Abstract
Despite the availability of effective antiretroviral therapies, cognitive impairment (CI) remains prevalent in HIV-infected (HIV+) individuals. Evidence from primarily cross-sectional studies, in predominantly male samples, implicates monocyte- and macrophage-driven inflammatory processes linked to HIV-associated CI. Thus, peripheral systemic inflammatory markers may be clinically useful biomarkers in tracking HIV-associated CI. Given sex differences in immune function, we focused here on whether mean and intra-individual variability in inflammatory marker-predicted CI in HIV+ and HIV− women. Seventy-two HIV+ (36 with CI) and 58 HIV− (29 with CI) propensity-matched women participating in the Women’s Interagency HIV Study completed a neuropsychological battery once between 2009 and 2011, and performance was used to determine CI status. Analysis of 13 peripheral immune markers was conducted on stored biospecimens at three time points (7 and 3.5 years before neuropsychological data collection and concurrent with data collection). HIV+ women showed alterations in 8 immune markers compared to HIV− women. The strongest predictors of CI across HIV+ and HIV− women were lower mean soluble tumor necrosis factor receptor I (sTNFRI) levels, higher mean interleukin (IL)-6 levels, and greater variability in C-reactive protein (CRP) and matrix metalloproteinase (MMP)-9 (p values < 0.05). Stratified by HIV, the only significant predictor of CI was greater variability in CRP for both HIV+ and HIV− women (p values < 0.05). This variability predicted lower executive function, attention/working memory, and psychomotor speed in HIV+ but only learning in HIV− women (p values < 0.05). Intra-individual variability in CRP levels over time may be a good predictor of CI in predominately minority low-socioeconomic status midlife women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
HIV-associated cognitive impairment (CI) remains a major clinical issue in HIV care despite combination antiretroviral therapy (cART). Although the incidence of dementia has markedly decreased in the cART era, 30–60% of individuals living with HIV will exhibit CI during their lifetime (Grant 2008). The pathophysiology of HIV-associated CI in the cART era remains incompletely understood and is a high-priority issue in HIV research.
HIV-associated CI may result from direct neurotoxic effect of the virus itself or via the shedding of viral proteins such as Tat and gp120 (D’Aversa et al. 2005; Haughey and Mattson 2002; Li et al. 2005). There is also compelling evidence that HIV-associated CI may result from indirect neurotoxic immunological processes mediated by cells of the monocyte/macrophage lineage (Burdo et al. 2013a; Hong and Banks 2015; Kaul et al. 2001; Langford and Masliah 2001; Valcour et al. 2010). Activated HIV-infected monocytes traffic across the blood–brain barrier, infecting microglia and macrophages which in turn lead to an overexpression of cytokines and chemokines and initiation of an astrocyte-induced inflammatory cascade (Eugenin et al. 2011; Kou et al. 2009; Vartak-Sharma et al. 2014; Wang et al. 2008). Even with suppressive cART, HIV-induced inflammation can result in brain injury (Vera et al. 2016).
The role of neuroinflammation as a major contributor of brain injury has been examined in studies where peripheral systemic inflammatory markers are used as indicators of neuroinflammation. In neuroimaging studies for example, plasma and CSF inflammatory biomarkers are strongly associated with adverse alterations in brain structure and function (Ances and Hammoud 2014; Anderson et al. 2015a, b; Bora et al. 2014). The state of the science is primarily based on largely male-dominant samples and cross-sectional studies. Given substantial evidence of sex differences in neuroimmune activation (Martin et al. 2013; Mathad et al. 2016; Ticona et al. 2015) and cognition (Failde-Garrido et al. 2008; Heaton et al. 2015; Robertson et al. 1996; Royal et al. 2016), previous findings might not be generalizable to HIV-infected women. Moreover, longitudinal studies are needed to enhance our understanding of the time course of CI in relation to altered immune function, including whether absolute levels and/or variability in these biomarkers over time relate to HIV-associated CI.
In a prospective, nested, case–control study, we examine the time course of inflammatory and immune biomarkers over a 6-year time period and the extent to which those biomarkers predict CI at the 6-year mark. We include four groups of women that differ in HIV serostatus (HIV+ versus HIV−) and CI (present or absent at 6 years). The selected biomarkers have shown differences by HIV serostatus, associations with cognitive performance in HIV+ individuals, and/or associations with cognitive performance in other individuals (Cohen et al. 2011; Correia et al. 2013; Koyama et al. 2013; Singh and Newman 2011). We hypothesized that regardless of HIV status, mean levels and variability in interleukin (IL)-6, IL-10, IL-16, IL-18, tumor necrosis factor (TNF)-α, C-reactive protein (CRP), interferon-gamma inducible protein (IP)-10, monocyte chemoattractant protein (MCP)-1, MCP-9, soluble TNFR(receptor) I and II, and macrophage inflammatory protein (MIP)-1β would be predictors of prevalent CI. However, we expected that mean levels and variability in IL-6, IL-10, TNF-α, and CRP would be stronger predictors of prevalent CI among HIV+ women than among HIV− women and that IL-1β and TNF-related apoptosis-inducing ligand (TRAIL) would be specific predictors of prevalent CI among HIV+ women.
