Abstract
Numerous insect species living in temperate regions survive adverse conditions, such as winter, in a state of developmental arrest. The most reliable cue for anticipating seasonal changes is the day-to-night ratio, the photoperiod. The molecular mechanism of the photoperiodic timer in insects is mostly unclear. Multiple pieces of evidence suggest the involvement of circadian clock genes, however, their role might be independent of their well-established role in the daily oscillation of the circadian clock. Furthermore, reproductive diapause is preferentially studied in females, whereas males are usually used for circadian clock research. Given the idiosyncrasies of male and female physiology, we decided to test male reproductive diapause in a strongly photoperiodic species, the linden bug Pyrrhocoris apterus. The data indicate that reproduction is not under circadian control, whereas the photoperiod strongly determines males’ mating capacity. Clock mutants in pigment dispersing factor and cryptochrome-m genes are reproductive even in short photoperiod. Thus, we provide additional evidence of the participation of circadian clock genes in the photoperiodic time measurement in insects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
We live in a periodic world, when the risk, resources, opportunities, and adversities often regularly alternate. The 24 daily oscillations are anticipated in organisms by the so-called circadian clock, whereas seasonal changes are orchestrated by a photoperiodic timer (also called the photoperiodic clock). The possible link(s) between the circadian clock and photoperiodic timer have been extensively debated and, at least in insects, remains only partially understood (Bradshaw and Holzapfel 2010; Koštál 2011; Dolezel 2015, Goto 2022).
From a mechanistic perspective, circadian clocks are formed from interconnected transcription/translation feedback loops (TTFL). Mutation of clock genes results in altered free-running period of the clock in constant darkness or even in complete arrhythmicity. A remarkable collection of Drosophila mutants has been instrumental for deciphering clock mechanisms (reviewed in Hall 2003). Much fewer mutations are available in non-Drosophila insects; these mutations have usually been created in a forward genetic approach, in which a candidate gene is modified or silenced by RNA-mediated interference.
Most of the clock TTFLs is conserved in insects, even though some lineage-specific variation can be identified (Tomioka and Matsumoto 2015; Dolezel 2023). In Drosophila melanogaster, the best-understood insect model, CLOCK (CLK) and CYCLE (CYC), two basic helix-loop-helix PAS (PER-ARNT-SINGLE MINDED) transcription factors, control mRNA expression of the so-called negative elements period (per) and timeless (tim). During the early night, PER and TIM proteins accumulate and later translocate to the nucleus, where they inhibit CLK and CYC (reviewed in Ozkaya and Rosato 2012; Hardin 2011; Peschel and Helfrich-Forster 2011). Drosophila CYC does not contain a Terminal Activation Domain (TAD) which is a typical feature of BMAL1, the mammalian homolog of CYC. Interestingly, most insect species contain BMAL1 (Chang et al. 2003; Tomioka and Matsumoto 2015), and the presence of BMAL1 correlates precisely with the presence of the mammalian type CRYPTOCHROME (CRY-m) (Thakkar et al. 2022). In contrast to CRY-m, the Drosophila-type of CRYPTOCHROME (CRY-d) is a deep brain receptor key for light-mediated entrainment and its removal result in aberrant rhythmicity at constant light (Stanewsky et al. 1998; Emery et al., 2000; Dolezelova et al. 2008) Several other TTFLs are well characterized in Drosophila (Cyran et al. 2003; Jaumouille et al. 2015) and most likely conserved across insects (Tomioka and Matsumoto 2015; Dolezel 2023; Tomioka 2014; Kotwica-Rolinska et al. 2022a).
CRY-m acts as a transcriptional repressor in cell lines (Yuan et al. 2007) and its role in the clock has been demonstrated by RNA-mediated interference (RNAi) in the bean bug Riptortus pedestris (Ikeno et al. 2011a), the linden bug Pyrrhocoris apterus (Kotwica-Rolinska et al. 2022a), the cricket Gryllus bimaculatus (Tokuoka et al. 2017), the cockroach Rhyparobia maderae (Werckenthin et al. 2020), and by stable modification in the monarch butterfly Dannaus plexippus (Zhang et al. 2017). However, while knocking down Clk or Bmal1 leads to almost all arrhythmic individuals (Kotwica-Rolinska et al. 2022a), a considerable proportion of animals with knocked down or even stably mutated cry-m are still weakly rhythmic, although the free-running period deviates from 24 h or even multiple free-running periods are detected (Werckenthin et al. 2020; Kotwica-Rolinska et al. 2022a).
In addition to TTFLs controlling oscillations even at the single-cell level, intracellular communication is also required for the circadian clock. In Drosophila, PIGMENT DISPERSING FACTOR (PDF) is a well-established neuropeptide responsible for clock function when pdf01 mutation results in severely abnormal free-running period and arrhythmicity (Renn et al. 1999). In P. apterus, genetic depletion of PDF leads to arrhythmicity (Kotwica-Rolinska et al. 2022b), confirming its evolutionarily conserved role in the circadian clock.
