Abstract
This study aimed to examine cognitive function in acute/early HIV infection over the subsequent 2 years. Fifty-six HIV+ subjects and 21 seronegative participants of the Chicago Early HIV Infection Study were evaluated using a comprehensive neuropsychological assessment at study enrollment and at 2-year follow-up. Cognitive performance measures were compared in the groups using t tests and mixed-effect models. Patterns of relationship with clinical measures were determined between cognitive function and clinical status markers using Spearman’s correlations. At the initial timepoint, the HIV group demonstrated significantly weaker performance on measures of verbal memory, visual memory, psychomotor speed, motor speed, and executive function. A similar pattern was found when cognitive function was examined at follow-up and across both timepoints. The HIV subjects had generally weaker performance on psychomotor speed, executive function, motor speed, visual memory, and verbal memory. The rate of decline in cognitive function across the 2-year follow-up period did not differ between groups. Correlations between clinical status markers and cognitive function at both timepoints showed weaker performance associated with increased disease burden. Neurocognitive difficulty in chronic HIV infection may have very early onset and reflect consequences of initial brain viral invasion and neuroinflammation during the intense, uncontrolled viremia of acute HIV infection. Further characterization of the changes occurring in initial stages of infection and the risk and protective factors for cognitive function could inform new strategies for neuroprotection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
HIV infection confers high risk of deterioration in cognitive function (Heaton et al. 2010; Heaton et al. 2011). The prevalence of overt dementia from HIV infection has declined in the contemporary treatment era (Antinori et al. 2007; Heaton et al. 2010; Heaton et al. 2011). Yet, despite long-term treatment characterized by sustained suppression of viral replication, HIV-associated neurocognitive disorder (HAND) is diagnosed in over 60% of infected individuals (Tozzi et al. 2007). HIV-infected individuals who underperform on neuropsychological evaluation, even if asymptomatic, are more likely to progress to clinically symptomatic cognitive impairment (Tozzi et al. 2007; Nightingale et al. 2014).
HIV-associated neurocognitive disorder is associated with impairment in everyday functioning, higher levels of dependence, unemployment, morbidity, and poorer outcome (Heaton et al. 2004; Scott et al. 2011; Doyle et al. 2013). Changes in brain structure and metabolites have been detected early in HIV infection (Lentz et al. 2011; Wang et al. 2011, Ragin et al. 2012, 2015). Neuropsychological manifestations of early brain changes have also been shown as early as within 100 days of initial infection (Ragin et al. 2015).
Whether these very early neuropsychological findings are transient in association with the immune disturbances of acute HIV and how they evolve across the early clinical course are not well understood. This longitudinal investigation examined the evolution of cognitive function across the first years of infection in participants of the Chicago Early HIV Infection Study. A comprehensive neuropsychological test battery was used to evaluate verbal memory, visual memory, visuoconstruction, psychomotor speed, reaction time, motor speed, and executive function in acute/early infection and then again at an average of 2 years later.
Methods
Standard protocol approvals, registrations, and patient consents. The Northwestern University Institutional Review Board approved this investigation. All subjects provided written informed consent.
Participants
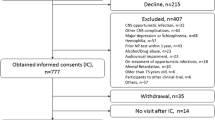
Fifty-six HIV (49 men, 7 women; mean age 32.9 ± 9.8 years) and 21 seronegative (16 men, 5 women; mean age 31.4 ± 8.8 years) participants were enrolled from similar urban Chicago areas. Forty-five HIV and 18 seronegative controls were available for follow-up of an average of 2.4 years later (mean follow-up 869.3 ± 320 days). Exclusion criteria included chronic neurologic disorder, head injury, radiation/chemotherapy (prior month), uncontrolled seizure disorder, experimental drugs or vaccination within the past 15 days, mental condition involving inability to understand, chronic/active alcohol abuse, chronic/active drug abuse, pregnancy, opportunistic infection, cancer, or medical condition (heart, liver, or kidney). HIV subjects were enrolled based on referrals from the emergency room, Infectious Disease Clinic, and Sexually Transmitted Disease Clinic of Northwestern Memorial Hospital or through self-referral. Participants with self-reported recent HIV infection were screened before study enrollment for an available prior negative test result or clinical history suggesting recent infection (e.g., symptoms consistent with acute HIV after unprotected high-risk sexual behavior or newly diagnosed HIV in a sexual partner). All infected participants reported sexual transmission as the method of infection. Seronegative subjects were recruited from the same Chicago urban areas. Demographic and clinical information are presented in Table 1. HIV and seronegative groups did not differ in age, gender, racial composition, educational level, and alcohol use. Marijuana use was higher in the HIV group in this observational cohort. Other substances did not differ, and IV drug use was not reported in either group (Table 1).