Methods
Study sample
Participants were enrolled in the Women’s Interagency HIV Study (WIHS), an ongoing longitudinal, multi-site cohort study of HIV+ and sociodemographically similar HIV− women (http://wihshealth.org). For this prospective, nested case/control study, we used participants enrolled in the first two waves who also subsequently completed baseline neuropsychological (NP) testing between 2009 and 2011 (for complete information regarding demographics, behavioral, and clinical characteristics, see Maki et al. 2015). The first wave of data collection occurred between October 1994 and November 1995 and the second between October 2001 and September 2002 at six sites (Brooklyn, Bronx, Chicago, DC, Los Angeles, and San Francisco). Detailed information regarding recruitment procedures, eligibility criteria, and study methods has been previously published (Bacon et al. 2005; Barkan et al. 1998).
To determine which cases were selected for analyses (see Supplemental Table 1 for the distribution of CI for HIV+ and HIV− women), we used propensity matching which reduces case–control selection bias (Walsh et al. 2012). A single logistic regression model was used to obtain propensity scores where the outcome was case status and predictors were HIV status; age; years of education; Wide Range Achievement Test (WRAT-R) reading subset score; race/ethnicity (African–American, Latina, White, Other), HCV status at baseline (HCV Ab[antibody]−, HCV Ab+ RNA−, HCV Ab+ RNA unknown, HCV Ab+ RNA+); body mass index; current smoker; recent marijuana use; recent crack, cocaine, and/or heroin use; alcohol use (none, light (0–7 drinks/week), moderate (7–12 drinks/week), heavy (> 12 drinks/week)); depressive symptoms (Center for Epidemiologic Studies Depression Scale (CES-D) 16 cutoff); self-reported antidepressant use; liver fibrosis (aspartate aminotransferase to platelet ratio index—APRI); and hepatic fibrosis (FIB-4). The matching was implemented separately by HIV status. Using a tolerance of 0.04, HIV+ cases (CI) were matched to HIV+ controls (no CI) and HIV− cases (CI) were matched to HIV− controls (no CI). The goal of propensity matching was to create four groups (HIV+ CI, HIV+ no CI, HIV− CI, HIV− no CI) balanced on all covariates included in the matching model which were factors known to be or potentially associated with CI.
Prior to propensity matching, cases were included/excluded from possible selection based on the following factors. Cases available for study inclusion were the following: (1) availability of specimens at the visit when NP testing occurred (2009–2011) and at one or two earlier visits (2003–2004 and/or 2006–2007), (2) valid completion of all neuropsychological tests in each of the seven domains, and (3) English speaking. A total of 341 HIV+ no CI, 104 HIV+ CI, 202 HIV− no CI, and 45 HIV− CI met these criteria. Cases excluded from study inclusion were the following: (1) seroconverters and other rare phenotypes (ART-naïve HAART initiator, long-term non-progressor (CD4 > 500 cells/mm3 for ≥ 5 years, no ART), elite controller (viral load < 80 cp/ml for ≥ 1.5 years, no ART), registry-confirmed incident cancer), (2) CD4 < 200 cells/mm3, (3) self-report of a physician diagnosis of dementia, (4) self-reported CVA/stroke, (5) self-reported use of antipsychotic or Alzheimer’s medications, (6) visual or health-related issues that could impact performance on the cognitive test battery, (7) staff note indicating that the participant was under the influence of drugs during testing, and (8) women self-reporting neuropathy or complaints of arthritis or damaged fingers which would invalidate performance on grooved pegboard. After exclusion criteria were applied, we were able to select from 167 HIV+ no CI, 36 HIV+ CI, 149 HIV− no CI, and 30 HIV− CI for propensity matching.
Measures
Multiplex cytokine and chemokine analysis
Serum samples were assayed for two or three longitudinal time points at approximately 3-year intervals using the standard-sensitivity Milliplex Map kit (Millipore) for IL-10, IL-1β, IL-6, IP-10, MCP-1, MIP-1β, and TNF-α; standard-sensitivity Panel II kit (Millipore) for IL-16 and TRAIL; Soluble Receptor kit (Millipore) for soluble TNF receptor types I (sTNFRI) and II (sTNFRII); Matrix Metalloproteinase panel Luminex (Millipore) for MMP-9; R&D Quantikine ELISA for CRP; and MBL International ELISA for IL-18. Standards and samples were tested in duplicate. Beads were acquired on a Labscan analyzer (Luminex) using Bio-Plex Manager 6.1 software (Bio-Rad). ELISA was read on a Molecular Devices Emax plate reader and acquired on Softmax Pro (version 5.4). High CV% between repeat samples were flagged and repeated for analyses (Keating et al. 2011). To avoid confounding of batch with time and group, all longitudinal samples were run on the same plate, with an equal proportion of the four groups (ratio 2:1 HIV+ to HIV−). The same two controls were run on every plate to evaluate the reliability of values across plates. Values that were determined to be out of range low were assigned 0.5, the lowest standard. The proportion of undetectable values for cytokines was as follows: IL-1β (56%), IL-6 (45%), IL-10 (20%), IL-16 (9%), CRP (2%), MIP-1β (1%), and TRAIL (1%). Values that were extrapolated beyond the standard curve were accepted as that value. All immune markers were log transformed and winsorized (< 1% of values changed to be equal to the highest or lowest value that was within 3 SD of the interquartile range) to normalize distributions.