While the circadian clock “keeps ticking” under constant conditions (in animals this is usually constant darkness, DD), the photoperiodic timer measures the day-to-night ratio (= photoperiod). Therefore, the photoperiodic timer and the circadian clock are by definition distinct and different devices, even though some of their components (e.g., proteins) may overlap. Before addressing the genetic aspects of the photoperiodic timer, we must briefly introduce diapause, which serves as a typical output. Organisms must cope with adverse conditions, such as changes in weather (access to food, low temperatures, dry seasons, etc.), and it is beneficial to anticipate these regular seasonal changes. Photoperiod is the most reliable cue that informs insects of the coming season, well before the onset of more severe adversity, such as low temperatures. For example, nymphs of P. apterus do not tolerate low winter temperatures. Because development from egg to adult takes 4–5 weeks at 25 °C and two to three months in the field (Socha 1993), it is critical to avoid oviposition at the end of summer, even if the weather is still pleasant at that time. Reproduction of P. apterus is strongly regulated by photoperiod (Saunders 1983), although the photoperiodic response curve is further shaped by temperature (Numata et al. 1993).
Diapause is terminated by low temperatures during winter, after which insects remain in a suppressed state called quiescence. The difference between diapause and quiescence can be well documented in experiments in which P. apterus females were brought from the field to ambient temperature and short photoperiod in the laboratory (Hodek 1971). Females collected and transferred in August or September did not reproduce and remained in diapause. However, females transferred later in winter (December, January, February) reproduced even during short photoperiods that supported diapause. Thus, although the long photoperiod in laboratory experiments may serve as a signal to break diapause, in the natural environment diapause is terminated by low temperatures. Thereafter, females are no longer photoperiod sensitive and can begin reproducing virtually immediately when environmental conditions become favorable. Due to a robust and clearly visible change in ovarian morphology (Smykal et al. 2014), females have been the preferred subject of diapause research in many insect species.
Although P. apterus males also enter diapause, certain sex-specific dimorphism connected to endocrine regulation exists in males (Urbanová et al. 2016; Hejníková et al. 2022). For example, females absolutely require juvenile hormone (JH) for reproduction, whereas its absence (or only very low levels) is considered a hallmark of reproductive diapause (Denlinger et al. 2012). In contrast, male diapause can be terminated by a long photoperiod even if JH signaling is experimentally impaired/abolished (Hejníková et al. 2022).
In the seminal work of Ikeno (Ikeno et al. 2010, 2011b, c, 2013), period, cyc, Clk, and cry-m were identified as regulators of photoperiod-induced reproduction in R. pedestris females. While RNAi-mediated knockdown of per and cry-m resulted in females reproducing even at short photoperiods, depletion of cyc or Clk resulted in photoperiod-independent reproductive arrest. The identical trend was observed in P. apterus after knockdown of cyc and Clk (Kotwica-Rolinska et al. 2017) and in cry-m gene mutants (Kotwica-Rolinska et al. 2022b). The possible involvement of pdf was tested in R. pedestris with rather ambiguous results: microsurgical removal identified a PDF-containing region as key for the photoperiodic timer, whereas RNAi silencing of pdf had no impact on diapause. This contrasts with data from pdf mutants generated by gene editing in P. apterus, where pdf depletion resulted in an altered photoperiodic response curve (PRC), with only a subset of pdf0 females entering diapause in SD photoperiod (Kotwica-Rolinska et al. 2022b).
Given the sex-specific differences in hormonal regulation of reproductive development in P. apterus, we decided to investigate the photoperiodic reproduction in males, to test whether mating is under circadian control, and to determine the impact of circadian clock mutants on male reproductive diapause.
Material and methods
Insects and rearing conditions
Pyrrhocoris apterus colonies were maintained under long-day conditions (LD, 18 h/6 h light/dark cycle), at a temperature of 25 ± 1 ˚C, with constant access to food (dry linden Tilia cordata seeds) and water. Nymphs in the last nymphal stage were divided into female and male groups and kept separately in small jars. The diapausing bugs were reared in a similar manner but kept under short-day conditions (SD, 12 h/12 h). The lines used were reference wild-type Oldrichovec (Oldr) and Roana (Ro) strains (described in Pivarciova et al. 2016), cry-m mutants (details in Kotwica-Rolinska et al. 2022a), and pdf mutants (described in Kotwica-Rolinska et al. 2022b). All mutants were backcrossed to the corresponding wild-type strain for at least 8 generations to minimize the effect of off-target editing (Kotwica-Rolinska et al. 2019). One of the cry-m mutants was additionally backcrossed to Ro for an additional 8 generations (Netušil et al. 2021). A set of experiments was performed on overwintered males collected on March 20, 2023, from the field in Ceske Budejovice, Czech Republic. For the locomotor activity experiments, which were performed at SD, a non-photoperiodic diapause Lyon strain (Lyon-rednpd) was used as a reference (Dolezel et al. 2005; Pivarciova et al. 2016; Supplementary Table 1).
