Abstract
Interaction between a predator and a parasitoid attacking ant-attended aphids was examined in a system on photinia plants, consisting of the aphid Aphis spiraecola, the two ants Lasius japonicus and Pristomyrmex pungens, the predatory ladybird beetle Scymnus posticalis, and the parasitoid wasp Lysiphlebus japonicus. The ladybird larvae are densely covered with waxy secretion and are never attacked by attending ants. The parasitoid females are often attacked by ants, but successfully oviposit by avoiding ants. The two ants differ in aggressiveness towards aphid enemies. Impacts of the predator larvae and attending ant species on the number of parasitoid adults emerging from mummies per aphid colony were assessed by manipulating the presence of the predator in introduced aphid colonies attended by either ant. The experiment showed a significant negative impact of the predator on emerging parasitoid numbers. This is due to consumption of healthy aphids by the predator and its predation on parasitized aphids containing the parasitoid larvae (intraguild predation). Additionally, attending ant species significantly affected emerging parasitoid numbers, with more parasitoids in P. pungens-attended colonies. This results from the lower extent of interference with parasitoid oviposition by the less aggressive P. pungens. Furthermore, the predator reduced emerging parasitoid numbers more when P. pungens attended aphids. This may be ascribed to larger numbers of the predator and the resulting higher levels of predation on unparasitized and parasitized aphids in P. pungens-attended colonies. In conclusion, a negative effect of the predator on the parasitoid occurs in ant-attended aphid colonies, and the intensity of the interaction is affected by ant species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many species of predators and parasitoids exploiting honeydew-producing homopterans such as aphids, scale insects and membracids are heavily attacked and excluded by ants attending the homopterans (e.g., Bartlett 1961; Banks 1962; Bristow 1984; Cushman and Whitham 1989; Vinson and Scarborough 1991; Jiggins et al. 1993; Itioka and Inoue 1996). However, some species of predators and parasitoids are able to utilize the homopterans that ants are guarding, through behavioral, chemical and/or morphological adaptations to avoid ant aggression (Pontin 1959; Eisner et al. 1978; Takada and Hashimoto 1985; Majerus 1989; Völkl 1992, 1995, 1997; Völkl and Vohland 1996; Völkl and Mackauer 2000; Barzman and Daane 2001). Additionally, honeydew-collecting ants provide such “ant-adapted” predators and parasitoids with protection from predators, parasitoids or hyperparasitoids (Völkl 1992, 1995; Cudjoe et al. 1993; Novak 1994; Kaneko 2002). Ant-adapted enemies exhibit preferential distribution for ant-attended homopteran colonies (e.g., Völkl 1992, 1995; Völkl and Stechmann 1998), possibly due to ant protection of their resources against their competitors and protection of themselves from their enemies. However, homopteran-attending ants are limited in number (Addicott 1978; Cushman and Addicott 1989; Cushman and Whitham 1991; Fischer et al. 2001), hence limiting the number of ant-attended homopteran colonies. Therefore, there is a possibility that ant-adapted predators and parasitoids compete for common resources in ant-attended homopteran colonies.
Predators and parasitoids sharing a prey/host insect species interact with each other in diverse manners (e.g., Fritz 1982; Abrahamson et al. 1989; Tscharntke 1992; Losey et al. 1997; Taylor et al. 1998). Parasitized insects that contain the immature stages (egg, larva and pupa) of parasitoids internally are often consumed by predators (e.g., Tostowaryk 1971; Rees and Onsager 1982; Jones 1987; Stark and Hopper 1988; Rosenheim et al. 1995). This type of predation is a form of intraguild predation (Polis et al. 1989). Intraguild predation on immature parasitoids has also been reported in many homopterans (Hagen and van den Bosch 1968; Wheeler et al. 1968; Quezada and DeBach 1973; Hoelmer et al. 1994; Novak 1994; Ferguson and Stiling 1996; Snyder and Ives 2001). Intraguild predation on immature parasitoids has a significant influence on parasitoid survival (Rosenheim et al. 1995; Brodeur and Rosenheim 2000; Meyhöfer and Hindayana 2000; Colfer and Rosenheim 2001; Snyder and Ives 2001). It is therefore hypothesized that the presence of ant-adapted predators reduces the number of parasitoid adults emerging from mummies in ant-attended homopteran colonies, through consumption of healthy homopterans as the parasitoids’ hosts and through intraguild predation on immature parasitoids.
