Abstract
This article presents the results of the investigation of the properties of phenol-formaldehyde resin, obtained using the phenol-replacing fraction. A two-step method was developed for phenol-replacing fraction separation from liquid pyrolysis products with a yield up to 15%, and this fraction was used in the phenol-formaldehyde resin synthesis. Then, a work was conducted for the removal of neutrals from the modified phenol-formaldehyde resin with organic solvents, n-hexane and benzene. As a result, benzene was defined as a more efficient solvent because it removed more aromatics, like ethers and substituted phenols, that cannot react and worsen the glue line water resistance. Benzene dissolved 3.2% weight of the resin, and n-hexane dissolved 2.5% weight. The removal of neutrals increased the water resistance coefficient by more than 60%, so neutrals have a considerable effect on the resin properties. The results can be used for production of resin from renewable feedstock with the similar properties with the traditional resin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Resol phenol-formaldehyde resins (PFR) are widely used in the wood processing industry. They are a product of phenol and formaldehyde polycondensation in alkaline medium with an excess of formaldehyde and are primarily used in the production of plywood and wood-particle boards [1]. Phenol today is produced on a large scale from petroleum-derived feedstocks. Accounting for 95% of production is the cumene process which involves the partial oxidation of cumene (isopropylbenzene) via the Hock rearrangement [2]. Taking into account the increased phenol demand and depletion of fossil resources, phenol replacement by renewable feedstock is becoming a topical issue [3].
About 25–40% of wood wastes are produced during wood processing, and these wastes sometimes do not find useful applications. One method for processing such a feedstock is fast ablative pyrolysis. While it is related to the traditional pyrolysis processes used for making charcoal, fast pyrolysis is a more advanced process that can be carefully controlled to give high yields of liquid [4]. Liquid products (pyrolysis liquid, bio-oil), with a yield of up to 70%, are produced in this process [5]. Fast pyrolysis is a thermal decomposition process without oxygen access in a temperature range 450–550 °C, during which a fast heating and fast removal of a gas-vapor mixture occur with its following condensation into a liquid product [6, 7]. Pyrolysis liquid (PL) includes acids, phenols [8], alcohols, hydroxyls, esters, aldehydes, and unsaturated hydrocarbons. The phenol content can be 12–30% [9,10,11]. Based on this data, the application of PL as a phenol substitute in PFR synthesis was suggested.
Obtaining new resin is interesting for the industry from both an ecological and economical point of view for the following reasons:
-
Feedstocks for PL are forestry and wood processing wastes, and their treatment decreases ecological impact.
-
PL application will reduce the price of PFR without decreasing its quality. The price of synthetic phenols is $10–12/kg, while the price of PL is $0.02–0.25/kg.
-
PL is a less toxic substance than phenol and will improve the ecological aspect of PFR production. However, the presence of neutral substances in PL and low functionality can lead to worse resin properties [12,13,14,15].
Many works have been published in the area of green adhesives from wood extractives [16,17,18].
Different types of PL and its fraction have been investigated as a phenol substitute: whole PL [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23], separated lignin [24], phenol-reach fraction [25,26,27], separated and cleaned phenols [28, 29], methylolated bio-oil [30], bio-oil from palm kernel shell [31], and bio-oil from kraft lignin [32, 33]. A deep review in phenolic resin production from biomass conversion liquids is presented in [34].
Such resins have some disadvantages (strength, odor, color, etc.) compared with the control of PFR, requiring additional attempts in this direction. Some of these disadvantages come from neutral compounds in PL which do not participate in the PFR synthesis.
This work presents one possible way for improving the properties of PFR. Investigation of PFR showed that it contains neutral substances, and neutrals and water-soluble substances can decrease the quality of resin and glue line. The neutrals are different substances produced during pyrolysis of main components of wood—cellulose, hemicelluloses, and lignin. They can be separated from bio-oil by distillation and solvent extraction. Therefore, the aim of this work was to study the influence of neutrals from PL on PFR resin, produced with a 40% phenol substitution. This substitution level was chosen basing on our previous work [12]. The influence was investigated according to an ASTM D5266-13 method by PFR glue line strength measurements before and after the extraction of neutrals by different solvents [35,36,37].
