Abstract
Most multivoltine insects in temperate zones enter diapause in response to short days. The photoperiod is evaluated in these organisms by a photoperiodic time measurement system, which involves the circadian clock, and activates or inactivates endocrine organs or cells to alter their physiological status. Although the physiological mechanisms underlying insect photoperiodism have been extensively studied, the molecular linkage between the circadian clock and endocrine signaling pathways remains unclear. In this study, we evaluated the bean bug Riptortus pedestris (F.) (Hemiptera: Alydidae), which enters adult (reproductive) diapause in response to short days. A gene encoding the insulin-like peptide ILP1, which is expressed in the pars intercerebralis in the brain, was upregulated and involved in fecundity under long days. Ilp1 appeared to function independently of the photoperiodic response controlled by juvenile hormone signaling. Cyp15, which encodes an epoxidase crucial for juvenile hormone biosynthesis, was upregulated and involved in ovarian development under long days. RNA interference targeted against the circadian clock gene per canceled the Ilp1 and Cyp15 suppression and allowed females to be reproductive even under diapause-inducing short days. Thus, the circadian clock may control the photoperiodic response by altering the expression of key elements in two independent endocrine pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photoperiodism is the ability to respond to photoperiods and enables organisms to coordinate their physiological status with annual changes in their biotic and abiotic environments (Danks 1987; Tauber et al. 1986). The most well-known photoperiodic event among insects is diapause, during which development or reproduction is suppressed or arrested and metabolic activity is extensively reduced. Diapause at the egg, larval (or nymphal), and pupal stages is characterized by developmental arrest or suspension, whereas the central feature of diapause at the adult stage is the cessation of reproduction (Denlinger 2022). For example, adults of the bean bug Riptortus pedestris (F.) (Hemiptera: Alydidae) allocate energy resources to the reproductive organs to develop these organs for reproductive activities under summer long-day conditions. By contrast, under autumnal short-day conditions, adults accumulate lipids and suppress reproductive organ development for overwintering (Morita et al. 1999; Numata and Hidaka 1982).
Insects assess day or night length using a photoperiodic time measurement system, which is a function of the circadian clock (Nelson et al. 2009). The circadian clock is a biological time-keeping system that controls organismal rhythms over approximately 24 h and is established by a dozen of circadian clock genes, including period (per), mammalian-type cryptochrome (cry-m), Clock (Clk), cycle (cyc), timeless, PAR-domain protein 1, vrille, and clockwork orange (Patke et al. 2020; Tomioka and Matsumoto 2019). Knockdown or knockout of circadian clock genes disrupts photoperiodic responses in various insect species (Goto and Nagata 2022; Ikeda et al. 2021; Meuti et al. 2015; Mukai and Goto 2016; Tamai et al. 2019; Zhu et al. 2019). For example, knockdown of per and cry-m induces ovarian development even under diapause-inducing short-day conditions in R. pedestris. By contrast, knockdown of cyc and Clk suppressed ovarian development even under diapause-averting long-day conditions (Ikeno et al. 2010, 2011a, b, 2013). Thus, the circadian clock composed of these circadian clock genes may be involved in photoperiodic time measurement (Numata et al. 2015).
Insect diapause is regulated by ecdysteroids, juvenile hormone (JH), diapause hormone, and insulin-like peptides (ILPs). Among them, JH and ILPs play major roles in adult diapause (Denlinger 2022). JH is an acyclic sesquiterpenoid that is synthesized in the endocrine organ, the corpus allatum (CA), and by a series of JH biosynthetic enzymes such as JH acid methyltransferase (JHAMT) and cytochrome P450 15 (CYP15) (Goodman and Cusson 2012). Analysis of the photoperiodic control of the JH titer, JH biosynthesis in the CA, and expression of JH biosynthetic enzymes revealed that inactivation of CA leads to a low JH hemolymph titer to induce adult diapause (Hejníková et al. 2022; de Kort et al. 1982; Larrere et al. 1993; Matsumoto et al. 2013; Okuda et al. 1996; Rankin and Riddiford 1978; Readio et al. 1999). Additionally, in R. pedestris, allatectomy suppressed reproduction even under diapause-averting long-day conditions (Morita and Numata 1997). Furthermore, topical application of JH III skipped bisepoxide (JHSB3), the JH of this species, and a JH analog induced expression of Vitellogenin-1 (Vg-1) and the hexameric yolk protein gene Cyanoprotein-α (CP-α) in the fat body as well as ovarian development (Ando et al. 2020; Hirai et al. 1998; Miura et al. 1998). Thus, JH causally regulates diapause in R. pedestris.
Studies of the mosquito Culex pipiens provided strong evidence that insulin signaling is also a pivotal component regulating photoperiodic adult diapause (Sim and Denlinger 2008, 2009; Sim et al. 2015). In Drosophila melanogaster, insulin signaling, initiated in insulin-producing cells in the brain, controls the insulin cascade in the CA, ultimately leading to ovarian development by stimulating JH biosynthesis (Kubrak et al. 2014; Ojima et al. 2018; Schiesari et al. 2016). Apart from the photoperiodic response, an axis from ILPs to vitellogenin gene expression through JH has been proposed in the red flour beetle Tribolium castaneum (Sheng et al. 2011). ILPs are expressed in the dorsocentral part of the brain (pars intercerebralis, PI) in various insect species (Barberà et al. 2019; Broughton et al. 2005; Cuti et al. 2021; Goltzené et al. 1992; Mizoguchi et al. 1987; Riehle et al. 2006; Vafopoulou and Steel 2012; Xu et al. 2015). Surgical removal of the PI disrupted photoperiodic ovarian development, supporting a crucial role for ILPs in insect photoperiodism (Hodková 1976; Hodková and Okuda 2019; Poras 1982; Shiga and Numata 2000).
However, the role of ILPs in R. pedestris would be different from these species. Surgical removal of the PI did not affect photoperiodic regulation of ovarian development in R. pedestris, although fecundity was affected under long-day conditions (Shimokawa et al. 2008, 2014). Silencing of genes encoding two ILPs, Ilp1 and Ilp2, affected fecundity but not ovarian development (Hasebe and Shiga 2021). Alternatively, ovarian development is regulated by JH (Ando et al. 2020; Morita and Numata 1997). These results suggest that the ILP signaling pathway does not reside upstream of the JH signaling pathway, in contrast to that in C. pipiens and D. melanogaster, rather these pathways independently regulate photoperiodic responses under the control of the photoperiodic time measurement system in R. pedestris. However, the photoperiodic regulation of ILPs, location of ILP-producing cells in the brain, and molecular linkage between the circadian clock and ILP and JH signaling pathways are unclear. Thus, an overall understanding of the molecular mechanisms underlying photoperiodism is lacking.
In this study, we examined the relationship between the ILP and JH signaling pathways in the photoperiodic response. We investigated the photoperiodic and temporal regulation of Ilp1 and Ilp2, location of cells expressing ILP1, and function of ILP1 in the photoperiodic response using RNA interference (RNAi). We also evaluated the molecular linkage between the circadian clock and two endocrine pathways. Many studies have shown that the circadian clock is involved in the photoperiodic induction of diapause; however, how the clock affects the endocrine effector is not well understood. To evaluate this, we investigated the photoperiodic and temporal regulation of Cyp15 and jhamt and the hemolymph JH titer. The function of Cyp15 in photoperiodic ovarian development was also investigated. Furthermore, we examined the effect of RNAi targeted to per on ovarian development, expression of Ilp1 and Cyp15, and hemolymph JH titer.