Different species of ants attending a homopteran species show different levels of aggressiveness against the homopteran’s enemies and thereby different extents of interference with prey consumption by predators and oviposition by parasitoids (Bristow 1984; Buckley and Gullan 1991; Cudjoe et al. 1993; Völkl and Mackauer 1993; Stechmann et al. 1996; Itioka and Inoue 1999; Kaneko 2003a, b). Therefore, the effect of ant-adapted predators on the number of emerging parasitoids may differ depending on the ant species attending the homopteran.
In order to test the two hypotheses stated above, a field experiment was conducted on the plant Fraser photinia Photinia × fraseri Dress. (Rosaceae) harboring an aphid-centered system consisting of the spirea aphid Aphis spiraecola Patch (Homoptera: Aphididae), the two ants Lasius japonicus Santschi and Pristomyrmex pungens Mayr (both Hymenoptera: Formicidae), the small-sized predatory ladybird Scymnus posticalis Sicard (Coleoptera: Coccinellidae), and the parasitoid wasp Lysiphlebus japonicus Ashmead (Hymenoptera: Aphidiidae). The ladybird larvae and the parasitoid females are observed foraging more often in ant-attended than in unattended aphid colonies (Kaneko 2002). The ladybird larvae, which are densely covered with waxy secretion, are never attacked by aphid-attending ants (Kaneko 2002). Foraging females of the parasitoid are often attacked by ants, but successfully oviposit into aphids by moving swiftly and avoiding encounters with approaching ants (Kaneko 2002). The ladybird larvae prey on living parasitized aphids, though they cannot attack mummified aphids (Kaneko 2002). Workers of the ant P. pungens are less aggressive than those of the ant Lasius japonicus (Itioka and Inoue 1999), so that the parasitoid females stay longer and lay more eggs in P. pungens-attended than in Lasius japonicus-attended aphid colonies (Kaneko 2003a). In addition, more larvae of the ladybird were found in P. pungens-attended aphid colonies (Kaneko 2003a). Thus, this system is suitable to examine interactions between ant-adapted predators and parasitoids in homopteran colonies attended by different ant species. In this paper, I assess impacts of the predatory ladybird larvae and attending ant species on the number of parasitoid adults emerging from mummies in aphid colonies by manipulating the presence of the ladybird larvae in aphid colonies attended by either ant.
Methods
Study organisms
The Fraser photinia Photinia × fraseri (variety “Red Robin”) was raised in New Zealand, as a hybrid between P. glabra (Thunb.) Maxim. and P. serratifolia (Desf.) Kalkman. It is a medium-to-large sized evergreen shrub often used as a hedge plant. Brilliant red young shoots emerge in spring to summer in central Japan.
The spirea aphid Aphis spiraecola infests various plant species and is known as a harmful pest of tree fruits such as citrus, apple and Japanese pear (Moritsu 1983). It feeds on the phloem sap of young shoots and forms dense colonies, consisting of nymphs and adults, on the upper parts of shoots and on the lower surface of expanding leaves. The aphid is attacked by many species of predators and parasitoids (Korenaga et al. 1992).
The two ants Lasius japonicus [formerly described as Lasius niger (Linnaeus) in Japan] and Pristomyrmex pungens attend various species of honeydew-producing insects, and both ants attack and exclude many enemies of honeydew-producers (e.g., Itioka and Inoue 1999; Kaneko 2003a, b; Katayama and Suzuki 2003). In this paper, the names of the ant Lasius japonicus and the parasitoid Lysiphlebus japonicus are not abbreviated to avoid confusion.
The parasitoid wasp Lysiphlebus japonicus is a solitary endoparasitoid and a generalist species that attacks chiefly aphids belonging to the genera Aphis and Toxoptera (Takada 1968; Takanashi 1990). The parasitoid larvae mummify spirea aphids 6–7 days after being deposited, and the adults emerge form the mummies 3–4 days after mummification in early summer (S. Kaneko, unpublished observation).
The predatory ladybird Scymnus posticalis attacks many species of aphids. The adult females lay eggs inside or in the vicinity of aphid colonies, and the larvae require 5–6 days to pupate in early summer (S. Kaneko, unpublished observation).
Experimental design
I experimentally verified the two hypotheses stated above by manipulating the presence of larvae of the ladybird S. posticalis in aphid colonies attended by the two ant species on photinia shoots. The experiment was conducted using a photinia hedge (1.5 m ×30 m) in Shizuoka City, central Japan. Some individuals of the aphid A. spiraecola were found on the hedge, but they were removed using a paintbrush on 16 June 2005. A total of 28 A. spiraecola colonies were then introduced onto young photinia shoots; the distance between the introduced shoots was 0.3–0.7 m and the number of aphids per colony ranged from 20 to 50. Of the 28 colonies, 14 colonies were attended by the ant Lasius japonicus and the remaining 14 colonies were attended by another ant, P. pungens.
