Abstract
The acute and early period of HIV-1 infection (AEH) is characterized by neuroinflammatory and immunopathogenic processes that can alter the integrity of neural systems and neurocognitive functions. However, the extent to which central nervous system changes in AEH confer increased risk of real-world functioning (RWF) problems is not known. In the present study, 34 individuals with AEH and 39 seronegative comparison participants completed standardized neuromedical, psychiatric, and neurocognitive research evaluations, alongside a comprehensive assessment of RWF that included cognitive symptoms in daily life, basic and instrumental activities of daily living, clinician-rated global functioning, and employment. Results showed that AEH was associated with a significantly increased risk of dependence in RWF, which was particularly elevated among AEH persons with global neurocognitive impairment (NCI). Among those with AEH, NCI (i.e., deficits in learning and information processing speed), mood disorders (i.e., Bipolar Disorder), and substance dependence (e.g., methamphetamine dependence) were all independently predictive of RWF dependence. Findings suggest that neurocognitively impaired individuals with AEH are at notably elevated risk of clinically significant challenges in normal daily functioning. Screening for neurocognitive, mood, and substance use disorders in AEH may facilitate identification of individuals at high risk of functional dependence who may benefit from psychological and medical strategies to manage their neuropsychiatric conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The acute and early period of HIV-1 infection (AEH) is characterized by significant levels of viral replication and immune activation (e.g., Pilcher et al. 2004). As early as 2 weeks after systemic infection, the virus crosses the blood–brain barrier and enters the central nervous system (CNS; An et al. 1999; Davis et al. 1992). While there is limited research regarding the nature and extent of AEH-related neural injury, preliminary evidence suggests that the CNS is subject to neuroinflammatory processes early in the course of infection (e.g., Brenchley et al. 2004). To date, four neuroimaging studies have provided evidence of altered brain structure and function during the AEH period. Ances et al. (2009) reported that AEH was associated with reduced resting cerebral blood flow in the lenticular nuclei and visual cortex as compared to seronegatives. Similarly, Lentz et al. (2009) observed altered levels of several cerebral metabolites (i.e., N-acetylaspartate, glutamate-glutamine, and choline) in the frontal cortex of persons with AEH. The same group later found evidence for altered basal ganglia metabolism due to elevated levels of myo-inositol, which peaked approximately two months post-seroconversion (Lentz et al. 2011). Most recently, Wang et al. (2011) reported fMRI evidence of reduced functional connectivity within the lateral occipital resting state network of an AEH group relative to a seronegative group, which correlated with poorer performance on tests of visual-motor coordination (i.e., fine-motor coordination and complex figure drawing).
Consistent with the emergence of studies revealing metabolic and structural changes in the AEH brain, there have also been three recent studies showing corresponding neurocognitive impairment (NCI) in individuals with AEH. First, Moore et al. (2011) reported that global neurocognitive performance of an AEH sample (n = 39; median duration of infection 16 weeks) was intermediate relative to chronically HIV-infected (median duration of infection 255 weeks) and HIV-uninfected groups. Second, the Wang et al. (2011) fMRI study referenced above also found cognitive deficits in the domains of information processing speed and working memory in a sample of individuals with AEH (n = 15; median duration of infection <1 year) relative to an HIV-uninfected group. Most recently, Weber et al. (2013a) reported that an AEH cohort (n = 46; median duration of infection 75 days) was nearly four times more likely to evidence global NCI than HIV-uninfected comparison subjects, which was largely driven by deficits in domains of information processing speed and verbal learning. Furthermore, the same study found that NCI in the AEH sample was significantly associated with problematic methamphetamine (MA) use, but not with other neuropsychiatric problems (e.g., current affective distress, alcohol use disorders), all of which are highly comorbid within AEH populations (Atkinson et al. 2009; Plankey et al. 2007).
Thus, these emerging data suggest that AEH is associated with alterations in CNS structure and function, and yet questions remain regarding the real-world implications of such changes. In chronic infection, while HIV-associated declines in real-world functioning (RWF) are highly multifactorial (see Blackstone et al. 2013a), NCI is a unique risk factor for dependence in instrumental activities of daily living (Heaton et al. 2004a; Woods et al. 2008), antiretroviral (ARV) non-adherence (Woods et al. 2009b), unemployment (Woods et al. 2011), automobile driving accidents (Marcotte et al. 1999, 2004, 2006), lower health-related quality of life (HRQoL; Doyle et al. 2012), and engagement in HIV transmission risk behaviors (Anand et al. 2010). Executive dysfunction (e.g., cognitive flexibility), episodic memory, and psychomotor speed are the most robust neurocognitive predictors of RWF problems in chronic infection in the cART era (Blackstone et al. 2013a). Given that these associations have been reliably reported in chronic HIV infection, it is reasonable to posit that individuals with AEH who experience NCI would also suffer from similar RWF deficits, though no prior studies have yet examined this topic.
