Abstract
Stable α-alumina-water-ethylene glycol (WEG) based nanofluids with a low viscosity requirement are preferable for promising engineering applications. Viscosity of nanofluids is a significant parameter that decides the flow characteristics and pumping pressure requirements. In this study, α-alumina nanoparticles (spherical morphology with 40 nm) dispersed in WEG mixture in a ratio of 50:50 (v/v) using an ultra-sonication process. Further analysis of the effects of process parameters on the viscosity of prepared nanofluid, including volume concentrations (0.01%–0.2%), temperatures (30-45 °C), and sonication times (0–4 h). A decrease in viscosity of 11.36% was observed for 0.2% volume concentration as sonication time increased from 0 to 3 h at a process temperature of 45 °C. The viscosity value of nanofluids approaches a stable value at 3 h of sonication. No significant sonication ‘null effect’ was required for lower concentrations irrespective of the temperature and sonication time, yielding low viscosity. At the same time, clusters were observed at a higher volume concentration under a minimal sonication time (1 h) resulting in a higher viscosity. On the other hand, the viscosity of nanofluid was reduced with the help of an increase in sonication duration and process temperature. Statistical analysis ranks a higher degree to volume concentration of nanoparticles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Conventional heat transfer fluids are considered energy-intensive and demanding costly thermal management systems and can cause environmental concerns. The concept of nanofluids started at the beginning of the nineteenth century, but there is still a need for comprehensive research, as the development of new types of nanoparticles and their exotic properties offer a solution for emerging engineering applications. Choi and Eastman [1] introduce the concept of nanofluids by suspending nanoparticles in the base fluid in order to create advantages over conventional fluids. Nanofluids are new-generation heat transfer fluids synthesized by dispersing metallic like copper, aluminium, etc., or non-metallic nanoparticles such as various forms of carbide, ceramics, and semiconductors or nanofibers/nanotubes of less than or equal to 100 nm size in base fluids [2,3,4].
Zarei et al. [5] have suggested the prospects of nanofluids as a working fluid for various heat transfer applications. Nanofluids have boosted thermal properties with better long-term stability, and offer low pressure drops and erosion during fluid pumping [2]. Nanofluids are prepared using either one-step or two-step techniques without/with the addition of a surfactant to increase its stability and prevent sedimentation. Generally, heat transfer fluids such as water, ethylene glycol, propylene glycol [6], tri-ethylene glycol [5], glycerol [7], and engine oil [8] are explored as base fluid. However, a mixture of water and ethylene glycol is widely used as a heat exchange medium in both heating and cooling systems to maximize the boiling point and minimize the freezing point, respectively [9]. In a nanoparticle group, alumina (α-Al2O3) is one of the non-metallic nanoparticles having unique favourable characteristics for the requirement of many heat transfer applications. Alumina (α-Al2O3) nanoparticle is a thermodynamically stable metal oxide with attractive features such as better specific heat, high thermal conductivity, and lower density [10].
Characteristics of nanofluid are governed by four essential thermophysical properties, such as thermal conductivity, viscosity, density, and specific heat [11]. Also, these properties are directly related to parameters such as type of nanoparticles, their volume concentration, mixture of base fluids, and process temperature [2].
A suitable combination of above-mentioned parameters is to be controlled to tailor the required properties of nanofluids. Thermal conductivity and viscosity of nanofluids are the most significant characteristics that describe the application of nanofluids in heating and cooling systems, nuclear reactors, pool boiling, solar heater, lubricant for machining processes, automotive cooling system, medical, and food industries [6]. Thermal conductivity and viscosity of the nanofluid increase with the addition of nanoparticles is unavoidable. However, the required heat transfer fluids must be balanced as low viscosity and high thermal conductivity for efficient use as coolants [12]. In terms of flow and heat transfer properties, viscosity is one of the essential properties of fluids and determines the selection of nanofluids for various applications [13]. Much research has been conducted on the thermal properties of nanofluids [14,15,16], especially thermal conductivity, stability and specific heat, with regard to the morphology and size of added nanoparticles. Few research studies have reported on the viscosity of nanofluids which consist mainly of a mono-type fluid [3]. Viscosity is also referred to as dynamic viscosity, which is an important behaviour of thermophysical properties of colloidal suspensions like nanofluid [17, 18]. However, there has been gradually increasing interest in the viscosity of nanofluids due to its impact on several other characteristics of nanofluid such as heat transfer, fluid flow, resistance to flow, specific heat capacity, and pumping pressure [19,20,21,22]. Several factors influence the viscosity of nanofluids, either directly or indirectly, including particle shape and size, volume concentration, base fluid properties, process temperature, and added surfactants [23, 24]. Usually, nanofluids have a higher viscosity as compared to their respective base fluids but the requirement of any nanofluid has as much as low viscosity to facilitate the fluid flow [3].
Likewise, temperature is a significant parameter that influences the viscosity of nanofluids. Normally, an increase in temperature significantly decreases the viscosity of nanofluids [25, 26]. In addition, Asadi et al. [27] stated in a study that nanofluids show a gradual reduction in viscosity up to critical temperature, after that the viscosity is dramatically reduced to a lower extent. Moreover, the prospect of nanofluid is also related to its processing conditions, which may extend stability of nanofluid.