2 Materials and methods
2.1 Properties of initial materials
PL was obtained from birch (Betula pendula) wood chips at the FPP02 plant [38]. The plant is able to process wood chips with a size less than 10 by 20 mm.
An FPP02 fast pyrolysis plant is designed for thermochemical processing and utilization of biomass and other organic polymeric wastes to produce liquid organic products and fine char. It allows processing wood wastes into a liquid fuel and fine biochar.
Appearance of the FPP02 plant is presented in Fig. 1.
A schematic diagram of the FPP02 plant is presented in Fig. 2.
The technological process at the FPP 02 plant is conducted in the following way: wood wastes crushed and dried to a moisture content of 10 ± 2 w% are fed to the feed hopper of the loading module, from where it is fed to the reactor through a two-valve sluice device. In the reactor, a mechanically activated thermal processing of the feedstock without oxygen occurs due to the heat generated in the energy module. Fast pyrolysis was completed at 450–550 °C. In the result, char and vapor-gas mixtures are produced.
The vapor-gas mixture is then fed to a purification module. In this module, the mixture is separated from the char dust. After that, the purified mixture is transported to a condensation module.
The fine char from the reactor is fed in a discharging module by a screw feeder. The discharging module carries out accumulation and distribution of the char. Char can be sent either to discharging or to combustion in the energy module.
Fast cooling and condensation of the vapor-gas mixture in the condensation module result in the separation of a liquid and a combustible gas. The gas is fed to combustion in the energy module.
The PL moisture content was measured on a Titration Compact V 20 Karl-Fischer apparatus, according to ASTM E 203-16 [39]. The pH value was determined at 25 ± 0.5 °C with a pH-150MI pH meter. PL viscosity was measured at 25 ± 0.5 °C using a VZ-246 viscometer with a 4-mm-diameter nozzle.
The chemical compositions of the liquids were investigated by gas chromatography-mass spectrometry (GCMS) on a Shimadzu QP2010 device with a HP-1 MS column under the following conditions: grade A helium carrier gas, 300 °C injector temperature, 1 mL/min flow rate through the column, and a thermostat temperature program at 80 °C for 2 min, followed by heating to 200 °C at 10 °C/min, and finally, holding the temperature at 200 °C for 6 min. Mass spectrometer parameters included an ion source at 270 °C, a scan mode, registered peaks in the range of 35–600, and a solvent elution time of 2 min. A 1-μL PL sample was taken with a Gilson precise syringe.
2.2 Biophenol separation from PL
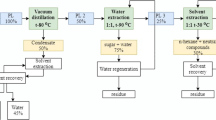
The biophenol (the phenol-substituting fraction (PSF) of bio-oil) separation process consisted of two stages. In the first stage, water, volatiles, and acetic acid were removed. For this, an IKA RV 10 digital V rotational evaporator was used. Distillation parameters were as follows: 80 °C, a residual pressure of 0.01–0.02 MPa, and a rotation rate of 45 min−1. In the second stage, non-volatile hydrocarbon compounds, like salts, oxyacids, and other water-soluble substances, which negatively influence the resin, were removed. A water extraction of PL was conducted for the removal of the non-volatile hydrocarbon compounds. This process is the diffusion of water-soluble substances from bio-oil into an aqueous solution, and its driving force is the concentration difference. Distilled PL was mixed with hot water in a 1:1 ratio; the mixture was settled in a dropping funnel and, then, was separated. The lower fraction, or biophenol, was used for the synthesis.
The diagram of PSF separation is presented in Fig. 3.
2.3 Resol PFR synthesis
The resin synthesis was carried out at a 1:1.75 molar ratio of phenol to formaldehyde; phenol substitution by PSF was 40%. Sodium hydroxide was added in two portions to maintain the alkaline medium. The first portion contained 65% weight of the total sodium hydroxide. The ratio of total phenol, including PSF, to sodium hydroxide was 1:1.1. The procedure also included water; its content was calculated based on the PSF water content.
A 0.5-L three-necked glass flask with a reflux condenser was used as the reactor. The flask was installed in a water bath, and the stirrer in the flask was set to 155 min−1. At the beginning, PSF, melted phenol, water, and the first portions of sodium hydroxide and formalin were put into the reactor. Then, continuous mixing at 50 °C was maintained for 1.5 h. After this stage, the solution was boiled at 94–99 °C. After boiling, the second portion of sodium hydroxide was added at 70–75 °C, and continuous mixing was, again, maintained for 15 min. A control PFR sample without phenol substitution was synthesized under identical conditions for reference.
