p-toluenesulfonic acid. Springerplus 2:429
Mo X, Lotero E, Lu C, Liu Y, Goodwin JG (2008) A novel sulfonated carbon composite solid acid catalyst for biodiesel synthesis. Catal Lett 123:1–6
Verma S, Baig RBN, Nadagouda MN, Lenc C, Verma RS (2017) Sustainable pathway to furanics from biomass via heterogeneous organo-catalysis. Green Chem 19:164–168
Baig RBN, Verma S, Nadagouda MN, Verma RS (2016) Room temperature synthesis of biodiesel using sulfonated graphitic carbon nitride. Sci Rep 6:39387
Verma S, Jain SL, Sain B (2011) An efficient biomaterial supported bifunctional organocatalyst (ES-SO3− C5H5NH+) for the synthesis of β-amino carbonyls. Org Biomol Chem 9:2314–2318
Khatri PK, Manchanda M, Gosh IK, Jain SL (2015) Polymer impregnated sulfonated carbon composite solid acid catalyst for alkylation of phenol with methyl tert butyl ether. RSC Adv 5:3286–3290
Khatri PK, Karanwal N, Kaul S, Jain SL (2015) Sulfonated polymer impregnated carbon composite as a solid acid catalyst for the selective synthesis of furfural from xylose. Tetrahedron Lett 56:1203–1206
Ferreiraa ARO, Alberob JS, Maierc ME, Ricardod NMPS, Cavalcante CL Jr, Lunaa FMT (2020) Sulfonated activated carbons as potential catalysts for biolubricant synthesis. Mol Catal 488:110888
Porwal J, Kumar S, Kaul S, Jain SL (2016) Guanidine based task-specific ionic liquids for the synthesis of biolubricant range esters under solvent-free condition. RSC Adv 6:93640–93644
Marques JPC, Rios IC, Parente EJS Jr, Quintella SA, Luna FMT, Cavalcante CL Jr (2019) Synthesis and characterization of potential bio-based lubricant basestocks via epoxidation process. J Am Oil Chem Soc 97:437–446
Marques JPC, Rios IC, Arruda TBMG, Rodrigues FEAR, Uchoa AFJ, de Luna FMT, Cavalcante CL Jr, Ricardo NMPS (2019) Potential bio-based lubricants synthesized from highly unsaturated soybean fatty acids: physicochemical properties and thermal degradation. Ind Eng Chem Res 58:17709–17717
Riosa IC, Cordeiroa JP, Arrudaa TBMG, Rodriguesb FEA, Uchoaa AFJ, Lunac FMT, Célio L, Cavalcante CL Jr, Ricardo NMPS (2020) Chemical modification of castor oil fatty acids (Ricinus communis) for biolubricant applications: an alternative for Brazil’s green market. Ind Crop Prod 145:112000
Acknowledgments
The authors gratefully acknowledge Director IIP for his kind permission to publish this work. We are thankful to the Analytical Science Division of the Institute for their support in the analysis of the samples.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Synthesis of the acid catalyst (P-C-SO3H catalyst), characterization of the catalyst (FT-IR, X-ray diffraction pattern, and thermogram) and catalytic parameters (temperature, catalyst loading, reaction time) are given in the supporting information file.
ESM 1
(DOC 973 kb)
Rights and permissions
About this article
Cite this article
Porwal, J., Khatri, P.K., Kaul, S. et al. Polymer-grafted sulfonated carbon-catalyzed synthesis of α-hydroxy ethers as bio-lubricants from waste vegetable oil. Biomass Conv. Bioref. 12, 4701–4708 (2022). https://doi.org/10.1007/s13399-020-01047-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01047-8