The effect of the ultrasonication process on the thermal conductivity and viscosity of nanofluid has been studied in order to understand its positive impact on the studying fluids [28, 29]. Ultrasonication can prevent the formation of agglomeration of nanoparticles and facilitate its uniform dispersion in the base fluid. Ultrasonication process controlled by parameters of power, duration, and frequency to make a dispersion effectively. However, sonication duration is an ultrasonication process parameter that effectively controls the nanofluid properties by a uniform dispersion of nanoparticles and its stability in a base fluid [30]. Further to understand the ultrasonication process, Mahbubul et al. [31] investigated the effect of ultrasonication energy on the viscosity of Al2O3 water nanofluid and reported the decrease in the number of clusters with an increase in ultrasonication duration but not considered to have a concentration effect and a process temperature.
As compared to low viscosity base fluids, higher viscosity base fluids containing nanoparticles need more sonication time (energy) to become homogeneous [32]. Kwak and Kim [33] found that an optimal duration as 9 h required to complete dispersion of nanoparticles for ethylene glycol-based CuO nanofluid from the experimental sonication duration of 1 to 30 h. Lee et al. [25] conducted the sonication of aqueous-based Al2O3 (30 nm) nanofluids up to 30 h and found that 5 h of sonication was an optimum. They asserted that an optimal ultrasonication period for the nanofluids depends on the size and zeta potential of alumina nanofluids. Buonomo et al. [34] and Gangadevi et al. [35] found optimum sonication duration for water based Al2O3 nanofluid as 2 h (40 nm) and 4 h (50 nm), respectively. At the same time, Adio et al. [36] conducted experiments with Al2O3 nanoparticles of different sizes such as 30, 80 and 100 nm in glycerol as base fluid and reported that nanofluid prepared with smaller particle size (30 nm) shows higher viscosity and requires more sonication energy when compared with nanofluid prepared with larger particle size (80 nm and 100 nm). He et al. [37] investigated the viscosity of TiO2 nanofluids with particle sizes ranging from 95 to 210 nm and found that the viscosity increases with increasing nanoparticle size; however, their experimental results contradicted Adio et al. findings.
Considering the above discussions, the requirement of efficient heat transfer nanofluids with optimum viscosity is required for the heat transfer applications. The present investigation aimed to study the effect of ultra-sonication duration, volume concentration of nanoparticles, and temperature on the dynamic and relative viscosity of the WEG based Al2O3 nanofluid to suit different engineering applications. Further, statistical analysis on viscosity of nanofluid has been performed to study the effect of considered parameters to determine its significance and their ranking.
2 Experimental Procedure
2.1 Source and Characteristics of Alumina Nanoparticles
Al2O3 nanoparticles are chemically stable, less toxic, cheaper, and commercially available. α-Al2O3 nanoparticles of less than 50 nm were purchased from Sigma-Aldrich, Germany. High-Resolution Transmission Electron Microscope (HR-TEM) was employed to study the morphological characteristics of Al2O3 nanoparticles.
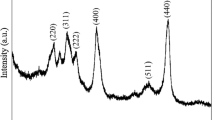
The morphology and average size of the nanoparticles, as shown in Fig. 1a, were almost spherical, and 40 nm. XRD analysis on the Al2O3 nanoparticles was carried out with an Empyrean X-ray diffractometer (PANalytical, Netherlands) with Cu anode (line and point focus) to confirm the crystalline structure of metal oxide. Figure 1b reveals the rhombohedral structure of Al2O3 nanoparticles and confirmed peaks with the help of JCPDS card number 1040LQS. Major XRD reflections observed were listed as follows: 25.59°, 35.14°, 37.78°, 43.36°, 52.55°, 57.52°, 59.8°, 61.23°, 66.55°, 68.20°, and 77.2°. The morphology, size, and structure of Al2O3 nanoparticles were identified to be suitable for the preparation of WEG based alumina nanofluid.
2.2 Calculation of Volume Fraction of Nanoparticle
The formula used to find the volume concentration of the nanoparticle [38] in the nanofluid is mentioned below Eq. (1).
where,
ϕ is the volume fraction of the nanoparticle.
VAl2O3 and VWEG are the weights of nanoparticle and WEG fluid, respectively.
ρAl2O3 and ρWEG are the densities of nanoparticles and WEG fluid, respectively.
2.3 Preparation of Nanofluids
The two-step procedure is the most widely used method for nanofluid preparation. Ethylene glycol has anti-freezing properties [39] and is miscible with water. Yu et al. [40] suggested that the suitable proportion of water and ethylene glycol (WEG) is 50: 50 for anti-freezing or anti-boiling applications. It is therefore considered to be a good base fluid for nanofluid preparation [41]. Nanofluid was prepared by dispersing the Al2O3 nanoparticles in the WEG fluid in the ratio of 50:50 (Ethylene glycol: water) using a two-step method [42], as follows:
-
1.
100 ml of a mixture of deionized water and ethylene glycol was stirred well over a magnetic stirrer (REMI 5MLH) at a constant speed of 420 rpm. Al2O3 nanoparticles were then added to the mixture and the stirring process continued at the same speed.
-
2.
Finally, the obtained suspension was again sonicated to required stability with an ultrasonication method (RS PRO Ultrasonic) with a facility to precisely control the temperature of the bath.
2.4 Examination of Stability of Al2O3 Nanofluids
Al2O3 nanoparticles were dispersed in the volume concentration of 0.01%, 0.05%, 0.1%, and 0.2% in ethylene glycol–water mixture. Prepared nanofluid samples were stored for one month in glass vials to determine the stability through visual observation. Figure 2a shows the photograph of nanofluid samples at the time of preparation. After one month, the photograph of samples was taken and is presented in Fig. 2b. No visual sediments were observed in all studied samples but there was a presence of slight sedimentation for higher volume concentration samples.