2.4 Neutrals removal from the resin
The synthesized resin was treated with two organic solvents using a liquid-liquid one-stage extraction. Two types of solvents were used, benzene and n-hexane. These solvents were chosen as typical solvents for aromatic and aliphatic hydrocarbons. The balance comparison could show us which type of the neutral hydrocarbons is dominant. The treatment method was similar for the both solvents. Resin and solvent were mixed in a reaction flask in a 1:1 ratio over 30 min at 30–40 °C. The mixture, then, was poured into a 500-mL dropping funnel and was allowed to settle for at least 3 h. Raffinate was exposed to vacuum drying at 30 °C for 1 h to remove the solvent. Solvent extracts were analyzed by GCMS as described in Section 2.1.
2.5 Sample preparation for GCMS
Three samples were collected for GCMS analysis: the water extract after hot water extraction of dried PL, n-hexane extract of modified PFR, and benzene extract of modified PFR. The hexane and benzene extracts were analyzed without pretreatment. The water extract sample was preliminarily dried to a constant weight, and then, the resulting residue was dissolved in acetone in a 1:1 ratio.
GCMS analysis was conducted as described in Section 2.1.
2.6 Gluing and destruction of the samples
Four resin types were used for gluing and strength testing of the glue line:
-
Control PFR without PSF
-
PFR modified with PSF
-
PFR modified with PSF and cleaned with benzene
-
PFR modified with PSF and cleaned with hexane
Wooden test samples, 7 by 2 by 1 cm in size, were prepared for glue line testing. Gluing was conducted in a Nordberg ECO N3620L laboratory press with mobile heating plates with the following parameters: gluing area, 2 cm2; temperature, 126–130 °C; pressure, 1.96–2.45 MPa; press time, 7 min. The samples were tested according to ASTM D5266-13 on a LDS.5.L.01 universal laboratory testing machine (producer Jinan Liangong Testing Technology Co., Ltd., China).
Samples were divided into two groups. The first group was destroyed after boiling and the second one without boiling. Boiling was conducted in a boiling water bath for 60 min. After that, the samples were kept at air for 10 min and, then, were destroyed at the testing machine [40]. The water resistance coefficient was calculated by the formula:
where Rw describes the wet samples’ shear strength and Rdr describes the dry samples’ shear strength, both in MPa.
3 Results and discussion
3.1 Properties of initial materials
The products yielded from the FPP02 fast pyrolysis plant are presented in Table 1. The yield was calculated as a percentage of a measured product mass to the initial feedstock mass.
Properties of water-insoluble and water-soluble fractions of PL are presented in Table 2.
3.2 Biophenol separation from PL
The material balance of the PSF separation from PL is presented in Table 3. The target fraction yield, which was used in the PFR synthesis, was 14.77% of PL or 8.27% of the dry wood weight.
The water extracted during the second stage of the PSF separation was 57.2% of the PL weight after distillation, and the yield of the water-insoluble fraction was 36.5% of the PL weight after distillation. Analysis of the water extract by GCMS showed that the extract contained significant amounts of anhydro-derivatives of carbohydrates, like 3,4-anhydro-d-galactozane and 2,3-anhydro-d-mannosane, but the main constituent was d-allose (C3 epimer of glucose), accounting for 50.28% of the peaks. These substances are products of cellulose decomposition. The reactions of their formation during cellulose decomposition can be found in [41]. The extract also contained phenols, which were dissolved in water because of the partial solubility.
3.3 Neutrals removal from the resin
Mass fractions of the compounds, which were dissolved in the solvents, were 2.5% for hexane and 3.2% for benzene.
The higher solubility of benzene shows that the neutrals include more aromatic compounds than aliphatic. We can assume that they include fully substituted phenols. The substituted phenols have lower acidity and less participate in the PFR synthesis reactions. These compounds disrupt the structure of the glue line and decrease its water resistance.
The compositions of hexane and benzene extracts are presented in Fig. 4. The organic compounds were classified by the type of functional groups.
