Abstract
A total of fifteen saturated fatty acid esters were newly identified from the secretions of an unidentified Anaulaciulus sp. (Julida: Julidae). The fatty acid components of the esters were composed of normal chain acids (from C10 to C14) and of branched chain acids (from iso-C12 to iso-C15 and anteiso-C15). The alcohol moieties were all composed of normal chain alcohols varying from n-butanol to n-octanol. The most abundant component found in the total esters was n-hexyl laurate (64.7%). Novel compounds identified from the millipede secretion extracts include six branched iso- and anteiso-fatty esters, an odd-numbered C11-fatty acid ester, a C13-fatty acid ester, and a C7-alcohol ester, all of which were previously undescribed natural products. In addition, a characteristic mixture of benzoquinones, such as 2-methyl-1,4-benzoquinone, 2-methoxy-3-methyl-1,4-benzoquinone, 2,3-dimethoxy-1,4-benzoquinone, 2-methoxy-6-methyl-1,4-benzoquinone, and 2,3-dimethoxy-5-methyl-1,4-benzoquinone were identified from the secretions, together with trace amounts of 1,4-benzoquinone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most millipedes (Diplopoda) possess defensive glands in the form of integumental sacs arranged segmentally along the length of the body, from which they discharge secretory fluids when disturbed. The fluids have been studied in a wide variety of millipedes, and the order specificity for the distribution of the major components has been elucidated (Eisner et al., 1978; Kuwahara, 1999). The major components identified include cyanogenic compounds (order Polydesmida) (Blum and Woodring, 1962; Ômura et al., 2002a), phenolic compounds (some polydesmid species) (Blum et al., 1973; Duffey et al., 1977; Ômura et al., 2002b), 1,4-benzoquinones (orders Julida, Spirobolida, and Spirostreptida) (Monro et al., 1962; Röper and Heyns, 1977; Kuwahara et al., 2002), and alkaloids (orders Glomerida and Polyzoniida) (Meinwald et al., 1966; Meinwald et al., 1975; Wood et al., 2000; Kuwahara et al., 2007). These compounds are adhesive, irritating, or toxic, thus contributing to the millipede’s chemical defense against various predators (Eisner and Meinwald, 1966; Pasteels et al., 1983).
Millipedes in genus Anaulaciulus are distributed in East Asia (Attems, 1909), and more than twenty species are known in Japan (Shinohara, 1990). In the present paper, we focused our research on a species known as “fuji yasude” in Japanese, Anaulaciulus sp. (Julida: Julidae). There is little published information on secretions of East Asian julid species. Here, we describe detailed characterization of a set of fatty esters by GC-MS, NMR and syntheses for Anaulaciulus sp., including new natural products comprised of branched or odd-numbered fatty acids and an odd-numbered alcohol. We also detected several 1,4-benzoquinone derivatives in the defensive volatile secretions. Excluding common defensive benzoquinones, secondary components such as aliphatic esters may be useful as chemotaxonomic characters for the classification of East Asian Julida.
Materials and Methods
General Procedures
The gas chromatography-mass spectroscopic (GC-MS) analyses were performed on a Network GC System (6890N; Agilent Technologies Inc.) coupled with a mass selective detector (5975 Inert XL; Agilent Technologies Inc.) operated at 70 eV, using an HP-5MS capillary column (Agilent Technologies Inc, 0.25 mm I.D. × 30 m, 0.25 μm film thickness). Helium was used as the carrier gas at a constant flow rate of 1.00 ml/min. Samples were analyzed in the splitless mode with temperature programmed to change from 60°C to 290°C at 10°C/min after an initial 2 min hold, and a final hold at 290°C for 5 min.
GC and GC-MS data were recorded using Chemstation (Agilent Technologies Inc.) with reference to an MS database (Agilent NIST05 mass spectral library, Agilent Technologies Inc.). 1H NMR spectra (400 MHz, TMS at δ = 0.00 as internal standard) and 13 C NMR spectra (100 MHz, CDCl3 at δ = 77.0 as internal standard) were recorded on a Bruker Biospin AC400M spectrometer. All chemicals and solvents used for analyses, extraction and syntheses, except CDCl3, were of reagent grade (Sigma-Aldrich Co., Ltd., Tokyo, Japan and Wako Pure Chemical Industries, Ltd., Osaka, Japan). Column chromatography was performed on Wakosil silica gel C-200 with the specified solvents.