Materials and methods
Insects
Colonies of R. pedestris were established using individuals captured in Osaka, Japan (34.59°N, 135.51°E) in 2018–2021. Their progeny (G1–G3) were reared in a cylindrical cage (15 cm high and 15 cm diameter) in groups from the egg stage under diapause-inducing short-day (12 h light and 12 h dark; SD) conditions at 25 ± 1 °C. Within 24 h after adult emergence, the females were individually separated into small plastic cases (4 cm high and 10 cm diameter) and maintained under diapause-averting long-day (16 h light and 8 h dark; LD) conditions or were continuously kept under SD conditions. The insects were supplied with water containing 0.05% sodium ascorbate and 0.025% l-cysteine (w/v), soybean grain, and red clover seeds (Kamano 1991). Day 0 was defined as the day of adult emergence.
Gene identification
We obtained the Ilp1, Ilp2, jhamt, and Cyp15 sequences from the R. pedestris RNA-seq data (PRJDB7548 and PRJDB10569). The amino acid sequences of DILP2, DILP6, and JHAMT of D. melanogaster, Bombyxin A-1 and Bombyx IGF-like peptide (BIGFLP) from Bombyx mori, and Cyp15 A-1 of Diploptera punctata were used as queries in tBLASTn searches with the Bioedit program (Hall 1999). The accession numbers of these query sequences and sequences of R. pedestris are shown in Table 1.
ILPs can be divided into three classes based on their homology and C-peptide domain lengths estimated from the positions of two potential dibasic cleavable sites (Grönke and Partridge 2010; Mizoguchi and Okamoto 2013). To distinguish the classes, the amino acid sequences of ILP1 and ILP2 of R. pedestris were compared with those of the above-mentioned ILPs using ClustalW (Thompson et al. 1994). The signal peptide was estimated by signalP 5.0 (https://services.healthtech.dtu.dk/service.php?SignalP-5.0).
RT-qPCR
Reverse-transcription quantitative PCR (RT-qPCR) was performed to estimate the relative amount of target mRNA in the whole head with the prothorax (WH + PT), dorsocentral part of the brain (PI), brain and subesophageal ganglion without the PI (Br + SOG-PI), corpora cardiaca and CA with a part of the aorta (CC + CA), and fat body. Samples were collected at ZT 6–12. Total RNA was extracted using Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA) and treated with deoxyribonuclease (RT-grade) for Heat stop (NIPPON GENE, Toyama, Japan). cDNA was synthesized using a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA). Quantitative PCR was performed using a CFX connect™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) according to the standard curve method with Go Taq qPCR Master Mix (Promega, Madison, WI, USA) and the primer sets shown in Table 2. β-Tubulin (tub) was used as a reference gene for normalization (Ikeno et al. 2011b).
Immunohistochemistry
We prepared a rabbit polyclonal antibody against a nonapeptide (CGGSYNSPF), which is the C-terminal region of the ILP1 B-chain of R. pedestris (Cosmo Bio, Tokyo, Japan). The antibody was diluted by 1:1000 in 10% normal goat serum in phosphate-buffered saline (PBS) containing 0.1% Triton X-100 (PBSTx). A goat anti-rabbit IgG H&L conjugated with Alexa Fluor® 488 (Abcam, Cambridge, UK) was used as a secondary antibody at a 1:500 dilution in 10% normal goat serum in PBSTx.
The whole brains were dissected from adult females on day 13 in ice-cold PBS and fixed with 4% paraformaldehyde in PBSTx overnight. The antigen was retrieved in citrate buffer (pH 6.0) at 95 °C for 10 min and cooled at room temperature (18–25 °C) for 10 min. After washing in PBSTx and preincubation in 10% normal goat serum, the brain samples were incubated with primary antisera solution for 7 days at 4 °C. The brains were washed in PBSTx, preincubated in 10% normal goat serum, and incubated with the secondary antibody solution for 7 days at 4 °C. The brains were then washed with PBSTx and incubated with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) at a final concentration of 100 ng/μL in PBS overnight. For fluorescence microscopy, the brains were dehydrated using an ethanol series (70%, 80%, 90%, 99%, and 100%) and cleared in methyl salicylate. The fluorescence of the samples was detected using confocal laser microscopy (DM6000CS, Leica Microsystems, Wetzlar, Germany) and analyzed with LAS X software (Leica Microsystems). The number of ILP1 immunoreactive (ILP1-ir) cells was estimated by counting the DAPI-stained nuclei in the ILP1-ir fluorescence images. Antibody specificity was confirmed in a preabsorption test, in which the primary antibody solution was preabsorbed with the synthesized nonapeptide at a final concentration of 10 μg/mL overnight at 4 °C.
RNA interference
RNAi-mediated gene silencing was performed by double-stranded (ds) RNA injection. Total RNA was extracted from adult females as described above. cDNA was synthesized with oligo (dT)12–18 primer and M-MLV reverse transcriptase (Thermo Fisher Scientific). The DNA template for dsRNA synthesis was amplified using the primers shown in Table 2 and Pwo Super Yield DNA polymerase (Roche, Basel, Switzerland). A T7 RiboMAX™ RNAi system (Promega) was used to synthesize the dsRNAs of per, Cyp15, and Ilp1 according to the manufacturer’s instruction. On day 0, the adults were anesthetized on ice, and their heads were injected with 1 μL of dsCyp15 (5 μg/μL), dsper (1 μg/μL), or dsIlp1 (1 μg/μL) RNA. The dsRNA of bacterial β-lactamase (bla), a control gene, was also synthesized and injected at the same concentration (1 or 5 μg/μL).
Fecundity and ovarian development
For the fecundity assay, adults were individually transferred to LD conditions on day 0. A single female and two males were placed in a single case on day 7, and the number of eggs deposited was recorded daily from days 8 to 20.
For ovarian development, adult females were dissected under a stereoscopic microscope and their ovaries were checked on day 13 or 20. The ovarian status was evaluated as described by Numata and Hidaka (1982): no oocytes as stage 0, one oocyte as stage I, two transparent oocytes as stage II, light-blue yolk deposition in the basal oocytes as stage III, light-blue yolk deposition in two oocytes as stage IV, and post-ovulation as stage V. Females in stages 0–II were considered to be in diapause and those in stages III–V were considered as non-diapause (Numata and Hidaka 1982).
Hemolymph JH quantification
To collect the hemolymph, a decapitated adult female was placed head-down in a perforated 0.5 mL tube inserted in a 1.5 mL silicon-coated tube. The tube was centrifuged at 100 × g for 5 min at 4 °C, and the collected hemolymph was stored at -80 °C until use.
Hemolymph sample preparation and ultra-performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) analysis were performed as described by Ando et al. (2020) and Lee et al. (2019). Briefly, 16–20 μL of hemolymph from 4 to 18 individuals was added to 200 pg of synthetic JH III (internal control; Sigma-Aldrich, St. Louis, MO, USA). The sample was mixed with 500 μL of methanol and incubated for 5 min on ice. After adding 500 μL of 2% NaCl solution (w/v), the sample was extracted with 200 μL of hexane, and the supernatant was transferred to another tube after centrifugation at 3000 × g for 5 s. This process was repeated 3 times. The pooled extract was dried in a centrifugal evaporator (CVE-2200, EYELA, Tokyo, Japan). The sample was suspended in 20 μL of methanol and transferred to a dedicated tube (Autosampler vial #186000385C, Waters, Milford, MA, USA). This process was repeated 2 times.