2.5 Particle Size Determination in Alumina Nanofluids
Dynamic Light Scattering (DLS) is the most common method that can be used to find the size of particles in the suspension or nanofluids. The alumina particle/cluster size of the prepared nanofluids was determined using a Dynamic Light Scattering (Model: Nanotrac Wave II) analyzer. After 3 days of nanofluid preparation, particle size analysis was performed at room temperature for lower (0.01%) and higher (0.2%) volume concentrations.
2.6 Viscosity Measurements
A rotational type digital viscometer (VISCO-895, ATAGO, Japan) was used to measure the dynamic viscosity of prepared samples. Some advantages of using this type over other viscometers were high accuracy (± 1%), small sample amount (16 mL), least count (~ 1mPas), and variable speed (0.5–250 rpm). The digital viscometer measures the viscosity of the liquid sample directly using the theory of measuring the shear stress (torque) between the cylindrical surface of the spinning cylinder immersed in the sample. The viscosity of samples was taken at a constant rotation speed of 250 rpm.
Prior to measurement, the instrument was calibrated using a standard liquid with known viscosity. The dynamic viscosity of WEG (50:50) is measured three times for the validation of the instrument, and the same is compared with the values of ASHRAE [43]. A k-type thermocouple was used to monitor changes in the temperature of nanofluids during viscosity measurements. Figure 3 shows a comparison of the WEG experimental results with the ASHRAE data. The observed results were in good agreement with ASHRAE data showing a variance of ± 5 per cent and thus verified the validity of the measurements.
For experimentation, process parameters such as ultrasonication time (0–4 h), temperature (30–45 °C), volume concentration (0.01%, 0.05%, 0.1%, 0.2%) of nanoparticles were considered to investigate the change in viscosity of WEG -based Al2O3 nanofluid. The selection of process parameters was based on the previous literature [19, 21]. This study is used to determine the variation in viscosity of WEG -based Al2O3 nanofluids without surfactant. The viscosity of each sample was measured every hour after sonication under investigation conditions and repeated an average of three trials.
2.7 Taguchi’s Statistical Design and Analysis for Ranking
Statistical analysis was conducted using a Taguchi method for ranking of considered parameters that are influencing the viscosity of nanofluids [44]. The present work aims to acquire minimum viscosity as the desired property of nanofluid, therefore the ‘smaller is better’ condition was chosen to obtain the optimum results. Experimentally obtained viscosity values and their corresponding parameters were used to construct the orthogonal array (L16) design. Experimental parameters and their levels for the L16 orthogonal model are shown in Table 1.
The ranking of the influencing parameters, such as temperature, nano-aluminium concentration and sonication time, and their optimum levels for achieving the minimum viscosity of the prepared nanofluid, were analysed using the Signal-to-Noise (S/N) ratio.
3 Results and Discussion
Experimental findings of the viscosity of WEG based alumina nanofluids were presented in this section, with the effect of ultrasonication time, volume concentration of nanoparticles, and process temperatures.
3.1 Effect of Ultra-sonication Time
The effect of ultrasonication time on dynamic viscosity of water-ethylene glycol (WEG)-based Al2O3 nanofluids is presented in Fig. 4a–d, as a function of various volume concentrations and process temperatures. It is noted that the dynamic viscosity of the nanofluid increases marginally for 1 h of sonication time. Thereafter, there is a decreasing trend with the sonication duration at all volume concentrations. A similar pattern in viscosity with a sonication duration has been observed for deionized water-based multi-walled carbon nanotubes nanofluid [45] and ethylene glycol-based carbon nanotubes nanofluid [46]. A small rise in viscosity was attributed to the presence of clusters of nanoparticles that provide resistance to the viscometer spindle. Later, it decreases due to the de-clustering of agglomerates with a sonication duration. At volume concentration of 0.01%, the rate of increment in viscosity for the sonication time of 1 h is lower at 45 °C compared to 30 °C. An increase in process temperature certainly reduces the increment of viscosity due to particle clusters. For given volume concentrations and temperatures, nanofluid viscosity values without sonication are referred to as the baseline values.
The baseline viscosity was 3.42 cP for 0.01% (lower volume concentration) at 30 °C, as the sonication time increased to 3 h the viscosity decreased by 1.75% to 3.36 cP. It is shown that ultrasonication does not alter the nanofluid viscosity considerably. Similarly, the decrease in viscosity was 10.6% for 0.2% (higher volume concentration). Ultrasonication has a major impact on the viscosity of WEG-based Al2O3 nanofluids. It is noted that there is an interrelation between the volume concentration of nanoparticles and the ultrasonication duration.
Likewise, at 45 °C, the sonication time increased from 0 to 3 h, and the viscosity value decreased by 3.8% for 0.01% (lower volume concentration). Similarly, a decrease in viscosity of 11.36% was observed for 0.2% (higher volume concentration). It is shown that concentration increased from 0.01 to 0.2%, at a higher temperature, the rate of reduction in viscosity was a combined effect of increased process temperature and ultrasonication duration. This combined effect apparently causes mobility of the particles and weakens the cohesive force between the particles [47].