Blood samples were collected from all subjects. HIV serostatus was determined by enzyme-linked immunosorbent assay and Western blot. An early infection assay (EIA) was used to assess duration of infection (Blood Systems Research Institute, San Francisco, CA). Antibody non-reactivity was used to define primary infection, estimated as less than 4 months post-infection (pi) (n = 15); 4–12 months pi (n = 15), infected >12–24 months (n = 26). For the HIV group, absolute CD4 T cell counts ranged from 139 to 1282 cells/μL (mean 546 ± 254.0 cells/μL); plasma viral load (log10) ranged from undetectable to 5.54 copies/mL (mean 3.34 ± 1.5 copies/mL). Plasma viral load was undetectable (<50 copies/mL) in 11 of the 56 HIV subjects, including 10 suppressed on antiretroviral therapy (ART) (0–4 months pi: n = 2; 4–12 months pi: n = 2, >12–24 months pi: n = 6). Thirty of the HIV subjects were ART naive and 26 had initiated treatment with subgroup distribution as follows: 0–4 months pi: 7 naive, 8 ART; 4–12 months pi: 11 naive, 4 ART; and >12–24 months pi: 12 naive; 14 ART.
Cognitive assessment
The neuropsychological test battery included tests that have been used in HIV neurologic outcome studies (Selnes et al. 1991; Sevigny et al. 2004). Cognitive measures included verbal memory using the Rey Auditory Verbal Learning Test (Selnes et al. 1990), visual memory using the Rey-Osterrieth Complex Figure Immediate Recall Test (Rey 1941), and visuoconstruction skills using the Ray Complex Figure Copy (Rey 1941). Psychomotor skills were assessed using the Digit Symbol Test (Wechsler 1981; Selnes et al. 1990); reaction times were measured using the California Computerized Assessment Package (CALCAP) (Miller et al. 1991); and motor skills were assessed using Grooved Pegboard (GP) for the dominant and non-dominant hands (Klove 1963; Selnes et al. 1990) and Timed Gait tests (Robertson et al. 2006). Executive function was assessed with the Controlled Oral Word Association Test (i.e., verbal fluency) (Benton and Hamsher 1976), Letter-Number Sequencing of the WAIS-III (Wechsler 1997), Trail Making Test (Bondy 1994; Tombaugh 2004), and Odd Man Out (Flowers and Robertson 1985) tests.
Statistical analysis
Initially, the cross-sectional data at baseline and at follow-up were analyzed separately. Group comparisons were accomplished with independent t tests. Longitudinal analyses considering all available data at both timepoints were accomplished with mixed-effect models using a subject-specific random intercept, HIV group effect, time (in days) in study, and HIV by time interaction. Wald tests were used for HIV main effects and HIV by time interactions. All subjects, with or without a follow-up, were included in the model. Between-group comparisons of cognitive measures between HIV and control groups were considered a priori analyses; a significance level of 0.05 was used. Spearman correlation coefficients were used to examine relationships of neuropsychological measures and clinical status measures. All analyses were executed in R.
Results
Cross-sectional univariate group comparisons
At baseline, the HIV group had generally poorer cognitive performance with significant differences identified for Digit Symbol (p < 0.0001), Rey Complex Figure Recall (p = 0.001), and Rey Auditory Verbal Learning (p = 0.010). Differences at baseline were also indicated for verbal fluency (p = 0.02), letter-number sequencing (p = 0.02), and Grooved Pegboard (dominant p = 0.05 and non-dominant p = 0.04). At follow-up, cognitive performance measures for the HIV group were generally poorer, with differences identified for Odd Man Out (p = 0.007); Digit Symbol (p = 0.02); verbal fluency (p = 0.02); Grooved Pegboard, dominant (p = 0.04); and Rey Complex Figure Recall (p = 0.05). Results are shown in Table 2.
Longitudinal analysis
Longitudinal analysis considering all available data at both timepoints was accomplished with mixed-effect models using a subject-specific random intercept, HIV group effect, follow-up time (in days), and HIV by time interaction. The HIV group had generally poorer cognitive performance than controls. Significant differences were indicated for Digit Symbol (p = 0.0005) and verbal fluency (p = 0.007). Differences were also indicated for Grooved Pegboard (dominant p = 0.03 and non-dominant p = 0.05) and Rey Complex Figure Recall (p = 0.03). Rey Auditory Verbal Learning was nearly significant (p = 0.058). When the rate of change over time in individual cognitive tests was compared in HIV and control groups, there was no significant group by time interactions. Results are shown in Table 3.