Millipedes
Anaulaciulus sp. millipedes were collected as nymphs and adults from leaf litter on the campus of Kyoto Gakuen University in the winter of 2008 (voucher specimens (6 males, 4 females and a juvenile) have been deposited as D5124 in Faculty of Education, Kumamoto University, Japan). The millipedes were fed and kept alive in the laboratory on decaying plant material.
Extraction of Secretions and Body Surface Components
The sex of each millipede was determined, and then they were individually treated as described below. Millipedes were squeezed with forceps causing emission of defensive secretion, which was collected in glass capillary tubes (0.5 mm id, hand-made), and extracted with hexane (0.1 ml) for 3 min. Body surface components of 1) frozen dead millipedes (-80°C for 1 h) and, 2) freshly decapitated millipedes were each extracted with 0.5 ml of hexane for 3 min. One μl portions of the resulting hexane extracts were analyzed by GC-MS.
Conventional Extraction and Collection of Ester Fraction
In order to isolate the ester fraction, twelve adults were dipped in 1 ml of hexane for 3 min, the extract was loaded onto a SiO2 (1.0 g) column, and eluted stepwise with the addition of 5 ml diethyl ether (1, 5, 10, 20, and 50%) in hexane. The resulting esters (2 mg) were recovered from the 5% ether-hexane fraction.
Syntheses of Standard Esters
1-Hexanol (1.0 g, 10.0 mmol) and lauric acid (100 mg, 0.5 mmol) were heated at 80°C for 1 h with a drop of sulfuric acid as a catalyst. After cooling to room temperature, the residual oil was chromatographed over SiO2 (10 g, hexane:ethyl acetate, 10:1) to give n-hexyl laurate (compound 7) (114 mg, 80%) as a colorless oil. GC-MS t R: 18.52 min. 1H NMR (CDCl3, δppm): 0.88 (t, 3H, J = 6.6 Hz, CH 3), 0.90 (t, 3H, J = 6.8 Hz, CH 3),1.26-1.36 (m, 22H, CH 2), 1.58-1.63 (m, 4H, CH 2), 2.29 (t, J = 7.6 Hz, 2H, CH 2CO), 4.06 (t, J = 6.6 Hz, 2H, CH 2O); 13 C NMR (CDCl3, δppm): 174.0, 64.4, 34.4, 31.9, 31.4, 29.6 (x 2), 29.5, 29.3 (x 2), 29.2, 28.6, 25,6, 25.0, 22.7, 22.5, 14.1, 14.0.
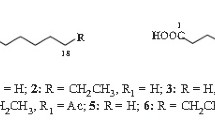
Syntheses of the remaining 14 esters were achieved using the same procedure employed in the synthesis of n-hexyl laurate (compound 7) via the reaction between the corresponding carboxylic acids and alcohols (see Table 2). The esters synthesized using this method included n-hexyl caprate (compound 1), n-butyl laurate (compound 2), n-hexyl n-undecanoate (compound 3), n-pentyl laurate (compound 4), n-pentyl 11-methyldodecanoate (compound 6), n-hexyl laurate (compound 7), n-hexyl 11-methyldodecanoate (compound 8), n-heptyl laurate (compound 9), n-hexyl n-tridecanoate (compound 10), n-hexyl 12-methyltridecanoate (compound 11), n-octyl laurate (compound 12), n-hexyl myristate (compound 13), n-hexyl 13-methyltetradecanoate (compound 14), and n-butyl 12-methyltetradecanoate (compound 15). The preparation of n-hexyl 10-methylundecanoate (compound 5) by the same synthetic procedure was not possible as 10-methylundecanoic acid was not commercially available. The ester products were subsequently identified by GC-MS analysis without silica gel column purification.