Accordingly, the current study seeks to evaluate the independent and additive effects of AEH and NCI on a battery of RWF outcomes, including cognitive symptoms in daily life, basic and instrumental activities of daily living, clinician-rated global functioning, and employment. Despite evidence of early CNS dysfunction and greater risk for neuropsychiatric disorders (i.e., substance dependence and affective distress) in AEH, this is the first study to our knowledge to characterize RWF in this high-risk population. Thus, we aim to add to an emerging literature by determining the nature, severity, and predictors of AEH-related RWF dependence relative to a demographically similar HIV-uninfected comparison group, in hopes of providing insight into the detection, treatment and prevention of everyday functioning deficits in AEH.
Methods
Participants
The study sample included 34 individuals with AEH and 39 HIV-uninfected participants who were enrolled in the Translational Methamphetamine Research Center (TMARC), a NIDA-funded cohort study on the CNS effects of HIV infection and MA use disorders. AEH infection was determined through one of the following methods: (a) positive HIV-1 RNA (Amplicor, Roche) with negative HIV EIA or rapid test; (b) HIV RNA-positive and Western blot indeterminate with no more than three positive bands; or (c) HIV RNA-positive, EIA-, or rapid test-positive and less sensitive (detuned) EIA consistent with very recent infection (OD < 0.3 by Vironostika or equivalent by Vitros ECi or OraQuick detuned). Duration of infection was estimated via an algorithm that evaluated the following data at study entry: (a) HIV-1 serologic tests, (b) detuned EIA testing, and (c) HIV RNA levels (Le et al. 2013). HIV disease characteristics for the AEH group were obtained via a standardized neuromedical evaluation by certified research staff and are displayed in Table 1. Of note, AEH individuals who were undergoing antiretroviral therapy (ART; i.e., approximately 32 %) at the time of evaluation had an estimated duration of infection range of 1.2–11.9 months (M = 5.9 months), and began treatment post-diagnosis (vs. pre-exposure prophylaxis), reflecting the current trend of early treatment initiation (Cohen et al. 2011). Exclusion criteria for both groups included a history of severe psychiatric (e.g., schizophrenia) or neurologic illness (e.g., seizure disorders, active CNS opportunistic infections) unrelated to HIV infection or MA, inability to provide informed consent, and a verbal IQ estimate of <80 based on the Wide Range Achievement Test—4th Edition (WRAT-IV; Wilkinson and Robertson 2006).
Table 1 illustrates the demographic, neurocognitive, and psychiatric characteristics of the study samples. Lifetime histories of psychiatric (i.e., mood disorders) and substance dependence were established by the Composite International Diagnostic Interview (CIDI v. 2.1; World Health Organization 1998), using criteria as determined by the DSM-IV (American Psychiatric Association 1994). For the present study, a history of lifetime mood disorder included meeting criteria for Major Depressive Disorder and/or Bipolar Disorder. The AEH sample had a greater proportion of males as compared to the HIV-uninfected sample, as well as significantly higher rates of lifetime mood disorder and lifetime alcohol and non-alcohol dependence (all ps < .05). Given the very small number of women in the AEH group, we did not include sex as a covariate in the statistical model; however, sex was not associated with RWF in either group (ps > .10), and we are unaware of any studies showing HIV by sex interactions on RWF.
Materials and procedures
The study procedures were approved by the human subjects institutional review board at the University of California, San Diego. After providing written, informed consent, participants completed an assessment of RWF alongside a comprehensive neuropsychological, psychiatric, and medical evaluation.
RWF assessment
Activities of daily living (ADL) scale (Lawton and Brody 1969).
Participants completed a modified version (Heaton et al. 2004a) of the original ADL Scale, in which they are asked to self-report perceived “current” and “best” levels of functioning among various ADLs. Items include 5 basic activities of daily living (BADLs; i.e., housekeeping, home repairs, bathing, dressing, and laundry), and 11 instrumental activities of daily living (IADLs; i.e., finance management, shopping, grocery shopping, understanding reading material/TV, planning social activities, communication, medication management, transportation, cooking, child care, and work). To enhance our sensitivity to AEH-related disability, the current study used only “current” BADL and IADL ratings, given that declines in functioning are not likely to have occurred yet within a relatively young population that only seroconverted on average 2.1 months prior to evaluation. Participants were classified as BADL- and/or IADL-dependent if they reported difficulties on 2 or more items within each BADL and IADL domain, separately (see Blackstone et al. 2013b).