In addition, there is an insignificant decrease in viscosity value with sonication time for 0.01% and 0.05%. Despite using ultrasonic energy to disperse nanoparticles, a large volume of base fluid with a low fraction of nanoparticles absorbs ultrasonic energy. Sonication was more beneficial by adding more nanoparticles to the base fluid. In particular, a higher volume concentration (0.2%) shows a substantial shift in viscosity with sonication time compared to lower volume concentrations.
Continuous reduction in nanofluid viscosity is observed up to a sonication duration of 3 h. It was evident that the de-clustering of agglomerates stimulates the mobility of nanoparticles, leading to a reduction in viscosity. After that, viscosity is approached by a stable value for a higher sonication duration. It's because nanofluids reach their level of homogeneity. It is observed that the prolonged sonication of nanofluid does not produce any desirable effect on viscosity. A similar pattern of viscosity is observed for the various processing temperatures and concentrations. Comparing all the results, the optimal sonication time to obtain the lowest nanofluid viscosity for the volume concentrations considered is 3 h.
3.2 Effect of Temperature
The effect of temperature on dynamic viscosity of water-ethylene glycol (WEG)-based Al2O3 nanofluids under various sonication times and volume concentrations is shown in Fig. 5a–d. All samples of WEG-based Al2O3 nanofluids show a steady decrease in viscosity with a rise in temperature from 30 to 45 °C. The increase in processing temperature of nanofluids reduces the strength of intermolecular attraction between base fluid and nanoparticles [3] and increases the Brownian motion of nanoparticles [45]. The addition of particles increases viscosity, but the decrease in viscosity is related to temperature. The increase in thermal energy of nanofluid helps to encourage Brownian motion, which leads to a decrease in the attractive forces of inter-particles and thus decreases viscosity of nanofluids.
For a volume concentration of 0.01%, a negligible shift in viscosity values with a volume concentration of 0.05% in volume at all temperatures for a sonication duration of 1 h is observed, as shown in Fig. 5a. It shows that an increase in temperature decreases the viscosity for all concentrations. However, for 0.01% and 0.05%, the difference in viscosity would be almost the same, but little change is observed due to a small amount of nanoparticles added. Conversely, there is a considerable increase in the viscosity of nanofluid with addition of nanoparticles (0.1% and 0.2%). This is due to an increase in the volume concentration of the number of dispersed nanoparticles in the base fluid, which contributes to an increase in viscosity regardless of the increase in temperature. It is noteworthy that the decrement trend of viscosity of nanofluid was observed with an increase in process temperature. It may reduce the viscosity, which has been increased by added nanoparticles. Thus, it brings the viscosity values down to desired value. Ruan and Jocabi [46] performed experiments with ethylene glycol nanofluids based on carbon nanotubes and reported similar effects of temperature on viscosity.
Decreased viscosity with processing temperature was observed for all concentrations regardless of the sonication time, as shown in Fig. 5b. As compared to Fig. 5a, b indicates a decrease in the magnitude of the viscosity due to the de-clustering of agglomerated nanoparticles in the base fluid. Further sonication time increased to 3 h, and the magnitude of the viscosity decreased to a certain level to all temperatures considered as shown in Fig. 5c. It seems that an effective sonication realized at 3 h. It is noted that the sonication effect was not much realized, but a small shift in the magnitude of viscosity is observed at lower concentrations. At the same time, the viscosity value of 0.2% volume concentration is moved further downwards and reached a closer viscosity value of 0.1% for studied temperatures. Similar pattern is also observed in Fig. 5d. This figure shows a negligible change in magnitude of the viscosity between 0.1 and 0.2% for the 4 h sonication duration. Experimental results show that there is a substantial decrease in the viscosity magnitude of WEG-based Al2O3 nanofluid for a 3 h sonication period. This means that WEG-based Al2O3 nanofluid achieves homogeneity at a sonication time of 3 h leading to a significant declustering of agglomerates. Asadi and Alarifi [30] found that extended sonication time might not have had any beneficial impact on nanofluid dispersion. It is derived from experimentation that the ultrasonication process was much needed to achieve lower viscosity for higher concentrations added nanofluid as compared to lower concentrations.
The distribution of nanoparticles in nanofluid under different conditions is presented schematically in Fig. 6. Null effect is characterized as an infinitesimal effect on the dispersion of nanoparticles due to sonication. Null effect was observed for lower concentrations of nanoparticles under 1 h and 3 h, irrespective of processing temperatures. Low-volume concentration does not take longer sonication time for dispersion of nanoparticles. At the same time, clusters were observed at a higher volume concentration under a minimal sonication time. This implies that the importance of the sonication effect would be needed to disperse higher concentrations of nanoparticles. This concludes that the concentration of alumina has a higher impact on the viscosity of nanofluids compared to process temperature and sonication time.
3.3 Effect of Cluster Size on Viscosity of Nanofluids
In order to study the effect of alumina cluster particle distribution on viscosity of WEG-based Al2O3 nanofluids, lower volume concentration (0.01%) and higher volume concentration (0.2%) at 1 h and 3 h sonicated conditions were considered. Figure 7a–d depicts the particle/cluster size of alumina-WEG nanofluids measured at room temperature by DLS method for sonication durations (1 h and 3 h) and volume concentrations (0.01 and 0.2%).