Results
The prominent feature from the GC analyses of the millipede extract was the presence of fifteen compounds (compounds 1–15), with multiple minor compounds recorded in the less-volatile range (t R > 16 min by GC-MS, 55.4% of total compound responses), together with seven compounds (compounds A–G) recorded in the volatile range (t R < 13 min by GC-MS, 44.6% of total compound responses) (Fig. 1). No differences were observed in profiles of components between sexes, nor between the whole body extracts and the defensive secretions. The extracts from frozen and decapitated millipedes did not contain any compounds (data not shown), indicating negligible hexane extractable components are present on the body surface. All components from the conventional hexane extract, therefore, represented the secretory compounds derived from the repugnatorial gland.
Typical gas chromatogram of a hexane extract from Anaulaciulus sp.: A, benzoquinone; B, 2-methyl-1,4-benzoquinone; C, 2-methoxy-3-methyl-1,4-benzoquinone; D, 2,3-dimethoxy-1,4-benzoquinone; E, unknown; F, 2-methoxy-6-methyl-1,4-benzoquinone; G, 2,3-dimethoxy-5-methyl-1,4-benzoquinone (see the text for details of compounds 1–15)
Compounds 1–15 eluting by GC in the less-volatile range were recovered from the 5% ether-hexane fraction using a SiO2 column, which is indicative of esters. Compound 7 (t R 18.52 min) was the most abundant product (64.7% of the ester fraction), was identified by NMR analysis with a mixture of minor esters also present. 1H NMR spectrum of the compound 7 indicated two ω-methyls at δH 0.88 ppm (t, 6.6 Hz) and 0.90 ppm (t, 6.8 Hz), an O-substituted methylene at δ 4.06 ppm (t, 6.6 Hz), and a carbonyl-substituted methylene at δ 2.29 ppm (t, 7.6 Hz), together with multiplet methylenes recorded around δ 1.3 and 1.6 ppm. 13 C NMR spectrum gave a carbonyl carbon at δC 174.0 ppm and O-substituted carbon at δ 64.4 ppm, with a total of 14 methylene carbons recorded within δ 20–40 ppm. Two ω-methyls appeared at δ 14.0 and 14.1 ppm. Mass spectral analysis of compound 7 showed ions at m/z 284 (M+) with the base ion at m/z 201 and a diagnostic ion at m/z 84. The base ion at m/z 201 was indicative of a fragment derived from a C12-fatty acid moiety. The diagnostic ion recorded at m/z 84 was the second most intense ion, and was concluded to be derived from a C6-alcohol moiety. The NMR spectra and GC-MS data of compound 7 were identical to those of synthetic n-hexyl laurate.
The GC-MS spectral data recorded for fifteen esters (compounds 1–15) are listed in Table 1. Esters containing a C6 alcohol moiety showed a fragment ion of m/z 84 as the base ion or as the second largest ion (compounds 1, 3, 5, 7, 8, 10, 11, 13, 14, and 15, underlined in Table 1). Similarly, m/z 56 (compound 2), m/z 70 (compounds 4 and 6), m/z 98 (compound 9) and m/z 112 (compound 12) corresponded to C4-, C5-, C7- and C8-alcohol moieties, respectively, as underlined in Table 1. The base ion of compound 7 recorded at m/z 201 was assigned as a C12 fatty acid, as previously mentioned (compounds 2, 4, 5, 9, and 12, dashed-underlined in Table 1). Ions recorded from the mass spectra (Table 1; dashed-underlined) at m/z 173 (compound 1), m/z 187 (compound 3), m/z 215 (compounds 6, 8, and 10), m/z 229 (compounds 11 and 13), and m/z 243 (compounds 14 and 15) suggest the presence of C10-, C11-, C13-, C14- and C15-fatty acid moieties, respectively. The results from the MS analyses suggest that all recorded compounds (1–15) are esters derived from C10-C15 saturated fatty acids and C4-C8 saturated alcohols, as summarized in Table 2. Consequently, compound 1 (t R 16.46 min) was assigned as n-hexyl caprate, 2 (t R 16.51 min) as n-butyl laurate, 3 (t R 17.49 min) as n-hexyl n-undecanoate, 4 (t R 17.52 min) as n-pentyl laurate, 7 (t R 18.52 min) as n-hexyl laurate, 9 (t R 19.40 min) as n-heptyl laurate, 10 (t R 19.43 min) as n-hexyl n-tridecanoate, 12 (t R 20.28 min) as n-octyl laurate, and 13 (t R 20.33 min) as n-hexyl myristate.
