Abstract
An aphidophagous ladybird, Platynaspidius maculosus (Weise) (Coleoptera: Coccinellidae), is originally distributed in China, Taiwan, and Vietnam. The ladybird has recently intruded into the southern and central parts of Japan. The present study found that the larvae of this ladybird preyed on three aphid species, Aphis spiraecola, Aphis gossypii, and Toxoptera citricidus (all Hemiptera: Aphididae), feeding on young shoots of various Citrus species in August to early October in Shizuoka Prefecture, central Japan. Laboratory rearing of the sampled larvae confirmed that the larvae completed their development (adult emergence) by consuming each of the three aphid species. The ladybird larvae were observed foraging in aphid colonies attended by one of the four ants, Lasius japonicus, Pristomyrmex punctatus, Formica japonica, and Camponotus japonicus (all Hymenoptera: Formicidae). Field observations revealed that the foraging/feeding larvae were almost completely ignored by honeydew-collecting ants even when they physically contacted each other. Thus, in Japan, the larvae of the exotic ladybird exploit colonies of the three aphid species attended by one of the four ant species on many Citrus species. On the basis of the results, I discuss the possibility of the ladybird’s reproduction on citrus trees in Japan, probable adaptations of the ladybird larvae to aphid-attending ants, and potential impacts of the ladybird on native insect enemies attacking ant-attended aphids on citrus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alien organisms have the potential to considerably diminish or even destroy populations of indigenous species occupying common ecological niches in the areas that they have invaded (e.g., Elton 1958; Mack et al. 2000; Williamson 1996). Establishment of exotic aphidophagous ladybirds and the following substantial reduction in the abundance of native ladybird species have been demonstrated in North America and Europe (e.g., Brown et al. 2011; Elliott et al. 1996; Evans 2004). Interspecific relationships between exotic and native aphidophagous insects, including ladybirds, lacewings, syrphids, and parasitoid wasps, are expected to be affected by their ecological characteristics, such as prey species, foraging habitats, seasonal occurrence, and tolerance to ants attending aphids.
An aphidophagous ladybird, Platynaspidius maculosus (Weise, 1910) (Coleoptera: Coccinellidae) is originally distributed in China, Taiwan, and Vietnam (Sasaji and Taniguchi 2003; Tao and Chiu 1971). The ladybird has recently intruded into the southern parts of Japan: Okinawa Island in 1989 (Matsubara et al. 1998) and then Kyushu Island in 2006 (Imasaka 2006). Thereafter, the ladybird was detected in Shizuoka City, located in the central portion of Honshu Island (the main island of Japan), in 2010 (Kaneko 2013). In addition, the capture of P. maculosus adults in 3 consecutive years, 2010–2012, in multiple citrus groves in Shizuoka City suggests that the ladybird has already become established in the area (Kaneko 2013). The exotic ladybird P. maculosus might exert negative impacts on the abundance of native aphidophagous insects in the areas that it has invaded. However, little has been elucidated about the ecological attributes of P. maculosus in Japan, including prey aphid species, host plant species, and interrelationships with aphid-attending ants.
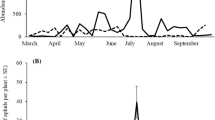
Monitoring using yellow sticky traps set inside citrus tree canopies in my previous surveys in 2010–2012 showed that P. maculosus adults occur continuously from late April through October in Shizuoka City, being more abundant in late spring (late April–mid June), mid-summer (early July–early August), and early autumn (early September–early October) (Kaneko 2013). During these periods, aphid densities on citrus trees are high because of abundant young citrus shoots available for the aphids (Korenaga et al. 1992). These facts imply that the exotic ladybird P. maculosus preys on aphids on citrus, thereby reproducing in citrus groves in Japan. However, the occurrence of the larvae of P. maculosus on citrus trees has not been confirmed in Japan, except a preliminary observation by Kaneko (2013). In addition, the prey aphid species of P. maculosus on citrus and the citrus species it utilizes have not been identified. Furthermore, many aphid species on citrus are frequently attended by honeydew-collecting ants in Japan (Kaneko 2003a, 2003b), but interactions between P. maculosus and aphid-attending ants have not been revealed.