A single second-instar larva of the predator S. posticalis was released into each of 14 aphid colonies (7 colonies for each ant species) at the start of the experiment (17 June 2005), and afterwards other naturally occurring S. posticalis larvae were allowed to intrude into the colonies; these colonies are referred to as “predator-present” colonies. On the other hand, foraging S. posticalis larvae were removed, when found, from the remaining 14 aphid colonies (7 colonies for each ant) using a paintbrush at 6:00 a.m. and 6:00 p.m. everyday during the experimental period; these are referred to as “predator-absent” colonies.
From 17 June, at 2-day intervals, the numbers of aphid-attending workers of the two ants, living aphids, foraging larvae of the predator S. posticalis, and ovipositing females of the parasitoid Lysiphlebus japonicus were counted in each aphid colony. Other large-sized predators such as the ladybird Harmonia axyridis Pallas were observed only rarely during the experimental period. Mummified aphids on the examined shoots were collected on 27 June. The mummies were then reared in the laboratory, and 11 days later the number of mummies from which adults of the parasitoid emerged was counted for each colony. As only a few adults of hyperparasitoids—Syrphophagus sp. (Hymenoptera: Encyrtidae) and Pachyneuron aphidis (Bouché) (Hymenoptera: Pteromalidae)—emerged from the mummies, the data on the hyperparasitoids was omitted from the analysis.
Results
No clear difference in the number of aphid-attending workers between predator-present and predator-absent aphid colonies was found for either ant species for the first 3 days of the experiment (Fig. 1a). On the other hand, for both ant species, the number of living aphids in predator-present colonies continued to decline from the start of the experiment, whereas aphid numbers in predator-absent colonies either increased slightly or did not change for the first 5 days and then declined (Fig. 1b).
Changes in the mean number of a attending ant workers and b living aphids per colony of the aphid Aphis spiraecola attended by the two ants Lasius japonicus or Pristomyrmex pungens in the presence or absence of the predator Scymnus posticalis larvae. Colonies: Filled circles predator-present, Lasius japonicus-attended; open circles predator-absent, Lasius japonicus-attended; filled triangles predator-present, P. pungens-attended; open triangles predator-absent, P. pungens-attended. Vertical bars +1 SE
The number of foraging larvae of the predatory ladybird S. posticalis per surviving aphid colony gradually decreased for the first 5 days in aphid colonies attended by each ant species (Fig. 2). Larval numbers then started to increase and reached a peak on the 8th day. More larvae were observed in P. pungens-attended than in Lasius japonicus-attended colonies on the 6th and 8th day, although there was no significant difference in larval numbers between these colonies on each day (P >0.05; t-test).
Changes in the mean number of foraging larvae of the predator S. posticalis per aphid colony attended by the two ants Lasius japonicus or P. pungens. Colonies: Filled circles predator-present, Lasius japonicus-attended; open circles predator-absent, Lasius japonicus-attended; filled triangles predator-present, P. pungens-attended; open triangles predator-absent, P. pungens-attended. Vertical bars ± 1 SE
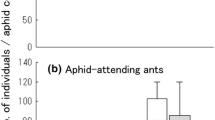
The cumulative number of ovipositing females of the parasitoid wasp Lysiphlebus japonicus recorded during the first 4 days was compared because only a small number of females was noted at each census. There was a tendency for more females to be found in P. pungens-attended than in Lasius japonicus-attended colonies (Fig. 3). However, no significant difference was detected for the effect of the presence of the predator, that of attending ant species, or that of interaction of the two factors (Table 1).
The effects of the presence of the predator S. posticalis larvae and attending ant species on the mean number of ovipositing females of the parasitoid Lysiphlebus japonicus per aphid colony. Colonies: Filled columns Lasius japonicus-attended; open columns P. pungens-attended. The cumulative number of females observed for the first 4 days of the experiment is shown. Vertical bars +1 SE
The presence of the predator larvae significantly reduced the number of adults of the parasitoid that emerged from the collected mummies per aphid colony (Fig. 4, Table 1). In addition, the attending ant species significantly affected emerging parasitoid numbers, with more parasitoids when P. pungens attended aphid colonies. Furthermore, the effect of interaction of the two factors was significant. Thus, the effect of the predator presence on emerging parasitoid numbers differed between the aphid colonies attended by the two ants, with the predator reducing parasitoid numbers more greatly in P. pungens-attended colonies.