The UPLC–MS/MS (ACQUITY UPLC H-Class, Xevo TQ-S micro, Waters) and a C18 column (ACQUITY UPLC BEH C18 Column, 2.1 × 100 mm, 1.7 µm particle size, Waters) were used to detect JHSB3 and JH III with MassLynks software (Waters). The solvent for the C18 column was water: methanol = 2:8, and the flow rate was 0.2 mL/min. MS/MS analysis revealed the fragment ions of JHSB3 and JH III at m/z = 283.2 > 233.2 and m/z = 267.3 > 43.0, respectively (Ando et al. 2020). The hemolymph JHSB3 concentration was corrected according to the peak area of the control JH III.
Statistical analysis
The statistical analysis was conducted in R Studio Version 2022.2.0.443 and R version 4.1.2.
Results
ILP classes
tBlastn searches detected two Ilp sequences, Ilp1 and Ilp2. Insect ILPs can be divided into 3 classes, i.e., ILP (sensu stricto), IGFLP, and DILP7-like (Grönke and Partridge 2010). To determine the classes of Ilp1 and Ilp2, their amino acid sequences and domain structures were compared with those of ILPs from other insect species (Fig. 1). The R. pedestris ILP1 amino acid sequence contained a long C-peptide, which is characteristic of members of the ILP (sensu stricto) class including DILP2 and Bombyxin A-1 (Fig. 1, upper panel). The positions of cysteine residues and dibasic cleavable sites were well conserved among species. The R. pedestris ILP2 amino acid sequence contained a short C-peptide, which is characteristic of members of the IGFLP class including DILP6 and BIGFLP (Fig. 1, lower panel). The positions of the cysteine residues were also conserved.
Structures and amino acid sequences of ILPs (sensu stricto) (upper panel) and IGFLPs (lower panel). Rped ILP1 and Rped ILP2 are from Riptortus pedestris, DILP2 and DILP6 are from Drosophila melanogaster, and Bombyxin A-1 and BIGFLP are from Bombyx mori. Predicted domains of the A- and B-chains, C-peptide, and signal peptide (SP) are shown in boxes. “S–S” indicates the disulfide bond. Asterisks indicate conserved amino acid residues. Potential cleavage sites estimated by dibasic amino acids are shown with shaded letters. Italicized “C” indicates conserved cysteine residue. For accession numbers, see Table 1
Expression of Ilp1 and Ilp2
We investigated the photoperiodic and temporal changes in Ilp expression (Fig. 2a). The relative amount of Ilp1 mRNA gradually increased from days 5 to 13 under LD conditions (4.1-fold increase compared to the median), whereas no temporal change was detected under SD conditions. The amount of Ilp1 mRNA was significantly higher in LD than in SD on day 13 (3.1-fold increase compared to the median; Student's t test, P < 0.05), but not on other days (Student's t test, P > 0.05). The relative amounts of Ilp2 mRNA did not change temporally and photoperiodically (day 5; Welch's t test, days 9 and 13; Student's t test, P > 0.05). RT-qPCR analysis revealed that Ilp1 was highly expressed in the PI (Fig. 2b).
Expression profiles of Ilp1 and Ilp2. a Amounts of Ilp1 and Ilp2 mRNAs in the whole head with the prothorax (WH + PT) of virgin adult females on days 5, 9, and 13 under short-day (SD) and long-day (LD) conditions. n = 6–7. *, The Student’s t test (P < 0.05). b Ilp1 mRNA amounts in the brain and subesophageal ganglion without the pars intercerebralis (Br + SOG-PI) and pars intercerebralis (PI) on day 13 under LD conditions. n = 8. Each plot indicates a sample from a single individual. The highest value was set at 1.0. The middle line in the box indicates the median, and the upper and lower lines of the box indicate the quartile
Immunohistochemical staining verified specific ILP1 expression in the PI; the antibody against R. pedestris ILP1 detected ILP1-ir cells in the dorsocentral part of the brain (Fig. 3a–c). Absorption control experiment for the ILP1 antibody revealed autofluorescence only in the retina, confirming the specificity of the ILP1 antibody (data not shown). The most frequent number of ILP1-ir cells was six in each hemisphere and was identical between photoperiodic conditions (Fig. 3d).
Immunofluorescence of ILP1 in the whole brain of adult female Riptortus pedestris. a ILP1 immunoreactive(ir)-cells in dorsal view of the whole brain. b Schematic drawing of a. c Enlarged photo inside of the dashed line in a. Twelve ILP1-ir cells were detected (arrowheads). d Number of ILP1-ir cells detected in the left (L) and right (R) brain hemispheres under short-day (SD) and long-day (LD) conditions. n = 5–7
Effects of Ilp1 RNAi on egg number and yolk protein expression
Injection of dsIlp1 effectively downregulated Ilp1 expression (91.3% reduction compared to the median; Welch's t test, P < 0.05) (Fig. 4a). Control females laid an average of 2.64–5.55 eggs every day. Females injected with dsIlp1 also laid eggs but their daily number was 1.77–4.08, which was significantly smaller than the number of control females (two-way ANOVA, P < 0.05) (Fig. 4b). Ilp1 RNAi did not affect the expression of the yolk proteins Vg-1 and CP-α in the fat body (Fig. 4c; Student's t test, P > 0.05), suggesting that the low fecundity of Ilp1 RNAi females was not caused by downregulation of yolk proteins.
Effects of Ilp1 dsRNA injection on Ilp1 mRNA amounts (a) number of eggs deposited (b), and Vg-1 and CP-α mRNA amounts (c) in adult females of Riptortus pedestris under long-day conditions. a Ilp1 mRNA amounts in the whole head with the prothorax (WH + PT) on day 20 are shown. Each plot indicates a sample from a single individual. An asterisk indicates a significant difference (Welch's t test, P < 0.05). n = 11–13. b Number of eggs deposited each day. Females were individually reared with two males, and the number of eggs was counted every day. Closed and open circles indicate the mean egg numbers in dsbla- (n = 11) and dsIlp1-injected (n = 13) females, respectively. The error bars indicate the standard errors. Two-way ANOVA revealed a significant difference between dsbla- and dsIlp1-injected females (P = 0.0075) but not among days (P = 0.427). No interaction was detected (P = 0.988). c Vg-1 and CP-α mRNA amounts in the fat body on day 20. Each plot indicates a sample from a single individual. n = 11–13. n.s., no significant difference (Student's t test, P > 0.05). The middle line in the box indicates the median, and the upper and lower lines of the box indicate the quartile in a and c
Hemolymph JHSB3 concentration and Cyp15 and jhamt expression
The photoperiodic and temporal profiles of JHSB3 concentrations in the hemolymph are shown in Fig. 5a. The JHSB3 concentration increased under LD conditions (two-way ANOVA, P < 0.05). The JHSB3 concentration increased from days 9–13 (3.6-fold increase compared to the average), but decreased on day 20 under LD conditions. By contrast, the JHSB3 concentration remained low under SD conditions, irrespective of the days after adult emergence (Fig. 5a).
