Abstract
Present work aims to study the dispersion characteristics of Al2O3 nano-dispersoid in water following different periods of ultrasonication and its impact on the thermal conductivity and viscosity of the nanofluid. Nanofluids with 0.5–2 vol% of Al2O3 nanoparticles have been prepared by ultrasonication for varying period. Al2O3 nanofluids reported a maximum thermal conductivity enhancement of 16.1% for 2 vol% of nanoparticle concentration, after an optimum ultrasonication of 2 h beyond which the thermal conductivity decreases with further ultrasonication. The optimum ultrasonication time required for uniform dispersion of nanoparticles increases with the increase in the Al2O3 volume fraction. For 1.5 vol% Al2O3 nanoparticle loading, the viscosity of nanofluid decreased by 33% with an increase in the sonication time from 30 to 90 min. Further increase in sonication time by 30 min resulted in 13% increase in the viscosity of Al2O3 nanofluid. This decrease in the thermal conductivity enhancement and increase in the viscosity beyond the optimum ultrasonication period have been attributed to the re-agglomeration of nanoparticles which are confirmed by TEM, and DLS results carried out after different instants of ultrasonication. The occurrence of re-agglomeration is explained in terms of the convective flow associated with the ultrasonication process. Various theoretical models like Maxwell or Hamilton–Crosser models which when used to predict the thermal conductivity of nanofluid, underestimate the thermal conductivity. A new correlation is, therefore, developed on the basis of experimental results. With an R2 value of 0.9924, the correlation showed a good agreement with the present thermal conductivity data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is an ever-increasing demand for faster heat dissipation in modern equipments such as computers, power electronics, high powered lasers, X-rays, etc., to maintain the desired level of performance. Hence, the idea of enhancement of heat transfer in industrial applications has led the researchers to look for an alternative cooling fluid or an alternative means of faster heat dissipation. While working at Argonne National Laboratory in 1995, Choi et al. [1] came up with a different fluid called nanofluid. It is a suspension of nanoparticles of a few nanometer (< 100 nm) in the base fluid (e.g. ethylene glycol, water, etc.). Following the pioneer work of Choi et al. [1], many researchers around the world have explored the field of nanofluid for different applications. Several types of nanoparticles have been reported in the literature to prepare nanofluids, which include metallic nanoparticles (Cu [2], Ag [3], and Au [4]), nonmetallic particles (Al2O3 [5], TiO2 [6], SiC [7], Fe3O4 [8], and AlN [9], BN [10]), and different particle morphologies such as carbon nanotubes [11], nanorods [12], etc. Alumina (Al2O3) comes under the commonly used nanoparticles in different experimental research works for dispersion in already available conventional base fluids. On the theoretical front, significant research has been carried out to understand the dispersion behavior of the nanoparticles in the base fluids such as the instantaneous interactions taking place amid nanoparticles and base fluid molecules [13, 14], the effects of local nanoparticle concentration and particle velocity [15,16,17,18,19]
Having a stable nanofluid with uniform dispersion is of utmost priority for all industrial applications. Different methods such as adding surfactants, applying ultrasonic vibration, etc. are implemented to achieve the maximum stability of nanofluids. The use of both the methods are observed in many works. Several researchers have studied the effect of sonication by varying its different parameters for attaining better dispersion of nanoparticles in the nanofluid [20,21,22,23,24,25,26]. The thermo-physical properties and stability were found to be dependent on parameters like sonication time, sonication energy, etc., to which the nanofluids have been subjected to during synthesis. Garg et al. [27] reported a twofold effect of ultrasonication time on the MWCNT nanofluids. Here, ultrasonication for 40 min was found to be optimum for achieving a better dispersion. However, ultrasonication beyond this period led to the breakage of the nanotubes thereby reducing the aspect ratio of CNTs. Also, ultrasonication beyond the optimum duration resulted in a negative influence on the maximum enhancement in thermal conductivity. Nguyen et al. [28] after studying the effect of ultrasonication time on the dispersion stability of alumina nanofluids suggested that prolonged ultrasonication does not lead to a significant reduction in particle rather it manifested a tendency of re-agglomeration. EG-based MWCNT nanofluids were investigated by Ruan et al. [29]. They reported an increase in thermal conductivity with increase in sonication time. The breakage of agglomerates with increasing sonication time was considered to be the main reason for the thermal conductivity improvement. Also, an initial increase in the viscosity is observed which later decreases with further sonication time approaching the viscosity of base fluid. Yet another study by Sonawane et al. [30] showed the optimum sonication period of 60 min was able to attain maximum thermal conductivity enhancement for TiO2-based nanofluids, but it started decreasing with further ultrasonication. It was proposed that beyond the optimum ultrasonication time a decrease in the effective surface area to volume ratio took place, which caused a reduction in the effective heat transfer area of nanoparticles. Asadi et al. [31] used Mg(OH)2 nanofluids to study the effects of sonication time on its thermal conductivity. They observed a fall in the relative thermal conductivity with increasing sonication time, which was more prominent at higher nanoparticle loading. A further detailed study on finding the optimal sonication period for nanofluids was recommended. Li et al. [32], found in their work on Cu nanofluids, that higher sonication time and temperature tend to increase the Brownian motion causing the larger clusters to collapse. Eventually, it led to a decrease in the viscosity of nanofluids. Buonomo et al. [33] pointed out that sonication time and energy are important factors in determining the maximum limit of thermal conductivity increase of alumina nanofluids. A simple method was also proposed to estimate the minimum required sonication time to yield a stable nanofluid. Chen et al. [34] examined the thermal conductivity of paraffin based Al2O3 nanofluids at different time intervals of stirring and sonication. They concluded that prolonging the sonication time initially increased then decreased the thermal conductivity. An optimum sonication and stirring time were determined on the basis of maximum thermal conductivity attained. Similar conclusions were also drawn by Shahsavar et al. [35], who used water-based Fe3O4/CNT hybrid nanofluids to study the sonication effects on its thermal conductivity. Xian et al. [36] obtained a better dispersion of graphene nanoplatelets–TiO2 (GnP–TiO2) hybrid nanoparticles in water-ethylene glycol mixture after only 90 min of sonication using cetyltrimethylammonium bromide (CTAB) as a surfactant. From aforesaid, it can be understood that, although the ultrasonication period is one of the important factors, its effect on the thermo-physical properties colloidal needs further investigation.