Primary outcome
CI status, the primary outcome variable, was based on a neuropsychological test battery completed once between 2009 and 2011. The battery included eight tests: Hopkins Verbal Learning Test-Revised (HVLT-R), Letter-Number Sequencing, Trail Making Test (TMT), Stroop Test, Symbol Digit Modalities Test (SDMT), Controlled Oral Word Association Test (COWAT), Category Fluency Test (Animals), and Grooved Pegboard (GPEG). Seven domains were assessed using these tests: learning (outcome = total learning across HVLR-T trials), memory (outcome = delayed free recall on HVLT-R), attention/working memory (outcomes = total correct on LNS control and experimental conditions), psychomotor speed (outcomes = total correct on SDMT, time to completion on Stroop Trial 2), executive function (outcomes = time to completion on TMT Part B and Stroop Trial 3), fluency (outcomes = total correct on COWAT and Category Fluency Test), and motor skills (outcomes = total time to completion for each hand on GPEG). Timed outcomes were log transformed to normalize distributions and reverse scored so higher values equated to better performance.
Consistent with previous large-scale HIV cohorts (Cysique et al. 2014; Heaton et al. 2004; Sacktor et al. 2016) including WIHS (Maki et al. 2015; Rubin et al. 2015, 2016), demographically adjusted T-scores were derived for each outcome and these T-scores were used to create domain scores (Supplemental Materials). CI was defined as scoring below the expected level of performance (T-score < 40) in at least three of seven domains. Although only two domains of CI are typically required for a diagnosis of HIV-associated neurocognitive disorder (HAND) (Antinori et al. 2007), we chose a more stringent definition because at the time we did not have measures necessary to determine HAND, including instrumental activities of daily living.
Statistical analysis
A series of conditional logistic regression models were conducted in SAS (version 9.4, Cary, NC) to assess whether average or fluctuation (standard deviation) in inflammatory biomarkers over all time points tested predicted CI in general (using overall sample) or whether they differentially predicted HIV-associated CI (stratified by HIV serostatus). Conditional logistic regression models were selected to handle the nested propensity-matched pair design (65 pairs). Unadjusted models were first conducted to examine the association of primary predictor variables to CI. Primary predictor variables were average levels and variability (standard deviation) of the 13 inflammatory markers from all time points. Based on these models, all predictors associated with the outcome at p < 0.10 were included in a stepwise model. The study design prohibited the inclusion of HIV status in models for the overall sample; the proportion of cases was exactly 50% in both HIV+ and HIV− women. Variables were retained in the stepwise models if p < 0.10. Odds ratios (ORs) and 95% CIs were calculated using maximum likelihood estimates from the conditional logistic regression models. When inflammatory markers were predictive of CI among HIV+ women, models were rerun including HIV-specific covariates (viral load, current and nadir CD4 count, and CD4/CD8 ratio). Additionally, when inflammatory markers were predictive of CI in either group, exploratory correlational analyses using Spearman’s rho were conducted to determine the specific domains contributing to the global effect. Because exploratory analyses were performed for heuristic purposes to examine the potential clinical significance for future larger scale studies, we did not correct for multiple comparisons. However, given the small sample sizes, when correlations were observed (p < 0.10), bootstrapping of the correlation coefficient (based on 1000 samples) was conducted to determine the strength of the associations and to ensure findings were not driven by outliers. The 95% CI from the bootstrapping procedure was used to determine statistical significance (95% CI does not include 0).
Results
Sample characteristics
The overall sample of 130 women was 67% African–American, non-Hispanic, and 20% Hispanic and ranged in age from 25 to 70 years (mean = 43.9, SD = 9.6), which is comparable to previously published large-scale WIHS studies (Maki et al. 2015; Rubin et al. 2016). The four groups were comparable across a range of sociodemographic, behavioral, and clinical characteristics (Table 1). Among those with CI, there was a similar prevalence of CI across all domains except psychomotor speed which was more prevalent among HIV+ compared to HIV− women (p = 0.03, Supplemental Table 2).
Serum immune biomarkers
Table 2 provides the average of each individual’s mean and intra-individual variability (SD) from the two (27 women contributed 54 observations) or three (103 women contributed 309 observations) time points of immune marker levels as a function of HIV status. There were no significant differences in immune marker levels (mean or SD) as a function of having two or three time points. Compared to HIV− women, HIV+ women showed higher mean levels of IL-18, sTNFRII, MCP-1, IP-10, TNF-α, and TRAIL and lower mean levels of IL-1β and IL-6 (p values < 0.05). After controlling the false discovery rate (FDR) using the Benjamini–Hochberg procedure, only two markers did not remain significant: TRAIL and IL-1β. HIV+ women also showed greater individual variability in sTNFRII and TRAIL compared with HIV− women (p < 0.05); however, these differences did not remain after controlling the FDR. Similar results were seen when comparing women with HIV RNA ≤ 80cp/ml and HIV− women. Of the markers showing HIV serostatus differences, higher viral load was associated with lower mean levels of TRAIL (r s = − 0.28, p = 0.02) and sTNFRII (r s = − 0.24, p = 0.04). Lower CD4/CD8 ratio was associated with higher mean levels of TNF-α (r s = − 0.42, p < 0.0001), IL-1β (r s = − 0.32, p = 0.007), IL-6 (r s = − 0.32, p = 0.007), and TRAIL (r s = − 0.27, p = 0.03). These markers were not significantly associated with current or nadir CD4 count (p values > 0.07).