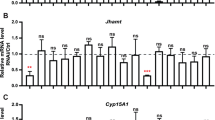
Hemolymph JHSB3 concentration and Cyp15 and jhamt expression profiles. a Hemolymph JHSB3 concentrations on days 9, 13, and 20 under short-day (SD) and long-day (LD) conditions. Each plot was obtained from 20 μL of hemolymph. n = 2. Two-way ANOVA revealed a significant difference between SD and LD (P < 0.05). b Amounts of Cyp15 and jhamt mRNAs in the whole head with the prothorax (WH + PT) of virgin adult females on days 5, 9, and 13 under short-day (SD) and long-day (LD) conditions. n = 6–7. A significant difference between SD and LD was detected in Cyp15 (Welch's t test, P < 0.05), but not in jhamt (Student's t test, P > 0.05). c Amounts of Cyp15 mRNA in the brain and subesophageal ganglion without the pars intercerebralis (Br + SOG-PI), pars intercerebralis (PI), and corpus allatum with the corpora cardiaca (CC + CA). n = 8. Each plot indicates a sample from a single individual in b and c. The highest value was set at 1.0, and the middle line in the box indicates the median and the upper and lower lines indicate the quartile in b and c
We further investigated temporal and photoperiodic regulation of Cyp15 and jhamt. Under LD conditions, Cyp15 expression increased from days 5 to 9 and remained high on day 13. The amounts were consistently higher under LD than SD on all dates (3.3–6.8-fold increase compared to the median; Welch's t test, P < 0.05) (Fig. 5b). The relative amounts of jhamt mRNA gradually increased from days 5–13, with no or little photoperiodic change (Student's t test, P > 0.05) (Fig. 5b). Cyp15 was highly expressed in the CC–CA complex (Fig. 5c).
Effects of Cyp15 RNAi on ovarian development
Cyp15 mRNA amounts were significantly lower in females injected with dsCyp15 than in control (dsbla-injected) females on day 13 under LD conditions (74% reduction compared to the median) (Fig. 6a, Student's t test, P < 0.05). Ovarian development was affected by Cyp15 RNAi; the proportion of females that had ovulated mature eggs (stage V) was 88.0% in the dsbla-injected group, whereas that in the dsCyp15 group was only 42.4% (Fig. 6b), which was a significant difference (Mann–Whitney U test; P < 0.05).
Effects of Cyp15 dsRNA injection on Cyp15 mRNA amounts (a) and ovarian development (b) in virgin adult females of Riptortus pedestris under long-day conditions on day 13. a Cyp15 mRNA amounts in the whole head with the prothorax (WH + PT). Each plot represents a sample from a single individual. n = 5–8. The middle line in the box indicates the median, and the upper and lower lines indicate the quartile. An asterisk indicates a significant difference (Student's t test, P < 0.05). b Ovarian stages. n = 25–33. The asterisk indicates a significant difference (Mann–Whitney U test, P < 0.001)
Effects of per RNAi on Ilp1 and Cyp15 expression
We focused on the role of the circadian clock in endocrine regulation. Injection of dsper weakly but significantly reduced per mRNA amounts (24% reduction compared to the median) (Fig. 7a, Welch's t test, P < 0.05). RNAi targeted to per increased the number of vitellogenic females (Fig. 7b, Fisher's exact test, P < 0.05). RNAi targeted to per also significantly induced Ilp1 and Cyp15 expression on day 20 under SD conditions (1.9- and 4.8-fold increment compared to the median, respectively) (Fig. 7c; Student's t test, P < 0.05), suggesting that these genes reside downstream of the circadian clock. Upregulation of Cyp15 via per RNAi was reflected as an increment in hemolymph JHSB3 concentrations (Fig. 7d, Welch's t test, P = 0.089).
Effect of per dsRNA injection on per mRNA amount (a), ovarian development (b), Ilp1 and Cyp15 mRNA amounts (c), and hemolymph JHSB3 concentration (d) in virgin adult females of Riptortus pedestris under short-day conditions. a per mRNA in the brain with the subesophageal ganglion on day 3. Each point represents a sample from a single individual. n = 9. b Ovarian development on day 13. The closed and open columns indicate non-vitellogenic (stages 0–II) and vitellogenic (stages III–V) individuals, respectively. c Cyp15 and Ilp1 mRNAs in the head with the prothorax (WH + PT) on day 13. Each point represents a sample from a single individual. n = 11–14. d Hemolymph JHSB3 concentration on day 13. Each plot represents JHSB3 concentration from 16 μL of the hemolymph. n = 3. The middle line in the box indicates the median, and the upper and lower lines indicate the quartile in a and c. A significant difference (indicated by an asterisk) between dsbla and dsper injection was detected in a (Welch's t test, P < 0.05), b (Fisher's exact test, P < 0.05), and c (Student's t test, P < 0.05), but not in d (Welch's t test, P = 0.089)
Discussion
Both ILP (sensu stricto) and IGFLP regulate systemic growth, but their expression sites and regulatory elements differ. ILPs are mainly expressed in the PI in the brain and regulated by sugars in the hemolymph, peptides from the peripheral tissues, and neurotransmitters in the brain. By contrast, IGFLPs are mainly expressed in the fat body and regulated by nutrients such as amino acids, lipids, and carbohydrates in the hemolymph (Nässel and Broeck 2015; Okamoto and Yamanaka 2015). We found that in R. pedestris, Ilp1, which belongs to the ILP (sensu stricto) class, was downregulated under SD conditions, whereas Ilp2, which belongs to the IGFLP class, was not regulated in a photoperiodic manner. Photoperiodic regulation of ILPs was also reported in other insect species. In C. pipiens, genes encoding ILP-1 and ILP-5 are downregulated in diapausing females, but a gene encoding ILP-2 is not downregulated (Sim and Denlinger 2009). In A. pisum, Ilp1 and Ilp4 are downregulated under SD conditions, which are sexual morphs producing conditions. (Barberà et al 2019; Cuti et al. 2021). We verified that ILP1 is specifically expressed in the PI of R. pedestris. The single-cell PCR also supported ILP1 expression in the PI of this species (Hasebe and Shiga 2021).
In C. pipiens, RNAi targeted to the genes encoding insulin-like receptor and ILP-1 suppressed photoperiodic ovarian development, whereas JH III and JH analogs rescued this suppression (Sim and Denlinger 2008, 2009). In D. melanogaster, in which dormancy is regulated by JH (Kurogi et al. 2021), ovarian development was found to be more retarded in Ilp2-3- and Ilp5-deficient mutants than in control flies (Kubrak et al. 2014). An extensive genetic dissection of the insulin signaling pathway verified that ILP2 and ILP5 are key antagonists of ovarian arrest (Schiesari et al. 2016). Ojima et al. (2018) further revealed in D. melanogaster that insulin signaling, initiated in insulin-producing cells in the brain, regulates the insulin cascade in the CA to induce yolk accumulation in the egg by stimulating JH biosynthesis. Thus, in these species, ILP resides upstream of the JH biosynthesis pathway and controls the JH biosynthetic process to regulate the photoperiodic response (Kurogi et al. 2021; Sim and Denlinger 2013a); however, this is not the case in R. pedestris. The present study revealed that Ilp1 regulated fecundity, but this effect was not caused by suppression of yolk protein expression. Hasebe and Shiga (2021) also demonstrated in R. pedestris that Ilp1 is important in promoting oviposition but plays no or little role in ovarian development. Previous studies revealed that yolk protein expression and ovarian development are regulated by JH in this species (Hirai et al. 1998; Miura et al. 1998). Furthermore, surgical removal of the PI, which contains insulin-producing cells, did not affect photoperiodic ovarian development but affected fecundity in R. pedestris (Shimokawa et al. 2008, 2014). Thus, in R. pedestris, ILP1 does not reside upstream of JH signaling. The ILP and JH signaling pathways independently regulate different photoperiodic events.