Compound 5 (t R 18.13 min) and compound 7 (t R 18.52 min) showed the same M+ ion at m/z 284, with a common alcohol moiety fragment recorded at m/z 84; these findings are suggestive of the isomeric relationship between the two compounds, indicating that compound 5 is n-hexyl isododecanoate on the basis of shorter GC t R. Similarly, compound 8 (t R 19.09 min, m/z 298 (M+), with the base ion at m/z 215, and the alcohol moiety at m/z 84) is elucidated as n-hexyl isotridecanoate, an isomer to n-hexyl n-tridecanoate (compound 10, t R 19.43 min). Compound 11 (t R 20.00 min, m/z 312 (M+), with the base ion at m/z 229, and the alcohol moiety at m/z 84) is n-hexyl isotetradecanoate, an isomer to n-hexyl myristate (compound 13, t R 20.33 min). Mass fragmentation patterns recorded for compound 14 (t R 20.87 min, m/z 326 (M+), with the second largest ion at m/z 243, and the base ion and the alcohol moiety at m/z 84); and for compound 15 (t R 20.95 min, m/z 326 (M+), with the second largest ion at m/z 243, and the base ion and the alcohol moiety at m/z 84) were similar to that of n-hexyl n-pentadecanoate. However, the GC t R for compound 14 and 15 were less than the calculated value (t R ca. 21.2 min), based on comparison of the t R observed from compound 3 (t R 17.49 min, n-hexyl n-undecanoate), compound 7 (t R 18.52 min, n-hexyl laurate), compound 10 (t R 19.43 min, n-hexyl n-tridecanoate), and compound 13 (t R 20.33 min, n-hexyl myristate). Therefore, compound 14 (t R 20.87 min) was assigned as n-hexyl isopentadecanoate, and compound 15 (t R 20.95 min) as n-hexyl anteisopentadecanoate. Compound 6 was assigned as n-pentyl isotridecanoate, based on the observation of the GC t R of compound 6 (t R 18.14 min, ion at m/z 284 (M+), with the second largest ion at m/z 215, and the base ion and the alcohol moiety at m/z 70) being less than the calculated value (t R ca. 18.55 min), which was determined by comparing the value expected for the corresponding ester between n-tridecanoic acid and n-pentyl alcohol.
The elucidated structures for compound 1 to 15, excluding compound 5, were verified by comparisons to synthetic standards prepared via acid-catalyzed esterifications between the corresponding fatty acids and alcohols. The compounds synthesized by this method exhibited identical GC t R and mass spectra values to those of the corresponding natural products.
Compounds A-G were identified as benzoquinone derivatives. The GC-MS analyses of these compounds by co-injection with authentic samples (Sigma-Aldrich Co., Ltd., Tokyo, Japan) revealed that compound A (t R 4.89 min, m/z 108 (M+, base ion)), B (t R 6.35 min, m/z 122 (M+, base ion)), and G (t R 12.28 min, m/z 182 (M+, 66%) and the base ion at m/z 137) corresponded to 1,4-benzoquinone, 2-methyl-1,4-benzoquinone, and 2,3-dimthoxy-5-methyl-1,4-benzoquinone, respectively. Compound C (t R 9.00 min, m/z 152 (M+), base ion) was identified as 2-methoxy-3-methyl-1,4-benzoquinone. Compound D (t R 10.95 min, m/z 168 (M+, 76%) and the base ion at m/z 123) was identified as 2,3-dimethoxy-1,4-benzoquinone (Kuwahara et al., 2002). Compound E (t R 11.25 min, m/z 152 (M+, 36%) and the base ion at m/z 124) is suggestive of a di- or tri-substituted 1,4-benzoquinone. Compound F (t R 11.33 min) also gave the same M+ ion as compound E, at m/z 152 (53%) with the base ion at m/z 69, and the fragmentation pattern was virtually identical to that of 2-methoxy-6-methyl-1,4-benzoquinone derived from SDBS mass spectrum (National Institute of Advanced Industrial Science and Technology, 2010).