The present study, therefore, explores the effects of the sonication period on the dispersion behavior and heat transfer characteristics of aqueous Al2O3 nanofluids. The experimental results are also compared with the literature and a possible explanation has been provided on the basis of experimental results. Moreover, a new correlation function for thermal conductivity with the volume fraction of dispersoid has been reported.
Experimental procedure
Materials
Al2O3 nanoparticles of average particle size 13 nm were procured from Sigma Aldrich. Polyvinylpyrrolidone (PVP) (MW: 40,000) was also obtained from the Sigma Aldrich company for use as a surfactant during the synthesis of the nanofluid. Distilled water was used as the base fluid in the synthesis of nanofluids.
Nanofluid synthesis
Water-based Al2O3 nanofluids have been synthesized using the two-step method. Although the two-step synthesis method of nanofluids has the advantages of being low cost and easily scalable, it does suffer from poor stability. Due to the very high surface area of nanoparticles, agglomeration takes place at a fast rate during the dispersion stage. Hence, the addition of a suitable surfactant and ultrasonication process [25] have been employed to improve the stability by de-agglomerating the nanoparticles in the base fluid. Based on the literature available on water-based Al2O3 nanofluids, surfactants like CTAB [37], SDS [38] SDBS [39], and PVP [40] have been used to maintain the stability. For water-based Al2O3 nanofluids, PVP is the widely used polymeric dispersant because of its higher stability when compared to other surfactants [5, 41]. The long polymer chains help to interact with the nanoparticle and restrict the formation of aggregates, thereby leading to a stable and more uniformly dispersed nanofluid. Furthermore, PVP can also be used at higher temperatures making it a suitable choice for high temperature applications.
Desired amounts of Al2O3 nanopowder, calculated using Eq. (1) were initially mixed in the base fluid and stirred by a magnetic stirrer for 1 h, which is followed by sonication for 3 h [29]. Ultrasonication was carried out with a probe type ultrasonic processor (SONOPROS PR-1000MP) having a frequency of 20 ± 3 kHz and power of 1000 W using the OSCAR ULTRASONICS equipment. To avoid excess heat generation during the sonication, a water bath is maintained at 27 °C and replaced at regular intervals with fresh water. The as-prepared Al2O3 nanofluid is kept aside for around 20 min to attain a steady state and equilibrium condition. For the thermal conductivity and viscosity measurements, required volume of nanofluid is collected from top section of nanofluid. Here,
where \(w_{{{\text{Al}}_{2} {\text{O}}_{3} }}\) and \(w_{{{\text{bf}}}}\) denote the weight of Al2O3 nanoparticles and base fluid in g; \(\rho_{{{\text{Al}}_{2} {\text{O}}_{3} }}\) and \(\rho_{{{\text{bf}}}}\) are the density of Al2O3 nanoparticles and base fluid in g/cm3.