In the present study, I surveyed the larvae of the ladybird P. maculosus in two citrus groves in Shizuoka Prefecture and recorded the prey aphid species that the larvae were feeding on, the citrus species being infested with the prey aphids, and the ant species attending the aphids. In addition, to examine developmental completion of P. maculosus larvae through preying on the aphid species, the larvae found in the citrus groves were sampled and then reared in the laboratory by providing them with the aphid species that they had been consuming on citrus trees. Behavioral interactions between P. maculosus larvae foraging in aphid colonies and honeydew-collecting ants in their encounters were also observed to assess the ability of P. maculosus to exploit ant-attended aphids. On the basis of the results, I discuss the possibility for the exotic ladybird P. maculosus to reproduce on citrus trees in Japan, probable adaptations of the ladybird larvae to various ants attending aphids, and potential impacts of the ladybird’s establishment on populations of native aphidophagous insects, especially those mainly attacking ant-attended aphids on citrus.
Materials and methods
Ladybird
The body of the P. maculosus adult is ca. 2–3 mm in length. The pronotum is black and the elytra are light red–orange, with two pairs of large black spots surrounding a smaller black spot (see the photo in Kaneko 2013). The larva has a peculiar feature: a flat, oval-shaped body, with the dorsum weakly convex and the entire outer margin very thin (Sasaji 1992, and see the photo in Kaneko 2013). The larva of P. maculosus resembles that of a native aphidophagous ladybird, Phymatosternus lewisii (Crotch); however, the two species can be distinguished by the presence of a pair of black spots on the pronotum of the P. maculosus larva (Sasaji 1998).
Occurrence of P. maculosus larvae on citrus trees and laboratory rearing
Larvae of P. maculosus were surveyed in a citrus grove cultivating Satsuma mandarin, Citrus unshiu Marcow. (Rutaceae), at Shizuoka Prefectural Fruit Tree Research Center (hereafter referred to as SPFTRC), located in Komagoe, Shizuoka City, in August to September in 2011–2012, and in a citrus grove containing various species and varieties, such as orange, C. sinensis (L.) Osbeck, and ‘Iyokan’, C. iyo Hort. ex Tan. (both Rutaceae), at Shizuoka Prefectural Izu Agriculture Research Center (hereafter referred to as SPIARC), located in Inatori, Higashi-Izu Town, in August to early October in 2013–2014.
When a second- or third-instar larva of P. maculosus (body length > 2 mm) was found, the aphid species that the larva was feeding on, the plant (citrus) species infested with the aphid colony, and the ant species attending the aphid colony were noted and then the larva was sampled. Each collected larva was reared in a plastic Petri dish (30 mm diameter, 10 mm depth) at an ambient temperature of 20–25 °C in the laboratory, with being provided daily with more than 20 nymphs of the aphid species that the ladybird larva had been preying on when it had been found. The aphids were collected from citrus trees in the groves where each aphid species was abundant enough to be used for the prey. Two to three weeks after the beginning of the rearing, adult emergence was determined for each larva. The proportion of the total (pooled) number of P. maculosus adults that emerged to the total (pooled) number of collected larvae (hereafter referred to as the adult emergence ratio) was calculated for each category (the study site, the plant species, the aphid species, and the ant species). Voucher specimens of the adults have been deposited at SPIARC.
Comparisons of the number of P. maculosus larvae per aphid colony were made between study sites, plant species, aphid species, and ant species using Mann–Whitney U test or Scheffe’s multiple comparison test with Kruskal–Wallis test.
Behavioral interactions between P. maculosus larvae and ants
I observed behavioral interactions between honeydew-collecting workers of each ant, Lasius japonicus Santschi and Pristomyrmex punctatus Smith (both Hymenoptera: Formicidae), and foraging or feeding larvae (third-instar) of P. maculosus when they encountered each other in colonies of the spirea aphid Aphis spiraecola Patch (Hemiptera: Aphididae) attended by either ant species on C. unshiu trees at SPFTRC in September 2011–2012. In addition, I noted behaviors of aphid-attending workers of each ant, Formica japonica Motschoulsky and Camponotus japonicus Mayr (both Hymenoptera: Formicidae), and P. maculosus larvae (third-instar) when encountering each other in colonies of the brown citrus aphid Toxoptera citricidus (Kirkaldy) (Hemiptera: Aphididae) attended by either ant species on citrus trees at SPIARC in August and September 2014.