Next, we focused on photoperiodic regulation of jhamt and Cyp15 in the JH biosynthesis pathway. Their important roles in the JH biosynthetic process have been verified in various insect species (Daimon et al. 2012; Helvig et al. 2004; Li et al. 2013; Marchal et al. 2011; Minakuchi et al. 2008; Niwa et al. 2008; Nouzova et al. 2021; Shinoda and Itoyama 2003). In the present study, photoperiodic regulation of jhamt was undetectable, in contrast to that of Cyp15. Cyp15 expression was suppressed under SD conditions compared to that under LD conditions, in which Cyp15 was expressed in the CC–CA complex. In addition, Cyp15 suppression was associated with a low hemolymph JHSB3 concentration under SD conditions. We also found that Cyp15 RNAi suppressed ovarian development. These results suggest that Cyp15 is the photoperiodic regulator in the JH biosynthesis cascade in the CA of R. pedestris. The roles of jhamt and Cyp15 in photoperiodic diapause have also been examined in several insect species. For example, both jhamt and Cyp15 are downregulated in diapause-destined females of the cabbage beetle Colaphellus bowringi (Tian et al. 2021). Similar results were obtained in diapausing Danaus plexippus (Zhan et al. 2011). In contrast to in R. pedestris, RNAi targeted to Cyp15 did not decrease ovarian development in C. bowringi, whereas RNAi targeted to jhamt did (Tian et al. 2021). The function of Cyp15 in the photoperiodic signaling pathway may be species-specific.
Although RNAi of circadian clock genes verified the involvement of the circadian clock in photoperiodism in various species (Goto 2022), it has been veiled how the clock regulates endocrine signaling pathways. The present study revealed that RNAi targeted to per induced Ilp1 and Cyp15 expression, boosted hemolymph JH concentrations, and finally induced ovarian development. These results indicate that the circadian clock governs the photoperiodic response by altering the expression of key elements in two endocrine pathways. In the brain of R. pedestris, two PER-ir cells are located in an anterior medial region of the medulla, which is close to the accessory medulla, and named as “lateral neuron lateral (LNl)” cells (Koide et al. 2021). Microsurgical removal of the region containing these PER-ir cells disrupted photoperiodic ovarian development, suggesting that LNl cells act as clock cells in the photoperiodic response (Ikeno et al. 2014; Koide et al. 2021). In R. pedestris, photoperiodic ovarian development is not controlled by a neuropeptide pigment-dispersing factor, but possibly by the neurotransmitter glutamate (Des Marteaux et al. 2022; Ikeno et al. 2014). The importance of glutamate as a circadian output element was previously verified in D. melanogaster (Collins et al. 2012; Guo et al. 2016). The circadian clock that includes per may use glutamate to control Cyp15 expression in the CA and regulates photoperiodic ovarian development through the hemolymph JH concentration, which requires further investigation.
Furthermore, studies are needed for neuroanatomical dissection and functional verification of the LNl cells and pars lateralis neurons, which innervate the CA and suppress CA activity (Shimokawa et al. 2008). It is also important to verify the CA regulatory factors in the pars lateralis neurons. Possible candidates would for these regulatory factors include allatotropin (Kang et al. 2014), allatostatin (Matsumoto et al. 2017; Tamai et al. 2019), myosuppressin (Miki et al. 2020), ecdysteroids, and ecdysis-triggering hormone (Guo et al. 2021). How the circadian clock regulates ILP1 expression remains unknown in R. pedestris, although other insect species provide some clues. In the kissing bug Rhodnius prolixus, both the production and axonal transport of ILPs show a daily rhythm and intimate associations with the ILP and pigment-dispersing factor axons in both the central brain and retrocerebral complex (Steel and Vafopoulou 2006; Vafopoulou and Steel 2012, 2014). The results suggest a neural connection. In D. melanogaster, a subset of circadian clock neurons, posterior dorsal neuron 1, makes synaptic contact with insulin-producing cells in the PI (Barber et al. 2016) and regulates circadian oogenesis with a neuropeptide allatostatin C (Zhang et al. 2021). In addition, two circadian output neuropeptides, pigment-dispersing factor and short neuropeptide F, synergistically inhibit reproductive dormancy, likely by modulating the activity of insulin-producing cells (Nagy et al. 2019). Studies are needed to investigate the neuroanatomy of LNl cells and PI cells expressing ILPs and the roles of these neuropeptides in fecundity in R. pedestris.
Here, we summarize the molecular cascade regulating photoperiodic adult diapause in R. pedestris, which is partially different from that in C. pipiens (Fig. 8). In C. pipiens, ILP-1 regulates reproduction under long days through two pathways; it resides upstream of the CA and regulates JH production and also regulates the physiological status of the output module directly (Readio et al. 1999; Sim and Denlinger 2008, 2009, 2013a, b). The circadian clock genes govern the photoperiodic response, but it is still unknown how they regulate downstream endocrine elements (Chang and Meuti 2020; Meuti et al. 2015; Peffers and Meuti 2022). In R. pedestris, ILP1 regulates fecundity and sugar homeostasis (Hasebe and Shiga, 2021; the present study), and Cyp15 regulates vitellogenesis and ovarian development through JHSB3 biosynthesis (Ando et al. 2020; the present study). ILP1 does not regulate JH-dependent vitellogenesis and ovarian development (Shimokawa et al. 2008; Hasebe and Shiga 2021; the present study). The circadian clock including per governs the photoperiodic response (Ikeno et al. 2010) and this clock regulates the expression of Ilp1 in the PI and Cyp15 in the CA (the present study). Our results provide insight into the molecular linkage between the circadian clock and endocrine effectors.