Mating chambers
Square plastic arenas (7 × 7 × 1 cm) covered on top with non-reflective transparent plastic were used to observe and record mating. A removable (sliding) divider separates the arena in the middle (Supplementary Fig. 1). Males and females were placed in the divided arenas and allowed to become familiar with the environment, then the divider was removed. A top-mounted webcam (ArduCAM 2MP OV2710 Camera Module with integral 650 IR filter, ArduCAM) connected to a computer recorded throughout the experiment (up to 24 h) activity via Dorgem software (version 2.1.0). Manual annotation of observed interactions (mating attempts and copulations) and the duration of copulation were carried out ‘frame-by-frame’ using VirtualDub (version 1.10.4). Mating attempts were defined as when the male approaches the female within one body length, taps with his antennae, and then jumps on top of the female with shaking movements. Copulation is defined as when there is a clear genital connection between the insects. Males were marked with a white dot to distinguish the sexes (using white non-toxic edding790 paint marker).
Mating assays
Females and males (age, strain, and conditions varied by the experiment, see below) were housed in the arenas and allowed to become familiar with the environment for 1–2 min, and then the divider was gently removed. During the first 30 min, activity was photographed every second (1 fps, frame per second), then one frame per minute (fpm) was taken for an additional 11.5 or 23.5 h.
To test the optimal age of females, only the Oldrichovec strain was used. Three to ten days after adult ecdysis (dAAE) virgin females were individually mixed with 7 d AAE males, and the activity recorded at 1 fps for the first 30 min and then at 1 fpm for an additional 11.5 h.
To test mating around the clock, 5 dAAE virgin females were individually mixed with 7 d AAE males (both from the Oldrichovec strain) at six different time points evenly spaced throughout the day. Males and females were housed in the arenas, allowed to familiarize themselves with the arena for 1–2 min, and then the divider was gently removed 30 min before the targeted ZT. Mating was scored manually for the next 30 min, and the observation finished exactly at ZT 2, ZT 6, ZT 10, ZT 14, ZT 18, and ZT 22 (ZT0 corresponds to the “light on” signal). The experiment was repeated in four biological replicates on different days (Table 1). The analysis of the potential circadian rhythm was performed using JTK-Cycle (Hughes et al. 2010).
The remaining mating experiments were performed with Oldrichovec, Roana, cry-m and pdf mutants, and with males collected in the field on March 20 (spring equinox). Males from LD were 7d AAE and females from LD were 5d AAE old. Males and females from SD conditions were 10d AAE. Pairs were housed in the arenas and the divider was removed at around ZT2, then activity was captured at 1 fps during the first 30 min and then at 1 fpm for an additional 23.5 h.
Locomotor activity assays
To measure the free-running period, males collected in the field and reference Lyon-rednpd males were individually placed into 15-cm glass test tubes with a small vial filled with water and a cotton stopper. At the other end of the test tube, a linden seed was mechanically fixed in a small mesh bag. Males were synchronized in a 12:12 light–dark regime for 5 days at 25 °C and then released into constant dark conditions at the same temperature. Their locomotor activity was recorded in 5-min bins for an additional 12 days in DD. The free-running period was determined from the first 10 days in DD, with the additional two days serving as a control for insect survival. Lomb-Scargle analysis in Actogram J (Schmid et al. 2011) was used to identify arrhythmic individuals, animals with multiple free-running periods or unstable periods, and animals with a stable free-running period for which the exact value can be determined. In principle, we followed the protocol of Kaniewska et al. (2020).
Methoprene application
10d AAE diapausing males from SD were combined with reproductive 5d AAE female virgins from LD and kept under diapause-inducing SD photoperiod at 25 °C and activity was recorded. The next day, males were anesthetized with CO2 and 2 µg juvenile hormone mimic methoprene dissolved in acetone was ectopically applied. As a control, only the vehicle (acetone) was applied. The male mating activity was then recorded, and fresh reproductive virgins were replaced every other day.
Software
Following software was used in this study:
Dorgem Software, Frank Fesevur (2006) https://dorgem.sourceforge.net/.
GraphPad Software, Inc. San Diego, California USA, https://www.graphpad.com/.
VirtualDub Software, Avery Lee (2013) https://www.virtualdub.org/index.html.
Results
Pilot experiments and age of animals
From previous studies we know that 7-day-old males (7 d AAE) are efficient in mating. Therefore, we tested how age affects female mating performance. While mating efficiency reached up to 90% on day 6, it gradually decreased thereafter, reaching a minimum of ~ 25% around day 9 (Supplementary Fig. 2). This pattern is consistent with the vitellogenic cycle and JH titer in P. apterus females (Hejnikova et al. 2022). Therefore, we used 5 d AAE females for the mating experiments.
Mating of P. apterus is not under circadian control
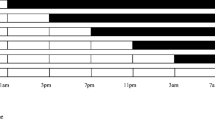
The first experiment addressed the question of whether or not the mating of P. apterus is tied to a specific time of day. First, it is interesting to see if this activity is controlled by the clock. Second, the possible time preference is important because we are using circadian clock mutants in the study. When males and females were paired at any of the six tested Zeitgeber times (ZT), ~ 40–60% of pairs mated within the next 30 min (Fig. 1). Although the sum of all four replicates (Table 1) indicates a small trough at ZT6, no oscillation can be detected in the biological replicates with JTK-Cycle. Thus, P. apterus mating is independent of time of day, at least in the simplified laboratory setup. However, it should be noted that both males and females were kept isolated from the second sex for seven and five days, respectively. In contrast, in the field, males and females of this species live in large colonies where individual bugs meet frequently. On the other hand, mating often lasts more than 12 h, so targeting this activity may not be as critical as if it were a brief event. The results of this laboratory experiment suggest that mating occurs independently of visual cues, as pairs also mate in the dark at ZT22.