Correlations with clinical status
Table 4 presents Spearman correlation coefficients for the neuropsychological measures and important markers of HIV clinical status (HIV RNA, CD4 count, CD4 nadir, and hemoglobin). At baseline, significant correlations were identified between CD4 count and California Computerized Assessment Package (CALCAP) Choice (rho = −0.29; p = 0.03), between CD4 nadir and CALCAP Choice (rho = −0.32; p = 0.02), and between hemoglobin and Trail B (rho = −0.27; p = 0.02). At follow-up, significant correlations with plasma HIV RNA level were identified for Rey Auditory Verbal Learning (rho = −0.33; p = 0.03) and Grooved Pegboard, non-dominant (rho = 0.31; p = 0.04); CD4 count was significantly correlated with CALCAP Sequential (rho = −0.44; p = 0.01). There were numerous significant correlations between hemoglobin and the cognitive measures at follow-up, including Rey Complex Figure Recall (rho = 0.27; p = 0.04), Rey Complex Figure Copy (rho = 0.28; p = 0.03), CALCAP Sequential (rho = −0.35; p = 0.01), Timed Gait (rho = −0.41; p = 0.001), Trail Making A (rho = −0.26; p = 0.04), and Odd Man Out (rho = 0.26; p = 0.05).
Discussion
The status of cognitive function following initial HIV infection and the changes that occur across the early clinical course are not well understood. This longitudinal study evaluated neuropsychological performance measures in participants within approximately the first year of infection, and then again 2 years later. At the initial evaluation, the HIV group had weaker performance on measures of psychomotor speed (Digit Symbol), visual memory (Rey Complex Figure Recall), verbal memory (Rey Auditory Verbal Learning), executive function (verbal fluency and letter-number sequencing), and motor speed (Grooved Pegboard dominant and non-dominant).
Information concerning cognitive status in early infection is very limited because often HIV is not diagnosed in initial stages. Neuropsychological performance measures are not routinely evaluated in those patients presenting with initial symptoms (i.e., acute HIV) or in primary infection. It is difficult to stage duration of infection in individual patients. Studies of cognitive changes have used different definitions of early disease, using asymptomatic participants characterized variously by CDC stage (i.e., pre-acquired immunodeficiency syndrome (AIDS)), neuropsychological test standards, or HAND diagnosis. This study based on duration of infection found differences on neuropsychological measures. The pattern is consistent with cognitive deficits in chronic HIV infection involving learning new information, delayed cognitive processing, and slower completion of tasks requiring psychomotor coordination (Miller et al. 1990; Lezak et al. 2004; Antinori et al. 2007; Moore et al. 2011). Other evidence supports early onset of cognitive deterioration in HIV infection. An investigation, at a 3.5-month duration (median), found that the majority of subjects had cognitive impairment in one or more domains (Paterson et al. 2010). Weaker psychomotor performance and a correlation with reduced putamen volume have been found in the first year of infection (Wright et al. 2016). Other studies of this period have found functional connectivity changes involving visual networks that correlate with visual-motor coordination (Wang et al. 2011) and diminished psychomotor speed, visual memory, executive function, and motor speed (Ragin et al. 2012). Diminished psychomotor speed and visual memory have been reported within 100 days of initial infection (Ragin et al. 2015). Early change in cognitive function is also supported by retrospective analyses of data available from large longitudinal studies for subgroups with estimated seroconversion dates. The CHARTER group found a “stair step” trend across seronegatives, acute/early (median 16 weeks), and chronic HIV (median 4.9 years) groups, suggesting early decline in executive functioning, information processing speed, learning, recall, and working memory (Moore et al. 2011). Rare data for cognitive function before and after seroconversion from a subsample of the Multicenter AIDS Cohort indicated significant change in executive function following HIV seroconversion (Vo et al. 2013). The MACS study did not find psychomotor losses within the first 2 years of infection. Differences in psychomotor findings may be due to sample composition, inclusion criteria, study design, specific neurocognitive measures, or timing of cognitive assessment. The retrospective MACS analysis included only male subjects with CD4 >500, spanning both pre- and post-ART treatment eras. The prospective ART era Chicago study did not use immune status as an inclusion criterion, and findings for cognitive status were based on comparison with seronegative controls.