The patient’s assessment of own functioning inventory (PAOFI; Chelune et al. 1986).
The PAOFI is a self-report questionnaire in which participants are asked to rate their current level of cognitive functioning in day-to-day life. PAOFI domains include memory, language and communication, higher-level cognitive and intellectual functioning, motor, sensory-perceptual ability, and recreation. Participants rated their perceived difficulty in such areas using a 6-point Likert-type scale, which ranges from 1 (“almost always”) to 6 (“almost never”). A total score was obtained by summing the number of items that were rated 3 or less (i.e., having difficulty with the task at least “fairly often”). Participants were classified as impaired on the PAOFI if they had a total score of 3 or greater (see Woods et al. 2004).
Employment status
The PAOFI also includes an item concerning the participant’s current employment status, which is classified as either: (a) not employed, (b) employed (part-time), or (c) employed (full-time). Participants were classified as “impaired” in employment if they indicated they were not employed.
Karnofsky scale of performance status (Karnofsky and Burchenal 1949).
A certified nurse assigned each participant an overall functional impairment rating via the Karnofsky Scale, with scores ranging from 100 (i.e., normal/no complaints/no evidence of disease) to 0 (i.e., death). A cutpoint score of <90 was used to determine at least mild impairment (Schag et al. 1984).
For our primary analyses, we created a composite variable to represent global functional impairment, which was comprised of the five functional domains stated above (i.e., BADLs, IADLs, PAOFI, Employment, and the Karnofsky Performance Scale; range = 0–5). A cutoff score of impairment in at least two domains was used to classify participants as RWF impaired (RWFI) or RWF normal (RWFN; see Blackstone et al. 2013b; Woods et al. 2004).
Neuropsychological assessment
Participants received a comprehensive neuropsychological test battery designed to assess neurocognitive domains sensitive to HIV-associated neurocognitive disorders (HAND; Antinori et al. 2007), including attention/working memory, executive functions, learning, memory, motor skills, information processing speed, and verbal fluency (see Heaton et al. 2010 for details). Raw scores from individual tests were converted into demographically adjusted T-scores using published normative standards (Heaton et al. 2004b; Norman et al. 2011; Woods et al. 2004), which were then converted into deficit scores (range 0–5, with higher scores indicating greater dysfunction). A global deficit score (GDS; see Carey et al. 2004) was calculated by averaging the individual deficit scores. A standard cutoff score of GDS ≥0.5 classified individuals as either evidencing neurocognitive impairment (NCI) or normal neurocognitive functioning (NCN; Carey et al. 2004).
Results
A logistic regression was conducted in order to investigate the individual and combined effects of AEH and NCI (i.e., GDS) on global RWF dependence, while controlling for lifetime histories of mood, alcohol, and other substance dependence (see Table 1). The overall model was significant (χ 2 = 46.1, p < .001), revealing main effects of AEH (χ 2 = 8.7, p = .003) and lifetime non-alcohol dependence (χ 2 = 4.8, p = .029), but not lifetime mood disorder (χ 2 = 2.6, p = .109). Additionally, a significant interaction emerged between AEH and NCI (χ 2 = 6.7, p = .009). Planned follow-up pairwise comparisons revealed that NCI in the AEH group was associated with a significantly higher rate of global RWF dependence (91.7 % vs. 36.4 % dependent; χ2 = 9.6, p < .001, OR = 19.3, confidence interval [CI] = 2.1–178). In contrast, there was no effect of NCI on RWF dependence in the HIV-uninfected group (6.5 % vs. 0.0 % dependent; χ 2 = .54, p = .461).