Characterization of nanofluids
The nanofluids of Al2O3 dispersoids have been characterized by different methods. The Al2O3 nanoparticles were characterized by Advance X-Ray Diffractometer (BRUKER D8, Germany) using Cu-Kα radiation (wavelength, λ = 1.54 Å). The powder sample for this analysis is placed on a Perspex slide in a groove of 1–2 mm depth and 10 mm × 10 mm area. The diffraction angle covered was 20°–80° at a scan speed of 0.05°. The phases present in the powder, have been identified by correlating the XRD spectrum with the XRD data compiled by International Center for Diffraction Data (ICDD). Philips X’pert High score Plus software has been used to identify the phases that are present in the diffraction pattern. Particle size and morphology of Al2O3 nanoparticles have been obtained from the bright field images of the powder particles observed by a JEOL JEM 2100 (JEOL, Japan) transmission electron microscope (TEM). It was operated at an accelerating voltage of 200 kV. A minute drop of the nanofluid is placed on a carbon coated copper grid of 3 mm size, which is later dried at room temperature. Using Zetasizer Nano ZS apparatus (Malvern instrument, UK) that operates on dynamic light scattering (DLS) principle, the particle size distribution of Al2O3 in the nanofluids is determined. A monochromatic light (laser) is incident onto the sample, and the light is scattered in all directions when it hits the nanoparticles undergoing Brownian motion in the fluid. The random changes in the intensity of the scattered light have been analyzed to calculate the size distribution of the dispersoids. All the measurements were done at 27 °C, and the time required for each measurement is 2 min. Viscosity measurements were performed using Physica MCR 101 rheometer (Anton Paar, Graz, Austria). Peltier system (P-PTD 200) at the lower plate was used for achieving constant temperature. A nanofluid sample of 20 ml was used for each viscosity measurements. The thermal conductivity of Al2O3 nanofluids have been measured using LAMBDA (Flucon fluid control GmbH, Germany) apparatus which works on the transient hot-wire (THW) principle. A Teflon-coated platinum wire (diameter = 100 μm) serves here as a temperature sensor and a heating element. A cylindrical shaped vial of length 90 mm and diameter 35 mm holds the nanofluid sample in which the platinum wire is immersed. The platinum wire is initially maintained at thermal equilibrium with its surrounding fluid. A voltage applied to the wire induces an electrical current flow through it which heats the fluid. The thermal conductivity is then calculated based on the transient temperature rise in the fluid, in a given time interval. The mathematical model that defines the temperature rise ∆T (r, t) over the platinum wire [42]:
where r is the diameter of the wire; q is constant heat produced per unit time and per unit length; a is the thermal diffusivity; C is the Euler’s constant. The thermal conductivity (k) is then calculated from the slope (c) of the linear section of the plot ∆T (r, t) vs ln (t).
For every thermal conductivity measurement, a total of ten readings were recorded. The uncertainty (u) was estimated using the mean and standard deviation (s) calculated from the set of measured thermal conductivity data using the following equation:
where n = total number of readings. The uncertainty was found to be well within 3%.
Table 1 gives a detailed summary of the experimental conditions used in the present study.
Results and discussion
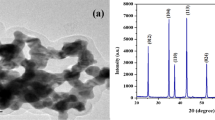
Figure 1 shows the XRD pattern of Al2O3 nanoparticles recorded in the range between 20° and 80°. The observed peaks in Fig. 1 correspond to the γ-Al2O3 phase which is in conformity with the JCPDS-ICDD data file no. 290063. The average crystallite size of the γ-Al2O3 calculated by the modified Williamson–Hall method [42] was ~ 7 nm.
To obtain an insight into the effect of ultrasonication time on the thermal conductivity of Al2O3 nanofluids, the thermal conductivity has been measured at different ultrasonication periods using the transient hot-wire method. Figure 2a shows the thermal conductivity improvement of Al2O3 nanofluids containing 0.5–2 vol% of Al2O3 with increasing ultrasonication time. In case of 0.5 vol% Al2O3 nanofluid, the thermal conductivity shows an improvement of 6.2% after 60 min of ultrasonication, but when the fluid is subjected to further sonication, the thermal conductivity enhancement deteriorated. A similar trend has also been observed for other particle concentrations of Al2O3 in the nanofluids. Moreover, it is also clear that with the increase in volume fraction of Al2O3 in the nanofluids, the ultrasonication time required for uniform distribution of nanoparticles to yield the maximum increment in thermal conductivity increases. For example, 2 vol% Al2O3 in nanofluid takes 120 min of sonication to obtain a 16.1% thermal conductivity enhancement, while for a lower concentration of Al2O3 like 1.5 vol%, the highest thermal conductivity enhancement is achieved after sonication for only 90 min. A similar phenomenon has been reported by other researchers [27, 30]. Figure 2b shows the maximum thermal conductivity enhancement of Al2O3 nanofluid attained for each Al2O3 volume concentration.
To further investigate why this kind of behavior of the thermal conductivity of Al2O3 nanofluids is manifested, the HR-TEM studies have been performed. 1.5 vol% Al2O3 nanofluid has been used for this purpose, which is then further diluted to get the desired concentration required for TEM studies. Figure 3 shows the TEM images of Al2O3 nanofluids at different instants of ultrasonication. It shows that with the increase in ultrasonication time homogenous dispersion is achieved after 90 min, and later the nano-Al2O3 starts re-agglomerating with further ultrasonication. A careful look reveals less clustering after 90 min of ultrasonication (Fig. 3b). These results can be related to the reduction in thermal conductivity enhancement as observed in Fig. 2a. Li et al. [32] also reported similar results for nano-CuO dispersion in EG-based nanofluids. They stated that the coalescing of nanoparticles due to their high surface energy had led to the formation of bigger clusters after longer ultrasonication times. These big clusters might be the reason for the decrease in thermal conductivity after longer ultrasonication.
DLS analysis has been performed to confirm the re-agglomeration of Al2O3 nanoparticles in water beyond an optimum ultrasonication time. A similar concentration of Al2O3 nanofluid used for TEM analysis has been used for conducting the DLS study. Figure 4 shows the particle size distribution of Al2O3 nanofluid measured at a constant interval of 30 min up to a total of 120 min of ultrasonication time. The initial 30 min of ultrasonication on the Al2O3 nanofluids gives a particle size distribution in the range of 68– 255 nm. The particle size distribution shows a shift towards the left i.e., lower particle size range with increasing ultrasonication period. After 90 min of ultrasonication, Al2O3 manifests a bimodal distribution with the first and second peak in the range 50–90 nm and 164–342 nm, respectively. The higher intensity for the second peaks is mainly due to the scattering intensity measured during DLS measurement being proportional to ~ r6 (r is the radius of the dispersed particle). Thus, even a slight presence of larger aggregate results in higher intensity. Finally, at 120 min, a wider range in the particle size distribution towards the higher particle size is observed (Fig. 4). The DLS confirms the re-agglomeration of Al2O3 nanoparticles during extended ultrasonication, and these results are in confirmity with the TEM data.