The effects of the presence of the predator S. posticalis larvae and attending ant species on the mean number of adults of the parasitoid Lysiphlebus japonicus that emerged from collected mummies per aphid colony. Colonies: Filled columns Lasius japonicus-attended; open columns P. pungens-attended.Vertical bars +1 SE
Discussion
The present study suggests that the more rapid reduction in aphid numbers in predator-present than in predator-absent colonies for both attending ant species (Fig. 1b) is due exclusively to predation by the ladybird S. posticalis larvae. This difference in aphid numbers would not be related to ant numbers because no clear difference was found in ant numbers between these colonies early in the experiment (Fig. 1a). The ladybird larval numbers in aphid colonies attended by each ant species changed greatly during the experimental period (Fig. 2). The gradual decrease in larval numbers during the first half of the experiment probably occurred because the released larvae had fully grown by feeding on aphids and then left the aphid colonies to pupate, whereas increased larval numbers during the latter half would be caused by intrusion of naturally occurring larvae into the aphid colonies.
This study experimentally revealed that larvae of the ladybird S. posticalis significantly reduce the number of emerging adults of the parasitoid Lysiphlebus japonicus in ant-attended aphid colonies (Table 1). Some studies have suggested that parasitoid females visit aphid colonies bearing predatory ladybirds for oviposition less preferentially, to avoid intraguild predation on their offspring (Taylor et al. 1998; Raymond et al. 2000; Nakashima and Senoo 2003). The present experiment, however, detected no significant difference in numbers of parasitoid females ovipositing in ant-attended aphid colonies, with respect to the presence of the ladybird larvae (Table 1). This result implies that the parasitoid females laid eggs even in the ladybird-present aphid colonies. Therefore, the greatly reduced emerging parasitoid numbers in the predator-present aphid colonies is considered to result from consumption by the ladybird not only of healthy aphids but also of parasitized aphids containing the parasitoid larvae. Thus, in addition to exploitative competition, intraguild predation on immature parasitoids seems to function between the predator and the parasitoid in ant-attended aphid colonies. In conclusion, this study supports the hypothesis stated above that ant-adapted predators reduce emerging parasitoid numbers in ant-attended homopteran colonies through competition for the homopterans and predation on the parasitoids.
One of the advantages that ant-adapted predators and parasitoids gain through exploiting ant-attended homopterans is the lower mortality risk for their immatures feeding in the homopteran colonies owing to protection by ants from their enemies, i.e., intraguild predators and hyperparasitoids (Völkl 1992, 1995; Cudjoe et al. 1993; Novak 1994). Kaneko (2002) also showed that ants attending the cotton aphid Aphis gossypii Glover provided immatures of the ant-adapted parasitoid Lysiphlebus japonicus inside parasitized aphids with protection from hyperparasitoids and intraguild predators such as the large ladybird H. axyridis. Aphid-attending ants also protect the ant-adapted ladybird S. posticalis larvae from intraguild predation by the ladybird Coccinella septempunctata bruckii Mulsant (Takizawa and Yasuda 2005). Another advantage for such ant-adapted enemies utilizing ant-attended homopterans is the higher prey/host availability resulting from exclusion of their potential competitors by ants from the homopteran colonies. Thus, ant-attended homopteran colonies are “enemy-free space (Jeffries and Lawton 1984)” and “competitor-free space” for ant-adapted enemies. However, the result of the present study, i.e., that the predator S. posticalis diminished the number of emerging adults of the parasitoid Lysiphlebus japonicus in ant-attended aphid colonies, suggests that ant-attended aphid colonies are not complete enemy/competitor-free space for the ant-adapted parasitoid.