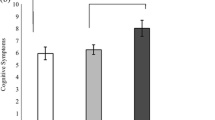
Figure 1 and Table 2 display the number and severity of global and domain-specific RWF dependence across three groups, that is, the AEH sample split by neurocognitive status (AEH/neurocognitive impairment = AEH/NCI; AEH neurocognitive normal = AEH/NCN) and the HIV-uninfected group, which was collapsed across cognitive status given the lack of an NCI effect on global RWF reported above. For the ADL measure, the AEH/NCI group was significantly more likely to be dependent on BADLs than both the AEH/NCN (Cohen’s d = .57, p = .043) and HIV-uninfected groups (Cohen’s d = .87, p = .001), though the AEH/NCN and HIV-uninfected groups did not differ from each other (Cohen’s d = .23, p = .520). A similar pattern emerged among the IADLs, in which the AEH/NCI group was significantly more dependent than both the AEH/NCN (Cohen’s d = .97, p = .004) and HIV-uninfected groups (Cohen’s d = 1.62, p < .001), with a trend-level difference between the AEH/NCN and HIV-uninfected groups (Cohen’s d = .60, p = .076). Regarding the Karnofsky Performance Scale, the AEH/NCI group was significantly more impaired than the AEH/NCN group (Cohen’s d = .72 p = .008) and the HIV-uninfected group (Cohen’s d = 1.15, p = .002), though the AEH/NCN and HIV-uninfected groups did not differ from each other (Cohen’s d = .13, p = .732). On the PAOFI, a significant stepwise pattern emerged, with the AEH/NCI group evidencing significantly greater impairment than the AEH/NCN (Cohen’s d = .52, p = .043) and HIV-uninfected (Cohen’s d = 1.51, p < .001) groups, as well as the AEH/NCN group being significantly more impaired than the HIV-uninfected group (Cohen’s d = .91, p = .014). Finally, the AEH/NCI group had significantly greater rates of unemployment than the HIV-uninfected group (χ 2 = 7.4, OR = 6.8, p = .042), but did not differ from the AEH/NCN group (χ 2 = .86, OR = 2.1, p = .369).
The next series of analyses were conducted to determine the clinical correlates of RWF in the AEH group. For these analyses, the AEH group was split by functional status (AEH/RWFI = AEH real-world functioning impaired; AEH/RWFN = AEH real-world functioning normal). We first conducted a series of independent-samples t tests on neurocognitive domain-based T-scores in order to determine which neurocognitive ability areas were driving the relationship between global NCI and RWF outcomes in AEH. Results revealed that the AEH/RWFI sample had significantly lower average T-scores than the AEH/RWFN sample in the domains of speeded information processing (AEH/RWFI: M = 46.9, SD = 9.0; AEH/RWFN: M = 53.5, SD = 7.3; p = .03; Cohen’s d = .80) and learning (AEH/RWFI: M = 42.1, SD = 8.0; AEH/RWFN: M = 50.2, SD = 8.4; p = .01; Cohen’s d = .99), but not for attention, memory, motor skills, executive functions, or verbal fluency (ps > .10). We then conducted an additional series of independent samples t tests (or chi-square analyses, as appropriate) examining differences between the AEH/RWFI and AEH/RWFN groups in regards to demographic, HIV disease, psychiatric, and substance dependence characteristics (see Table 1). Results showed that the AEH/RWFI group had higher rates of lifetime mood and non-alcohol substance dependence, but groups were similar on all other factors, including HIV disease variables (ps > .10). When these two variables were entered into a logistic regression along with global NCI (i.e., GDS) predicting functional impairment, all three variables emerged as significant, independent predictors (GDS: χ 2 = 6.4, p = .01; lifetime mood disorder: χ 2 = 3.9, p = .05; lifetime non-alcohol dependence: χ 2 = 6.3, p = .01). In order to explore which specific mood and substance disorders could be driving these findings, we conducted another series of chi-square tests in which results revealed the AEH/RWFI group to have significantly higher rates of lifetime Bipolar Disorder (χ 2 = 5.8, p = .02), MA dependence (χ 2 = 9.7, p < .01), and cannabis dependence (χ 2 = 4.2, p = .04) as compared to the AEH/RWFN group, but no other mood or substance use variables were associated with RWF (ps > .10).
Discussion
The present study sought to examine the nature and extent of multi-modal RWF impairment in the context of AEH. Results revealed a significant interaction between the risk factors of AEH and global NCI, independent of other important comorbidities, such that RWF impairment rates were disproportionately high in the AEH group with NCI. Specifically, whereas the HIV- and AEH/NCN groups were classified as RWF normal at rates of 95 % and 64 %, respectively; only 9 % of AEH/NCI individuals was RWF normal (see Fig. 1). This rate of functional impairment in the AEH group is elevated as compared to rates found in chronic infection studies (e.g., Heaton et al. 2004a; Blackstone et al. 2013b), which could be in part due to other possible AEH factors, including the physical burden of significant levels of neuroinflammatory and immunopathogenic processes, NCI due to factors other than AEH, and psychological and/or social turmoil. Speaking to the breadth of the RWF findings in AEH, an adverse effect of NCI was observed within all five domains (i.e., basic and instrumental activities of daily living, everyday cognitive complaints, employment status, and clinician-rated performance). These data support the literature regarding the RWF impact of NCI in chronic HIV infection (e.g., Heaton et al. 2004a; Woods et al. 2008, 2009b, Woods et al. 2011; Doyle et al. 2012; Marcotte et al. 1999, 2004, 2005; Anand et al. 2010), and extend it to a unique, high-risk subset of the HIV population which has been largely unexplored in terms of its neurocognitive and functional outcomes.