Table 3 provides the results from the unadjusted conditional logistic regression models across HIV+ and HIV− groups to assess general predictors of CI as well as stratified by serostatus to assess for specific predictors of HIV-associated CI. In unadjusted analyses, the strongest predictors of CI in the overall sample were lower mean levels of sTNFRI (p = 0.009) and greater variability in CRP (p = 0.003), with trends for higher mean levels of IL-6 (p = 0.08) and greater variability in MMP-9 (p = 0.08). In adjusted analyses, all four of these biomarkers were significant predictors of CI: mean levels of sTNFRI (OR 0.07, 95% CI 0.01–0.46, p = 0.006), mean levels of IL-6 (OR 1.54, 95% CI 1.09–2.18, p = 0.01), variability in CRP (OR 4.66, 95% CI 1.52–14.24, p = 0.007), and variability in MMP-9 (OR 16.85, 95% CI 1.98–143, p = 0.009). In unadjusted analyses in HIV+ women only, the only significant predictor of CI was increased variability in CRP levels (p = 0.04). CRP remained significant (p = 0.04) after adjusting for viral load, CD4 count (current and nadir), and the CD4/CD8 ratio (p values NS). In unadjusted analyses in HIV− women, the strongest predictors of CI were lower mean levels of sTNFRI (p = 0.04) and increased variability in CRP (p = 0.03), but only increased variability in CRP remained significant in adjusted analyses (OR 4.21, 95% CI 1.14–15.49).
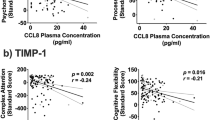
Follow-up exploratory analyses in the overall sample indicated that a lower mean level of sTNFRI was associated with lower performance in psychomotor speed (r s = 0.18, p = 0.04, 95% CI 0.01–0.35), attention/working memory (r s = 0.25, p = 0.005, 95% CI 0.10–0.41), and fluency (r s = 0.28, p = 0.001, 95% CI 0.11–0.44). Greater variability in MMP-9 levels was associated with lower performance on fluency (r s = − 0.18, p = 0.04, 95% CI − 0.37 to − 0.01) whereas greater variability in CRP levels was associated with worse executive function (95% CI − 0.41 to − 0.07), psychomotor speed (95% CI − 0.38 to − 0.07), attention/working memory (95% CI − 0.37 to − 0.04), and learning (95% CI − 0.36 to − 0.04) (Fig. 1). Greater variability in CRP levels was the strongest correlate of psychomotor speed, attention/working memory, and executive function in HIV+ women (p values < 0.05, 95% CI ≤ 0.01) and learning in HIV− women (p = 0.04, 95% CI − 0.51 to − 0.02) (Fig. 1). Similar results were seen among HIV+ women after adjusting for viral load and CD4 count (current and nadir) (p values NS).
Mean variability in log CRP levels predicted cognitive performance. a Executive function. b Psychomotor speed. c Attention/working memory. d Learning. WM, working memory. Mean variability in log CRP levels predicted performance on all of these domains in the overall sample (p values < 0.05). CRP is the strongest predictor of attention/working memory, executive function, and psychomotor speed for HIV+ women, whereas for HIV− women, CRP is the strongest predictor of learning
Discussion
Extending previous cross-sectional studies in predominantly male samples, we conducted a prospective, nested case–control study to determine if peripheral inflammatory markers over time predict CI in HIV+ women and HIV− women. Our conclusions support the finding of consistent immune alterations despite ART. Consistent with previous studies, serum levels of cytokines (IL-6, IL-18, TNF-α, IL-1β) and their receptors (sTNFRII, TRAIL) as well as chemokines (MCP-1, IP-10) differed between a sample of predominately cART-treated (96%) and virologically suppressed (93%) HIV+ women and HIV− women (Cohen et al. 2011; Correia et al. 2013; Deeks et al. 2013; Neuhaus et al. 2010; Ronsholt et al. 2013). Importantly, there was no bias in which group contributed two or three samples. We not only found robust HIV serostatus differences when examinng mean levels, but also found differences when examining intra-individual variability in immune markers (sTNFRII, TRAIL; CRP and IL-18 trends) a metric that can only be examined with multiple measurements.
Although most studies report HIV serostatus differences in immune markers, the patterns and markers themselves are not always consistent across studies. Compared to HIV− women, HIV+ women in the present study showed lower levels of IL-6 and IL-1β and higher levels of IL-18, TNF-α, sTNFRII, TRAIL, MCP-1, and IP-10. These findings are partially consistent with those of previous studies (Cohen et al. 2011; Correia et al. 2013; Neuhaus et al. 2010; Ronsholt et al. 2013). For example, Neuhaus et al. (2010) found that ART-treated men and women had approximately 40 to 60% higher IL-6 levels compared to controls. Others have reported lower levels of TNF-α in HIV+ compared to HIV− individuals, no differences in TRAIL, and elevated levels of MIP-1β (Cohen et al. 2011; Correia et al. 2013). Inconsistencies across studies could be due to numerous factors such as the examination of mixed samples of men and women, using a single measurement versus our use of average levels across multiple time points, or the inclusion of individuals co-infected with HCV. This study includes individuals co-infected with HCV, albeit a similar proportion across the four groups.