Recently, many studies have reported the presence of predators and parasitoids (also hyperparasitoids) that can exploit honeydew-producing homopterans protected by ants (Völkl 1992, 1995; Völkl et al. 1994, 1996; Völkl and Vohland 1996; Sloggett et al. 1998; Barzman and Daane 2001; Kaneko 2002). It is therefore expected that many other species of ant-adapted enemies exist and that a single homopteran species is utilized by multiple species of ant-adapted enemies. Competition and intraguild predation between predators and parasitoids in ant-attended homopteran colonies might occur more commonly than we have previously thought. Kaneko (2002) indicated that numbers of mummies formed by the parasitoid Lysiphlebus japonicus in ant-attended colonies of the cotton aphid A. gossypii was not significantly different between colonies where the ladybird S. posticalis larvae were abundant and colonies where they were present in small numbers. The ladybird prefers the spirea aphid A. spiraecola to other aphids such as A. gossypii and the brown citrus aphid Toxoptera citricidus (Kirkaldy) on citrus shoots (Komazaki 2004). Therefore, the number of aphid individuals consumed by the ladybird larvae may differ between the two aphids and this difference may lead to the different results obtained. Thus, the effect of prey homopteran species on the intensity of interaction between ant-adapted predators and parasitoids needs to be considered.
This study indicated that numbers of emerging adults of the parasitoid Lysiphlebus japonicus differed significantly between A. spiraecola colonies attended by the two ants, with more parasitoids in P. pungens-attended than in Lasius japonicus-attended colonies (Table 1). The same result was obtained in a different aphid: A. gossypii (Kaneko 2003a). Itioka and Inoue (1999) showed that P. pungens workers were less aggressive against enemies of mealybugs that they attended than were workers of Lasius japonicus. Kaneko (2003a) reported that P. pungens workers attending A. gossypii attacked and disturbed ovipositing females of the parasitoid Lysiphlebus japonicus less frequently than did Lasius japonicus, so that females stayed longer and laid more eggs in P. pungens-attended aphid colonies. In addition, more parasitoid females were observed foraging in P. pungens-attended colonies (Kaneko 2003a); the same tendency was found in the present experiment (Fig. 3). Thus, the levels of aggressiveness of the attending ant species have a strong influence on the parasitoid’s oviposition success and the resulting offspring numbers in aphid colonies.
Larvae of the ladybird S. posticalis foraging in A. gossypii colonies are ignored and are never attacked by either Lasius japonicus or P. pungens workers attending the aphids (Kaneko 2002, 2003a). Pope (1979) proposed that the wax covering of Scymnus larvae was an adaptation against ant aggression. Nevertheless, the present study showed that ladybird larval numbers differed between A. spiraecola colonies attended by the two ants during the latter half of the experiment, with more larvae in P. pungens-attended colonies (Fig. 2). A similar result was found in A. gossypii colonies (Kaneko 2003a). This difference in ladybird larval numbers might be because the ladybird adult females, which are often attacked by aphid-attending ants (S. Kaneko, unpublished observation), deposit more eggs in aphid colonies attended by less aggressive P. pungens workers, which would interfere with ladybird oviposition to a lower extent. Alternatively, the more aggressive and exclusive Lasius japonicus workers may be more likely to remove or consume the deposited ladybird eggs. Thus, the numbers of ladybird larvae and their prey consumption in aphid colonies might also be affected by the difference in aggressiveness between the two ants.
The present experiment supported another hypothesis, i.e., that impact of ant-adapted predators on emerging parasitoid numbers varies depending on the ant species attending the aphids; ladybird larvae reduced emerging parasitoid numbers to a greater extent when P. pungens attended aphids than when Lasius japonicus did (Fig. 4). This is possibly caused by the larger numbers of ladybird larvae (Fig. 2) and the resulting higher levels of predation on both healthy aphids and parasitized aphids containing the parasitoid larvae in colonies attended by the less aggressive P. pungens. Thus, foraging or oviposition activities of the predator and the parasitoid are higher in P. pungens-attended than in Lasius japonicus-attended aphid colonies due to the lower degree of interference by P. pungens and, consequently, more intense competitive interaction seems to occur between these enemies in P. pungens-attended colonies (Fig. 5). This result suggests that the levels of aggressiveness of aphid-attending ant species influence the intensity of competition between ant-adapted predators and parasitoids indirectly through affecting prey consumption or oviposition success of each enemy in aphid colonies, and therefore that more intense competition may occur in aphid colonies attended by less aggressive ants that impose a smaller reduction in prey/host availability for the enemies.
Many studies have documented that the levels of aggressiveness of homopteran-attending ants affect their defensive abilities against arthropod homopteran enemies, thereby influencing homopteran population density and dynamics (e.g., Addicott 1979; Bristow 1984; Buckley and Gullan 1991; Cudjoe et al. 1993; Itioka and Inoue 1999). Kaneko (2003a) showed that the less aggressive ant P. pungens less effectively excluded ovipositing hyperparasitoid females from attended A. gossypii colonies than did the ant Lasius japonicus, leading to lower survival of the parasitoid Lysiphlebus japonicus larvae caused by higher hyperparasitism. This result of Kaneko (2003a) and the result of the present study suggest that levels of ant aggressiveness might play an important role in determining the abundance of predators and parasitoids exploiting ant-attended homopterans, not only by directly affecting foraging/oviposition success of each enemy but also indirectly through changing the intensity of interactions between the enemies. The effect of ant aggressiveness levels on abundance of homopteran enemies is worthy of more attention and should be examined in various ant-associated homopterans such as aphids, mealybugs and scale insects.