At the cognitive domain level, impairment in learning (e.g., immediate recall of a word list and simple geometric figures) and speeded information processing (SIP; e.g., timed tests of matching numbers to symbols and drawing lines between consecutive numbers) were the neurocognitive ability areas that were most closely linked to poorer RWF in the AEH cohort. Interestingly, deficits in learning and SIP have been among the most commonly reported in prior AEH cohorts (e.g., Weber et al. 2013a; Wang et al. 2011). Deficits in these domains could additionally confer elevated risk for problems in other RWF outcomes not measured here, such as engagement in HIV transmission risk behaviors; for example, learning dysfunction could preclude an individual from adequately acquiring psychoeducation regarding HIV prevention, whereas SIP dysfunction may manifest itself as risky decision making in rapidly evolving social situations (see Blackstone et al. 2013a). Future studies may wish to delineate such domains into their component processes (e.g., learning = encoding, maintenance, recency/primacy effects; SIP = psychomotor speed, visual search and sequencing) in order to investigate specific mechanisms of impairment that could be driving RWF dependence (see Woods et al. 2009a). Additionally, it may prove useful to focus neurocognitive rehabilitation efforts on improving such cognitive abilities, as improvement could confer better functioning outcomes and/or reduced risk of disease transmission (see Weber et al. 2013b).
Findings from this study also suggest that lifetime psychiatric and/or mood disorders may play an important role in adverse functional outcomes in AEH. These non-HIV-related comorbidities are highly prevalent within AEH (Weber et al. 2013a; Moore et al. 2011; Plankey et al. 2007), which is of considerable clinical concern given their clear detrimental influence on successful RWF functioning of this group. While these neuropsychiatric factors did not trump the effect of NCI on functional status in this study, histories of lifetime non-alcohol dependence and mood disorder were nevertheless themselves unique predictors of global RWF status with the AEH group. Post hoc analyses revealed that lifetime histories of non-alcohol dependence were driven by high rates of MA and cannabis dependence, while histories of mood disorder were driven by increased diagnoses of Bipolar Disorder. In concordance with these post hoc findings, recent studies have also reported deleterious additive effects of MA and HIV, which are highly comorbid, on neurocognitive deficits (Weber et al. 2012) and daily functioning outcomes (Blackstone et al. 2013b). Similarly, Bipolar Disorder occurs at higher rates in HIV, and there are several studies elucidating its role in functional decline within HIV cohorts (e.g., Moore et al. 2012; Blackstone et al. 2012a). However, as Bipolar Disorder is highly complex and multifactorial (e.g., Pacchiarotti et al. 2013), it remains to be seen which aspects of the disorder may be most involved in functional impairment (e.g., irritability, dysphoria, hedonism, and activation). Nonetheless, these results are consistent with previous research identifying the detrimental effect of negative neuropsychiatric factors on RWF outcomes in HIV, including global functional impairment, ARV nonadherence, increased risk behaviors, and lower HRQoL (Blackstone et al. 2013b; Avants et al. 2000; Arnsten et al. 2001; Doyle et al. 2012). Further exploration of other aspects of neuropsychiatric distress, including for example, apathy (Kamat et al. 2012), may be worthwhile. Such efforts are of potential clinical value as many of these common comorbidities are remediable (Sherr et al. 2011; Whetten et al. 2006), and thus early detection and intervention may mitigate RWF problems for individuals with AEH.
A unique aspect of this AEH study was the lack of associations observed between RWF outcomes and HIV disease severity. In chronically infected samples, poorer functional status reliably corresponds to AIDS status and lower nadir CD4 counts (e.g., Heaton et al. 2004a). However, AEH samples have ostensibly not suffered CD4 declines to the level at which one might expect them to carry real-world complications. Findings also diverge from Weber et al. (2013a), who reported that higher plasma RNA levels were correlated with NCI in AEH. Nevertheless, Wilson and Cleary’s (1995) influential model of disability suggests that such biomarkers are several stages “upstream” from RWF, which are more closely linked to symptoms (e.g., neurocognitive deficits) that may arise from biological influences. It is also possible that the lack of such associations is an artifact of the relatively small sample size of the present study.