The mean cluster size of 0.01% nanofluid at 1 h of sonication was 150 nm, which decreased to 135 nm as the sonication time increased to 3 h. As the concentration of nano-alumina increased to 0.2%, the mean cluster size at 1 h of sonication was 215 nm, which decreased substantially to 175 nm after 3 h of sonication. Similarly, the observed viscosity of nanofluid was higher for 1 h sonicated samples relative to 3 h sonicated samples, as previously shown in this study. The obtained results suggest that increasing the cluster size of nanoparticles increases the dynamic viscosity of prepared nanofluids. This result is consistent with the findings of Nguyen et al. [26] and Jarahnejad et al. [48], who analysed the viscosity of Al2O3/water nanofluids with cluster/particle sizes ranging from 36 to 300 nm and found that the viscosity of the nanofluid increased with the size of the nanoparticles. This may be because the molecular structure of nanofluids has changed. It is noted that the mean cluster size of nanofluids was substantially bigger than the primary nanoparticle size (average size 40 nm).
3.4 Relative Viscosity of WEG Based Al2O3 Nanofluid
Figure 8a–d describes the relative viscosity of WEG (50:50) based Al2O3 nanofluids with different volume concentrations, temperatures, and sonication durations. Loading nanoparticles in the base fluid increases the density of nanofluids, which increases the viscosity of nanofluids [8]. Generally, higher nanoparticle loading reduces the inter-particle distance resulting in increased interaction between the nanoparticles.
From the observation, a maximum rise in relative viscosity is observed at a volume concentration of 0.2% under all considered sonication durations and temperatures. This indicates that the volume concentration contribution to the viscosity was large compared to other variables, including process temperature and ultrasonication time. Esfe and Saedodin [49] and Kole and Dey [50] have reported that the viscosity of nanofluids increases linearly with an increase in the volume fraction or concentration of nanoparticles.
It can also be shown from Fig. 8a–d that the relative viscosity decreases linearly with an increase in sonication time. This is the outcome of the ultrasonication effect on nanofluids arising from the de-clustering of nanoparticle agglomerates in WEG nanofluid. By comparing all cases, the minimum relative viscosity observed was 1.13, 1.14, 1.29, and 1.32 for 0.01%, 0.05%, 0.1%, and 0.2% of the volume concentration at 3 h and 45 °C, respectively.
3.5 S/N Ratios for Viscosity Measurements
The S/N ratio was used to determine the statistical parameters affecting the viscosity of WEG-based Al2O3 nanofluid. Table 2 presents the S/N ratio of parameters considered for dynamic viscosity of nanofluids including their ranks.
Figure 9 shows the main effect plot of the viscosity of WEG -based Al2O3 nanofluid for the considered parameters using an L16 Orthogonal design. The goal of the present work is to minimize the viscosity of the prepared nanofluid, which can be done at a temperature of 45 °C with a S/N ratio of-10.81. The addition of heat energy to nanofluid increases the random motion of nanoparticles and reduces the resistance of fluids, thus increasing the distance between particles. This leads to a major decrease in nanofluid viscosity. The findings obtained are similar to previous experimental studies on nanofluids [51, 52]. The minimum viscosity of the prepared nanofluid was achieved at a S/N ratio of -10.23 for the lowest aluminium concentration of 0.01%. Normally, a smaller quantity of nanoparticles may not have had a significant effect on the viscosity of the base fluid. Ultrasonication process offers good dispersion of nanoparticles by invoking de-clustering of agglomerates in an alumina based WEG nanofluid. Based on the results, the minimum viscosity of nanofluid was achieved at a S/N ratio of -10.88 for a sonication period of 3 h. The optimal sonication time was 3 h from both experimental and statistical analysis.
Based on the present statistical design, the desired combination of parameters to obtain a minimum nanofluid viscosity was a high processing temperature (45 °C), a low aluminium concentration (0.01%) and an intermittent sonication time (3 h). Other thermophysical properties have also been considered during the selection of parameters for the preparation of nanofluids for specific engineering applications. However, the present research is limited to the investigation of viscosity, which is one of the essential characteristics of nanofluids.
The delta value for each parameter was determined by the difference in the maximum and minimum S/N ratio values. Based on delta value, rank for influencing parameters on viscosity of nanofluid was determined. The current statistical study lists the order of parameters that follows alumina concentration > temperature > sonication time.
4 Conclusion
The present study reveals the variation in viscosity of α-Al2O3-WEG based nanofluids without surfactant. Influence of parameters such as ultra-sonication duration (0–4 h), process temperature (30–45 °C), and volume concentration (0.01%, 0.05%, 0.1%, 0.2%) of nanoparticles on the dynamic viscosity and relative viscosity of α-Al2O3-WEG based nanofluids was studied through experimental and statistical analysis. The following observations are made from this study:
-
1.
TEM results of α-Al2O3 reveal spherical morphology and are free from impurities. XRD results of α-Al2O3 show the characteristic peaks of rhombohedral Al2O3 structure.
-
2.
Dynamic viscosity of the nanofluid increases as a result of nanoparticle clusters due to the nature of inter-particle attraction requiring strong ultrasonication. However, viscosity decreases with an increase in sonication duration due to the de-clustering effect of agglomerates, increasing inter-particle distance.
-
3.
Sonication time increased from 0 to 3 h, a decrease in viscosity of 11.36% was observed for 0.2% volume concentration with precondition of higher process temperature (45 °C). After sonication time of 3 h, viscosity value becomes almost stable and nanofluids reach homogeneity for all considered temperatures and volume concentrations.
-
4.
A low concentration nanoparticle-containing nanofluid requires a smaller ultrasonication, in other words, null effect was seen for lower concentrations irrespective of the temperature and sonication time. At the same time, clusters were observed at a higher volume concentration under a minimal sonication time (1 h).