Regulatory pathways of photoperiodic responses in Culex pipiens (left panel) and Riptortus pedestris (right panel). ILPs regulate the JH pathway in C. pipiens but not in R. pedestris, in which ILP and JH independently regulate reproduction. CA corpus allatum, Cyp15 cytochrome P450 15 gene, ILP insulin-like peptide, IPC ILP-producing cell, per, period gene, PI pars intercerebralis. For further explanation, see the main text
References
Ando Y, Matsumoto K, Misaki K et al (2020) Juvenile hormone III skipped bisepoxide, not its stereoisomers, as a juvenile hormone of the bean bug Riptortus pedestris. Gen Comp Endocrinol 289:113394. https://doi.org/10.1016/j.ygcen.2020.113394
Barber AF, Erion R, Holmes TC, Sehgal A (2016) Circadian and feeding cues integrate to drive rhythms of physiology in Drosophila insulin-producing cells. Genes Dev 30:2596–2606. https://doi.org/10.1101/gad.288258.116
Barberà M, Cañas-Cañas R, Martínez-Torres D (2019) Insulin-like peptides involved in photoperiodism in the aphid Acyrthosiphon pisum. Insect Biochem Mol Biol 112:103185. https://doi.org/10.1016/j.ibmb.2019.103185
Broughton SJ, Piper MDW, Ikeya T et al (2005) Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci USA 102:3105–3110. https://doi.org/10.1073/pnas.0405775102
Chang V, Meuti ME (2020) Circadian transcription factors differentially regulate features of the adult overwintering diapause in the Northern house mosquito Culex Pipiens. Insect Biochem Mol Biol 121:103365. https://doi.org/10.1016/j.ibmb.2020.103365
Collins B, Kane EA, Reeves DC, Akabas MH, Blau J (2012) Balance of activity between LNVs and glutamatergic dorsal clock neurons promotes robust circadian rhythms in Drosophila. Neuron 74:706–718. https://doi.org/10.1016/j.neuron.2012.02.034
Cuti P, Barberà M, Veenstra JA, Martínez-Torres D (2021) Progress in the characterization of insulin-like peptides in aphids: Immunohistochemical mapping of ILP4. Insect Biochem Mol Biol 136:103623. https://doi.org/10.1016/j.ibmb.2021.103623
Daimon T, Kozaki T, Niwa R et al (2012) Precocious metamorphosis in the juvenile hormone–deficient mutant of the silkworm, Bombyx mori. Plos Genet 8:e1002486. https://doi.org/10.1371/journal.pgen.1002486
Danks HV (1987) Insect Dormancy: An Ecological Perspective. Biological Survey of Canada, Ottawa
de Kort CAD, Bergot BJ, Schooley DA (1982) The nature and titre of juvenile hormone in the Colorado potato beetle, Leptinotarsa decemlineata. J Insect Physiol 28:471–474. https://doi.org/10.1016/0022-1910(82)90077-4
Denlinger DL (2022) Insect Diapause. Cambridge University Press, Cambridge
Des Marteaux L, Xi J, Mano G, Goto SG (2022) Circadian clock outputs regulating insect photoperiodism: A potential role for glutamate transporter. Biochem Biophys Res Commun 589:100–106. https://doi.org/10.1016/j.bbrc.2021.12.014
Goltzené F, Holder F, Charlet M et al (1992) Immunocytochemical localization of Bombyx-PTTH-like molecules in neurosecretory cells of the brain of the migratory locust, Locusta migratoria. Cell Tissue Res 269:133–140. https://doi.org/10.1007/BF00384733
Goodman WG, Cusson M (2012) The Juvenile Hormones. In: Gilbert LI (ed) Insect Endocrinology. Academic Press, Oxford, pp 310–365
Goto SG (2022) Photoperiodic time measurement, photoreception, and circadian clocks in insect photoperiodism. Appl Entomol Zool 57:193–212. https://doi.org/10.1007/s13355-022-00785-7
Goto SG, Nagata M (2022) The circadian clock gene (Clock) regulates photoperiodic time measurement and its downstream process determining maternal induction of embryonic diapause in a cricket. Eur J Entomol 119:12–22. https://doi.org/10.14411/EJE.2022.002
Grönke S, Partridge L (2010) The Functions of Insulin-like Peptides in Insects. In: Clemmons D, Robinson I, Christen Y (Eds) IGFs:Local Repair and Survival Factors Throughout Life Span. Research and Perspectives in Endocrine Interactions. Springer, Heidelberg, pp 105–124. https://doi.org/10.1007/978-3-642-04302-4_9
Guo S-S, Zhang M, Liu T-X (2016) Insulin-related peptide 5 is involved in regulating embryo development and biochemical composition in pea aphid with wing polyphenism. Front Physiol 9:7–31. https://doi.org/10.3389/fphys.2016.00031
Guo S, Tian Z, Wu Q-W et al (2021) Steroid hormone ecdysone deficiency stimulates preparation for photoperiodic reproductive diapause. PLoS Genet 17:e1009352. https://doi.org/10.1371/journal.pgen.1009352
Hall TA (1999) BIOEDIT: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/ NT. Nucl Acids Symp Ser 41:95–98
Hasebe M, Shiga S (2021) Oviposition-promoting pars intercerebralis neurons show period-dependent photoperiodic changes in their firing activity in the bean bug. Proc Natl Acad Sci USA 118:e2018823118. https://doi.org/10.1073/pnas.2018823118
Hejníková M, Nouzova M, Ramirez CE et al (2022) Sexual dimorphism of diapause regulation in the hemipteran bug Pyrrhocoris apterus. Insect Biochem Mol Biol 142:103721. https://doi.org/10.1016/j.ibmb.2022.103721
Helvig C, Koener JF, Unnithan GC, Feyereisen R (2004) CYP15A1, the cytochrome P450 that catalyzes epoxidation of methyl farnesoate to juvenile hormone III in cockroach corpora allata. Proc Natl Acad Sci USA 101:4024–4029. https://doi.org/10.1073/pnas.0306980101
Hirai M, Yuda M, Shinoda T, Chinzei Y (1998) Identification and cDNA cloning of novel juvenile hormone responsive genes from fat body of the bean bug, Riptortus clavatus by mRNA differential display. Insect Biochem Mol Biol 28:181–189. https://doi.org/10.1016/S0965-1748(97)00116-1
Hodková M (1976) Nervous inhibition of corpora allata by photoperiod in Pyrrhocoris apterus. Nature 263:521–523. https://doi.org/10.1038/263521a0
Hodková M, Okuda T (2019) Three kinds of regulatory signals for production of juvenile hormone in females of the linden bug, Pyrrhocoris apterus. J Insect Physiol 113:17–23. https://doi.org/10.1016/j.jinsphys.2019.01.002
Ikeda K, Daimon T, Shiomi K et al (2021) Involvement of the clock gene period in the photoperiodism of the silkmoth Bombyx mori. Zoolog Sci 38:523–530. https://doi.org/10.2108/zs210081
Ikeno T, Tanaka SI, Numata H, Goto SG (2010) Photoperiodic diapause under the control of circadian clock genes in an insect. BMC Biol 8:116. https://doi.org/10.1186/1741-7007-8-116
Ikeno T, Numata H, Goto SG (2011a) Circadian clock genes period and cycle regulate photoperiodic diapause in the bean bug Riptortus pedestris males. J Insect Physiol 57:935–938. https://doi.org/10.1016/j.jinsphys.2011.04.006
Ikeno T, Numata H, Goto SG (2011b) Photoperiodic response requires mammalian-type cryptochrome in the bean bug Riptortus pedestris. Biochem Biophys Res Commun 410:394–397. https://doi.org/10.1016/j.bbrc.2011.05.142
Ikeno T, Ishikawa K, Numata H, Goto SG (2013) Circadian clock gene Clock is involved in the photoperiodic response of the bean bug Riptortus pedestris. Physiol Entomol 38:157–162. https://doi.org/10.1111/phen.12013
Ikeno T, Numata H, Goto SG, Shiga S (2014) The involvement of the brain region containing pigment-dispersing factor-immunoreactive neurons in the photoperiodic response of the bean bug Riptortus pedestris. J Exp Biol 217:453–462. https://doi.org/10.1242/jeb.091801