Our findings also provide support for the role of inflammation in CI in HIV− individuals. In the overall sample, IL-6, CRP, sTNFRI, and MMP-9 predicted CI. Among population studies of aging, IL-6, CRP, and TNF-α are among the most studied markers of adverse outcomes including cognitive decline (see Singh and Newman 2011, for review). Many, but not all studies, demonstrate associations between these general markers of systemic low-grade inflammation and cognitive performance and/or decline as well as all-cause dementia. For example, findings from the Northern Manhattan Study, a large community-based prospective cohort of older HIV-uninfected socioeconomically and ethnically diverse sample of men and women, demonstrated that elevated IL-6 levels were associated with lower cognitive performance (Wright et al. 2006) and greater cognitive decline (Economos et al. 2013).
Our strongest effects of predictors of prevalent CI were with variability in CRP levels. While Economos et al. (2013) did not demonstrate an association between CRP and cognitive decline, others have demonstrated this association with absolute levels of CRP (Noble et al. 2010; Puzianowska-Kuznicka et al. 2016; Roberts et al. 2009; Yaffe et al. 2003) and with variability in CRP levels (Metti et al. 2014). In a meta-analysis, CRP was associated with a 45% increase in all-cause dementia and IL-6 was associated with a 32% increase (Koyama et al. 2013). Variability in CRP has been observed in other settings (Bogaty et al. 2013) and might be related to the presence of and variability in inflammation and immune status, weight gain, physical activity, vascular and metabolic conditions, and/or psychological symptoms (Metti et al. 2014) as CRP is associated with each of these comorbidities (DeGoma et al. 2012; Koenig et al. 2003; Ladwig et al. 2005; Puzianowska-Kuznicka et al. 2016). Larger longitudinal studies with more frequent measurements are needed to identify any particularly meaningful pattern in variability in CRP and any mediators (e.g., vascular) of these associations. As with other biomarkers shown to predict HIV-related CI (e.g., neuroimaging biomarkers), CRP may have limited clinical utility. Rather, the value of these findings is in identifying the mechanisms contributing to HIV-associated CI so that they can be targeted through appropriate interventions.
Greater variability in MMP-9 levels was also a significant predictor of CI in the overall sample. MMP-9, a major secretion product of macrophages, is implicated in the breakdown and remodeling of the extracellular matrix in normal and pathological inflammatory processes (Klein and Bischoff 2011). HIV can both downregulate and upregulate MMP-9 production (Ciborowski et al. 2004; Muratori et al. 2007). Neurotoxic viral proteins gp120 and Tat increase expression of MMP-9 and consequently induce blood–brain barrier permeability by degrading vascular tight junction proteins in endothelial cells (Louboutin et al. 2010; Xu et al. 2012). Thus, MMP-9-related disruption of the blood–brain barrier may play a critical role in CI generally and in the pathogenesis of HAND (Avison et al. 2004).
In stratified analyses, only variability in CRP levels remained a significant predictor of CI in HIV+ and HIV− women. In exploratory analyses in HIV+ women, greater variability in CRP was associated with lower performance on a broad range of cognitive abilities including psychomotor speed, attention/working memory, and executive function. Conversely, in HIV− women, greater CRP variability was associated only with lower learning. CRP may influence cognitive performance differently in HIV+ compared with HIV− individuals. The robustness of these differences and the mechanisms leading to different patterns warrant further investigation.
Limitations of the present study include the relatively small sample size and unknown cognitive status of participants before 2009. Most likely, we are examining prevalent and not incident cases of CI. No peripheral monocyte-driven immune activation markers such as soluble CD163 and CD14 were studied but have shown associations with CI (Burdo et al. 2013b; Royal et al. 2016) including in the WIHS (Imp et al. 2017). While the use of propensity matching yielded four groups that were balanced on a number of sociodemographic, clinical, and behavioral characteristics known to be associated with cognitive impairment, this approach controls only for measured confounders and unmeasured factors can still bias results. Finally, the standard sensitivity assay measured most of the analytes required for this analysis but can cause loss of values at the lower end of detection; however, this did not greatly impact the significant markers found in this study to predict prevalent CI. Sensitivity was more of an issue for IL-1β, IL-6, IL-10, and IL-16 in the present study. A deeper investigation impact of low levels of these cytokines on CI will need to be conducted using more sensitive assays.
In sum, our longitudinal findings in women provide further support for the adverse role of persistent residual immune activation despite cART in HIV+ individuals. Alterations in immune processes predicted CI among both HIV+ and HIV− women. Although variability in CRP, a more general marker of low-grade systemic inflammation, was the strongest predictor in both HIV+ and HIV− women, the specific cognitive correlates of CRP differed across the groups with much broader associations in HIV+ women. Findings warrant further study into possible peripheral immune signatures examined over a longer duration that may differentially predict the patterns of CI over time generally and in HIV+ individuals specifically.