-
5.
Sonication effect was much required to disperse higher concentrations of nanoparticles effectively. This concludes that the concentration of alumina has a higher impact on the viscosity of nanofluids compared to process temperature and sonication time.
-
6.
The addition of heat energy to nanofluid by increasing process temperature reduces the resistance of fluids which decrease the viscosity of nanofluid for all concentrations, regardless of the sonication time.
-
7.
The current study revealed that the clustering effect of alumina nanoparticles increases the viscosity of Al2O3-WEG based nanofluids.
-
8.
The minimum relative viscosity observed was 1.13, 1.14, 1.29, and 1.32 for 0.01%, 0.05%, 0.1%, and 0.2% of the volume concentration at 3 h and 45 °C, respectively. Since the loading of nanoparticles in WEG base fluid increases the interaction between nanoparticles and clustering resulting in increased relative viscosity of nanofluid.
-
9.
In addition, based on the S/N ratio, the order of parameters affecting the viscosity value was the alumina concentration > temperature > sonication time. The inference obtained from the statistical analysis is in line with experimental results.
References
Choi, S.U.S.; Eastman, J.A.: Enhancing thermal conductivity of fluids with nanoparticles. Am. Soc. Mech. Eng. Fluids Eng. Div. FED. 231, 99–105 (1995)
Ghadimi, A.; Saidur, R.; Metselaar, H.S.C.: A review of nanofluid stability properties and characterization in stationary conditions. Int. J. Heat Mass Transf. 54, 4051–4068 (2011). https://doi.org/10.1016/j.ijheatmasstransfer.2011.04.014
Murshed, S.M.S.; Estellé, P.: A state of the art review on viscosity of nanofluids. Renew. Sustain. Energy Rev. 76, 1134–1152 (2017). https://doi.org/10.1016/j.rser.2017.03.113
Gbadeyan, J.A.; Titiloye, E.O.; Adeosun, A.T.: Effect of variable thermal conductivity and viscosity on Casson nanofluid flow with convective heating and velocity slip. Heliyon 6, e03076 (2020). https://doi.org/10.1016/j.heliyon.2019.e03076
Zarei, J.M.; Keshavarz, P.; Zerafat, M.M.; Sabbaghi, S.: Experimental investigation on the thermal conductivity of Triethylene Glycol-Water-CuO nanofluids as a desiccant for dehydration process. Int. J. Nano Dimens. 11, 74–87 (2020)
Babar, H.; Sajid, M.; Ali, H.: Viscosity of hybrid nanofluids: a critical review. Therm. Sci. 23, 1713–1754 (2019). https://doi.org/10.2298/tsci181128015b
Sharifpur, M.; Adio, S.A.; Meyer, J.P.: Experimental investigation and model development for effective viscosity of Al2O3–glycerol nanofluids by using dimensional analysis and GMDH-NN methods. Int. Commun. Heat Mass Transf. 68, 208–219 (2015)
Mishra, P.C.; Mukherjee, S.; Nayak, S.K.; Panda, A.: A brief review on viscosity of nanofluids. Int. Nano Lett. 4, 109–120 (2014). https://doi.org/10.1007/s40089-014-0126-3
Sekrani, G.; Poncet, S.: Ethylene- and propylene-glycol based nanofluids: a litterature review on their thermophysical properties and thermal performances. Appl. Sci. (2018). https://doi.org/10.3390/app8112311
Saleemi, M.; Vanapalli, S.; Nikkam, N., et al.: Classical behavior of alumina (Al2O3) nanofluids in antifrogen N with experimental evidence. J. Nanomater. 2015, 1–7 (2015)
Okonkwo, E.C., Wole-Osho, I., Almanassra, I.W. et al.: An updated review of nanofluids in various heat transfer devices. J. Therm. Anal. Calorim. 1–56 (2020)
Aishwarya, V.; Suganthi, K.S.; Rajan, K.S.: Transport properties of nano manganese ferrite-propylene glycol dispersion (nanofluids): New observations and discussion. J. Nanoparticle Res. (2013). https://doi.org/10.1007/s11051-013-1774-3
Patra, A.K.; Nayak, M.K.; Misra, A.: Viscosity of nanofluids-a review. Int. J. Thermofluid Sci. Technol. 7, 70202 (2020)
Thomas, S.; Sobhan, C.B.P.: A review of experimental investigations on thermal phenomena in nanofluids. Nano Res. Lett. 6, 377 (2011)
Murshed, S.M.S.; De. Castro, C.A.N.: Superior thermal features of carbon nanotubes-based nanofluids–a review. Renew Sustain. Energy Rev. 37, 155–167 (2014)
Aybar, H.Ş; Sharifpur, M.; Azizian, M.R., et al.: A review of thermal conductivity models for nanofluids. Heat Transf. Eng. 36, 1085–1110 (2015)
Estellé, P.; Halelfadl, S.; Maré, T.: Lignin as dispersant for water-based carbon nanotubes nanofluids: impact on viscosity and thermal conductivity. Int. Commun. Heat Mass Transf. 57, 8–12 (2014)
Qiu, L.; Zhu, N.; Feng, Y., et al.: A review of recent advances in thermophysical properties at the nanoscale: from solid state to colloids. Phys. Rep. 843, 1–81 (2020). https://doi.org/10.1016/j.physrep.2019.12.001