In encounters between an ant worker and a P. maculosus larva, their behaviors were recorded as follows. The ant displayed either of the following two different behaviors to the larva: ignoring–the ant physically contacted the larva with the antennae or legs but otherwise did not respond to it, and antennal tapping–the ant made physical contact with the larva and tapped it with the antennae for a short period of time (5 to 10 s). The larva responded to the ant with either of the two distinct behaviors: ignoring–the larva continued its activities (feeding on aphids or walking) after the contact with the ant, and cowering–the larva pressed its body tightly against the plant surface when touched by the ant and then remained motionless for a short period of time (5 to 10 s).
A total of seven P. maculosus larvae were observed for each ant species, L. japonicus and P. punctatus, and five and four larvae for F. japonica and C. japonicus, respectively. Behavioral interactions were noted in ten encounters in a row for each P. maculosus larva; a total of 20–40 encounters per larva, with intervals of approximately 10 min between each 10–encounter set. In total 220, 200, 130, and 100 encounters were recorded for L. japonicus, P. punctatus, F. japonica, and C. japonicus, respectively.
Results
Occurrence of P. maculosus larvae on citrus trees and laboratory rearing
A total of 29 P. maculosus larvae (second– or third–instar) were collected in a citrus grove at SPFTRC in Komagoe, Shizuoka City, in 2011–2012, and a total of 91 larvae (second- or third-instar) were sampled in a citrus grove at SPIARC in Inatori, Higashi-Izu Town, in 2013–2014 (Table 1). The larvae recorded in 2011–2014 were found on a total of seven species of citrus (C. unshiu, C. sinensis, C. natsudaidai Hayata, C. hanaju Siebold ex Shirai, C. iyo, C. maxima Merr., and C. canaliculata Y. Tanaka) with colonies of the three aphid species (the cotton aphid Aphis gossypii Glover, A. spiraecola, and T. citricidus), which were being attended by one of the four ant species (L. japonicus, P. punctatus, F. japonica, and C. japonicus).
The number of P. maculosus larvae per aphid colony showed no significant difference between the two study sites (Table 2; p = 0.44, Mann–Whitney U test). The adult emergence ratio was almost the same between the two sites.
There were no clear differences among the seven plant species in the larval numbers per aphid colony (no significant difference among C. sinensis, C. unshiu, and C. iyo; p = 0.89, Kruskal–Wallis test) and in the adult emergence ratio (Table 3).
The larval numbers per aphid colony did not differ significantly between T. citricidus and A. spiraecola colonies (Table 4; p = 0.89, Mann–Whitney U test). The adult emergence ratio was high in colonies of both aphid species.
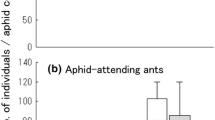
All the P. maculosus larvae were noted in aphid colonies attended by one of the four ant species (Table 5). The larval numbers per aphid colony was significantly smaller when being attended by L. japonicus than when attended by P. punctatus (p < 0.05, Scheffe’s multiple comparison test with Kruskal–Wallis test). The adult emergence ratio was similar with respect to the ant species that had been attending the aphid colonies from which the larva had been collected.
Behavioral interactions between P. maculosus larvae and ants
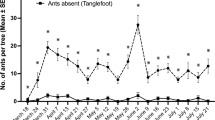
In 218 out of 220 encounters (99.1%) between a honeydew-collecting L. japonicus worker and a foraging/feeding P. maculosus larva (third-instar) in aphid colonies, the ant ignored the larva without exhibiting any aggressive behavior and the larva also ignored the ant, continuing the activity. In just two out of the 220 encounters (0.9%), a L. japonicus worker tapped a P. maculosus larva with the antennae for 5–10 s, and the larva cowered and remained motionless during the period, and then the ant walked away without showing any aggression. In all 200 encounters, a worker of P. punctatus ignored a P. maculosus larva and the larva also ignored the ant. A worker of F. japonica or C. japonicus ignored a P. maculosus larva that also ignored the ant in all 130 or 100 encounters, respectively. Thus, none of the four ant species treated P. maculosus larvae aggressively.
Discussion
Many studies have shown so far the occurrence of the adults of the exotic ladybird P. maculosus in Japan (Imasaka 2006; Ishikawa 2011; Kaneko 2013; Kido 2010; Kusakabe 2013; Matsubara et al. 1998; Sasaji and Taniguchi 2003). However, the larvae of P. maculosus have not been recorded in this country, except a preliminary observation by Kaneko (2013). The present survey detected a large number of P. maculosus larvae foraging in two citrus groves in central Japan (Table 1). Thus, in practice, this is the first study that unequivocally confirmed the occurrence of P. maculosus larvae in Japan.