Imaging studies of acute and early infections have reported differences in brain metabolites, including increased choline in the frontal gray matter and white matter (Lentz et al. 2011), changes in functional connectivity (Wang et al. 2011; Ragin et al. 2012), volumetric reductions in gray matter and alterations in basal ganglia (Sailasuta et al. 2012), putamen (Wright et al. 2016), lenticular nucleus (Ances et al. 2009), occipital gray matter (Ances et al. 2009; Sailasuta et al. 2012), and subcortical structures (Ances and Ellis 2007). Structural and microstructural brain changes have been detected within the first 100 days of infection, including volume reduction in parenchyma, enlargement of third ventricle, loss of white matter integrity in corpus callosum, and diffusion alterations in caudate (Ragin et al. 2015). Taken together, this evidence supports early onset of change in cognitive function.
This longitudinal study extends prior results from the Chicago Early HIV Infection cohort by evaluating cognitive status across the subsequent 2 years. In cross-sectional comparison of the groups at follow-up (Table 2), the HIV subjects showed weaker performance on measures of psychomotor speed, visual memory, motor speed, and executive function. New findings emerged for a measure of executive function (Odd Man Out). Group differences between HIV and controls on select measures of verbal memory, motor speed (Grooved Pegboard—non-dominant), and executive function (letter-number sequencing), that were observed at baseline, were no longer significant at follow-up. When all available cognitive function measures were examined in longitudinal analysis across both time periods adjusting for follow-up time, the HIV group had weaker HIV performance on visual memory, psychomotor speed, motor speed, and executive function (Table 3). There were no differences between the groups in rate of decline in the cognitive performance measures over the 2-year follow-up. These findings are consistent with the clinical course of HAND involving indolent progression across time (Antinori et al. 2007) and with evidence that risk of cognitive impairment increases with duration of infection (Rao et al. 2014).
Psychomotor speed, as measured by the Digit Symbol Test, showed the most marked difference from controls at both time periods. The findings indicate psychomotor slowing early in infection, and this is sustained over the subsequent clinical course. While still diminished compared to controls, some improvement in psychomotor speed was observed between baseline assessment in early infection and follow-up (p = 0.03). There was no significant change in psychomotor performance over the same period in the seronegative controls (supplemental Fig. 1). These results highlight the dynamic nature of neurocognitive change in early HIV. Decreases in psychomotor speed have been associated with systemic viremia (Sacktor et al. 2003). Other evidence indicates reduced psychomotor speed in early infection (Wright et al. 2016), suggesting potential utility as a measure of neurological vulnerability and progression (Sacktor et al. 1996, 2003). Tasks that assess psychomotor functioning are among the most sensitive for discriminating symptomatic HIV+ subjects from controls (Miller et al. 1990). Psychomotor speed may represent a sensitive measure for monitoring response to treatment. Virologic suppression has been associated with improved performance on psychomotor tasks (Sacktor et al. 2003).
Comparison of HIV subgroups classified for marijuana use indicated greater impairment only for Grooved Pegboard at the initial timepoint (data not shown). Whereas marijuana use may amplify impairment on this cognitive performance measure in early HIV, it does not appear to account for the more global pattern of cognitive differences observed at both timepoints and across time. Because pre-infection cognitive data are not available for the HIV participants, the possibility that the HIV-infected population had lower cognitive function prior to infection cannot be excluded. Lower pre-morbid cognitive function is unlikely to account for findings in the HIV group. There was no systematic bias in recruitment of subjects. HIV and control participants were recruited from similar urban Chicago areas to balance the groups for background. In addition, a relatively large sample of participants in early infection was evaluated (n = 56). The dynamic changes that were observed, involving improvement in some domains and decline in others, mirror the turbulent initial stages of infection, involving symptomatic acute HIV, intense viremia, and cytokine storm followed by a more quiescent asymptomatic period with the mounting of host response (Rao et al. 2014). Vo et al. found change in executive function before and after HIV seroconversion (Vo et al. 2013). Finally, our findings are consistent with imaging evidence of brain alterations early in HIV infection (Ances and Ellis 2007; Ances et al. 2009; Paterson et al. 2010; Lentz et al. 2011; Moore et al. 2011; Wang et al. 2011; Ragin et al. 2012; Sailasuta et al. 2012; Ragin et al. 2015; Wright et al. 2016).