Despite these interesting findings, this study has several limitations that warrant consideration. First, given that the AEH population is relatively small and difficult to recruit as compared to the chronically infected population, our AEH sample size was limited (n = 34), and future studies would benefit from obtaining a larger, well-characterized AEH sample. Second, the majority of indices used in our global functional status variable were gathered from self-report measures, which can be biased by mood and social desirability factors (Blackstone et al. 2012b). Future research would be enhanced by the use of objective functional measures, such as laboratory performance-based assessments (e.g., financial management), health literacy, and electronic medication adherence tracking, a functional outcome which is likely very relevant within this population given recent studies showing the potential benefits of early antiretroviral therapy. A third limitation of the current study is its cross-sectional nature; given the highly dynamic nature of the AEH period, it may prove advantageous to conduct well-controlled longitudinal research in order to investigate the relationships between NCI, neuropsychiatric factors, and functional status over time, especially given the highly variable trajectories of each.
Collectively, these findings indicate significant levels of NCI-driven RWF impairment during the early period of HIV infection. Previous HIV-associated neurocognitive and RWF outcomes research has been broadly focused on chronically infected individuals, and only recently has the AEH period of infection been garnering research attention within these areas. Although this is only the first study to examine RWF impairment and the role of NCI in AEH, these findings may inform rehabilitation efforts targeted towards AEH individuals who are suffering from functional decline. Furthermore, it is reasonable to posit that the combination of NCI and RWF impairment in these early stages of infection could not only represent risk for current adverse consequences (i.e., transmission), but also future consequences, including continued neuropsychiatric problems (e.g., substance use, mood disorders), exacerbated cognitive and functional decline, and lower HRQoL. As such, starting to initiate neurocognitive rehabilitation efforts so early on in the disease process may prove to be of great benefit to the AEH population both in the early and later stages of disease.
References
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn. Washington, DC
An SF, Groves M, Gray F, Scaravilli F (1999) Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J Neuropathol Exp Neurol 58(11):1156–1162
Anand P, Springer SA, Copenhaver MM, Altice FL (2010) Neurocognitive impairment and HIV risk factors: a reciprocal relationship. AIDS Behav 14(6):1213–1226
Ances BM, Sisti D, Vaida F, Liang CL, Leontiev O, Perthen JE, HNRC Group (2009) Resting cerebral blood flow: a potential biomarker of the effects of HIV in the brain. Neurology 73(9):702–708
Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Wojna VE (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69(18):1789–1799
Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Chang CJ, Schoenbaum EE (2001) Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis 33(8):1417–1423
Atkinson JH, Higgins JA, Vigil O, Dubrow R, Remien RH, Steward WT, Grant I (2009) Psychiatric context of acute/early HIV infection. The NIMH Multisite Acute HIV Infection Study: IV. AIDS Behav 13(6):1061–1067
Avants SK, Warburton LA, Hawkins KA, Margolin A (2000) Continuation of high-risk behavior by HIV-positive drug users. Treatment implications. J Subst Abuse Treat 19(1):15–22
Blackstone K, Moore DJ, Heaton RK, Franklin DR Jr, Woods SP, Clifford DB, CNS HIV Antiretroviral Therapy Effects Research (CHARTER) Group (2012a) Diagnosing symptomatic HIV-associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. J Int Neuropsychol Soc 18(1):79–88
Blackstone K, Tobin A, Posada C, Gouaux B, Grant I, Moore DJ, The HIV Neurobehavioral Research Program (HNRP) (2012b) HIV-infected persons with bipolar disorder are less aware of memory deficits than HIV-infected persons without bipolar disorder. J Clin Exp Neuropsychol 34(7):773–781
Blackstone K, Iudicello JE, Morgan EE, Weber E, Moore DJ, Franklin DR, Grant I, Woods SP, the Translational Methamphetamine AIDS Research Center Group (2013a) Human immunodeficiency virus infection heightens concurrent risk of functional dependence in persons with long-term methamphetamine use. J Addict Med 7(4):255–263
Blackstone K, Weber E, Iudicello JE, Woods SP (2013b) Real-world impact of HIV-associated neurocognitive impairment. In: Chiaravalloti N, Goverover Y (eds) Changing Brain, Changes in Daily Life. Springer, New York
Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Douek DC (2004) CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 200(6):749–759
Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, HNRC Group (2004) Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 26(3):307–319
Chelune GJ, Heaton RK, Lehman RAW (1986) Patients’ complaints of disability. Psychother Priv Pract 4(4):45–50
Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Fleming TR (2011) Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 356(6):493–505
Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Wiley CA (1992) Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology 42(9):1736–1739
Doyle K, Weber E, Atkinson JH, Grant I, Woods SP, HIV Neurobehavioral Research Program (HNRP) Group (2012) Aging, prospective memory, and health-related quality of life in HIV infection. AIDS Behav 16(8):2309–2318
Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore DJ, Bentley H, HNRC Group (2004a) The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc 10(3):317–331
Heaton RK, Miller SW, Taylor MJ, Grant I (2004b) Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults Scoring Program. Psychological Assessment Resources, Inc., Odessa, Florida
Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, HNRC Group (2010) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17(1):3–16
Kamat R, Woods SP, Marcotte TD, Ellis RJ, Grant I, HIV Neurobehavioral Research Program (HNRP) Group (2012) Implications of apathy for everyday functioning outcomes in persons living with HIV infection. Arch Clin Neuropsychol 27(5):520–531
Karnofsky DA, Burchenal JH (1949) The clinical evaluation of chemo-therapeutic agents in cancer. In: Maclead CM (ed) Evaluation of Chemotherapeutic Agents. Columbia University Press, New York, pp 191–205
Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9(3):179–186
Le T, Wright EJ, Smith DM, He W, Catano G, Okulicz JF, Ahuja SK (2013) Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med 368(3):218–230
Lentz MR, Kim WK, Lee V, Bazner S, Halpern EF, Venna N, González RG (2009) Changes in MRS neuronal markers and T cell phenotypes observed during early HIV infection. Neurology 72(17):1465–1472
Lentz MR, Kim WK, Kim H, Soulas C, Lee V, Venna N, González RG (2011) Alterations in brains metabolism during the first year of HIV infection. J Neurovirol 17(3):220–229
Marcotte TD, Heaton RK, Wolfson T, Taylor MJ, Alhassoon O, Arfaa K, Grant I (1999) The impact of HIV-related neuropsychological dysfunction on driving behavior. The HNRC Group. J Int Neuropsychol Soc 5(7):579–592
Marcotte TD, Wolfson T, Rosenthal TJ, Heaton RK, Gonzalez R, Ellis RJ, HIV Neurobehavioral Research Center Group (2004) A multimodal assessment of driving performance in HIV infection. Neurology 63(8):1417–1422
Marcotte TD, Lazzaretto D, Scott JC, Roberts E, Woods SP, Letendre S, HNRC Group (2006) Visual attention deficits are associated with driving accident in cognitively-impaired HIV-infected individuals. J Clin Exp Neuropsychol 28(1):13–28
Moore DJ, Letendre SL, Morris S, Umlauf A, Deutsch R, Smith DM, Heaton RK, Ellis RJ, Atkinson JH, Grant I, CHARTER Group (2011) Neurocognitive functioning in acute or early HIV infection. J Neurovirol 17(1):50–57
Moore DJ, Posada C, Parikh M, Arce M, Vaida F, Riggs PK, HIV Neurobehavioral Research Program (HNRP) (2012) HIV-infected individuals with co-occuring bipolar disorder evidence poor antiretroviral and psychiatric medication adherence. AIDS Behav 16(8):2257–2266
Norman MA, Moore DJ, Taylor M, Franklin D Jr, Cysique L, Ake C, HNRC Group (2011) Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J Clin Exp Neuropsychol 33(7):793–804
Pacchiarotti I, Nivoli AM, Mazzarini L, Kotzalidis GD, Sani G, Koukopoulos A, Colom F (2013) The symptom structure of bipolar acute episodes: in search for the missing link. J Affect Disord 149(1–3):56–66
Pilcher CD, Tien HC, Eron JJ Jr, Vernazza PL, Leu SY, Stewart PW, Duke-UNC-Emory Acute HIV Consortium (2004) Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infec Dis 189(10):1785–1792
Plankey MW, Ostrow DG, Stall R, Cox C, Li X, Peck JA, Jacobson LP (2007) The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr 45(1):85–92
Schag CC, Heinrich RL, Ganz PA (1984) Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 2(3):187–193
Sherr L, Clucas C, Harding R, Sibley E, Catalan J (2011) HIV and depression – a systematic review of interventions. Psychol Health Med 16(5):493–527
Wang X, Foryt P, Ochs R, Chung JH, Wu Y, Parrish T, Ragin AB (2011) Abnormalities in resting-state functional connectivity in early human immunodeficiency virus infection. Brain Connect 1(3):207–217