Discussion
In the julid millipede, Anaulaciulus sp. that we analyzed, there were no differences recognized in the composition of the conventional hexane extract from whole body and of the defensive secretion directly sampled by a glass capillary tube, indicating no significant additional hexane extractable compounds are present on the body surface. This is the first report of C16-21 aliphatic esters in the defensive secretion of a julid millipede.
n-Hexyl laurate (compound 7) was the major component of the millipede secretions. In the present study ca. 95.5% of total esters were identified; of these, 76.7% were n-hexyl esters. The remaining 4.1% of unknown esters are probably saturated branched-chain and monoenoic fatty acid esters. The identified branched iso- and anteiso-fatty esters (compounds 5, 6, 8, 11, 14, and 15) are novel products, while the odd-numbered C11-fatty acid ester (compound 3), C13-fatty acid ester (compound 10), and C7-fatty alcohol ester (compound 9) had not been previously described as natural products. Other major components of the millipede secretions were 2-methyl-1,4-benzoquinone (compound B) and 2-methoxy-3-methyl-1,4-benzoquinone (compound C), which are known as the most common defensive compounds in quinone-producing millipedes. In addition, one hitherto undescribed compound, 2-methoxy-6-methyl-1,4-benzoquinone (compound F), was characterized from the defensive secretion.
Julid species reportedly produce various 1,4-benzoquinones (Behal and Phisalix, 1900; Weatherston and Percy, 1969; Röper and Heyns, 1977; Eisner et al., 1978). Among them, the defensive secretion of a millipede, Uroblaniulus canadensis (Say), was shown to consist of a mixture of benzoquinones and unidentified aliphatic compounds (Weatherston and Percy, 1969). In quinone-producing spirobolid species, an aliphatic compound from Rhinocricus insulatus has been characterized as trans-2-dodecenal (Wheeler et al., 1964), while uncharacterized components have been reported for two species, Narceus annularis and Floridobolus penneri (Monro et al., 1962). The spirobolid millipede species, Rhinocricus padbergi, produces long-chain hydrocarbons C21-C30 together with benzoquinones and alkaloids (Arab et al., 2003), but the glandular origin of the aliphatic compounds are uncertain.
Most arthropod defensive secretions that employ benzoquinones as major components also contain liquid hydrocarbons such as pentadecane and (Z,Z)-7,9-heptadecadiene, which may serve as solvents for the irritating quinones (Eisner et al., 2000). These hydrocarbons are effective carriers of benzoquinones, and surfactants promoting spread of secretion over the body following glandular discharge. In tests with insects, both hydrocarbons and benzoquinones proved to be repellent and irritating (Peschke and Eisner, 1987). Thus, the combination of benzoquinones and esters could be a more effective millipede defense than either type of compound alone. Excluding the new esters described hitherto, the remaining esters identified have been generally reported as chemical components of many cosmetics. In bioactivity studies, the honeybee Apis mellifera has been reported as producing the compounds associated with compounds 1, 7, 12, and 13, which are utilized in the queen fecal pheromones involved in nestmate recognition (Breed et al., 1992). Recently, the two laurates described as compounds 7 and 9 were evaluated for in vitro antimicrobial activity against the panel of Gram positive and Gram negative bacterial and fungal strains in quantitative structure activity relationship (QSAR) studies (Sarova et al., 2011). n-Hexyl myristate, described as compound 13, has been found to function as a species recognition signal and is reported to possess low-to-moderate aphrodisiac activity in two sulfur butterflies: Colias eurytheme and C. philodice (Grula and Taylor, 1979; Grula et al., 1980).