To gain an insight into the effect of sonication time on the dispersion behavior of Al2O3 nanoparticles in water, the viscosity measurements have been carried out on 1.5 vol% Al2O3 nanofluid over shear rates varying from 0 to 122/s. Figure 5 shows the viscosity of 1.5 vol% Al2O3 nanofluids measured at increasing ultrasonication periods from 30 to 120 min and at a temperature of 27 °C. It is clear from Fig. 5 that the viscosity of the nanofluid reduces with an increase in the ultrasonication time from 30 to 90 min. With further increase in the ultrasonication time, the viscosity increases as also observed in other works [43,44,45,46]. This can also be explained by the TEM images shown in Fig. 3. From the TEM images, it is clear that a homogeneous dispersion is achieved at 90 min of ultrasonication. The Al2O3 nanoparticle cluster splits into smaller particles due to the additional ultrasonic energy as the sonication time increases from 0 to 90 min. Such smaller particles offer less resistance to flow in nanofluids, which results in low viscosity. These smaller particles tend to re-agglomerate because of high surface energy, with further increase in sonication time. Due to re-agglomeration, more force is required for the nanoparticle to overcome the flow resistance, thus resulting higher viscosity. Precisely, the viscosity of the nanofluid decreases significantly by 33% with an increase in the sonication time from 30 to 90 min. However, further extension of sonication by 30 min causes the viscosity of Al2O3 nanofluid to increase by 13%. Therefore, it can be said that for a 1.5 vol% Al2O3 nanoparticles, an optimum sonication period of 90 min will achieve uniform dispersion and thereby better stability.
A possible explanation for the observed variation in the particle size distribution, thermal conductivity, and viscosity with ultrasonication time can be put forward as follows: the process of ultrasonication not only involves deagglomeration in the close vicinity of the ultrasonication probe, but also a convective flow of the nanofluid which brings agglomerated clusters closer to the probe as depicted in Fig. 6.
It is well known that the viscosity of nanofluid greatly depends on the average particle size of the dispersed nanoparticles [47,48,49,50]. With the increase in the sonication time, the state of agglomeration and the average particle size decreases significantly, thereby decreasing the viscosity [27, 29, 51]. But beyond an optimum ultrasonication period, viscosity is observed to increase as shown in Fig. 5. This increase in the viscosity is expected to obstruct the convective flow during the ultrasonication and keep away from the probe a substantial portion of the nanofluid. This portion of nanoparticles which remains away from the probe is where re-agglomeration is likely to occur, resulting in an increase in the average particle size which ultimately leads to a decrease in the thermal conductivity of the nanofluid.
Comparison of present experimental results with literature
Results of different researchers [37, 52,53,54,55,56,57,58,59,60] on water-based Al2O3 nanofluids have been compiled and compared with the present experimental results in Fig. 7. From Fig. 7, it is evident that the thermal conductivity enhancement of the nanofluids increases with the increase in volume fraction of nano-Al2O3. The following points emerge from the comparison with the present experimental results.
-
A simple two-step method for the synthesis of nanofluids was adopted by the majority of researchers and the THW technique was used for measuring the thermal conductivity of Al2O3 nanofluids.
-
It is observed that the results of Masuda et al. [60], Pak et al. [55], and Mojarrad et al. [54] closely follow the present experimental results. The nanoparticle size used by them was in the range of 13–30 nm, which is closer to the size of the nanoparticles used in the present study. It is evident that in the case of Esfe et al. [53], the use of very small size (5 nm) Al2O3 nanoparticles did not result in higher thermal conductivity enhancement when compared with others. This might be due to the phonon scattering taking place because of the very small size of Al2O3 nanoparticles leading to the reduced thermal conductivity of the nanoparticle resulting in a decrease in the effective thermal conductivity of nanofluids [61]. Moreover, using very small sized nanoparticles increases the probability of aggregation due to increased Van der Waals force, which further reduces the thermal conductivity of nanofluids.
-
The other set of researchers like Patel et al. [56], Xie et al. [57], Mintsa et al. [58] have used Al2O3 nanoparticles of comparatively bigger size like 150 nm, 47 nm, and 60.4 nm, respectively. The decreased surface area, as well as, the Brownian velocity for such larger nanoparticles, might be the reason for the decreased thermal conductivity [62].
-
Although the basic synthesis procedure (two-step method) was similar for all the works, other detailed factors like techniques used for achieving the stability vis., sonication period, sonication power, etc. varied from one researcher to the other.