References
Ances BM, Hammoud DA (2014) Neuroimaging of HIV-associated neurocognitive disorders (HAND). Curr Opin HIV AIDS 9:545–551
Anderson AM, Fennema-Notestine C, Umlauf A, Taylor MJ, Clifford DB, Marra CM, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Simpson DM, Morgello S, Grant I, Letendre SL (2015a) CSF biomarkers of monocyte activation and chemotaxis correlate with magnetic resonance spectroscopy metabolites during chronic HIV disease. J Neuro-Oncol 21:559–567
Anderson AM, Harezlak J, Bharti A, Mi D, Taylor MJ, Daar ES, Schifitto G, Zhong J, Alger JR, Brown MS, Singer EJ, Campbell TB, McMahon DD, Buchthal S, Cohen R, Yiannoutsos C, Letendre SL, Navia BA (2015b) Plasma and cerebrospinal fluid biomarkers predict cerebral injury in HIV-infected individuals on stable combination antiretroviral therapy. J Acquir Immune Defic Syndr 69:29–35
Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69:1789–1799
Avison MJ, Nath A, Greene-Avison R, Schmitt FA, Greenberg RN, Berger JR (2004) Neuroimaging correlates of HIV-associated BBB compromise. J Neuroimmunol 157:140–146
Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, Gange S, Barranday Y, Holman S, Weber K, Young MA (2005) The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 12:1013–1019
Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J (1998) The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology 9:117–125
Bogaty P, Dagenais GR, Joseph L, Boyer L, Leblanc A, Belisle P, Brophy JM (2013) Time variability of C-reactive protein: implications for clinical risk stratification. PLoS One 8:e60759
Bora A, Ubaida Mohien C, Chaerkady R, Chang L, Moxley R, Sacktor N, Haughey N, McArthur JC, Cotter R, Nath A, Graham DR (2014) Identification of putative biomarkers for HIV-associated neurocognitive impairment in the CSF of HIV-infected patients under cART therapy determined by mass spectrometry. J Neuro-Oncol 20:457–465
Burdo TH, Lackner A, Williams KC (2013a) Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev 254:102–113
Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC (2013b) Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS 27:1387–1395
Ciborowski P, Enose Y, Mack A, Fladseth M, Gendelman HE (2004) Diminished matrix metalloproteinase 9 secretion in human immunodeficiency virus-infected mononuclear phagocytes: modulation of innate immunity and implications for neurological disease. J Neuroimmunol 157:11–16
Cohen RA, de la Monte S, Gongvatana A, Ombao H, Gonzalez B, Devlin KN, Navia B, Tashima KT (2011) Plasma cytokine concentrations associated with HIV/hepatitis C coinfection are related to attention, executive and psychomotor functioning. J Neuroimmunol 233:204–210
Correia S, Cohen R, Gongvatana A, Ross S, Olchowski J, Devlin K, Tashima K, Navia B, Delamonte S (2013) Relationship of plasma cytokines and clinical biomarkers to memory performance in HIV. J Neuroimmunol 265:117–123
Cysique LA, Heaton RK, Kamminga J, Lane T, Gates TM, Moore DM, Hubner E, Carr A, Brew BJ (2014) HIV-associated neurocognitive disorder in Australia: a case of a high-functioning and optimally treated cohort and implications for international neuroHIV research. J Neuro-Oncol 20:258–268
D'Aversa TG, Eugenin EA, Berman JW (2005) NeuroAIDS: contributions of the human immunodeficiency virus-1 proteins Tat and gp120 as well as CD40 to microglial activation. J Neurosci Res 81:436–446
Deeks SG, Tracy R, Douek DC (2013) Systemic effects of inflammation on health during chronic HIV infection. Immunity 39:633–645
DeGoma EM, French B, Dunbar RL, Allison MA, Mohler ER 3rd, Budoff MJ (2012) Intraindividual variability of C-reactive protein: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 224:274–279
Economos A, Wright CB, Moon YP, Rundek T, Rabbani L, Paik MC, Sacco RL, Elkind MS (2013) Interleukin 6 plasma concentration associates with cognitive decline: the Northern Manhattan Study. Neuroepidemiology 40:253–259
Eugenin EA, Clements JE, Zink MC, Berman JW (2011) Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism. J Neurosci 31:9456–9465
Failde-Garrido JM, Alvarez MR, Simon-Lopez MA (2008) Neuropsychological impairment and gender differences in HIV-1 infection. Psychiatry Clin Neurosci 62:494–502
Grant I (2008) Neurocognitive disturbances in HIV. Int Rev Psychiatry 20:33–47
Haughey NJ, Mattson MP (2002) Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr 31(Suppl 2):S55–S61
Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I, Group H (2004) The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc 10:317–331
Heaton RK, Franklin DR Jr, Deutsch R, Letendre S, Ellis RJ, Casaletto K, Marquine MJ, Woods SP, Vaida F, Atkinson JH, Marcotte TD, McCutchan JA, Collier AC, Marra CM, Clifford DB, Gelman BB, Sacktor N, Morgello S, Simpson DM, Abramson I, Gamst AC, Fennema-Notestine C, Smith DM, Grant I, Group C (2015) Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis 60:473–480