References
Abrahamson WG, Sattler JF, McCrea KD, Weis AE (1989) Variation in selection pressures on the goldenrod gall fly and the competitive interactions of its natural enemies. Oecologia 79:15–22
Addicott JF (1978) Competition for mutualists: aphids and ants. Can J Zool 56:2093–2096
Addicott JF (1979) A multispecies aphid-ant association: density dependence and species-specific effects. Can J Zool 57:558–569
Banks CJ (1962) Effects of the ant Lasius niger (L.) on insects preying on small populations of Aphis fabae Scop. on bean plants. Ann Appl Biol 50:669–679
Bartlett BR (1961) The influence of ants upon parasites, predators, and scale insects. Ann Entomol Soc Am 54:543–551
Barzman MS, Daane KM (2001) Host-handling behaviours in parasitoids of the black scale: a case for ant-mediated evolution. J Anim Ecol 70:237–247
Bristow CM (1984) Differential benefits from ant attendance to two species of Homoptera on New York ironweed. J Anim Ecol 53:715–726
Brodeur J, Rosenheim JA (2000) Intraguild interactions in aphid parasitoids. Entomol Exp Appl 97:93–108
Buckley RC, Gullan P (1991) More aggressive ant species (Hymenoptera: Formicidae) provide better protection for soft scales and mealybugs (Homoptera: Coccidae, Pseudococcidae). Biotropica 23:282–286
Colfer RG, Rosenheim JA (2001) Predation on immature parasitoids and its impact on aphid suppression. Oecologia 126:292–304
Cudjoe AR, Neuenschwander P, Copland MJW (1993) Interference by ants in biological control of the cassava mealybug Phenacoccus manihoti (Hemiptera: Pseudococcidae) in Ghana. Bull Entomol Res 83:15–22
Cushman JH, Addicott JF (1989) Intra- and interspecific competition for mutualists: ants as a limited and limiting resource for aphids. Oecologia 79:315–321
Cushman JH, Whitham TG (1989) Conditional mutualism in a membracid-ant association: temporal, age-specific, and density-dependent effects. Ecology 70:1040–1047
Cushman JH, Whitham TG (1991) Competition mediating the outcome of a mutualism: protective services of ants as a limiting resource for membracids. Am Nat 138:851–865
Eisner T, Hicks K, Eisner M, Robson DS (1978) “Wolf-in-sheep’s-clothing” strategy of a predaceous insect larva. Science 199:790–794
Ferguson KI, Stiling P (1996) Non-additive effects of multiple natural enemies on aphid populations. Oecologia 108:375–379
Fischer MK, Hoffmann KH, Völkl W (2001) Competition for mutualists in an ant-homopteran interaction mediated by hierarchies of ant attendance. Oikos 92:531–541
Fritz RS (1982) Selection for host modification by insect parasitoids. Evolution 36:283–288
Hagen KS, van den Bosch R (1968) Impact of pathogens, parasites, and predators on aphids. Annu Rev Entomol 13:325–384
Hoelmer KA, Osborne LS, Yokomi RK (1994) Interactions of the whitefly predator Delphastus pusillus (Coleoptera: Coccinellidae) with parasitized sweetpotato whitefly (Homoptera: Aleyrodidae). Environ Entomol 23:136–139
Itioka T, Inoue T (1996) The consequences of ant-attendance to the biological control of the red wax scale insect Ceroplastes rubens by Anicetus beneficus. J Appl Ecol 33:609–618
Itioka T, Inoue T (1999) The alternation of mutualistic ant species affects the population growth of their trophobiont mealybug. Ecography 22:169–177
Jeffries MJ, Lawton JH (1984) Enemy free space and the structure of ecological communities. Biol J Linn Soc 23:269–286
Jiggins C, Majerus MEN, Gough U (1993) Ant defence of colonies of Aphis fabae Scopoli (Hemiptera: Aphididae), against predation by ladybirds. Br J Entomol Nat Hist 6:129–137
Jones RE (1987) Ants, parasitoids, and the cabbage butterfly Pieris rapae. J Anim Ecol 56:739–749
Kaneko S (2002) Aphid-attending ants increase the number of emerging adults of the aphid’s primary parasitoid and hyperparasitoids by repelling intraguild predators. Entomol Sci 5:131–146