In summary, we demonstrated the presence of aliphatic esters, mainly composed of n-hexyl laurate, in the defensive secretion of a julid millipede, Anaulaciulus sp. These esters are discharged as a mixture of benzoquinones in response to disturbance. The composition and amount of newly identified esters was not influenced by seasonal change (data not shown). Furthermore, Anaulaciulus sp. extracts collected in Kameoka-shi, Kyoto prefecture, were identical to those collected from the three other Kansai regions (i.e. Kyoto-shi, Osaka, and Wakayama prefectures; data not shown). Further studies are necessary in order to elucidate ester profile differences in other julid species that may have chemotaxonomic value.
References
Arab, A., Zacarin, G. G., Fontanetti, S. C., Camargo-Mathias, M. I., dos Santos, G. M., and Cabrera, C. A. 2003. Composition of the defensive secretion of the Neotropical millipede Rhinocricus padbergi Verhoeff 1938 (Diplopoda: Spirobolida: Rhinocricidae). Entomotropica. 18:79–82.
Attems, C. 1909. Die myriopoden der vega expedition. Ark. Zool. 5:1-85.
Behal, A. and Phisalix, M. 1900. La quinone, principe actif du venium du Julus terrestris. Bull. Mus. Nat. His. Natur. (Paris) 6:388-390.
Blum, M. S. and Woodring, J. P. 1962. Secretion of benzaldehyde and hydrogen cyanide by the millipede Pachydesmus crassicutis (Wood). Science. 138:512-513.
Blum, M. S., Macconnell, J. G., Brand, J. M., Duffield, R. M., and Fales, H. M. 1973. Phenol and benzaldehyde in the defensive secretion of a strongylosomid millipede. Ann. Entomol. Soc. Am. 66:234-235.
Breed, M. D., Stiller, T. M., Blum, M. S., and Page, R. E. 1992. Honeybee nestmate recognition: effects of queen fecal pheromones. J. Chem. Ecol. 18:1633–1640.
Duffey, S. S., Blum, M. S., Fales, H. M., Evans, S. L., Roncadori, R. W., Tiemann, D. L., and Nakagawa, Y. 1977. Benzoyl cyanide and mandelonitrile benzoate in the defensive secretions of millipedes. J. Chem. Ecol. 3:101-113.
Eisner, T. and Meinwald, J. 1966. Defensive secretions of arthropods. Science. 153:1341–1350.
Eisner, T., Alsop, D., Hicks, K., and Meinwald, J. 1978. Defensive secretions of millipedes, pp 41–72, in S. Bettini (ed.), Arthropod venoms. Handbook of experimental pharmacology, vol. 48. Springer-Verlag, Berlin.
Eisner, T., Aneshansley, D. J., Eisner, M., Attygalle, A. B., Alsop, D., and Meinwald, J. 2000. Spray mechanism of the most primitive bombardier beetle (Metrius contractus). J. Experm.Biol. 203:1265–1275.
Grula, J. W. and Taylor, O. R. 1979. The inheritance of pheromone production in the sulphur butterflies Colias eurytheme and C. philodice. Heredity 42:359–371.
Grula, J. W., Mcchesney, J. D., and Taylor, O. R. 1980. Aphrodisiac pheromones of the sulphur butterflies Colias eurytheme and C. philodice (Lepidoptera, Pieridae). J. Chem. Ecol. 6:241–256.
Kuwahara, Y. 1999. Chemical defenses of millipedes, pp 291–298, in T. Hidaka, Y. Matsumoto, K. Honda, H. Honda and S. Tatsuki (ed.), Environmental entomology, behavior, physiology and chemical ecology. University of Tokyo Press, Tokyo (in Japanese).
Kuwahara, Y., Noguchi, S., Mori, N., and Higa, Y. 2002. Identification of benzoquinones and hydroquinones as the secretory compounds from three species of Okinawan millipedes. Jpn. J. Environ. Entomol. Zool. 13:117–124.