Hong S, Banks WA (2015) Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun 45:1–12
Imp BM, Rubin LH, Tien PC, Plankey MW, Golub ET, French AL, Valcour VG (2017) Monocyte activation is associated with worse cognitive performance in HIV-infected women with virologic suppression. J Infect Dis 215:114–121
Kaul M, Garden GA, Lipton SA (2001) Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410:988–994
Keating SM, Golub ET, Nowicki M, Young M, Anastos K, Crystal H, Cohen MH, Zhang J, Greenblatt RM, Desai S, Wu S, Landay AL, Gange SJ, Norris PJ (2011) The effect of HIV infection and HAART on inflammatory biomarkers in a population-based cohort of women. AIDS 25:1823–1832
Klein T, Bischoff R (2011) Physiology and pathophysiology of matrix metalloproteases. Amino Acids 41:271–290
Koenig W, Sund M, Frohlich M, Lowel H, Hutchinson WL, Pepys MB (2003) Refinement of the association of serum C-reactive protein concentration and coronary heart disease risk by correction for within-subject variation over time: the MONICA Augsburg studies, 1984 and 1987. Am J Epidemiol 158:357–364
Kou W, Banerjee S, Eudy J, Smith LM, Persidsky R, Borgmann K, Wu L, Sakhuja N, Deshpande MS, Walseth TF, Ghorpade A (2009) CD38 regulation in activated astrocytes: implications for neuroinflammation and HIV-1 brain infection. J Neurosci Res 87:2326–2339
Koyama A, O'Brien J, Weuve J, Blacker D, Metti AL, Yaffe K (2013) The role of peripheral inflammatory markers in dementia and Alzheimer’s disease: a meta-analysis. J Gerontol A Biol Sci Med Sci 68:433–440
Ladwig KH, Marten-Mittag B, Lowel H, Doring A, Koenig W (2005) C-reactive protein, depressed mood, and the prediction of coronary heart disease in initially healthy men: results from the MONICA-KORA Augsburg Cohort Study 1984-1998. Eur Heart J 26:2537–2542
Langford D, Masliah E (2001) Crosstalk between components of the blood brain barrier and cells of the CNS in microglial activation in AIDS. Brain Pathol 11:306–312
Li W, Galey D, Mattson MP, Nath A (2005) Molecular and cellular mechanisms of neuronal cell death in HIV dementia. Neurotox Res 8:119–134
Louboutin JP, Agrawal L, Reyes BA, Van Bockstaele EJ, Strayer DS (2010) HIV-1 gp120-induced injury to the blood-brain barrier: role of metalloproteinases 2 and 9 and relationship to oxidative stress. J Neuropathol Exp Neurol 69:801–816
Maki PM, Rubin LH, Valcour V, Martin E, Crystal H, Young M, Weber KM, Manly J, Richardson J, Alden C, Anastos K (2015) Cognitive function in women with HIV: findings from the Women’s Interagency HIV Study. Neurology 84:231–240
Martin GE, Gouillou M, Hearps AC, Angelovich TA, Cheng AC, Lynch F, Cheng WJ, Paukovics G, Palmer CS, Novak RM, Jaworowski A, Landay AL, Crowe SM (2013) Age-associated changes in monocyte and innate immune activation markers occur more rapidly in HIV infected women. PLoS One 8:e55279
Mathad JS, Gupte N, Balagopal A, Asmuth D, Hakim J, Santos B, Riviere C, Hosseinipour M, Sugandhavesa P, Infante R, Pillay S, Cardoso SW, Mwelase N, Pawar J, Berendes S, Kumarasamy N, Andrade BB, Campbell TB, Currier JS, Cohn SE, Gupta A (2016) Sex-related differences in inflammatory and immune activation markers before and after combined antiretroviral therapy initiation. J Acquir Immune Defic Syndr 73:123–129
Metti AL, Yaffe K, Boudreau RM, Simonsick EM, Carnahan RM, Satterfield S, Harris TB, Ayonayon HN, Rosano C, Cauley JA (2014) Trajectories of inflammatory markers and cognitive decline over 10 years. Neurobiol Aging 35:2785–2790
Muratori C, Sistigu A, Ruggiero E, Falchi M, Bacigalupo I, Palladino C, Toschi E, Federico M (2007) Macrophages transmit human immunodeficiency virus type 1 products to CD4-negative cells: involvement of matrix metalloproteinase 9. J Virol 81:9078–9087
Neuhaus J, Jacobs DR Jr, Baker JV, Calmy A, Duprez D, La Rosa A, Kuller LH, Pett SL, Ristola M, Ross MJ, Shlipak MG, Tracy R, Neaton JD (2010) Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 201:1788–1795
Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA (2010) Association of C-reactive protein with cognitive impairment. Arch Neurol 67:87–92
Puzianowska-Kuznicka M, Owczarz M, Wieczorowska-Tobis K, Nadrowski P, Chudek J, Slusarczyk P, Skalska A, Jonas M, Franek E, Mossakowska M (2016) Interleukin-6 and C-reactive protein, successful aging, and mortality: the PolSenior study. Immun Ageing 13:21
Roberts RO, Geda YE, Knopman DS, Boeve BF, Christianson TJ, Pankratz VS, Kullo IJ, Tangalos EG, Ivnik RJ, Petersen RC (2009) Association of C-reactive protein with mild cognitive impairment. Alzheimers Dement 5:398–405