The neuropsychological measures were also examined for relationships with important HIV clinical status markers (Table 4). At baseline, lower CD4 and CD4 nadir were correlated with longer reaction times and lower hemoglobin was associated with delayed completion of Trail Making B, which is considered a measure of executive function. More extensive relationships with clinical status were observed at follow-up, particularly with lower hemoglobin. At follow-up, higher viral load was associated with weaker scores on verbal learning and visual-motor coordination and lower CD4 correlated with longer reaction time. Lower hemoglobin correlated with weaker scores on numerous cognitive measures, including psychomotor, visual memory, visuoconstruction, motor performance, spatial skills, and executive function. To evaluate prognostic significance, clinical status measures at baseline were also correlated with neuropsychological measures at follow-up (data not shown). Hemoglobin level at baseline correlated with performance on the letter-number sequencing task, a measure of working memory, at follow-up (rho = 0.301; p = 0.018). Other studies have reported prognostic significance for the hemoglobin and the related markers, IL-6 and hematocrit, for neurological disease progression in HIV infection (McArthur et al. 1993; Hamlyn et al. 2014). IL-6 and hematocrit have been associated with brain alterations quantified with neuroimaging in the earliest period of infection (Cao et al. 2015).
The early, natural history of HIV infection is marked by rapid increase in viremia and widespread dissemination to tissues in the body, followed by an adaptive immune response that rapidly brings down plasma viral load, usually to undetectable levels within the first 3 months. Nevertheless, the virus continues to replicate in cells that are minimally activated, and these cells are able to persist even after ART (Stevenson 2003). Early viral tropism and CNS compartmentalization allow viral replication and adaptation specifically in the CNS environment (Zayyad and Spudich 2015). This time period, marked by dramatic fluctuation in viral load and in loss of CD4 (Stevenson 2003), may be relevant to an understanding of neurocognitive changes in initial stages of infection. While transient, it has been shown that the extent of initial viremia predicts long-term outcomes including progression to AIDS and duration of survival (Mellors et al. 1996).
Diminished cognitive function in early HIV infection may reflect consequences of viral invasion of the brain in association with the intense, uncontrolled viremia that occurs soon after transmission (Stevenson 2003). Virus can be detected in the cerebrospinal fluid within 8 days of infection (Spudich et al. 2011), and patients with acute HIV may present with headache and symptoms of meningoencephalitis, which is consistent with early brain involvement (Lamers et al. 2011). HIV may gain ingress to the brain in this period either as free virus or through infected monocytes (Price 2000; Rao et al. 2014), and neuroinflammation is evident early in infection (Zayyad and Spudich 2015).
Mechanisms underlying neuronal injury have not been elucidated, particularly in the earliest stages. Various lines of evidence indicate that HIV-associated neurocognitive disorder (HAND) has a basis in neuronal and axonal injury, owing to viral proteins and an inflammatory milieu of elevated levels of monocyte-derived macrophages, cytokines, chemokines, and other neurotoxic factors (Rao et al. 2014). Biomarkers such as neurofilament light chain that have been used to detect inflammation and neuronal injury in chronic infection (Abdulle et al. 2007; Canizares et al. 2014) have been found in acute infection (Peluso et al. 2013). Brain metabolites like choline and creatine are elevated both in acute and in chronic infections (Sailasuta et al. 2012). Prolonged immune activation, involving increased permeability of the blood brain barrier and accelerated monocyte trafficking to the brain, may have deleterious consequences, leading to neural injury (Price 2000; Rao et al. 2014).
When interpreting these findings, it is important to appreciate that the results are based on scores on neuropsychological tests. These subjects may not present symptomatic evidence of cognitive difficulties or impaired function in early infection. HAND is diagnosed as asymptomatic neurocognitive impairment (ANI), HIV-associated mild neurocognitive disorder (MND), and HIV-associated dementia (HAD). ANI is defined by performance at least one standard deviation below the mean of demographically adjusted normative scores in at least two cognitive areas, with a minimum of five total cognitive areas observed (Antinori et al. 2007).
The findings of this study are consistent with the pattern of weaker performance observed in advanced infection on neuropsychological tests, such as Digit Symbol; Rey Auditory Verbal Learning; Grooved Pegboard, dominant and non-dominant; and Trail Making (Miller et al. 1990). Studies of chronic HIV infection have found differences involving impaired verbal functioning, memory, information processing, learning, and motor function (Arendt et al. 1994; Cherner et al. 2002). Impairment in certain cognitive functions persists even with successful therapy and suppression of viral load (Tozzi et al. 2007; Heaton et al. 2010; Heaton et al. 2011). Diminished cognitive function has implications for functional capacity in daily living independence and unemployment (Heaton et al. 2004; Antinori et al. 2007; Scott et al. 2011).