Kamano S (1991) Riptorus clavatus (Thunberg) (Bean bug). In: Yushima T, Kamano S, Tamaki Y (eds) Rearing methods of insects. Japan Plant Protection Association, Tokyo, pp 46–49. (in Japanese)
Kang DS, Denlinger DL, Sim C (2014) Suppression of allatotropin simulates reproductive diapause in the mosquito Culex pipiens. J Insect Physiol 64:48–53. https://doi.org/10.1016/j.jinsphys.2014.03.005
Koide R, Xi J, Hamanaka Y, Shiga S (2021) Mapping PERIOD-immunoreactive cells with neurons relevant to photoperiodic response in the bean bug Riptortus pedestris. Cell Tissue Res 385:571–583. https://doi.org/10.1007/s00441-021-03451-6
Kubrak OI, Kučerová L, Theopold U, Nässel DR (2014) The sleeping beauty: How reproductive diapause affects hormone signaling, metabolism, immune response and somatic maintenance in Drosophila melanogaster. PLoS ONE 9:e113051. https://doi.org/10.1371/journal.pone.0113051
Kurogi Y, Mizuno Y, Imura E, Niwa R (2021) Neuroendocrine regulation of reproductive dormancy in the fruit fly Drosophila melanogaster: A review of juvenile hormone-dependent regulation. Front Ecol Evol 9:715029. https://doi.org/10.3389/fevo.2021.715029
Larrere M, Lavenseau L, Tasei J-N, Couillaud F (1993) Juvenile hormone biosynthesis and diapause termination in Bombus terrestris. Invertebr Reprod Dev 23:7–14. https://doi.org/10.1080/07924259.1993.9672288
Lee J, Kim C-H, Jang HA et al (2019) Burkholderia gut symbiont modulates titer of specific juvenile hormone in the bean bug Riptortus pedestris. Dev Comp Immunol 99:103399. https://doi.org/10.1016/j.dci.2019.103399
Li W, Huang ZY, Liu F et al (2013) Molecular cloning and characterization of juvenile hormone acid methyltransferase in the honey bee, Apis mellifera, and its differential expression during caste differentiation. PLoS ONE 8:e68544. https://doi.org/10.1371/journal.pone.0068544
Marchal E, Zhang J, Badisco L et al (2011) Final steps in juvenile hormone biosynthesis in the desert locust, Schistocerca gregaria. Insect Biochem Mol Biol 41:219–227. https://doi.org/10.1016/j.ibmb.2010.12.007
Matsumoto K, Numata H, Shiga S (2013) Role of the brain in photoperiodic regulation of juvenile hormone biosynthesis in the brown-winged green bug Plautia stali. J Insect Physiol 59:387–393. https://doi.org/10.1016/j.jinsphys.2013.01.007
Matsumoto K, Suetsugu Y, Tanaka Y et al (2017) Identification of allatostatins in the brown-winged green bug Plautia stali. J Insect Physiol 96:21–28. https://doi.org/10.1016/j.jinsphys.2016.10.005
Meuti ME, Stone M, Ikeno T, Denlinger DL (2015) Functional circadian clock genes are essential for the overwintering diapause of the Northern house mosquito, Culex pipiens. J Exp Biol 218:412–422. https://doi.org/10.1242/jeb.113233
Miki T, Shinohara T, Chafino S et al (2020) Photoperiod and temperature separately regulate nymphal development through JH and insulin/TOR signaling pathways in an insect. Proc Natl Acad Sci USA 117:5525–5531. https://doi.org/10.1073/pnas.1922747117
Minakuchi C, Namiki T, Yoshiyama M, Shinoda T (2008) RNAi-mediated knockdown of juvenile hormone acid O-methyltransferase gene causes precocious metamorphosis in the red flour beetle Tribolium castaneum. FEBS J 275:2919–2931. https://doi.org/10.1111/j.1742-4658.2008.06428.x
Miura K, Shinoda T, Yura M et al (1998) Two hexameric cyanoprotein subunits from an insect, Riptortus clavatus. Sequence, phylogeny and developmental and juvenile hormone regulation. Eur J Biochem 258:929–940. https://doi.org/10.1046/j.1432-1327.1998.2580929.x
Mizoguchi A, Okamoto N (2013) Insulin-like and IGF-like peptides in the silkmoth Bombyx mori: discovery, structure, secretion, and function. Front Physiol 4:217. https://doi.org/10.3389/fphys.2013.00217
Mizoguchi A, Ishizaki H, Nagasawa H et al (1987) A monoclonal antibody against a synthetic fragment of bombyxin (4K-prothoracicotropic hormone) from the silkmoth, Bombyx mori: characterization and immunohistochemistry. Mol Cell Endocrinol 51:227–235. https://doi.org/10.1016/0303-7207(87)90032-3
Morita A, Numata H (1997) Role of the neuroendocrine complex in the control of adult diapause in the bean bug, Riptortus clavatus. Arch Insect Biochem Physiol 35:347–355. https://doi.org/10.1002/(SICI)1520-6327(199705)35:3%3c347::AID-ARCH8%3e3.0.CO;2-Q
Morita A, Soga K, Hoson T et al (1999) Changes in mechanical properties of the cuticle and lipid accumulation in relation to adult diapause in the bean bug, Riptortus clavatus. J Insect Physiol 45:241–247. https://doi.org/10.1016/S0022-1910(98)00119-X
Mukai A, Goto SG (2016) The clock gene period is essential for the photoperiodic response in the jewel wasp Nasonia vitripennis (Hymenoptera: Pteromalidae). Appl Entomol Zool 51:185–194. https://doi.org/10.1007/s13355-015-0384-1
Nagy D, Cusumano P, Andreatta G et al (2019) Peptidergic signaling from clock neurons regulates reproductive dormancy in Drosophila melanogaster. PLoS Genet 15:e1008158. https://doi.org/10.1371/journal.pgen.1008158
Nässel DR, Broeck JV (2015) Insulin/IGF signaling in Drosophila and other insects: factors that regulate production, release and post-release action of the insulin-like peptides. Cell Mol Life Sci 73:271–290. https://doi.org/10.1007/s00018-015-2063-3
Nelson RJ, Denlinger DL, Somers DE (2009) Photoperiodism. Oxford University Press, New York
Niwa R, Niimi T, Honda N et al (2008) Juvenile hormone acid O-methyltransferase in Drosophila melanogaster. Insect Biochem Mol Biol 38:714–720. https://doi.org/10.1016/j.ibmb.2008.04.003
Nouzova M, Edwards MJ, Michalkova V et al (2021) Epoxidation of juvenile hormone was a key innovation improving insect reproductive fitness. Proc Natl Acad Sci USA 118:e2109381118. https://doi.org/10.1073/pnas.2109381118
Numata H, Hidaka T (1982) Photoperiodic control of adult diapause in the bean bug, Riptortus clavatus Thunberg (Heteroptera : Coreidae). I. Reversible induction and termination of diapause. Appl Entomol Zool 17:530–538. https://doi.org/10.1303/aez.17.530
Numata H, Miyazaki Y, Ikeno T (2015) Common features in diverse insect clocks. Zool Lett 1:10. https://doi.org/10.1186/s40851-014-0003-y
Ojima N, Hara Y, Ito H, Yamamoto D (2018) Genetic dissection of stress-induced reproductive arrest in Drosophila melanogaster females. PLoS Genet 14:e1007434. https://doi.org/10.1371/journal.pgen.1007434
Okamoto N, Yamanaka N (2015) Nutrition-dependent control of insect development by insulin-like peptides. Curr Opin Insect Sci 11:21–30. https://doi.org/10.1016/j.cois.2015.08.001
Okuda T, Tanaka S, Kotaki T, Ferenz H-J (1996) Role of the corpora allata and juvenile hormone in the control of imaginal diapause and reproduction in three species of locusts. J Insect Physiol 42:943–951. https://doi.org/10.1016/0022-1910(96)00055-8
Patke A, Young MW, Axelrod S (2020) Molecular mechanisms and physiological importance of circadian rhythms. Nat Rev Mol Cell Biol 21:67–84. https://doi.org/10.1038/s41580-019-0179-2