Platynaspidius maculosus has been described as an aphidophagous coccinellid in Taiwan (Tao and Chiu 1971), but no information is available on its prey aphid species. The present study identified the prey aphid species of P. maculosus larvae in Japan, which were A. gossypii, A. spiraecola, and T. citricidus, on the basis of field observations in citrus groves and laboratory rearing (Table 1). The ladybird larvae were found almost exclusively in colonies of A. spiraecola and T. citricidus in August to early October. These two aphids are predominant among aphids on citrus trees in summer to early autumn, when A. gossypii is less abundant, in Japan (Korenaga et al. 1992). The same was true of the two citrus groves examined in this study (S. Kaneko, personal observation). These facts suggest that these two aphids are the main prey of P. maculosus larvae on citrus in summer to early autumn. On the other hand, in late April to early June, A. gossypii and A. spiraecola occur more abundantly on citrus (Korenaga et al. 1992), so P. maculosus larvae might exploit A. gossypii and A. spiraecola on citrus in late spring to early summer. The species composition of native aphidophagous insects on citrus trees differs among the three aphid species and the seasons (Kaneko 2003a, 2003b; S. Kaneko, personal observation). Therefore, the main prey aphid species of P. maculosus larvae on citrus and its seasonal differences need to be documented to assess the potential impact of P. maculosus on each native aphid enemy. On the other hand, the proportion of P. maculosus larvae that finally became adults did not differ largely between when being fed with A. spiraecola and with T. citricidus (Table 4). This result implies that the larvae complete their development at an almost equal possibility when consuming each of the two aphids. The nutritional values of the three aphid species as prey for P. maculosus larvae need to be compared through laboratory rearing.
Foraging P. maculosus larvae were recorded on a total of seven species of Citrus (Table 1). The three aphids noted as prey of P. maculosus larvae feed on various Citrus species and commonly occur on citrus trees from late April through October in Japan, although the seasonal prevalence in the occurrence differs among the three aphid species, as described above (Korenaga et al. 1992). These facts suggest that plants belonging to Citrus, on which the prey aphids of the ladybird are abundant for a relatively long period of time, are one of foraging habitats of the ladybird larvae in Japan. Furthermore, the adults of P. maculosus were caught on sticky traps set inside C. unshiu tree canopies almost continuously from late April through October in Japan (Kaneko 2013). The present study showed that P. maculosus larvae that were attacking aphids on citrus in August to early October could successfully become adults (Table 1). These results suggest that P. maculosus reproduces on citrus trees by preying on aphids, at least in mid-summer to early autumn in Japan. Also in late spring to early summer, P. maculosus might reproduce by exploiting A. gossypii and A. spiraecola, as stated above. Thus, the ladybird might utilize citrus trees for foraging and reproduction in late spring to early autumn in Japan. On the other hand, A. spiraecola and A. gossypii infest a wide range of plant species, including many agricultural crops (Moritsu 1983). It is therefore necessary to survey the occurrence of P. maculosus larvae on various plants other than citrus, as well as its prey aphid species, in Japan in order to determine its foraging/reproduction habitats in Japan that it has recently invaded.
The present study found that P. maculosus larvae foraged in aphid colonies that were attended by one of the four ants, L. japonicus, P. punctatus, F. japonica, and C. japonicus (Table 1). In addition, the field observations revealed that the larvae were almost completely ignored by honeydew-collecting workers of each ant species even when being touched by the ants’ antennae or legs. These results suggest that the ladybird larvae adopt some strategies to avoid being recognized as an aphid enemy by aphid-attending ants, irrespective of the ant species. To verify this suggestion, field observations on behavioral interactions between the ladybird larvae and aphid-attending ants in the ladybird’s original areas are needed. The larvae of two aphidophagous coccinellids, Platynaspis luteorubra Goeze and Phymatosternus lewisii are characterized by a flat, ‘coccid-like’ body shape and very short legs completely hidden under the thorax, just like P. maculosus larvae (Kaneko 2007a; Völkl 1995). Völkl (1995) documented that P. luteorubra larvae were totally disregarded by aphid-attending workers of the ant Lasius niger L. even when they physically contacted each other, suggesting that chemical camouflage of the larvae might contribute to the avoidance of ant aggression. The larvae of P. lewisii foraging in ant-attended aphid colonies are also never attacked by P. punctatus workers (Kaneko 2007a). The larvae of the three ladybirds are expected to have several strategies for preventing ant detection in common, including chemical (mimicry or camouflage), morphological (flat body and short legs), and behavioral (inconspicuous movements) adaptations to ants. Chemical substances on the larval body surface, such as epicuticular hydrocarbon, might act an important role in behavioral interactions with ants, as demonstrated for some aphid parasitoids (Akino and Yamaoka 1998; Liepert and Dettner 1993; Völkl et al. 1996). Comparison of the larval hydrocarbon compositions across the three ladybirds might lead to the identification of key substances that function for deceiving aggressive ants.