The circadian profile of mating behavior in WT Oldrichovec P. apterus males. The dots represent the percentage of copulating pairs. The floating numbers above dots are the number of copulating versus tested pairs. The bar on the bottom axis depicts the photoperiod—LD 18:6—white stands for light, black represents darkness. The mating of the linden bug is not under circadian control (p > 0.05, JTK-Cycle test)
Photoperiod is a strong signal regulating male reproductive diapause
Since there is no daily preference in P. apterus mating, we started the experiments at ZT2 for all tested combinations of genotypes (wt and mutant animals) and physiological states (males and females from SD and LD). Males of both wild-type lines mated at LD with > 75% success in 24 h (Fig. 2, Supplementary Table 2, Supplementary Fig. 3). When diapausing males from SD were combined with either diapausing (SD) or reproductive females (LD), no copulation was detected. However, ~ 42% of reproductive males copulated with diapausing females. Moreover, the mating was successful: when these females were transferred from diapause-inducing/maintaining conditions to reproduction-promoting LD, they began to lay eggs that were fertilized in ~ 73% of the crosses (see Supplementary Table 3 for a detailed description of the results). The approximately three-week delay in egg laying is a clear indication that females were in diapause during copulation, as reproductively mature females only require approximately one week to lay eggs (for details on delays in egg laying associated with the switch from diapause to reproduction, see Dolezel et al. 2007).
Photoperiodic timer and circadian clock in P. apterus males. (a) Reproductive status of male strongly influences mating. All used virgin females belong to the WT Oldrichovec line. Males are either Oldrichovec (no footnote), WT Roana (Ro), or overwintered bugs (OW) collected in the field in Ceske Budejovice, Czech Republic. The (OW*) group are the insects from (OW) that were kept under recovery conditions for three days (SD, 25 °C, with food and water ad libitum). The WT lines were reared in long day (LD) or short day (SD) conditions. Numbers next to the bars indicate the total number of pairs corresponding to the category. The copulation success of reproductive males (LD) is high for both WT lines, the reproductive male can even force the diapausing female into copulation (42%). Under the SD conditions, males are in diapause and do not copulate. Around 14% of overwintered males (OW) copulated upon transfer from the field. Copulating success raised to 92% after three-day recovery (OW*). See supplementary table 2 for detailed values. (b) The circadian clock is functional even in non-photoperiodic males in which diapause was terminated by low temperatures during overwintering (OW). The Lyonnpd strain was used as a reference. The difference in free-running period reflects the different geographic origin of the lines compared. The circadian clock of the tested males is characterized as the percentage of males exhibiting strong rhythmicity, complex rhythmicity, and arrhythmicity. For each male with strong rhythmicity, individual free-running period values are shown as dots. The red bars represent means ± SEM
Finally, we examined the mating performance of males collected in the field exactly on spring equinox. 14% of freshly collected over-wintered (OW) males mated immediately after the transfer from the field. When they were exposed to SD, 25 °C, and food and water ad libitum (recovery conditions) for three days, their performance increased to 92%. The mating was successful, as 89% of the crosses produced fertilized eggs (see Supplementary Table 3 for details).
Photoperiod-insensitive males possess a functioning circadian clock
The results described above confirmed that the males collected in the field were photoperiod insensitive and reproduced successfully. Therefore, we tested whether their circadian clock was still functioning. Because the conditions of SD (necessary for entrainment) induce a diapause state in photoperiod-sensitive linden bugs, for which low locomotor activity is characteristic (Hodková et al. 2003), we used the non-photoperiod diapause (npd) mutant strain Lyon-rednpd (Dolezel et al. 2005) as a control. In agreement with published data, Lyon-rednpd males remained highly rhythmic with a typical free-running period of 21.9 h (Pivarciova et al. 2016). OW males from Ceske Budejovice (‘Budweis’) were also rhythmic, with a free-running period of 24.4 h (Fig. 2B; Supplementary Fig. 4). Thus, the animals with inactive photoperiodic timer still have functioning circadian clock.
mCRY and PDF mutant males mate even at short photoperiods
Given the clear impact of photoperiod on mating, we investigated how circadian clock genes influence male reproductive diapause. Three stable genetic mutants of pdf were tested (Fig. 3A): pdfRK, a mutant in which two basic amino acids were introduced immediately downstream of the preprohormone cleavage site; pdf03L, a mutant in which a conserved leucine was removed; and pdf04, a strain in which half of the PDF peptide was replaced by a completely different amino acid sequence. As a control, we used Roana wt, a strain in which all pdf mutations were engineered.