Kuwahara, Y., Mori, N., and Tanabe, T. 2007. Detection of a neotropical frog alkaloid spiropyrrolizidine 236 from a Japanese polyzoniid millipede Kiusiozonium okai as a major defense component together with polyzonimine and nitropolyzonamine. Jpn. J. Environ. Entomol. Zool. 18:91–95.
Meinwald, J., Smolanoff, J., Mcphail, A. T., Miller, R. W., Eisner, T., and Hicks, K. 1975. Nitropolyzonamine: a spirocyclic nitro compound from the defensive glands of a milliped (Polyzonium rosalbum). Tetrahedron Lett. 16:2367-2370.
Meinwald, Y. C., Meinwald, J., and Eisner, T. 1966. 1,2-Dialkyl-4(3H)-quinazolinones in the defensive secretion of a millipede (Glomeris marginata). Science. 154:390-391.
Monro, A., Chadha, M., Meinwald, J., and Eisner, T., 1962. Defensive mechanisms of arthropods VI. Para-benzoquinones in the secretion of five species of millipedes. Ann. Entomol. Soc. Am. 55:261–262.
National Institute of Advanced Industrial Science and Technology, The spectral data base of organic compounds. Website accessed February 2010, http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi
Ômura, H., Kuwahara, Y., Tanabe, T. 2002a. Species-specific chemical compositions of defense secretions from Parafontaria tonominea Attems and Riukiaria semicircularis semicircularis Takakuwa (Polydesmida: Xystodesmidae). Appl. Entomol. Zool. 37:73–78.
Ômura, H., Kuwahara, Y., Tanabe, T. 2002b. 1-Octen-3-ol together with geosmin: New secretion compounds from a polydesmid millipede, Niponia nodulosa. J. Chem. Ecol. 28:2601–2612.
Pasteels, J. M., Grégoire, J. C., and Rowell-Rahier, M. 1983. The chemical ecology of defense in arthropods. Annu. Rev. Entomol. 28:263–289.
Peschke, K. and Eisner, T. 1987. Defensive secretion of the tenebrionid beetle, Blaps mucronata: Physical and chemical determinants of effectiveness. J. Comp. Physiol. A. 161:377–388.
Röper, H. and Heyns, K. 1977. Trace analysis of p-benzoquinone- and hydroquinone derivatives by gas-liquid chromatography and gas-liquid chromatography/mass-spectrometry. Identification of defensive secretion components from European Julids. Z. Naturforsch. 32C:61–66.
Sarova, D., Kapoor, A., Narang, R., Judge, V., and Narasimhan, B. 2011. Dodecanoic acid derivatives: synthesis, antimicrobial evaluation and development of one-target and multi-target QSAR models. Med. Chem. Res. 20:769–781.
Shinohara, K. 1990. Three new species of the genus Anaulaciulus (Diplopoda: Julidae) from Japan. Edaphologia 42:21-25.
Weatherston, J. and Percy, J. E. 1969. Studies of physiologically active arthropod secretions. III. Chemical, morphological, and histological studies of the defence mechanism of Uroblaniulus canadensis (Say) (Diplopoda: Julida). Can. J. Zool. 47:1389–1394.
Wheeler, J. W., and Meinwald, J., Hurst, J. J., and Eisner, T. 1964. Trans-2-dodecenal and 2-methyl-1,4-quinone produced by a millipede. Science. 144:540–541.
Wood, W. F., Hanke, F. J., Kubo, I., Carroll, J. A., and Crews, P. 2000. Buzonamine, a new alkaloid from the defensive secretion of the millipede, Buzonium crassipes. Biochem. Syst. Ecol. 28:305–312.
Acknowledgements
This work was supported in part by Agricultural Chemical Research Foundation and by a Grant-in-Aid for Young Scientists (B) [No.20780086 for NS] from the Japan society for the promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shimizu, N., Kuwahara, Y., Yakumaru, R. et al. n-Hexyl Laurate and Fourteen Related Fatty Acid Esters: New Secretory Compounds from the Julid Millipede, Anaulaciulus sp.. J Chem Ecol 38, 23–28 (2012). https://doi.org/10.1007/s10886-012-0063-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-012-0063-4