Bashirnezhad, K.; Bazri, S.; Safaei, M.R., et al.: Viscosity of nanofluids: a review of recent experimental studies. Int. Commun. Heat Mass Transf. 73, 114–123 (2016). https://doi.org/10.1016/j.icheatmasstransfer.2016.02.005
Meyer, J.P.; Adio, S.A.; Sharifpur, M.; Nwosu, P.N.: The Viscosity of nanofluids: a review of the theoretical, empirical, and numerical models. Heat Transf. Eng. 37, 387–421 (2016). https://doi.org/10.1080/01457632.2015.1057447
Yang, L.; Xu, J.; Du, K.; Zhang, X.: Recent developments on viscosity and thermal conductivity of nanofluids. Powder Technol 317, 348–369 (2017). https://doi.org/10.1016/j.powtec.2017.04.061
Routbort, J.L.; Singh, D.; Timofeeva, E.V.; Yu, W.; France, D.M.: Pumping power of nanofluids in a flowing system. J. Nanoparticle Res. 13, 931–937 (2011). https://doi.org/10.1007/s11051-010-0197-7
Soto, A.; Gaster, T.; Golden, C.; Vafaei, S.: Theoretical investigation of thermal conductivity and viscosity of nanofluids. Proc. Therm. Fluids Eng. Summer Conf. (2020). https://doi.org/10.1615/TFEC2020.nma.032014
Suganthi, K.S.; Anusha, N.; Rajan, K.S.: Low viscous ZnO–propylene glycol nanofluid: a potential coolant candidate. J. Nanoparticle Res. 15, 1–16 (2013)
Lee, J.H.; Hwang, K.S.; Jang, S.P.; Lee, B.H.; Kim, J.H.; Choi, S.U.S.; Choi, C.J.: Effective viscosities and thermal conductivities of aqueous nanofluids containing low volume concentrations of Al2O3 nanoparticles. Int. J. Heat Mass Transf. 51, 2651–2656 (2008)
Nguyen, C.T.; Desgranges, F.; Roy, G., et al.: Temperature and particle-size dependent viscosity data for water-based nanofluids–hysteresis phenomenon. Int. J. heat fluid flow. 28, 1492–1506 (2007)
Asadi, A.; Pourfattah, F.; Miklósszilágyi, I., et al.: Effect of sonication characteristics on stability, thermophysical properties, and heat transfer of nanofluids: a comprehensive review. Ultrason. Sonochem. (2019). https://doi.org/10.1016/j.ultsonch.2019.104701
Chen, Z.; Shahsavar, A.; Al-Rashed, A.; Afrand, M.: The impact of sonication and stirring durations on the thermal conductivity of alumina-liquid paraffin nanofluid: an experimental assessment. Powder Technol. 360, 1134–1142 (2020). https://doi.org/10.1016/j.powtec.2019.11.036
Sonawane, S.S.; Khedkar, R.S.; Wasewar, K.L.: Effect of sonication time on enhancement of effective thermal conductivity of nano TiO2–water, ethylene glycol, and paraffin oil nanofluids and models comparisons. J. Exp. Nanosci. 10, 310–322 (2015). https://doi.org/10.1080/17458080.2013.832421
Asadi, A.; Alarifi, I.M.: Effects of ultrasonication time on stability, dynamic viscosity, and pumping power management of MWCNT-water nanofluid: an experimental study. Sci. Rep. 10, 1–10 (2020). https://doi.org/10.1038/s41598-020-71978-9
Mahbubul, I.M.; Chong, T.H.; Khaleduzzaman, S.S.; Shahrul, I.M.; Saidur, R.; Long, B.D.; Amalina, M.A.: Effect of ultrasonication duration on colloidal structure and viscosity of alumina-water nanofluid. Ind. Eng. Chem. Res. 53, 6677–6684 (2014). https://doi.org/10.1021/ie500705j
Yang, J.C.; Li, F.C.; Zhou, W.W.; He, Y.R.; Jiang, B.C.: Experimental investigation on the thermal conductivity and shear viscosity of viscoelastic-fluid-based nanofluids. Int. J. Heat Mass Transf. 55, 3160–3166 (2012). https://doi.org/10.1016/j.ijheatmasstransfer.2012.02.052
Kwak, K.Y.; Kim, C.Y.: Viscosity and thermal conductivity of copper oxide nanofluid dispersed in ethylene glycol. Korea-Australia Rheol. J. 17, 35–40 (2005)
Buonomo, B.; Manca, O.; Marinelli, L.; Nardini, S.: Effect of temperature and sonication time on nanofluid thermal conductivity measurements by nano-flash method. Appl. Therm. Eng. 91, 181–190 (2015). https://doi.org/10.1016/j.applthermaleng.2015.07.077
Gangadevi, R.; Vinayagam, B.K.; Senthilraja, S.: Effects of sonication time and temperature on thermal conductivity of CuO/water and Al2O3/water nanofluids with and without surfactant. Mater. Today Proc. 5, 9004–9011 (2018). https://doi.org/10.1016/j.matpr.2017.12.347
Adio, S.A.; Sharifpur, M.; Meyer, J.P.: Influence of ultrasonication energy on the dispersion consistency of Al2O3–glycerol nanofluid based on viscosity data, and model development for the required ultrasonication energy density. J. Exp. Nanosci. 11, 630–649 (2016). https://doi.org/10.1080/17458080.2015.1107194
He, Y.; Jin, Y.; Chen, H.; Ding, Y.; Cang, D.; Lu, H.: Heat transfer and flow behaviour of aqueous suspensions of TiO2 nanoparticles (nanofluids) flowing upward through a vertical pipe. Int. J. Heat Mass Transfer. 50, 2272–2281 (2007). https://doi.org/10.1016/j.ijheatmasstransfer.2006.10.024