Proposed correlation
The thermal conductivity of Al2O3 nanofluids of present work has been compared with the results from other theoretical models such as Maxwell [63], Hamilton–Crosser [64] Mintsa [58], Timofeeva [65], Buongiorno [66], and Maiga [67] in Fig. 8a. From the observations of Fig. 8a, it is clear that these models underestimate the experimental thermal conductivity results. Some possible explanation may be put forward, e.g., H–C model does not consider different factors such as the effect of Brownian motion, particle size, nanolayer formation, particle clustering, etc., which play significant role in enhancing the effective thermal conductivity of nanofluids. Different researchers such as Chen et al. [68], Das et al. [69], and Mushed et al. [70] have suggested a similar outcome and have gone ahead and proposed new models and correlations. As shown in the figure below, even after considering the uncertainty of ~ ± 3% (as shown by error bar) to the measured thermal conductivity, it is observed that the deviation of the measured thermal conductivity from the predictions of H–C and other models is significant.
Hence, a new correlation is proposed to estimate the thermal conductivity of Al2O3 nanofluids for different volume fractions. This correlation (Eq. (5)), developed by the curve fitting method can estimate the thermal conductivity with an R2 value of 0.9924. The thermal conductivity estimated from the proposed correlation and the present experimental results are shown in Fig. 8b.
where φ is the volume fraction of nanoparticles in the nanofluid.
Conclusion
The present work aims to analyze the effect of ultrasonication period on the thermal conductivity and viscosity of aqueous Al2O3 nanofluids. Al2O3 nanofluids having particle concentrations varying from 0.5 to 2 vol% have been synthesized using a two-step method. Following conclusions are drawn based on the present results:
-
Thermal conductivity enhancement of Al2O3 dispersed aqueous nanofluids having 0.5–2 vol% nanoparticles is measured after ultrasonication for 30–150 min showed an increasing thermal conductivity up to an optimum period of ultrasonication beyond which it shows a decline. The maximum thermal conductivity enhancement of 14.6% is attained for 1.5 vol% Al2O3 after ultrasonication for 90 min apparently due to the uniform dispersion of Al2O3 nanoparticles in water.
-
The optimum ultrasonication time for attainting maximum thermal conductivity enhancement increased with increasing particle concentration.
-
With increase in the ultrasonication time beyond an optimum period, the particle size distribution begins to shift towards the higher particle size range which indicates re-agglomeration of nanoparticles taking place during prolong ultrasonication.
-
HR-TEM analysis reveals this re-agglomeration of Al2O3 nanoparticles beyond the optimum ultrasonication period.
-
The viscosity of 1.5 vol% aqueous Al2O3 nanofluids measured over shear rates 0–122/s at fixed time intervals (30 min) decreases by 33% with the increase in ultrasonication time from 30 to 90 min. However, with a further increase in the ultrasonication time, the viscosity shows an increase.
-
Re agglomeration occurs apparently due to the hindrance to convective flow caused by increase in viscosity after extended ultrasonication. This in turn leads to reduced thermal conductivity enhancement.
-
A new correlation function has been proposed to predict the thermal conductivity of Al2O3 nanofluids with an R2 value of 0.9924. The proposed correlation showed good agreement with the measured thermal conductivity data.
Abbreviations
- a :
-
Thermal diffusivity, m2/s
- C :
-
Euler’s constant
- c :
-
Slope of the linear section of the plot ∆T (r, t) vs ln (t)
- k :
-
Thermal conductivity, W/m K
- n :
-
No. of readings
- q :
-
Constant heat produced per unit time and per unit length, J/m s
- r :
-
Diameter of platinum wire, m
- s :
-
Standard deviation
- t :
-
Time, s
- u :
-
Uncertainty
- W :
-
Mass, kg
- ∆T:
-
Temperature rise over the platinum wire, K
- ρ :
-
Density, kg/m3
- ϕ :
-
Volume fraction, %
- λ :
-
Wavelength, Å
- bf:
-
Base fluid
- nf:
-
Nanofluid
References
Choi, S.U.S., Eastman, J.: Enhancing thermal conductivity of fluids with nanoparticles. In: International Mechanical Engineering Congress and Exposition, San Francisco, USA. pp. 99–105 (1995)
Li, D., Xie, W., Fang, W.: Preparation and properties of copper-oil-based nanofluids. Nanoscale Res. Lett. 6, 1–7 (2011)