Robertson K, Fiscus S, Wilkins J, van der Horst C, Hall C (1996) Viral load and neuropsychological functioning in HIV seropositive individuals:a preliminary descriptive study. J NeuroAIDS 1:7–15
Ronsholt FF, Ullum H, Katzenstein TL, Gerstoft J, Ostrowski SR (2013) Persistent inflammation and endothelial activation in HIV-1 infected patients after 12 years of antiretroviral therapy. PLoS One 8:e65182
Royal W 3rd, Cherner M, Burdo TH, Umlauf A, Letendre SL, Jumare J, Abimiku A, Alabi P, Alkali N, Bwala S, Okwuasaba K, Eyzaguirre LM, Akolo C, Guo M, Williams KC, Blattner WA (2016) Associations between cognition, gender and monocyte activation among HIV infected individuals in Nigeria. PLoS One 11:e0147182
Rubin LH, Cook JA, Weber KM, Cohen MH, Martin E, Valcour V, Milam J, Anastos K, Young MA, Alden C, Gustafson DR, Maki PM (2015) The association of perceived stress and verbal memory is greater in HIV-infected versus HIV-uninfected women. J Neuro-Oncol 21:422–432
Rubin LH, Pyra M, Cook JA, Weber KM, Cohen MH, Martin E, Valcour V, Milam J, Anastos K, Young MA, Alden C, Gustafson DR, Maki PM (2016) Post-traumatic stress is associated with verbal learning, memory, and psychomotor speed in HIV-infected and HIV-uninfected women. J Neuro-Oncol 22:159–169
Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, Ragin A, Levine A, Miller E (2016) Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 86:334–340
Singh T, Newman AB (2011) Inflammatory markers in population studies of aging. Ageing Res Rev 10:319–329
Ticona E, Bull ME, Soria J, Tapia K, Legard J, Styrchak SM, Williams C, Mitchell C, La Rosa A, Rosa AL, Coombs RW, Frenkel LM (2015) Biomarkers of inflammation in HIV-infected Peruvian men and women before and during suppressive antiretroviral therapy. AIDS 29:1617–1622
Valcour VG, Shiramizu BT, Shikuma CM (2010) HIV DNA in circulating monocytes as a mechanism to dementia and other HIV complications. J Leukoc Biol 87:621–626
Vartak-Sharma N, Gelman BB, Joshi C, Borgamann K, Ghorpade A (2014) Astrocyte elevated gene-1 is a novel modulator of HIV-1-associated neuroinflammation via regulation of nuclear factor-kappaB signaling and excitatory amino acid transporter-2 repression. J Biol Chem 289:19599–19612
Vera JH, Guo Q, Cole JH, Boasso A, Greathead L, Kelleher P, Rabiner EA, Kalk N, Bishop C, Gunn RN, Matthews PM, Winston A (2016) Neuroinflammation in treated HIV-positive individuals: a TSPO PET study. Neurology 86:1425–1432
Walsh MC, Trentham-Dietz A, Newcomb PA, Gangnon R, Palta M (2012) Using propensity scores to reduce case-control selection bias. Epidemiology 23:772–773
Wang T, Gong N, Liu J, Kadiu I, Kraft-Terry SD, Mosley RL, Volsky DJ, Ciborowski P, Gendelman HE (2008) Proteomic modeling for HIV-1 infected microglia-astrocyte crosstalk. PLoS One 3:e2507
Wright CB, Sacco RL, Rundek T, Delman J, Rabbani L, Elkind M (2006) Interleukin-6 is associated with cognitive function: the Northern Manhattan Study. J Stroke Cerebrovasc Dis 15:34–38
Xu R, Feng X, Xie X, Zhang J, Wu D, Xu L (2012) HIV-1 Tat protein increases the permeability of brain endothelial cells by both inhibiting occludin expression and cleaving occludin via matrix metalloproteinase-9. Brain Res 1436:13–19
Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T (2003) Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology 61:76–80
Acknowledgements
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). WIHS (Principal Investigators): UAB-MS WIHS (Michael Saag, Mirjam-Colette Kempf, and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen), U01-AI-034993; Metropolitan Washington WIHS (Mary Young), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Alexandra Levine and Marek Nowicki), U01-HD-032632 (WIHS I–WIHS IV).
Funding
Dr. Rubin’s effort was supported by Grant Number 1K01MH098798-01 from the National Institute of Mental Health (NIMH) and by Grant Number K12HD055892 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institutes of Health Office of Research on Women’s Health (ORWH). Dr. Valcour’s work was supported by his K24MH098759. This grant is also supported in part by the Chicago Developmental Center for AIDS Research (D-CFAR), an NIH-funded program (P30 AI 082151), which is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, and NCCAM. The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Electronic supplementary material
ESM 1
(DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Rubin, L.H., Benning, L., Keating, S.M. et al. Variability in C-reactive protein is associated with cognitive impairment in women living with and without HIV: a longitudinal study. J. Neurovirol. 24, 41–51 (2018). https://doi.org/10.1007/s13365-017-0590-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-017-0590-4