Mating behavior in pdf mutant lines. All used virgin females are from the Oldrichovec strain. a Schematic illustrating the positions of mutations in the protein sequence of pdf in relation to WT. The red color represents the preprohormone cleavage sites, black—no change in the protein sequence, blue—a difference in the amino acid sequence based on the in-frame insertion (pdfRK) and deletion (pdf03L), green—amidation of Glycine, yellow—changed sequence from out-of-frame deletion resulting in the termination of the protein (asterisk). b Copulation success in wt and mutants in LD 18:6 photoperiod. Numbers next to bars indicate the number (n) of pairs in each category. c Numbers of attempts by male bugs performed without success (U) and resulting in a copulation (S). Each dot represents a measurement from a single individual; the red lines represent the mean ± SEM. d Copulation success in bugs from SD 12:12 photoperiod. Each empty space corresponds to no individuals fulfilling requirements (none mated). e Numbers of unsuccessful and successful attempts in lines from SD. See supplementary Table 4 for detailed values
Males of all four lines mated under LD photoperiod, although the efficiency of pdfRK reached only 46% in 24 h, while the controls mated with 76% and pdf04 even with 84% success (Fig. 3B, Supplementary Fig. 5). In SD, both wt control and pdfRK did not mate at all, whereas both pdf04 and pdf03L mutants were reproductive (Fig. 3D, Supplementary Fig. 6). In addition to plotting mating success and total duration of mating (Supplementary Figs. 5 and 6), we also recorded the number of attempts that resulted in mating and the number of attempts in unsuccessful males. The values indicate that wt and pdfRK do not even attempt to mate at SD. Under the reproduction-inducing LD, and for pdf04 and pdf03L mutants at SD, unsuccessful males perform more attempts than the successful ones (Fig. 3C, E).
An identical analysis was performed for cry-m mutants, comparing the complete null mutant cry-m05 and the in-frame insertion mutant cry-m9in (Fig. 4A) with wt controls. Since cry-m9in was backcrossed to Roana for 8 generations, both Old and Ro wt strains were used as reference. Both cry mutants and wt controls mated at LD with greater than 60% success. At SD, a subset of both mutants reproduced, albeit at different levels (Fig. 4, Supplementary Figs. 7 and 8).
Mating behavior in cry-m mutant lines. All used females are from the Oldrichovec strain. a Schematic comparison of CRY-m protein sequences with highlighted domains in WT and mutants. The grey color corresponds to the positions of mutations. b Copulation success in wt and mutants in LD 18:6 photoperiod. Numbers next to bars indicate the number (n) of pairs corresponding to the category. c A number of attempts by male bugs performed without success (U) and resulting in a copulation (S). Each dot represents a measurement from a single individual, the red lines represent the mean ± SEM. d Copulation success in bugs from SD 12:12 photoperiod. Every blank space corresponds to no individuals fulfilling requirements (none mated). e Numbers of unsuccessful and successful attempts in lines from SD. See supplementary Table 4 for detailed values
Discussion
The endocrine regulation of reproductive diapause is different for males and females in P. apterus, as in many other insects. While females are absolutely dependent on JH signaling (Smykal et al. 2014), males can reproduce even without JH (Urbanová et al. 2016; Hejníková et al. 2022). However, JH is still capable of inducing reproduction in males, as shown by JH-mimic methoprene (Hejníková et al. 2022, and Supplementary Fig. 9). It would therefore be interesting to see if a similar situation, in which photoperiod acts independently of JH signaling in males, exists in additional species. One possible role of JH is that this hormone serves as a master switch downstream of the clock in peripheral tissues, as has been shown for tissue-autonomous expression of some circadian clock genes (Bajgar et al. 2013a, b; Dolezel et al. 2008).
The data presented here show that cry-m and pdf play comparable roles in photoperiodic induction of diapause in both sexes. In the natural environment, diapause is terminated by low temperature, after which insects are no longer photoperiodically sensitive. As indicated by the rhythmic activity of overwintered (OW) bugs at DD, the circadian clock remains functional even in non-photoperiodic males. The exact mechanism of how diapause is terminated by low temperature remains to be elucidated; clearly, this is likely a mechanism downstream of the photoperiodic timer. Termination of diapause by low temperature provides several advantages. First, all overwintered bugs can synchronously enter reproduction and mate as soon as environmental conditions become favorable. Furthermore, favorable spring conditions could also prevail during photoperiods shorter than the critical daylength required to initiate diapause in the autumn.
While diapause is energetically demanding for P. apterus (Kostál et al. 2008), the observation described here that reproductive males effectively mate with and fertilize diapausing females is puzzling. Socha reported that a certain percentage of females overwinter fertilized and then reproduce successfully (Socha 2010). Thus, males that maximize mating even in the autumn have a higher chance of passing their genes to the next generation, and at the same time, mating is less energetically demanding for males. Nevertheless, P. apterus males go into diapause. One possible (and at this point entirely speculative) explanation is mating competition. If the last mating determines the majority of offspring, surviving the winter in diapause and mating in spring is more advantageous than pre-diapause mating in autumn.