Kaneko S (2003a) Different impacts of two species of aphid-attending ants with different aggressiveness on the number of emerging adults of the aphid’s primary parasitoid and hyperparasitoids. Ecol Res 18:199–212
Kaneko S (2003b) Impacts of two ants, Lasius niger and Pristomyrmex pungens (Hymenoptera: Formicidae), attending the brown citrus aphid, Toxoptera citricidus (Homoptera: Aphididae), on the parasitism of the aphid by the primary parasitoid, Lysiphlebus japonicus (Hymenoptera: Aphidiidae), and its larval survival. Appl Entomol Zool 38:347–357
Katayama N, Suzuki N (2003) Bodyguard effects for aphids of Aphis craccivora Koch (Homoptera: Aphididae) as related to the activity of two ant species, Tetramorium caespitum Linnaeus (Hymenoptera: Formicidae) and Lasius niger L: (Hymenoptera: Formicidae). Appl Entomol Zool 38:427–433
Komazaki S (2004) The ladybird Scymnus posticalis. In: Nousan Gyoson bunka kyoukai (eds) A dictionary of natural enemies in Japan: ecology and exploitation (in Japanese). Nousan Gyoson bunka kyoukai, Tokyo, pp 637–640
Korenaga R, Koizumi M, Ushiyama K, Furuhashi K (eds) (1992) A handbook of diseases and insect pests of fruit trees, vol 1: citrus, loquat and kiwifruit (in Japanese). Japan Plant Protection Association, Tokyo
Losey JE, Ives AR, Harmon J, Ballantyne F, Brown C (1997) A polymorphism maintained by opposite patterns of parasitism and predation. Nature 388:269–272
Majerus MEN (1989) Coccinella magnifica (Redtenbacher)—a myrmecophilous ladybird. Br J Entomol Nat Hist 2:97–106
Meyhöfer R, Hindayana D (2000) Effects of intraguild predation on aphid parasitoid survival. Entomol Exp Appl 97:115–122
Moritsu M (1983) Aphids of Japan in colors (in Japanese). Zenkoku Noson Kyoiku Kyokai, Tokyo
Nakashima Y, Senoo N (2003) Avoidance of ladybird trails by an aphid parasitoid Aphidius ervi: active period and effects of prior oviposition experience. Entomol Exp Appl 109:163–166
Novak H (1994) The influence of ant attendance on larval parasitism in hawthorn psyllids (Homoptera: Psyllidae). Oecologia 99:72–78
Polis GA, Myers CA, Holt R (1989) The evolution and ecology of intraguild predation: competitors that eat each other. Annu Rev Ecol Syst 20:297–330
Pontin AJ (1959) Some records of predators and parasites adapted to attack aphids attended by ants. Entomol Mon Mag 95:154–155
Pope RD (1979) Wax production by coccinellid larvae (Coleoptera). Syst Entomol 4:171–196
Quezada JR, DeBach P (1973) Bioecological and population studies of the cottony-cushion scale, Icerya purchasi Mask., and its natural enemies, Rodolia cardinalis Mul. and Cryptochaetum iceryae Will., in southern California. Hilgardia 41:631–688
Raymond B, Darby AC, Douglas AE (2000) Intraguild predators and the spatial distribution of a parasitoid. Oecologia 124:367–372
Rees NE, Onsager JA (1982) Influence of predators on the efficiency of the Blaesoxipha spp parasites of the migratory grasshopper. Environ Entomol 11:426–428
Rosenheim JA, Kaya HK, Ehler LE, Marois JJ, Jaffee BA (1995) Intraguild predation among biological-control agents: theory and evidence. Biol Control 5:303–335
Sloggett JJ, Wood RA, Majerus MEN (1998) Adaptations of Coccinella magnifica Redtenbacher, a myrmecophilous coccinellid, to aggression by wood ants (Formica rufa group). I. Adult behavioral adaptation, its ecological context and evolution. J Insect Behav 11:889–904
Snyder WE, Ives AR (2001) Generalist predators disrupt biological control by a specialist parasitoid. Ecology 82:705–716
Stark SB, Hopper KR (1988) Chrysoperla carnea predation on Heliothis virescens larvae parasitized by Microplitis croceipes. Entomol Exp Appl 48:69–72
Stechmann DH, Völkl W, Starý P (1996) Ant-attendance as a critical factor in the biological control of the banana aphid Pentalonia nigronervosa Coq. (Hom. Aphididae) in Oceania. J Appl Entomol 120:119–123