Ghosh, M.M., Ghosh, S., Pabi, S.K.: On synthesis of a highly effective and stable silver nanofluid inspired by its multiscale modeling. Nanosci. Nanotechnol. Lett. 4, 843–848 (2012)
Moreira, L.M., Carvalho, E.A., Bell, M.J.V., Anjos, V., SantAna, A.C., Alves, A.P.P., Fragneaud, B., Sena, L.A., Archanjo, B.S., Achete, C.A.: Thermo-optical properties of silver and gold nanofluids. J. Therm. Anal. Calorim. 114, 557–564 (2013)
Aparna, Z., Michael, M.M., Pabi, S.K., Ghosh, S.: Diversity in thermal conductivity of aqueous Al2O3- and Ag-nanofluids measured by transient hot-wire and laser flash methods. Exp. Therm. Fluid Sci. 94, 231–245 (2018)
Yang, L., Hu, Y.: Toward TiO2 nanofluids—part 1: preparation and properties. Nanoscale Res. Lett. 12, 417 (2017)
Lee, S.W., Park, S.D., Kang, S., Bang, I.C., Kim, J.H.: Investigation of viscosity and thermal conductivity of SiC nanofluids for heat transfer applications. Int. J. Heat Mass Transf. 54, 433–438 (2011)
Zhu, H., Zhang, C., Liu, S., Tang, Y., Yin, Y.: Effects of nanoparticle clustering and alignment on thermal conductivities of Fe3O4 aqueous nanofluids. Appl. Phys. Lett. 89, 023123 (2006)
Liu, D., Zhou, Y., Yang, Y., Zhang, L., Jin, F.: Characterization of high performance AIN nanoparticle-based transformer oil nanofluids IEEE Trans. Dielectr. Electr. Insul. 23, 2757–2767 (2016)
Michael, M., Zagabathuni, A., Ghosh, S., Pabi, S.K.: Thermo-physical properties of pure ethylene glycol and water–ethylene glycol mixture-based boron nitride nanofluids. J. Therm. Anal. Calorim. 137, 369–380 (2019)
Soltanimehr, M., Afrand, M.: Thermal conductivity enhancement of COOH-functionalized MWCNTs/ethylene glycol–water nanofluid for application in heating and cooling systems. Appl. Therm. Eng. 105, 716–723 (2016)
Yang, B., Han, Z.H.: Temperature-dependent thermal conductivity of nanorod-based nanofluids. Appl. Phys. Lett. 89, 083111 (2006)
Peng, Z., Joshi, J.B., Moghtaderi, B., Khan, M.S., Evans, G.M., Doroodchi, E.: Segregation and dispersion of binary solids in liquid fluidised beds: a CFD-DEM study. Chem. Eng. Sci. 152, 65–83 (2016)
Khan, M.S., Mitra, S., Ghatage, S.V., Doroodchi, E., Joshi, J.B., Evans, G.M.: Segregation and dispersion studies in binary solid-liquid fluidised beds: a theoretical and computational study. Powder Technol. 314, 400–411 (2017)
Arifuzzaman, S.M., Biswas, P., Mehedi, M.F.U., Al-Mamun, A., Ahmmed, S.F., Khan, M.S.: Analysis of unsteady boundary layer viscoelastic nanofluid flow through a vertical porous plate with thermal radiation and periodic magnetic field. J. Nanofluids 7, 1122–1129 (2018)
Arifuzzaman, S.M., Khan, M.S., Mehedi, M.F.U., Rana, B.M.J., Ahmmed, S.F.: Chemically reactive and naturally convective high speed MHD fluid flow through an oscillatory vertical porous plate with heat and radiation absorption effect. Eng. Sci. Technol. Int. J. 21, 215–228 (2018)
Arifuzzaman, S.M., Uddin Mehedi, M.F., Al-Mamun, A., Biswas, P., Islam, M.R., Khan, M.S.: Magnetohydrodynamic micropolar fluid flow in presence of nanoparticles through porous plate: a numerical study. Int. J. Heat Technol. 36, 936–948 (2018)
Khan, M.S., Evans, G.M., Nguyen, A.V., Mitra, S.: Analysis of particle dispersion coefficient in solid–liquid fluidised beds. Powder Technol. 365, 60–73 (2020)
Arifuzzaman, S.M., Khan, M.S., Al-Mamun, A., Reza-E-Rabbi, S., Biswas, P., Karim, I.: Hydrodynamic stability and heat and mass transfer flow analysis of MHD radiative fourth-grade fluid through porous plate with chemical reaction. J. King Saud Univ. Sci. 31, 1388–1398 (2019)
Mahbubul, I.M., Elcioglu, E.B., Amalina, M.A., Saidur, R.: Stability, thermophysical properties and performance assessment of alumina–water nanofluid with emphasis on ultrasonication and storage period. Powder Technol. 345, 668–675 (2019)
Afzal, A., Khan, S.A., Ahamed Saleel, C.: Role of ultrasonication duration and surfactant on characteristics of ZnO and CuO nanofluids. Mater. Express 6, 1150d8 (1150d)
Afzal, A., Nawfal, I., Mahbubul, I.M., Kumbar, S.S.: An overview on the effect of ultrasonication duration on different properties of nanofluids. J. Therm. Anal. Calorim. 135, 393–418 (2019)
Rajendiran, G., Kuppusamy, V.B., Shanmugasundaram, S.: Experimental investigation of the effects of sonication time and volume concentration on the performance of PVT solar collector. IET Renew. Power Gener. 12, 1375–1381 (2018)
Shah, J., Ranjan, M., Gupta, S.K., Sonvane, Y.: Ultrasonication effect on thermophysical properties of Al2O3 nanofluids. AIP Conf. Proc. 1951, 1–4 (2018)