This observational study included both ART-initiated (n = 26) and naive (n = 30) subjects at the baseline timepoint. To gain insights concerning effects of viral infection independent of treatment, the ART naive subgroup was also compared to seronegative controls (data not shown). The pattern of neuropsychological change was similar to results for the larger cohort. ART naive subjects had significantly weaker performance on psychomotor speed (Digit Symbol; p = 0.0002; Grooved Pegboard, non-dominant; p = 0.03; and Grooved Pegboard, dominant; p = 0.05) and executive function (verbal fluency; p = 0.01) at the baseline timepoint. There were no differences in cognitive status between ART and naive HIV+ subgroups at baseline. When these subgroups were compared at follow-up, the subgroup on ART at baseline had significantly stronger scores on Grooved Pegboard non-dominant task (p = 0.03) compared to those who were ART naive at baseline (data not shown). Change in cognitive function was also evaluated before and after ART in the subgroup of subjects (n = 19) who were naive at baseline and then subsequently initiated antiretroviral treatment (Table 5). Significant improvement was quantified on measures of verbal memory (Rey Auditory Verbal Learning average; p = 0.02) and executive function (Trail A; p = 0.02) following ART. There were no differences across time in the HIV subgroup on ART at baseline. The cognitive measures were also correlated with a measure of CNS penetration in the ART subgroup (Letendre et al. 2008). This analysis indicated a significant inverse correlation with verbal fluency (r = −0.42; p = 0.03) at baseline, suggesting that higher CNS penetration correlated with weaker performance. At follow-up, however, significant correlations were identified with verbal memory (r = 0.37; p = 0.02) and Timed Gait, a measure of motor speed (r = −0.48; p = 0.002), suggesting improved function with higher CNS penetration. Further studies that include analysis of CSF in early infection may be particularly informative for clarifying the relationship between the CNS viral reservoir, ART penetration, and how timing of treatment initiation may modify cognitive outcome.
Conclusion
These findings indicate early changes in specific cognitive functions that are sustained across the subsequent course. While there may be some degree of recovery following the immune perturbances of seroconversion, function in specific domains may never return to pre-infection levels. These findings underscore the necessity of focusing on early infection for neuroprotective intervention. Further characterization of the changes occurring in initial stages of infection and the risk and protective factors for cognitive function could inform new strategies for neuroprotection.
References
Abdulle S, Mellgren A, Brew BJ, Cinque P, Hagberg L, Price RW, Rosengren L, Gisslen M (2007) CSF neurofilament protein (NFL)—a marker of active HIV-related neurodegeneration. J Neurol 254:1026–1032
Ances BM, Ellis RJ (2007) Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol 27:86–92
Ances BM, Sisti D, Vaida F, Liang CL, Leontiev O, Perthen JE, Buxton RB, Benson D, Smith DM, Little SJ, Richman DD, Moore DJ, Ellis RJ, group H (2009) Resting cerebral blood flow: a potential biomarker of the effects of HIV in the brain. Neurology 73:702–708
Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69:1789–1799
Arendt G, Hefter H, Hilperath F, von Giesen HJ, Strohmeyer G, Freund HJ (1994) Motor analysis predicts progression in HIV-associated brain disease. J Neurol Sci 123:180–185
Benton A, Hamsher K (1976) Multilingual Aphasia Examination. University of Iowa, Iowa City
Bondy KN (1994) Assessing cognitive function: a guide to neuropsychological testing. Rehabilitation nursing: the official journal of the Association of Rehabilitation Nurses 19:24–30 36
Canizares S, Cherner M, Ellis RJ (2014) HIV and aging: effects on the central nervous system. Semin Neurol 34:27–34
Cao B, Kong X, Kettering C, Yu P, Ragin A (2015) Determinants of HIV-induced brain changes in three different periods of the early clinical course: a data mining analysis. NeuroImage Clinical 9:75–82
Cherner M, Masliah E, Ellis RJ, Marcotte TD, Moore DJ, Grant I, Heaton RK (2002) Neurocognitive dysfunction predicts postmortem findings of HIV encephalitis. Neurology 59:1563–1567
Doyle KL, Morgan EE, Morris S, Smith DM, Little S, Iudicello JE, Blackstone K, Moore DJ, Grant I, Letendre SL, Woods SP, Translational Methamphetamine ARCG (2013) Real-world impact of neurocognitive deficits in acute and early HIV infection. Journal of neurovirology 19:565–573