Weber E, Blackstone K, Iudicello JE, Morgan EE, Grant I, Moore DJ, Translational Methamphetamine AIDS Research Center (TMARC) Group (2012) Neurocognitive deficits are associated with unemployment in chronic methamphetamine users. Drug Alcohol Depend 125(1–2):146–153
Weber E, Blackstone K, Woods SP (2013a) Cognitive neurorehabilitation of HIV-associated neurocognitive disorders: a qualitative review and call to action. Neuropsychol Rev 23(1):81–98
Weber E, Morgan EE, Iudicello JE, Blackstone K, Grant I, Ellis RJ, TMARC Group (2013b) Substance use is a risk factor for neurocognitive deficits and neuropsychiatric distress in acute and early HIV infection. J Neurovirol 19(1):65–74
Whetten K, Reif S, Ostermann J, Pence BW, Swartz M, Whetten R, Eron J (2006) Improving health outcomes among individuals with HIV, mental illness, and substance use disorders in the Southeast. AIDS Care 18(Suppl 1):S18–S26
Wilkinson GS, Robertson GJ (2006) Wide range achievement test-4: Professional manual. Psychological Assessment Resources, Inc., Lutz, Florida
Wilson IB, Cleary PD (1995) Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA 273(1):59–65
Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, Heaton RK (2004) Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol 26(6):759–778
Woods SP, Iudicello JE, Moran LM, Carey CL, Dawson MS, Grant I, HIV Neurobehavioral Research Center Group (2008) HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology 22(1):110–117
Woods SP, Dawson MS, Weber E, Gibson S, Grant I, Atkinson JH, HIV Neurobehavioral Research Center Group (2009a) Timing is everything: antiretroviral nonadherence is associated with impairment in time-based prospective memory. J Int Neuropsychol Soc 15(1):42–52
Woods SP, Moore DJ, Weber E, Grant I (2009b) Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev 19(2):152–168
Woods SP, Weber E, Weisz BM, Twamley EW, Grant I, HIV Neurobehavioral Research Programs Group (2011) Prospective memory deficits are associated with unemployment in persons living with HIV infection. Rehabil Psychol 56(1):77–84
World Health Organization (1998) Composite International Diagnostic Interview (CIDI, Version 2.1). World Health Organization, Geneva, Switzerland
Acknowledgments
The Translational Methamphetamine AIDS Research Center (TMARC) group is affiliated with the University of California, San Diego (UCSD) and the Sanford-Burnham Medical Research Institute. The TMARC is comprised of: Director: Igor Grant, M.D.; Co-Directors: Ronald J. Ellis, M.D., Ph.D., Cristian Achim, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Steven Paul Woods, Psy.D.; Aaron Carr (Assistant Center Manager); Clinical Assessment and Laboratory Core: Scott Letendre, M.D. (P.I.), Ronald J. Ellis, M.D., Ph.D., Rachel Schrier, Ph.D.; Neuropsychiatric Core: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Mariana Cherner, Ph.D., Thomas Marcotte, Ph.D.; Neuroimaging Core: Gregory Brown, Ph.D. (P.I.), Terry Jernigan, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D., Miriam Scadeng, Ph.D., Christine Fennema-Notestine, Ph.D., Sarah L. Archibald, M.A.; Neurosciences and Animal Models Core: Cristian Achim, M.D., Ph.D., Eliezer Masliah, M.D., Stuart Lipton, M.D., Ph.D.; Participant Unit: J. Hampton Atkinson, M.D., Rodney von Jaeger, M.P.H. (Unit Manager); Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D., Clint Cushman (Unit Manager); Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D., Reena Deutsch, Ph.D., Anya Umlauf, M.S.; Project 1: Arpi Minassian, Ph.D. (P.I.), William Perry, Ph.D., Mark Geyer, Ph.D., Brook Henry, Ph.D.; Project 2: Amanda B. Grethe, Ph.D. (P.I.), Martin Paulus, M.D., Ronald J. Ellis, M.D., Ph.D.; Project 3: Sheldon Morris, M.D., M.P.H. (P.I.), David M. Smith, M.D., M.A.S., Igor Grant, M.D.; Project 4: Svetlana Semenova, Ph.D. (P.I.), Athina Markou, Ph.D.; Project 5: Marcus Kaul, Ph.D. (P.I.).The authors report no conflicts of interest. This research was supported by National Institutes of Health grants P50-DA026306, P30-MH62512, T32-DA31098, DA-034510, F31-DA035708, AI-090970, AI-100665, AI-080353, MH-097520, DA-034978, MH-83552, MH-62512, AI-74621, AI-43638, AI-36214, TW-008908, AI-69432, AI-096113, and AI-47745. This work was additionally supported by the Department of Veterans Affairs and grants awarded from the International AIDS Vaccine Initiative (IAVI), the National Science Foundation DMS0714991, and the James B. Pendleton Charitable Trust. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Doyle, K.L., Morgan, E.E., Morris, S. et al. Real-world impact of neurocognitive deficits in acute and early HIV infection. J. Neurovirol. 19, 565–573 (2013). https://doi.org/10.1007/s13365-013-0218-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-013-0218-2