When we compare the solubility of different chemical groups in two solvents, we see that benzene extracts more aromatics than n-hexane. The hexane extract contains more alkanes (tetradecane, dodecane, pentadecane, eicosane, etc.). Benzene extracts aromatic compounds like xylol (up to 9.2%) and 2,3,5-trimethylphenol (up to 0.55%). The hexane extract contained aromatics like phenanthrene (up to 3.27%) and 2,6-dimethoxy-4-(2-propenyl)-phenol (up to 1.49%). Generally, the main portion of the aromatics found in the extracts represented 3-substituted phenols, which have low reactivity and practically do not participate in the PFR resin synthesis reactions. Both solvents extract methyl ether of palmitic acid (0.28% in benzene and 1.14% in hexane) and 2,4-di-tert-butylphenol (2.08% in benzene and 3.11% in hexane). Hexane extracted acetophenol (2.49%), which was not detected in benzene. However, the benzene extract contained 1,2,4-trimethylphenol (10.87%), which was not found in the hexane extract.
3.4 Gluing and destruction of the samples
Results of strength tests of the glue line before and after boiling and water resistance coefficients are presented in Fig. 5.
The presented data show that the shear strength of the samples glued with PFR and modified with PSF but not treated by solvent (sample 2, Fig. 5a) significantly decreased after boiling. It can be concluded that neutral compounds in this resin distinctly influence the water resistance of this resin, which is the lowest one (0.56; Fig. 5b). Shear strength before and after boiling stays almost the same for resins cleaned from neutrals by solvents (samples 3 and 4, Fig. 5a), and their water resistance coefficients (0.91 and 1.04, respectively) are close to the control resin (1.02).
The next step in this work will be a preliminary PSF treatment by solvent and investigation of the formation or decomposition of neutrals in alkali media during synthesis.
4 Conclusion
The results presented in the article show a significant influence of neutral substances from fast pyrolysis liquid products on the quality of resole PFR modified by PSF. PFR modified by PSF had the lowest water resistance coefficient of all the studied resins, meaning that the strength properties of the resin dramatically decrease after boiling. Separation of the neutral compounds by organic solvents increased the water resistance coefficient to 0.91 for hexane and 1.04 for benzene.
The results demonstrated a usefulness of PFR treatment by organic solvents benzene or hexane, for increasing water resistance of the glue line. However, it should be mentioned that the glue line strength of PFR modified by PSF without boiling is almost equal to that of the control resin. This could potentially be caused by a plastifying effect of some neutrals.
Also, the data show that benzene is a more effective solvent than hexane; mass fractions of the compounds, which were dissolved in the solvents, were 2.5% for hexane and 3.2% for benzene. The higher solubility of benzene shows that the neutrals include more aromatic compounds than aliphatic. We can assume that they include fully substituted phenols. The substituted phenols have lower acidity and less participate in the PFR synthesis reactions. These compounds disrupt the structure of the glue line and decrease its water resistance. Strength values of the resin cleaned by benzene stay stable before and after boiling, 3.17 MPa and 3.31 MPa, respectively, and the water resistance coefficient of this resin is comparable to that of the control resin.
Many extracted compounds are aromatics. This can lead to a decrease of PFR smell, which is a positive aspect.
Generally, it can be concluded that the PSF made from bio-oil included neutrals which decreased the properties of PFR. Separation of these compounds increased the water resistance and decreased the smell of the resin. As they are represent products of cellulose pyrolysis, additional studies in neutrals removal during PSF production should be interesting for further application.