In this study, we explored the impact of circadian clock genes that may be involved in the photoperiodic timer. Our data suggest that both clock mutants, cry-m and pdf, exhibit impaired measurement of photoperiod (with the exception of pdfRK, which is comparable to the wild type). In principle, the observed phenotypes in males are consistent with the impact of pdf and cry-m depletion in P. apterus females (Kotwica-Rolinska et al. 2022b), except that a complete phase response curve was determined only in females. As we have previously shown, mutants in these two genes severely disrupt rhythmic activity at DD (Kotwica-Rolinska et al. 2022a, b). Interestingly, the mating of cry-m05 is lower at SD compared with LD (Fig. 4), which could be interpreted as a partially functional photoperiodic timer. Indeed, a subset of cry-m mutants showed highly aberrant but still (weakly) rhythmic activity with multiple non-24-h circadian components (Kotwica-Rolinska et al. 2022a). Furthermore, when photoperiodic response curve was determined in females, all pdf mutants still discriminated between photoperiods, albeit to a lesser extent. However, the diapause incidence was much lower for cry-m mutant females at all short photoperiods (Kotwica-Rolinska et al. 2022a). Comparable “partial” phenotypes were observed when RNAi was used to silence Clk and cyc in P. apterus (Kotwica-Rolinska et al. 2017). While the result of an RNAi experiment can always be interpreted as only partial gene silencing, the homozygous mutants provide clear confirmation that the photoperiodic timer functions with the circadian clock product completely removed. The involvement of pdf suggests that photoperiodic timer may be a network property, rather than a device that runs autonomously at the level of the individual cell. Overall, the data presented here provide further evidence for the involvement of circadian clock genes in photoperiodic timing in insects. However, the exact mechanism of how the photoperiodic timer functions remains to be elucidated.
Data availability
All data are available in the manuscript and supplementary materials.
References
Bajgar A, Jindra M, Dolezel D (2013a) Autonomous regulation of the insect gut by circadian genes acting downstream of juvenile hormone signaling. Proc Natl Acad Sci USA 110:4416–4421. https://doi.org/10.1073/pnas.1217060110
Bajgar A, Dolezel D, Hodkova M (2013b) Endocrine regulation of non-circadian behavior of circadian genes in insect gut. J Insect Physiol 59:881–886. https://doi.org/10.1016/j.jinsphys.2013.06.004
Bradshaw WE, Holzapfel CM (2010) What season is it anyway? Circadian tracking vs. photoperiodic anticipation in insects. J Biol Rhythms 25:155–165. https://doi.org/10.1177/0748730410365656
Chang DC, McWatters HG, Williams JA et al (2003) Constructing a feedback loop with circadian clock molecules from the silkmoth, Antheraea pernyi. J Biol Chem 278:38149–38158. https://doi.org/10.1074/jbc.M306937200
Cyran SA, Buchsbaum AM, Reddy KL et al (2003) vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell 112:329–341. https://doi.org/10.1016/s0092-8674(03)00074-6
Denlinger DL, Yocum GD, Rinehart JP (2012) Hormonal control of diapause. In: Gilbert LI (ed) insect endocrinology. Academic Press, San Diego, pp 430–463. https://doi.org/10.1016/B978-0-12-384749-2.10010-X
Dolezel D (2015) Photoperiodic time measurement in insects. Curr Opin Insect Sci 7:98–103. https://doi.org/10.1016/j.cois.2014.12.002
Dolezel D (2023) Molecular mechanism of the circadian clock. In: Numata H, Tomioka K (eds) Insect chronobiology, 1st edn. Springer, Singapore
Dolezel D, Vanecková H, Sauman I, Hodkova M (2005) Is period gene causally involved in the photoperiodic regulation of reproductive diapause in the linden bug, Pyrrhocoris apterus? J Insect Physiol 51:655–659. https://doi.org/10.1016/j.jinsphys.2005.01.009
Dolezel D, Sauman I, Koštál V, Hodkova M (2007) Photoperiodic and food signals control expression pattern of the clock gene, period, in the linden bug, Pyrrhocoris apterus. J Biol Rhythms 22:335–342. https://doi.org/10.1177/0748730407303624
Dolezel D, Zdechovanova L, Sauman I, Hodkova M (2008) Endocrine-dependent expression of circadian clock genes in insects. Cell Mol Life Sci 65:964–969. https://doi.org/10.1007/s00018-008-7506-7
Dolezelova E, Dolezel D, Hall JC (2007) Rhythm defects caused by newly engineered null mutations in Drosophila's cryptochrome gene. Genet 177(1):329–345. https://doi.org/10.1534/genetics.107.076513
Emery P, Stanewsky R, Helfrich-Förster C et al (2000) Drosophila CRY Is a Deep Brain Circadian Photoreceptor. Neuron 26(2):493–504. https://doi.org/10.1016/S0896-6273(00)81181-2