Mahbubul, I.M., Elcioglu, E.B., Saidur, R., Amalina, M.A.: Optimization of ultrasonication period for better dispersion and stability of TiO2–water nanofluid. Ultrason. Sonochem. 37, 360–367 (2017)
Su, Y., Gong, L., Chen, D.: Dispersion stability and thermophysical properties of environmentally friendly graphite oil-based nanofluids used in machining Introduction. Res. Artic. Adv. Mech. Eng. 8, 1–11 (2016)
Garg, P., Alvarado, J.L., Marsh, C., Carlson, T.A., Kessler, D.A., Annamalai, K.: An experimental study on the effect of ultrasonication on viscosity and heat transfer performance of multi-wall carbon nanotube-based aqueous nanofluids. Int. J. Heat Mass Transf. 52, 5090–5101 (2009)
Nguyen, V.S., Rouxel, D., Hadji, R., Vincent, B., Fort, Y.: Effect of ultrasonication and dispersion stability on the cluster size of alumina nanoscale particles in aqueous solutions. Ultrason. Sonochem. 18, 382–388 (2011)
Ruan, B., Jacobi, A.M.: Ultrasonication effects on thermal and rheological properties of carbon nanotube suspensions. Nanoscale Res. Lett. 7, 1–11 (2012)
Sonawane, S.S., Khedkar, R.S., Wasewar, K.L.: Effect of sonication time on enhancement of effective thermal conductivity of nano TiO2–water, ethylene glycol, and paraffin oil nanofluids and models comparisons. J. Exp. Nanosci. 10, 310–322 (2015)
Asadi, A., Asadi, M., Siahmargoi, M., Asadi, T., Gholami Andarati, M.: The effect of surfactant and sonication time on the stability and thermal conductivity of water-based nanofluid containing Mg(OH)2 nanoparticles: an experimental investigation. Int. J. Heat Mass Transf. 108, 191–198 (2017)
Li, F., Li, L., Zhong, G., Zhai, Y., Li, Z.: Effects of ultrasonic time, size of aggregates and temperature on the stability and viscosity of Cu-ethylene glycol (EG) nanofluids. Int. J. Heat Mass Transf. 129, 278–286 (2019)
Buonomo, B., Manca, O., Marinelli, L., Nardini, S.: Effect of temperature and sonication time on nanofluid thermal conductivity measurements by nano-flash method. Appl. Therm. Eng. 91, 181–190 (2015)
Chen, Z., Shahsavar, A., Al-Rashed, A.A.A.A., Afrand, M.: The impact of sonication and stirring durations on the thermal conductivity of alumina-liquid paraffin nanofluid: an experimental assessment. Powder Technol. 360, 1134–1142 (2019)
Shahsavar, A., Salimpour, M.R., Saghafian, M., Shafii, M.B.: An experimental study on the effect of ultrasonication on thermal conductivity of ferrofluid loaded with carbon nanotubes. Thermochim. Acta 617, 102–110 (2015)
Xian, H.W., Sidik, N.A.C., Saidur, R.: Impact of different surfactants and ultrasonication time on the stability and thermophysical properties of hybrid nanofluids. Int. Commun. Heat Mass Transf. 110, 104389 (2020)
Murshed, S.M.S., Leong, K.C., Yang, C.: Investigations of thermal conductivity and viscosity of nanofluids. Int. J. Therm. Sci. 47, 560–568 (2008)
Ravikumar, S.V., Haldar, K., Jha, J.M., Chakraborty, S., Sarkar, I., Pal, S.K., Chakraborty, S.: Heat transfer enhancement using air-atomized spray cooling with water-Al2O3 nanofluid. Int. J. Therm. Sci. 96, 85–93 (2015)
Das, P.K., Islam, N., Santra, A.K., Ganguly, R.: Experimental investigation of thermophysical properties of Al2O3–water nanofluid: role of surfactants. J. Mol. Liq. 237, 304–312 (2017)
Xia, G., Jiang, H., Liu, R., Zhai, Y.: Effects of surfactant on the stability and thermal conductivity of Al2O3/de-ionized water nanofluids. Int. J. Therm. Sci. 84, 118–124 (2014)
Mingzheng, Z., Guodong, X., Jian, L., Lei, C., Lijun, Z.: Analysis of factors influencing thermal conductivity and viscosity in different kinds of surfactant solutions. Exp. Therm. Fluid Sci. 36, 22–29 (2012)
Williamson, G.K., Hall, W.H.: X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1, 22–31 (1953)
Garg, J., Poudel, B., Chiesa, M., Gordon, J.B., Ma, J.J., Wang, J.B., Ren, Z.F., Kang, Y.T., Ohtani, H., Nanda, J., McKinley, G.H., Chen, G.: Enhanced thermal conductivity and viscosity of copper nanoparticles in ethylene glycol nanofluid. J. Appl. Phys. 103, 074301 (2008)
Kole, M., Dey, T.K.: Thermophysical and pool boiling characteristics of ZnO-ethylene glycol nanofluids. Int. J. Therm. Sci. 62, 61–70 (2012)
Enomoto, N., Maruyama, S., Nakagawa, Z.: Agglomeration of silica spheres under ultrasonication Naoya. J. Mater. Res. 12, 1410–1415 (1997)
Suganthi, K.S., Rajan, K.S.: Temperature induced changes in ZnO-water nanofluid: zeta potential, size distribution and viscosity profiles. Int. J. Heat Mass Transf. 55, 7969–7980 (2012)
Sergis, A., Hardalupas, Y.: Anomalous heat transfer modes of nanofluids: a review based on statistical analysis. Nanoscale Res. Lett. 6, 1–37 (2011)