Peffers C, Meuti ME (2022) Characterizing the relative abundance of circadian transcription factors in diapausing and nondiapausing Northern house mosquitoes. J Insect Physiol 140:104404. https://doi.org/10.1016/j.jinsphys.2022.104404
Poras M (1982) Le Contrôle endocrinien de la diapause imaginale des femelles de Tetrix undulata (Sowerby, 1806) (Orthoptere, Tetrigidae). Gen Comp Endocrinol 46:200–210. https://doi.org/10.1016/0016-6480(82)90202-7
Rankin MA, Riddiford LM (1978) Significance of haemolymph juvenile hormone titer changes in timing of migration and reproduction in adult Oncopeltus fasciatus. J Insect Physiol 24:31–38. https://doi.org/10.1016/0022-1910(78)90008-2
Readio J, Chen M-H, Meola R (1999) Juvenile hormone biosynthesis in diapausing and nondiapausing Culex pipiens (Diptera: Culicidae). J Med Entomol 36:355–360. https://doi.org/10.1093/jmedent/36.3.355
Riehle MA, Fan Y, Cao C, Brown MR (2006) Molecular characterization of insulin-like peptides in the yellow fever mosquito, Aedes aegypti: expression, cellular localization, and phylogeny. Peptides 27:2547–2560. https://doi.org/10.1016/j.peptides.2006.07.016
Schiesari L, Andreatta G, Kyriacou CP et al (2016) The insulin-like proteins dILPs-2/5 determine diapause inducibility in Drosophila. PLoS ONE 11:e0163680. https://doi.org/10.1371/journal.pone.0163680
Sheng Z, Xu J, Bai H et al (2011) Juvenile hormone regulates vitellogenin gene expression through insulin-like peptide signaling pathway in the red flour beetle, Tribolium castaneum. J Biol Chem 286:41924–41936. https://doi.org/10.1074/jbc.M111.269845
Shiga S, Numata H (2000) The role of neurosecretory neurons in the pars intercerebralis and pars lateralis in reproductive diapause of the blowfly, Protophormia terraenovae. Naturwissenschaften 87:125–128. https://doi.org/10.1007/s001140050689
Shimokawa K, Numata H, Shiga S (2008) Neurons important for the photoperiodic control of diapause in the bean bug, Riptortus pedestris. J Comp Physiol A 194:751–762. https://doi.org/10.1007/s00359-008-0346-y
Shimokawa K, Numata H, Shiga S (2014) Pars intercerebralis promotes oviposition in the bean bug, Riptortus pedestris (Heteroptera: Alydidae). Appl Entomol Zool 49:525–528. https://doi.org/10.1007/s13355-014-0281-z
Shinoda T, Itoyama K (2003) Juvenile hormone acid methyltransferase: A key regulatory enzyme for insect metamorphosis. Proc Natl Acad Sci USA 100:11986–11991. https://doi.org/10.1073/pnas.2134232100
Sim C, Denlinger DL (2008) Insulin signaling and FOXO regulate the overwintering diapause of the mosquito Culex pipiens. Proc Natl Acad Sci USA 105:6777–6781. https://doi.org/10.1073/pnas.0802067105
Sim C, Denlinger DL (2009) A shut-down in expression of an insulin-like peptide, ILP-1, halts ovarian maturation during the overwintering diapause of the mosquito Culex pipiens. Insect Mol Biol 18:325–332. https://doi.org/10.1111/j.1365-2583.2009.00872.x
Sim C, Denlinger DL (2013a) Insulin signaling and the regulation of insect diapause. Front Physiol 4:189. https://doi.org/10.3389/fphys.2013.00189
Sim C, Denlinger DL (2013b) Juvenile hormone III suppresses forkhead of transcription factor in the fat body and reduces fat accumulation in the diapausing mosquito, Culex pipiens. Insect Mol Biol 22:1–11. https://doi.org/10.1111/j.1365-2583.2012.01166.x
Sim C, Kang DS, Kim S et al (2015) Identification of FOXO targets that generate diverse features of the diapause phenotype in the mosquito Culex pipiens. Proc Natl Acad Sci USA 112:3811–3816. https://doi.org/10.1073/pnas.1502751112
Steel CGH, Vafopoulou X (2006) Circadian orchestration of developmental hormones in the insect, Rhodnius prolixus. Comp Biochem Physiol 144:351–364. https://doi.org/10.1016/j.cbpa.2006.02.018
Tamai T, Shiga S, Goto SG (2019) Roles of the circadian clock and endocrine regulator in the photoperiodic response of the brown-winged green bug Plautia stali. Physiol Entomol 44:43–52. https://doi.org/10.1111/phen.12274
Tauber MJ, Tauber CA, Masaki S (1986) Seasonal Adaptations of Insects. Oxford University Press, New York
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680. https://doi.org/10.1093/nar/22.22.4673
Tian Z, Guo S, Li J-X et al (2021) Juvenile hormone biosynthetic genes are critical for regulating reproductive diapause in the cabbage beetle. Insect Biochem Mol Biol 139:103654. https://doi.org/10.1016/j.ibmb.2021.103654
Tomioka K, Matsumoto A (2019) The circadian system in insects: Cellular, molecular, and functional organization. In: Jurenka R (Ed) Advances in Insect Physiology, vol 56. Academic Press, Oxford, UK, pp 73–115. https://doi.org/10.1016/bs.aiip.2019.01.001
Vafopoulou X, Steel CGH (2012) Insulin-like and testis ecdysiotropin neuropeptides are regulated by the circadian timing system in the brain during larval-adult development in the insect Rhodnius prolixus (Hemiptera). Gen Comp Endocrinol 179:277–288. https://doi.org/10.1016/j.ygcen.2012.08.018
Vafopoulou X, Steel CGH (2014) Synergistic induction of the clock protein PERIOD by insulin-like peptide and prothoracicotropic hormone in Rhodnius prolixus (Hemiptera): implications for convergence of hormone signaling pathways. Front Physiol 5:41. https://doi.org/10.3389/fphys.2014.00041
Xu H-J, Xue J, Lu B et al (2015) Two insulin receptors determine alternative wing morphs in planthoppers. Nature 519:464–467. https://doi.org/10.1038/nature14286
Zhan S, Merlin C, Boore JL, Reppert SM (2011) The monarch butterfly genome yields insights into long-distance migration. Cell 147:1171–1185. https://doi.org/10.1016/j.cell.2011.09.052
Zhang C, Daubnerova I, Jang Y-H et al (2021) The neuropeptide allatostatin C from clock-associated DN1p neurons generates the circadian rhythm for oogenesis. Proc Natl Acad Sci USA 118:e2016878118. https://doi.org/10.1073/pnas.2016878118
Zhu L, Tian Z, Guo S et al (2019) Circadian clock genes link photoperiodic signals to lipid accumulation during diapause preparation in the diapause-destined female cabbage beetles Colaphellus bowringi. Insect Biochem Mol Biol 104:1–10. https://doi.org/10.1016/j.ibmb.2018.11.001
Acknowledgements
We appreciate Dr. Tetsuro Shinada (Osaka Metropolitan University) for supplying the synthesized JHSB3. We also appreciate Dr. Akira Mizoguchi (Aichi Gakuin University) for his advice on the target sequence of the anti-ILP1 antibody. We would like to thank Dr. Taro Fuchikawa (Osaka Metropolitan University) for his advice on immunostaining.
Funding
This work was supported by JST SPRING (JPMJSP2139) to GM and Grant-in-Aid for Scientific Research B (22H02361) to SGG. The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
Authors contributed to the study conception and design. Material preparation, data collection, analysis, and first draft of the manuscript were performed by Genyu Mano. Authors commented on previous versions of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mano, G., Goto, S.G. Photoperiod controls insulin and juvenile hormone signaling pathways via the circadian clock in the bean bug Riptortus pedestris (Hemiptera: Alydidae). Appl Entomol Zool 57, 363–377 (2022). https://doi.org/10.1007/s13355-022-00795-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-022-00795-5