Goto SG (2022) Photoperiodic time measurement, photoreception, and circadian clocks in insect photoperiodism. Appl Entomol Zool 57:193–212. https://doi.org/10.1007/s13355-022-00785-7
Hall JC (2003) Genetics and molecular biology of rhythms in Drosophila and other insects. Genet Circad Rhy 48:1–280. https://doi.org/10.1016/s0065-2660(03)48000-0
Hardin PE (2011) Chapter 5—molecular genetic analysis of circadian timekeeping in Drosophila. In: Brody S (ed) Advances in genetics. Academic Press, Hoboken, pp 141–173. https://doi.org/10.1016/B978-0-12-387690-4.00005-2
Hejníková M, Nouzova M, Ramirez CE et al (2022) Sexual dimorphism of diapause regulation in the hemipteran bug Pyrrhocoris apterus. Insect Biochem Mol Biol 142:103721. https://doi.org/10.1016/j.ibmb.2022.103721
Hodek I (1971) Termination of adult diapause in Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae) in field. Entomol Exp Appl 14:212–222. https://doi.org/10.1111/j.1570-7458.1971.tb00158.x
Hodková M, Syrová Z, Dolezel D, Šauman I (2003) Period gene expression in relation to seasonality and circadian rhythms in the linden bug, Pyrrhocoris apterus (Heteroptera). Eur J Entomol 100:267–273. https://doi.org/10.14411/eje.2003.042
Hughes ME, Hogenesch JB, Kornacker K (2010) JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J Biol Rhythms 25(5):372–380. https://doi.org/10.1177/0748730410379711
Ikeno T, Tanaka SI, Numata H, Goto SG (2010) Photoperiodic diapause under the control of circadian clock genes in an insect. BMC Biol 8:116. https://doi.org/10.1186/1741-7007-8-116
Ikeno T, Katagiri C, Numata H, Goto SG (2011a) Causal involvement of mammalian-type cryptochrome in the circadian cuticle deposition rhythm in the bean bug Riptortus pedestris. Insect Mol Biol 20:409–415. https://doi.org/10.1111/j.1365-2583.2011.01075.x
Ikeno T, Numata H, Goto SG (2011b) Photoperiodic response requires mammalian-type cryptochrome in the bean bug Riptortus pedestris. Biochem Biophys Res Commun 410:394–397. https://doi.org/10.1016/j.bbrc.2011.05.142
Ikeno T, Numata H, Goto SG (2011c) Circadian clock genes period and cycle regulate photoperiodic diapause in the bean bug Riptortus pedestris males. J Insect Physiol 57:935–938. https://doi.org/10.1016/j.jinsphys.2011.04.006
Ikeno T, Ishikawa K, Numata H, Goto SG (2013) Circadian clock gene Clock is involved in the photoperiodic response of the bean bug Riptortus pedestris. Physiol Entomol 38:157–162. https://doi.org/10.1111/phen.12013
Jaumouillé E, Machado Almeida P, Stähli P et al (2015) Transcriptional regulation via nuclear receptor crosstalk required for the Drosophila circadian clock. Curr Biol 25:1502–1508. https://doi.org/10.1016/j.cub.2015.04.017
Kaniewska MM, Vaněčková H, Doležel D, Kotwica-Rolinska J (2020) Light and temperature synchronizes locomotor activity in the linden bug, Pyrrhocoris apterus. Front Physiol 11:242. https://doi.org/10.3389/fphys.2020.00242
Kostál V, Tollarová M, Dolezel D (2008) Dynamism in physiology and gene transcription during reproductive diapause in a heteropteran bug, Pyrrhocoris apterus. J Insect Physiol 54:77–88. https://doi.org/10.1016/j.jinsphys.2007.08.004
Koštál V (2011) Insect photoperiodic calendar and circadian clock: independence, cooperation, or unity? J Insect Physiol 57:538–556. https://doi.org/10.1016/j.jinsphys.2010.10.006
Kotwica-Rolinska J, Pivarciova L, Vaneckova H, Dolezel D (2017) The role of circadian clock genes in the photoperiodic timer of the linden bug Pyrrhocoris apterus during the nymphal stage. Physiol Entomol 42:266–273. https://doi.org/10.1111/phen.12197
Kotwica-Rolinska J, Chodakova L, Chvalova D et al (2019) CRISPR/Cas9 genome editing introduction and optimization in the non-model insect Pyrrhocoris apterus. Front Physiol 10:891. https://doi.org/10.3389/fphys.2019.00891
Kotwica-Rolinska J, Chodáková L, Smýkal V et al (2022a) Loss of timeless underlies an evolutionary transition within the circadian clock. Mol Biol Evol 39:1. https://doi.org/10.1093/molbev/msab346
Kotwica-Rolinska J, Damulewicz M, Chodakova L et al (2022b) Pigment Dispersing Factor is a circadian clock output and regulates photoperiodic response in the linden bug, Pyrrhocoris apterus. Front Physiol 13:884–909. https://doi.org/10.3389/fphys.2022.884909
Netušil R, Tomanová K, Chodáková L et al (2021) Cryptochrome-dependent magnetoreception in a heteropteran insect continues even after 24 h in darkness. J Exp Biol 224(19):jeb243000. https://doi.org/10.1242/jeb.243000
Numata H, Saulich HA, Volkovich AT (1993) Photoperiodic responses of the linden bug, Pyrrhocoris apterus, under conditions of constant temperature and under thermoperiodic conditions. Zool Sci 10:521–527