References
Doronin YG, Svitkina MM, Miroshnichenko SN (1979) Synthetic resins in wood processing: a guide. Lesnaya promyshlennost’. 208 p. (in Russian)
Weber M, Weber M, Kleine-Boymann M (2004) Phenol/Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCN. doi:https://doi.org/10.1002/14356007.a19_299.pub2
Grachev AN, Zabelkin SA, Iakovleva AY, Fayzrahmanova GM, Bashkirov VN (2014) Resole phenol-formaldehyde resin glue line strength when modified by wood pyrolysis piqued products. Vestnik Kazanskogo Technologicheskogo Universiteta 16:27–29 (In Russian)
Bridgwater AV, Peacocke GVC (2000) Fast pyrolysis processes for biomass. Renew Sust Energ Rev 4(1):1–73. https://doi.org/10.1016/S1364-0321(99)00007-6
Stücker A, Schütt F, Saake B, Lehnen R (2016) Lignins from enzymatic hydrolysis and alkaline extraction of steam refined poplar wood: utilization in lignin-phenol-formaldehyde resins. Ind Crop Prod 85:300–308
Varfolomeev MA, Emel'yanenko VN, Musin TR, Gerasimov AV, Nurgaliev DK, Grachev AN, Makarov AA, Zabelkin SA (2015) Thermal analysis and calorimetric study of the combustion of hydrolytic wood lignin and products of its pyrolysis. Chem Technol Fuels Oils 51(1):140–145
Fayzrakhmanova GM, Zabelkin SA, Grachev AN, Bashkirov VN (2016) A study of the properties of a composite asphalt binder using liquid products of wood fast pyrolysis. Polymer Science, Series D: Glues and Sealing Materials 9(2):181–184
Amen-Chen C, Pakdel H, Roy C (2001) Production of monomeric phenols by thermochemical conversion of biomass: a review. Bioresour Technol 79:277–299
Fardhyanti DS, Triwibowo B, Prasetiawan H, Chafidz A, Andriyani S, Cahyani NN (2019) Improving the quality of bio-oil produced from rice husk pyrolysis by extraction of its phenolic compounds. Jurnal Bahan Alam Terbarukan JBAT 8(2):90–100
La Ifa, Setiawati Yani, Mandasini, Zakir Sabara, Nurjannah Nurjannah, & Andi Rusnaenah. Production of phenol from liquid smoke resulted by the pyrolysis of cashew nut shells. IOP Conf. Series: Earth and Environmental Science 2018 IOP Conf. Ser.: Earth Environ. Sci. 175 012033.
Awasthia A, Dhyania V, Biswasa B, Kumara J, Bhaskar T (2019) Production of phenolic compounds using waste coir pith: estimation of kinetic and thermodynamic parameters. Bioresour Technol 274:173–179
Zabelkin SA, Grachev AN, Bikbulatova GM, Yakovleva AE, Makarov AA, Bashkirov VN (2018) Resole-type phenol–formaldehyde resin with neutralized liquid products of fast pyrolysis of birch wood. Polymer Science, Series D 11:131–134
Zhang W, Ma Y, Wang C, Li S, Zhang M, Chu F (2013) Preparation and properties of lignin–phenol–formaldehyde resins based on different biorefinery residues of agricultural biomass. Ind Crop Prod 43:326–333
Zabelkin S, Grachev A, Fayzrakhmanova G, Makarov A, Bashkirov V (2016) Application of the water-insoluble pyrolysis oil fraction as an organic binder. Constr Build Mater 102:59–64
Grachev AN, Varfolomeev MA, Emel’yanov DA, Zabelkin SA, Gilfanov MF, Nuriyakhmetov RA (2017) Joint thermal treatment of heavy oil and liquid products of fast wood pyrolysis for producing fuels and chemicals. Chem Technol Fuels Oils 53:638–645
Lee JH, Jeon J, Kim SJ (2011) Green adhesives using tannin and cashew nut shell liquid for environment-friendly furniture materials. Korean Furniture Soc 22:219–229
Bisanda ETN, Ogola WO, Tesha JV (2003) Characterisation of tannin adhesive blends for particle board applications. Cem Concr Res 25:593–598
Vazquez G, Antorrena G, Gonzalez J et al (1996) Tannin based adhesives for bonding high moisture eucalyptus veneers: influence of tannin extraction and press conditions. Holz Roh Werkst 54:93–97
Uvarov IP, Gordon LV (1962) Wood resins (synthetic products based on wood chemical phenols). Moscow: Goslesbumizdat. 84 p. (In Russian)
Nakos P, Tsiantzi S, Athanassiadou E. (2001) Wood adhesives made with pyrolysis oils. In: Proceedings of 3rd European Wood-based Panel Symposium, Sep 12–14; European Panel Federation & Wilhelm Klauditz Institute, Hannover, pp. 1–8