Significantly fewer P. maculosus larvae were observed in aphid colonies attended by L. japonicus than in those attended by P. punctatus (Table 5). A similar pattern has been reported for the larvae of the ladybird Scymnus posticalis Sicard, which are also ignored by the two ants in colonies of the three aphids (Kaneko 2003a, 2003b, 2007b). These studies suggested that the difference in S. posticalis larval numbers between the aphid colonies attended by each of the two ants would be caused by the difference between the two ants in aggressiveness against S. posticalis adult females laying eggs in/nearby aphid colonies and in the consequent exclusion effects on the adult females. Also in the case of P. maculosus, the two ants might exhibit different intensities of defensive responses against ovipositing adult females of the ladybird. To verify this hypothesis, behavioral interactions between aphid-attending ant workers and P. maculosus adult females in aphid colonies need to be compared between the two ants.
The present study implies that P. maculosus larvae primarily prey on aphids being attended by ants, just as the larvae of the ladybirds, P. luteorubra and P. lewisii, do (Kaneko 2007a; Völkl 1995). Völkl (1995) suggested that the more frequent occurrence of P. luteorubra larvae in ant-attended aphid colonies than in unattended colonies could be attributed chiefly to the protection of the larvae by ants against their parasitoids and intraguild predators and to the resulting preference of the ladybird for ant-attended aphids. On citrus trees in Japan, ant-attended colonies of the three prey aphids of P. maculosus (A. spiraecola, A. gossypii, and T. citricidus) are frequently exploited by the ladybird P. lewisii and other native enemies that are capable of avoiding ant attacks, including the ladybird S. posticalis and the parasitoid wasp Lysiphlebus japonicus Ashmead (Kaneko 2002, 2003b). Therefore, the colonization of citrus groves in Japan by P. maculosus might cause severe competition for ant-attended aphids between the exotic ladybird and these native, ant-adapted enemies, thereby significantly depressing the abundance of the natives. In fact, P. maculosus larvae and these enemies occurred in the same aphid colonies in the examined citrus groves (S. Kaneko, personal observation). Direct and indirect interactions between P. maculosus and the indigenous enemies in ant-attended aphid colonies on citrus trees need to be elucidated by performing detailed field observations and laboratory experiments.