Flowers KA, Robertson C (1985) The effect of Parkinson’s disease on the ability to maintain a mental set. J Neurol Neurosurg Psychiatry 48:517–529
Hamlyn E, Fidler S, Stohr W, Cooper DA, Tambussi G, Schechter M, Miro JM, McClure M, Weber J, Babiker A, Porter K, Investigators ST (2014) Interleukin-6 and D-dimer levels at seroconversion as predictors of HIV-1 disease progression. AIDS 28:869–874
Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, McCutchan JA, Reicks C, Grant I, Group H (2004) The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society: JINS 10:317–331
Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75:2087–2096
Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C, Group H (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of neurovirology 17:3–16
Klove H (1963) The medical clinics of North America. The Medical Clinics of North America, New York
Lamers SL, Gray RR, Salemi M, Huysentruyt LC, McGrath MS (2011) HIV-1 phylogenetic analysis shows HIV-1 transits through the meninges to brain and peripheral tissues. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases 11:31–37
Lentz MR, Kim WK, Kim H, Soulas C, Lee V, Venna N, Halpern EF, Rosenberg ES, Williams K, Gonzalez RG (2011) Alterations in brain metabolism during the first year of HIV infection. Journal of neurovirology 17:220–229
Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ, Group C (2008) Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 65:65–70
Lezak MD, Howieson DB, Loring DW (2004) Neuropsychological assessment. Oxford University Press, New York
McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, Graham NM, McArthur JH, Selnes OA, Jacobson LP et al (1993) Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology 43:2245–2252
Mellors JW, Rinaldo CR Jr, Gupta P, White RM, Todd JA, Kingsley LA (1996) Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science (New York, NY) 272:1167–1170
Miller EN, Selnes OA, McArthur JC, Satz P, Becker JT, Cohen BA, Sheridan K, Machado AM, Van Gorp WG, Visscher B (1990) Neuropsychological performance in HIV-1-infected homosexual men: the Multicenter AIDS Cohort Study (MACS). Neurology 40:197–203
Miller EN, Satz P, Visscher B (1991) Computerized and conventional neuropsychological assessment of HIV-1-infected homosexual men. Neurology 41:1608–1616
Moore DJ, Letendre SL, Morris S, Umlauf A, Deutsch R, Smith DM, Little S, Rooney A, Franklin DR, Gouaux B, Leblanc S, Rosario D, Fennema-Notestine C, Heaton RK, Ellis RJ, Atkinson JH, Grant I, Group C (2011) Neurocognitive functioning in acute or early HIV infection. Journal of neurovirology 17:50–57
Nightingale S, Winston A, Letendre S, Michael BD, McArthur JC, Khoo S, Solomon T (2014) Controversies in HIV-associated neurocognitive disorders. The Lancet Neurology 13:1139–1151
Paterson J, Lee E, Hecht F, Price R, Robertson K, Spudich S (2010) Neurocognitive performance during primary HIV-1 infection. Conference on retroviruses and opportunistic infections. San Francisco, CA
Peluso MJ, Meyerhoff DJ, Price RW, Peterson J, Lee E, Young AC, Walter R, Fuchs D, Brew BJ, Cinque P, Robertson K, Hagberg L, Zetterberg H, Gisslen M, Spudich S (2013) Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. The Journal of infectious diseases 207:1703–1712
Price RW (2000) The two faces of HIV infection of cerebrospinal fluid. Trends Microbiol 8:387–391
Ragin AB, Du H, Ochs R, Wu Y, Sammet CL, Shoukry A, Epstein LG (2012) Structural brain alterations can be detected early in HIV infection. Neurology 79:2328–2334
Ragin AB, Wu Y, Gao Y, Keating S, Du H, Sammet C, Kettering CS, Epstein LG (2015) Brain alterations within the first 100 days of HIV infection. Annals of clinical and translational neurology 2:12–21
Rao VR, Ruiz AP, Prasad VR (2014) Viral and cellular factors underlying neuropathogenesis in HIV associated neurocognitive disorders (HAND). AIDS Res Ther 11:13
Rey A (1941) L’examen psychologique dans les cas d’encephalopathie traumatique. Arch Psychol 28:286–340
Robertson KR, Parsons TD, Sidtis JJ, Hanlon Inman T, Robertson WT, Hall CD, Price RW (2006) Timed Gait test: normative data for the assessment of the AIDS dementia complex. J Clin Exp Neuropsychol 28:1053–1064
Sacktor NC, Bacellar H, Hoover DR, Nance-Sproson TE, Selnes OA, Miller EN, Dal Pan GJ, Kleeberger C, Brown A, Saah A, McArthur JC (1996) Psychomotor slowing in HIV infection: a predictor of dementia, AIDS and death. Journal of neurovirology 2:404–410