Chaouch M, Diouf PN, Laghdir A, Yin S (2014) Bio-oil from whole-tree feedstock in resol-type phenolic resins. Journal of Applied Polymer Science. 10.1002/app.40014
Cui Y, Chang J, Wang W (2016) Fabrication of glass fiber reinforced composited based on bio-oil phenol formaldehyde resin. Materials 9:886. https://doi.org/10.3390/ma9110886
Lee W, Tseng I, Kao Y, Lee Y, Hu M (2014) Synthesis of alcohol-soluble resins from pyrolysis oil of Cunninghamia lanceolate wood and properties of molding plates made of resin-impregnated materials. Holzforschung. 68(2):217–222. https://doi.org/10.1515/hf-2013-0068
Sukhbaatar B, Steele PH, Kim MG (2009) Use of lignin separated from bio-oil in oriented strand board binder phenol-formaldehyde adhesives. BioResources. 4:789–804
Aslan M, Özbay G, Ayrilmis N (2015) Adhesive characteristics and bonding performance of phenol formaldehyde modified with phenol-rich fraction of crude bio-oil. J Adhes Sci Technol 29:2679–2691. https://doi.org/10.1080/01694243.2015.1080474
Guzelciftci B, Park K, Kim J(2020) Production of phenol-rich bio-oil via a two-stage pyrolysis of wood. Energy 200. doi: https://doi.org/10.1016/j.energy.2020.117536
Kim J (2014) Production, separation and applications of phenolic-rich bio-oil – A review. Bioresour Technol 178:90–98. https://doi.org/10.1016/j.biortech.2014.08.121
Chum HL, Black SK (1990) Process for fractionating fast-pyrolysis oils and products derived from, USA patent 4, 942, 269. July 17
Suzuki T, Hiroshi N, Yamada T, Homma T (1992) Preparation of wood tar-based phenol-resin adhesives. Mokuzai Gak 38:321–324
Cheng S, Yuan Z, Anderson M, Leitch M, Xu C (2012) Synthesis of biobased phenolic resins/adhesives with methylolated wood-derived bio-oil. J Appl Polym Sci 126:E431–E441. https://doi.org/10.1002/app.35655
Choi G, Oh S, Lee S, Kim J (2015) Production of bio-based phenolic resin and activated carbon from bio-oil and biochar derived from fast pyrolysis of palm kernel shells. Bioresour Technol 178:99–107
Farag S, Chaouki J (2014) Economics evaluation for on-site pyrolysis of kraft lignin to value-added chemicals. Bioresour Technol 175:254–261. https://doi.org/10.1016/j.biortech.2014.10.096
Vithanage AE, Chowdhury E, Alejo LD, Pomeroy PC, DeSisto WJ, Frederick BG, Gramlich WM (2017) Renewably sourced phenolic resins from lignin bio-oil. J Appl Polym Sci, DOI: 10.1002/APP.44827
Effendi A, Gerhauser H, Bridgwater AV (2007) Production of renewable phenolic resins by thermochemical conversion of biomass: a review. Renew Sustain Energy Rev. doi: 10.1016/j.rser.2007.04.008
Zhang W, Ma Y, Xu Y, Wang C, Chu F (2013) Lignocellulosic ethanol residue-based lignin–phenol–formaldehyde resin adhesive. Int J Adhes Adhes 40:11–18
Feghali E. et al. (2020) Thermosetting polymers from lignin model compounds and depolymerized lignins. In: Serrano L., Luque R., Sels B. (eds) Lignin chemistry. Topics in Current Chemistry Collections. Springer, Cham
Valeeva AR, Grachev AN, Zabelkin SA, Bashkirov VN, Sabirzyanova AI (2020) Determination of phenol substitution level influence by wood pyrolysis liquid products on phenol-formaldehyde resin strength. Derevoobrabatyvayuschaya Promyshlennost 16:88–95 (In Russian)
Grachev AN et al. A fast ablative pyrolysis plant for decentralized processing of biomass into biochar and biooil. EUBCE 2019; 27TH European Biomass Conference and Exhibition. Lisbon – Portugal: 164
ASTM E 203-16 Standard test method for water using volumetric Karl Fischer titration.
ASTM D5266-13 Standard practice for estimating the percentage of wood failure in adhesive bonded joints
Dahiya A (2015) Bioenergy. Biomass to biofuels. 1st Edition. Academic Press
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zabelkin, S., Valeeva, A., Sabirzyanova, A. et al. Neutrals influence on the water resistance coefficient of phenol-formaldehyde resin modified by wood pyrolysis liquid products. Biomass Conv. Bioref. 12, 5563–5570 (2022). https://doi.org/10.1007/s13399-020-01025-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01025-0