Ozkaya O, Rosato E (2012) The circadian clock of the fly: a neurogenetics journey through time. Adv Genet 77:79–123. https://doi.org/10.1016/B978-0-12-387687-4.00004-0
Peschel N, Helfrich-Förster C (2011) Setting the clock—by nature: circadian rhythm in the fruitfly Drosophila melanogaster. FEBS Lett 585:1435–1442. https://doi.org/10.1016/j.febslet.2011.02.028
Pivarciova L, Vaneckova H, Provaznik J et al (2016) Unexpected geographic variability of the free running period in the linden bug Pyrrhocoris apterus. J Biol Rhythms 31:568–576. https://doi.org/10.1177/0748730416671213
Renn SC, Park JH, Rosbash M et al (1999) A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99:791–802. https://doi.org/10.1016/s0092-8674(00)81676-1
Saunders DS (1983) A diapause induction-termination asymmetry in the photoperiodic responses of the linden bug, Pyrrhocoris apterus and an effect of near-critical photoperiods on development. J Insect Physiol 29:399–405. https://doi.org/10.1016/0022-1910(83)90067-7
Schmid B, Helfrich-Förster C, Yoshii T (2011) A new ImageJ plug-in “ActogramJ” for chronobiological analyses. J Biol Rhythms 26:464–467. https://doi.org/10.1177/0748730411414264
Smykal V, Bajgar A, Provazník J et al (2014) Juvenile hormone signaling during reproduction and development of the linden bug, Pyrrhocoris apterus. Insect Biochem Mol Biol 45:69–76. https://doi.org/10.1016/j.ibmb.2013.12.003
Socha R (1993) Pyrrhocoris apterus (Heteroptera)—an experimental model species: a review. Eur J Entomol 90:241–286
Socha R (2010) Pre-diapause mating and overwintering of fertilized adult females: new aspects of the life cycle of the wing-polymorphic bug Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae). Eur J Entomol 107:521–525. https://doi.org/10.14411/eje.2010.059
Stanewsky R, Kaneko M, Emery P et al (1998) The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 95(5):681–692. https://doi.org/10.1016/S0092-8674(00)81638-4
Thakkar N, Giesecke A, Bazalova O et al (2022) Evolution of casein kinase 1 and functional analysis of new doubletime mutants in Drosophila. Front Physiol 13:1062632. https://doi.org/10.3389/fphys.2022.1062632
Tokuoka A, Itoh TQ, Hori S et al (2017) cryptochrome genes form an oscillatory loop independent of the per/tim loop in the circadian clockwork of the cricket Gryllus bimaculatus. Zool Lett 3:5. https://doi.org/10.1186/s40851-017-0066-7
Tomioka K (2014) Chronobiology of crickets: a review. Zool Sci 31:624–632. https://doi.org/10.2108/zs140024
Tomioka K, Matsumoto A (2015) Circadian molecular clockworks in non-model insects. Curr Opin Insect Sci 7:58–64. https://doi.org/10.1016/j.cois.2014.12.006
Urbanová V, Bazalová O, Vaněčková H, Dolezel D (2016) Photoperiod regulates growth of male accessory glands through juvenile hormone signaling in the linden bug, Pyrrhocoris apterus. Insect Biochem Mol Biol 70:184–190. https://doi.org/10.1016/j.ibmb.2016.01.003
Werckenthin A, Huber J, Arnold T et al (2020) Neither per, nor tim1, nor cry2 alone are essential components of the molecular circadian clockwork in the Madeira cockroach. PLoS ONE 15:1–26. https://doi.org/10.1371/journal.pone.0235930
Yuan Q, Metterville D, Briscoe AD, Reppert SM (2007) Insect cryptochromes: gene duplication and loss define diverse ways to construct insect circadian clocks. Mol Biol Evol 24:948–955. https://doi.org/10.1093/molbev/msm011
Zhang Y, Markert MJ, Groves SC et al (2017) Vertebrate-like CRYPTOCHROME 2 from monarch regulates circadian transcription via independent repression of CLOCK and BMAL1 activity. Proc Natl Acad Sci USA 114:E7516–E7525. https://doi.org/10.1073/pnas.1702014114
Acknowledgements
We thank Hana Vaněčková for technical support, and all current laboratory members for the excellent working atmosphere.
Funding
This work was supported by European Research Council (ERC) under the European Union’s Horizon 2020 Program Grant Agreement 726049 to DD. MMK has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 765937 (CINCHRON).
Author information
Authors and Affiliations
Contributions
MMK and DD designed the experiments. MMK performed the experiments, analyzed data, and drew the figures with input from DD. MMK and DD interpreted the data and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Charlotte Helfrich-Förster.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaniewska, M.M., Chvalová, D. & Dolezel, D. Impact of photoperiod and functional clock on male diapause in cryptochrome and pdf mutants in the linden bug Pyrrhocoris apterus. J Comp Physiol A 210, 575–584 (2024). https://doi.org/10.1007/s00359-023-01647-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-023-01647-5