Sacktor N, Skolasky RL, Tarwater PM, McArthur JC, Selnes OA, Becker J, Cohen B, Visscher B, Miller EN, Multicenter ACS (2003) Response to systemic HIV viral load suppression correlates with psychomotor speed performance. Neurology 61:567–569
Sailasuta N, Ross W, Ananworanich J, Chalermchai T, DeGruttola V, Lerdlum S, Pothisri M, Busovaca E, Ratto-Kim S, Jagodzinski L, Spudich S, Michael N, Kim JH, Valcour V, teams RSp (2012) Change in brain magnetic resonance spectroscopy after treatment during acute HIV infection. PLoS One 7:e49272
Scott JC, Woods SP, Vigil O, Heaton RK, Schweinsburg BC, Ellis RJ, Grant I, Marcotte TD (2011) A neuropsychological investigation of multitasking in HIV infection: implications for everyday functioning. Neuropsychology 25:511–519
Selnes OA, Miller E, McArthur J, Gordon B, Munoz A, Sheridan K, Fox R, Saah AJ (1990) HIV-1 infection: no evidence of cognitive decline during the asymptomatic stages. The Multicenter AIDS Cohort Study Neurology 40:204–208
Selnes OA, Jacobson L, Machado AM, Becker JT, Wesch J, Miller EN, Visscher B, McArthur JC (1991) Normative data for a brief neuropsychological screening battery. Multicenter AIDS Cohort Study Perceptual and motor skills 73:539–550
Sevigny JJ, Albert SM, McDermott MP, McArthur JC, Sacktor N, Conant K, Schifitto G, Selnes OA, Stern Y, McClernon DR, Palumbo D, Kieburtz K, Riggs G, Cohen B, Epstein LG, Marder K (2004) Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology 63:2084–2090
Spudich S, Gisslen M, Hagberg L, Lee E, Liegler T, Brew B, Fuchs D, Tambussi G, Cinque P, Hecht FM, Price RW (2011) Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. The Journal of infectious diseases 204:753–760
Stevenson M (2003) HIV-1 pathogenesis. Nat Med 9:853–860
Tombaugh TN (2004) Trail Making Test A and B: normative data stratified by age and education. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists 19:203–214
Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, Vlassi C, Giulianelli M, Galgani S, Antinori A, Narciso P (2007) Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. Journal of acquired immune deficiency syndromes (1999) 45:174–182
Vo QT, Cox C, Li X, Jacobson LP, McKaig R, Sacktor N, Selnes OA, Martin E, Becker JT, Miller EN (2013) Neuropsychological test performance before and after HIV-1 seroconversion: the Multicenter AIDS Cohort Study. Journal of neurovirology 19:24–31
Wang X, Foryt P, Ochs R, Chung JH, Wu Y, Parrish T, Ragin AB (2011) Abnormalities in resting-state functional connectivity in early human immunodeficiency virus infection. Brain connectivity 1:207–217
Wechsler D (1981) Wechsler Adult Intelligence Scale revised. The Psychological Corporation, New York
Wechsler D (1997) Wechsler Adult Intelligence Scale-III. Psychology, San Antonio, TX
Wright PW, Pyakurel A, Vaida FF, Price RW, Lee E, Peterson J, Fuchs D, Zetterberg H, Robertson KR, Walter R, Meyerhoff DJ, Spudich SS, Ances BM (2016) Putamen volume and its clinical and neurological correlates in primary HIV infection. AIDS.
Zayyad Z, Spudich S (2015) Neuropathogenesis of HIV: from initial neuroinvasion to HIV-associated neurocognitive disorder (HAND). Current HIV/AIDS reports 12:16–24
Authorship contributions
This work was supported by the National Institutes of Health (R01-MH080636). A.P. participated in data analysis and interpretation and manuscript preparation. J.H. conducted statistical analysis and contributed to Methods and Results. L.L. advised on statistical analysis. Y.G. advised on statistical analysis and database management. C.K. conducted participant visits, data management, and manuscript preparation. A.B.R. is the principal investigator of the Chicago Early HIV Study and contributed to study design, imaging procedures, statistical analysis, interpretation of findings, and manuscript preparation. A.B.R. is funded by NIH grants R01AG034852, R01CA159178, R25NS080949, R01HL115828, and R01HL117888.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Standard protocol approvals, registrations, and patient consents. The Northwestern University Institutional Review Board approved this investigation. All subjects provided written informed consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding for this work
NIH MH 080636R01
Electronic supplementary materials
ESM 1
(DOCX 27 kb)
Rights and permissions
About this article
Cite this article
Prakash, A., Hou, J., Liu, L. et al. Cognitive function in early HIV infection. J. Neurovirol. 23, 273–282 (2017). https://doi.org/10.1007/s13365-016-0498-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-016-0498-4