Nwosu, P.N., Meyer, J., Sharifpur, M.: A review and parametric investigation into nanofluid viscosity models. J. Nanotechnol. Eng. Med. 5, 031008 (2014)
Bashirnezhad, K., Bazri, S., Safaei, M.R., Goodarzi, M., Dahari, M., Mahian, O., Dalkiliça, A.S., Wongwises, S.: Viscosity of nanofluids: a review of recent experimental studies. Int. Commun. Heat Mass Transf. 73, 114–123 (2016)
Khodabandeh, R., Jarahnejad, M., Haghighi, E.B., Nikkam, N., Palm, B., Toprak, M.S., Muhammed, M., Saleemi, M.: Experimental investigation on viscosity of water-based Al2O3 and TiO2 nanofluids. Rheol. Acta 54, 411–422 (2015)
Mahbubul, I.M., Chong, T.H., Khaleduzzaman, S.S., Shahrul, I.M., Saidur, R., Long, B.D., Amalina, M.A.: Effect of ultrasonication duration on colloidal structure and viscosity of alumina-water nanofluid. Ind. Eng. Chem. Res. 53, 6677–6684 (2014)
Roy, G.C., Nguyen, C.T., Doucet, D., Suiro, S., Mare, T.: Temperature dependent thermal conductivity of alumina based nanofluid. In: de Vahl Davis, G., Leonardi, E. (Eds.), Proceedings of the 13th International Heat Transfer Conference (Begell House), 4 (2006)
Esfe, M.H., Saedodin, S., Mahian, O., Wongwises, S.: Thermal conductivity of Al2O3/water nanofluids: measurement, correlation, sensitivity analysis, and comparisons with literature reports. J. Therm. Anal. Calorim. 117, 675–681 (2014)
Mojarrad, M.S., Keshavarz, A., Ziabasharhagh, M., Raznahan, M.M.: Experimental investigation on heat transfer enhancement of alumina/water and alumina/water-ethylene glycol nanofluids in thermally developing laminar flow. Exp. Therm. Fluid Sci. 53, 111–118 (2014)
Pak, B.C., Cho, Y.I.: Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. 11, 151–170 (1998)
Patel, H.E., Sundararajan, T., Das, S.K.: An experimental investigation into the thermal conductivity enhancement in oxide and metallic nanofluids. J. Nanopart. Res. 12, 1015–1031 (2010)
Xie, H., Wang, J., Xi, T., Liu, Y., Ai, F., Wu, Q.: Thermal conductivity enhancement of suspensions containing nanosized alumina particles. J. Appl. Phys. 91, 4568–4572 (2002)
Mintsa, H.A., Roy, G., Nguyen, C.T., Doucet, D.: New temperature dependent thermal conductivity data for water-based nanofluids. Int. J. Therm. Sci. 48, 363–371 (2009)
Zhang, X., Gu, H., Fujii, M.: Effective thermal conductivity and thermal diffusivity of nanofluids containing spherical and cylindrical nanoparticles. Exp. Therm. Fluid Sci. 31, 593–599 (2007)
Masuda, H., Ebata, A., Teramae, K., Hishinuma, N.: Alteration of thermal conductivity and viscosity of liquid by dispersing ultra-fine particles. Dispersion of Al2O3, SiO2 and TiO2 ultra-fine particles. Netsu Bussei 7, 227–233 (2012)
Beck, M.P., Yuan, Y., Warrier, P., Teja, A.S.: The effect of particle size on the thermal conductivity of alumina nanofluids. J. Nanopart. Res. 11, 1129–1136 (2009)
Philip, J., Shima, P.D.: Thermal properties of nanofluids. Adv. Colloid Interface Sci. 183–184, 30–45 (2012)
James Clerk, M.: A treatise on electricity and magnetism. Claderon press, London (1881)
Hamilton, R.L., Crosser, O.K.: Thermal conductivity of heterogeneous two-component systems. Ind. Eng. Chem. Fundam. 1, 187–191 (1962)
Timofeeva, E.V., Gavrilov, A.N., McCloskey, J.M., Tolmachev, Y.V., Sprunt, S., Lopatina, L.M., Selinger, J.V.: Thermal conductivity and particle agglomeration in alumina nanofluids: experiment and theory. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 76, 061203 (2007)
Buongiorno, J.: Convective transport in nanofluids. J. Heat Transf. 128, 240–250 (2006)
Maïga, S.E.B., Nguyen, C.T., Galanis, N., Roy, G.: Heat transfer behaviours of nanofluids in a uniformly heated tube. Superlattices Microstruct. 35, 543–557 (2004)
Chen, H., Yang, W., He, Y., Ding, Y., Zhang, L., Tan, C., Lapkin, A.A., Bavykin, D.V.: Heat transfer and flow behaviour of aqueous suspensions of titanate nanotubes (nanofluids). Powder Technol. 183, 63–72 (2008)
Das, S.K., Choi, S.U.S., Patel, H.E.: Heat transfer in nanofluids—a review. Heat Transf. Eng. 27, 3–19 (2006)
Murshed, S.M.S., Leong, K.C., Yang, C.: Enhanced thermal conductivity of TiO2—water based nanofluids. Int. J. Therm. Sci. 44, 367–373 (2005)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Michael, M., Zagabathuni, A., Sikdar, S. et al. Effect of dispersion behavior on the heat transfer characteristics of alumina nanofluid: an experimental investigation and development of a new correlation function. Int Nano Lett 10, 207–217 (2020). https://doi.org/10.1007/s40089-020-00306-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40089-020-00306-w