References
Akino T, Yamaoka R (1998) Chemical mimicry in the root aphid parasitoid Paralipsis eikoae Yasumatsu (Hymenoptera: Aphidiidae) of the aphid-attending ant Lasius sakagamii Yamauchi & Hayashida (Hymenoptera: Formicidae). Chemoecology 8:153–161
Brown PMJ, Frost R, Doberski J, Sparks T, Harrington R, Roy HE (2011) Decline in native ladybirds in response to the arrival of Harmonia axyridis: early evidence from England. Ecol Entomol 36:231–240
Elliott N, Kieckhefer R, Kauffman W (1996) Effects of an invading coccinellid on native coccinellids in an agricultural landscape. Oecologia 105:537–544
Elton CS (1958) The ecology of invasions by animals and plants. Methuen, London
Evans EW (2004) Habitat displacement of North American ladybirds by an introduced species. Ecology 85:637–647
Imasaka S (2006) New record of Platynaspidius maculosus from Kyushu Island in Japan. Gekkan-Mushi 425:45 (in Japanese)
Ishikawa H (2011) New record of Platynaspidius maculosus from Shizuoka Prefecture in Japan. Gekkan-Mushi 487:46 (in Japanese)
Kaneko S (2002) Aphid-attending ants increase the number of emerging adults of the aphid’s primary parasitoid and hyperparasitoids by repelling intraguild predators. Entomol Sci 5:131–146
Kaneko S (2003a) Different impacts of two species of aphid-attending ants with different aggressiveness on the number of emerging adults of the aphid’s primary parasitoid and hyperparasitoids. Ecol Res 18:199–212
Kaneko S (2003b) Impacts of two ants, Lasius niger and Pristomyrmex pungens (Hymenoptera: Formicidae), attending the brown citrus aphid, Toxoptera citricidus (Homoptera: Aphididae), on the parasitism of the aphid by the primary parasitoid, Lysiphlebus japonicus (Hymenoptera: Aphidiidae), and its larval survival. Appl Entomol Zool 38:347–357
Kaneko S (2007a) Larvae of two ladybirds, Phymatosternus lewisii and Scymnus posticalis (Coleoptera: Coccinellidae), exploiting colonies of the brown citrus aphid Toxoptera citricidus (Homoptera: Aphididae) attended by the ant Pristomyrmex pungens (Hymenoptera: Formicidae). Appl Entomol Zool 42:181–187
Kaneko S (2007b) Predator and parasitoid attacking ant-attended aphids: effects of predator presence and attending ant species on emerging parasitoid numbers. Ecol Res 22:451–458
Kaneko S (2013) Occurrence of the exotic predatory ladybird Platynaspidius maculosus (Coleoptera: Coccinellidae) in citrus groves in Shizuoka City, Central Japan: seasonal prevalence of adults captured on sticky traps. Appl Entomol Zool 48:189–194
Kido K (2010) Overwintering by Platynaspidius maculosus in Fukuoka Prefecture in Japan. Gekkan-Mushi 475:47 (in Japanese)
Korenaga R, Koizumi M, Ushiyama K, Furuhashi K (1992) A handbook of diseases and insect pests of fruit trees, vol 1. Citrus, loquat, and kiwifruit. Japan Plant Protection Association, Tokyo (in Japanese)
Kusakabe Y (2013) New record of Platynaspidius maculosus from Kanagawa Prefecture in Japan. Sayabane New Ser 12:59 (in Japanese)
Liepert C, Dettner K (1993) Recognition of aphid parasitoids by honeydew-collecting ants: the role of cuticular lipids in a chemical mimicry system. J Chem Ecol 19:2143–2153
Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Matsubara Y, Ueno T, Sasaji H (1998) Platynaspidius maculosus found in Okinawa Island: new record from Japan. Gekkan-Mushi 332:38 (in Japanese)
Moritsu M (1983) Aphids of Japan in colors. Zenkoku Noson Kyoiku Kyokai, Tokyo (in Japanese)
Sasaji H (1992) Descriptions of four coccinellid larvae of Formosa, with the phylogenetic importance (Coleoptera: Coccinellidae). Memo Facul Educ Fukui Univ Ser II (Nat Sci) 42:1–11
Sasaji H (1998) Natural history of the ladybirds. University of Tokyo Press, Tokyo (in Japanese)
Sasaji H, Taniguchi M (2003) New record of Platynaspidius maculosus (Weise, 1910) from Japan. Gekkan-Mushi 391:13 (in Japanese)
Tao C, Chiu SC (1971) Biological control of citrus, vegetables and tobacco aphids. Taiwan Agricultural Research Institute Special Publication No. 10
Völkl W (1995) Behavioral and morphological adaptations of the coccinellid Platynaspis luteorubra for exploiting ant-attended resources (Coleoptera: Coccinellidae). J Insect Behav 8:653–670
Völkl W, Liepert C, Birnbach R, Hübner G, Dettner K (1996) Chemical and tactile communication between the root aphid parasitoid Paralipsis enervis and trophobiotic ants: consequences for parasitoid survival. Experientia 52:731–738
Williamson M (1996) Biological invasions. Chapman and Hall, London
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaneko, S. Larvae of the exotic predatory ladybird Platynaspidius maculosus (Coleoptera: Coccinellidae) on citrus trees: prey aphid species and behavioral interactions with aphid-attending ants in Japan. Appl Entomol Zool 53, 85–91 (2018). https://doi.org/10.1007/s13355-017-0531-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-017-0531-y