Takada H (1968) Aphidiidae of Japan (Hymenoptera). Insect Matsum 30:67–124
Takada H, Hashimoto Y (1985) Association of the root aphid parasitoids Aclitus sappaphis and Paralipsis eikoae (Hymenoptera: Aphidiidae) with the aphid-attending ants Pheidole fervida and Lasius niger (Hymenoptera: Formicidae). Kontyû 53:150–160
Takanashi M (1990) Development and reproductive ability of Lysiphlebus japonicus Ashmead (Hymenoptera: Aphidiidae) parasitizing the citrus brown aphid, Toxoptera citricidus (Kirkaldy) (Homoptera: Aphididae) (in Japanese with English summary). Jpn J Appl Entomol Zool 34:237–243
Takizawa T, Yasuda H (2005) Relative strength of direct and indirect interactions of mutualistic ants and a large sized ladybird on the fate of two small sized ladybirds. In: Hirose Y, et al. (eds) Proceedings of international symposium on biological control of aphids and coccids 2005. Faculty of Agriculture, Yamagata University, Yamagata, pp 134–136
Taylor AJ, Müller CB, Godfray HCJ (1998) Effect of aphid predators on oviposition behavior of aphid parasitoids. J Insect Behav 11:297–302
Tostowaryk W (1971) Relationship between parasitism and predation of diprionid sawflies. Ann Entomol Soc Am 64:1424–1427
Tscharntke T (1992) Cascade effects among four trophic levels: bird predation on galls affects density-dependent parasitism. Ecology 73:1689–1698
Vinson SB, Scarborough TA (1991) Interactions between Solenopsis invicta (Hymenoptera: Formicidae), Rhopalosiphum maidis (Homoptera: Aphididae), and the parasitoid Lysiphlebus testaceipes Cresson (Hymenoptera: Aphidiidae). Ann Entomol Soc Am 84:158–164
Völkl W (1992) Aphids or their parasitoids: who actually benefits from ant-attendance? J Anim Ecol 61:273–281
Völkl W (1995) Behavioral and morphological adaptations of the coccinellid Platynaspis luteorubra for exploiting ant-attended resources (Coleoptera: Coccinellidae). J Insect Behav 8:653–670
Völkl W (1997) Interactions between ants and aphid parasitoids: patterns and consequences for resource utilization. In: Dettner K, Bauer G, Völkl W (eds) Vertical food web interactions, ecological studies, vol 130. Springer, Berlin Heidelberg New York, pp 225–240
Völkl W, Mackauer M (1993) Interactions between ants attending Aphis fabae ssp. cirsiiacanthoidis on thistles and foraging parasitoid wasps. J Insect Behav 6:301–312
Völkl W, Mackauer M (2000) The oviposition behaviour of aphidiine wasps (Hymenoptera: Braconidae, Aphidiinae): morphological adaptations and evolutionary traits. Can Entomol 132:197–212
Völkl W, Stechmann DH (1998) Parasitism of the black bean aphid (Aphis fabae) by the Lysiphlebus fabarum (Hym., Aphidiidae): the influence of host plant and habitat. J Appl Entomol 122:201–206
Völkl W, Vohland K (1996) Wax covers in larvae of two Scymnus species: do they enhance coccinellid larval survival? Oecologia 107:498–503
Völkl W, Hübner G, Dettner K (1994) Interactions between Alloxysta brevis (Hymenoptera, Cynipoidea, Alloxystidae) and honeydew-collecting ants: how an aphid hyperparasitoid overcomes ant aggression by chemical defense. J Chem Ecol 20:2901–2915
Völkl W, Liepert C, Birnbach R, Hübner G, Dettner K (1996) Chemical and tactile communication between the root aphid parasitoid Paralipsis enervis and trophobiotic ants: consequences for parasitoid survival. Experientia 52:731–738
Wheeler AG, Hayes JT, Stephens JL (1968) Insect predators of mummified pea aphids. Can Entomol 100:221–222
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kaneko, S. Predator and parasitoid attacking ant-attended aphids: effects of predator presence and attending ant species on emerging parasitoid numbers. Ecol Res 22, 451–458 (2007). https://doi.org/10.1007/s11284-006-0025-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-006-0025-9