Hamed Mosavian, M.T.; Zeinali Heris, S.; Etemad, S.G.; Nasr Esfahany, M.: Heat transfer enhancement by application of nano-powder. J. Nanoparticle Res. 12, 2611–2619 (2010). https://doi.org/10.1007/s11051-009-9840-6
Ashrae, A.: Handbook-Fundamentals. Atlanta, USA (2005)
Yu, W.; Xie, H.; Chen, L.; Li, Y.: Enhancement of thermal conductivity of kerosene-based Fe3O4 nanofluids prepared via phase-transfer method. Colloids Surf. A Physicochem. Eng. Asp. 355, 109–113 (2010)
Sawicka, D.; Cieśliński, J.T.; Smolen, S.: A comparison of empirical correlations of viscosity and thermal conductivity of water-ethylene glycol-Al2O3 nanofluids. Nanomaterials 10, 1–24 (2020). https://doi.org/10.3390/nano10081487
Beck, M.P.; Yuan, Y.; Warrier, P.; Teja, A.S.: The thermal conductivity of alumina nanofluids in water, ethylene glycol, and ethylene glycol + water mixtures. J. Nanoparticle Res. 12, 1469–1477 (2010). https://doi.org/10.1007/s11051-009-9716-9
Afrand, M.; Abedini, E.; Teimouri, H.: How the dispersion of magnesium oxide nanoparticles effects on the viscosity of water-ethylene glycol mixture: experimental evaluation and correlation development. Phys. E Low-Dimensional Syst. Nanostruct. 87, 273–280 (2017). https://doi.org/10.1016/j.physe.2016.10.027
Abadeh, A.; Passandideh-Fard, M.; Maghrebi, M.J.; Mohammadi, M.: Stability and magnetization of Fe3O4/water nanofluid preparation characteristics using Taguchi method. J. Therm. Anal. Calorim. 135, 1323–1334 (2019)
Sadri, R.; Ahmadi, G.; Togun, H.; Dahari, M.; Kazi, S.N.; Sadeghinezhad, E.; Zubir, N.: An experimental study on thermal conductivity and viscosity of nanofluids containing carbon nanotubes. Nanoscale Res. Lett. 9, 4–13 (2014). https://doi.org/10.1186/1556-276X-9-151
Ruan, B.; Jacobi, A.M.: Ultrasonication effects on thermal and rheological properties of carbon nanotube suspensions. Nanoscale Res. Lett. 7, 1–14 (2012). https://doi.org/10.1186/1556-276X-7-127
Mahbubul, I.M.; Saidur, R.; Amalina, M.A.; Niza, M.E.: Influence of ultrasonication duration on rheological properties of nanofluid: an experimental study with alumina–water nanofluid. Int. Commun. Heat Mass Transf. 76, 33–40 (2016)
Jarahnejad, M.; Haghighi, E.B.; Saleemi, M.; Nikkam, N.; Khodabandeh, R.; Palm, B.; Toprak, M.S.; Muhammed, M.: Experimental investigation on viscosity of water-based Al2O3 and TiO2 nanofluids. Rheol. Acta. 54, 411–422 (2015). https://doi.org/10.1007/s00397-015-0838-y
Hemmat Esfe, M.; Saedodin, S.: An experimental investigation and new correlation of viscosity of ZnO-EG nanofluid at various temperatures and different solid volume fractions. Exp. Therm. Fluid Sci. 55, 1–5 (2014). https://doi.org/10.1016/j.expthermflusci.2014.02.011
Kole, M.; Dey, T.K.: Effect of aggregation on the viscosity of copper oxide-gear oil nanofluids. Int. J. Therm. Sci. 50, 1741–1747 (2011). https://doi.org/10.1016/j.ijthermalsci.2011.03.027
Hemmat Esfe, M.; Rahimi Raki, H.; Sarmasti Emami, M.R.; Afrand, M.: Viscosity and rheological properties of antifreeze based nanofluid containing hybrid nano-powders of MWCNTs and TiO2 under different temperature conditions. Powder Technol. 342, 808–816 (2019). https://doi.org/10.1016/j.powtec.2018.10.032
Toghraie, D.; Mokhtari, M.; Afrand, M.: Molecular dynamic simulation of copper and platinum nanoparticles Poiseuille flow in a nanochannels. Phys. E Low-dimensional Syst. Nanostruct. 84, 152–161 (2016)
Acknowledgements
The authors would like to thank Sri Sivasubramaniya Nadar College of Engineering, Chennai for providing financial support through the SSN internal funding. We also acknowledge the technical support given by Prof. Dr. P. Ramasamy, Dean, SSN Research Centre.
Funding
This work is not funded by any agency.
Author information
Authors and Affiliations
Contributions
RP—Resources, Supervision, Validation and Visualization; LC—Conceptualization, Investigation, Data acquisition; KR—Supervision, Writing- Review and Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
About this article
Cite this article
Prakash, R., Chilambarasan, L. & Rajkumar, K. Process Parameters Effect Investigations on Viscosity of Water-ethylene Glycol-based α-alumina Nanofluids: An Ultrasonic Experimental and Statistical Approach. Arab J Sci Eng 46, 11909–11921 (2021). https://doi.org/10.1007/s13369-021-05790-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-021-05790-6