Abstract
Type 1 diabetes (T1D) is characterized by the progressive destruction of insulin-producing beta cells in the pancreas. Despite improvements in insulin monitoring techniques, there remains no cure for T1D. Individuals with T1D require lifelong insulin therapy and some develop life-threatening complications. T1D is a complex, multifactorial, autoimmune condition. Understanding why people get T1D and how it progresses has advanced our knowledge of the disease and led to the discovery of specific targets that can be therapeutically manipulated to halt or reverse the course of T1D. Scientists investigating the potential of immunotherapy treatment for the treatment have recently had some encouraging results. Teplizumab, an anti-CD3 monoclonal antibody that has been approved by the FDA, delays the onset of clinical T1D in patients ≥ 8 years of age with preclinical T1D and improves beta cell function. Therapies targeting beta cell health, vitality, and function are now thought to be an essential component of successful combination therapy for T1D. The idea that the beta cells themselves may influence their own destruction during the development of T1D is a notion that has recently been gaining acceptance in the field. Researchers have recently made remarkable strides in beta cell replacement therapy and beta cell regeneration techniques. This review offers a detailed exploration of the pathophysiological mechanisms of T1D. It discusses the intricate interplay of factors leading to T1D development and the innovative approaches being explored to discover new treatments and a cure for the millions of people living with T1D worldwide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Physiological function of the pancreas
The pancreas is the organ which produces insulin, one of the main hormones that helps to regulate blood glucose levels. The pancreas is located behind the lower part of the stomach, in front of the spine. It measures about 15 cm in length and is divided into the head, body, and tail, with the head adjacent to the duodenum and the tail near to the spleen. It is vascularized by branches of the celiac and superior mesenteric arteries, with a microvasculature that supports and regulates beta cell functions, including insulin production [4]. Additionally, it is richly innervated by sympathetic and parasympathetic nerves [6]. The pancreas plays a part in both the endocrine system and the exocrine system. The endocrine system includes organs which produce hormones, which are delivered via the blood to help regulate our mood, growth, metabolism, and reproduction. In the pancreas, the endocrine component is organized into the Islets of Langerhans, and includes various hormone-producing cells such as alpha cells (production of glucagon), beta cells (insulin), delta cells (somatostatin), pancreatic polypeptide cells (pancreatic polypeptide), and epsilon cells (ghrelin), which are essential for blood glucose regulation and metabolic balance [7]. The exocrine system is comprised of glands which release substances such as sweat (to the skin), saliva (in the mouth) or, in the case of the pancreas, digestive enzymes such as amylase, lipase, and proteases which assist with carbohydrate, fat, and protein digestion.
Susceptibility to T1D
A combination of genetic predisposition and environmental triggers determines an individual’s susceptibility to develop T1D [8]. Specific genes, notably those in the human leukocyte antigen (HLA) complex class II, are strongly associated with the risk of developing T1D. In addition, overexpression of HLA class I is thought to predispose islet cells for recognition by immune cells such as CD8+ T cells. The occurrence of increased HLA class I expression on insulin-positive beta cells is a characteristic feature of both T1D and its pre-diabetic stages [9]. The upregulation of HLA class I in patients with T1D occurs prior to immune cell infiltration and the cause, although as yet unknown, is thought to be due to environmental factors [10]. There is a striking correlation between the levels of STAT1 measured in the beta cells of patients with T1D and HLA class I hyperexpression. STAT1 is elevated in beta cells soon after T1D diagnosis but its expression declines with disease duration, thereby correlating with a similar decline in HLA class I. It is possible that the two may be coordinately regulated or that increased production of HLA class I in beta cells occurs as a consequence of enhanced STAT1 expression. STAT1 is a critical protein involved in mediating antiviral responses to interferons, and its early upregulation in the progression of T1D would be expected to place beta cells in a heightened state of responsiveness to these cytokines [10]. The upregulation of HLA class I affects the immune system’s ability to distinguish self from non-self, making individuals more prone to autoimmune reactions against their own beta cells. Epigenetic modifications (such as changes in DNA methylation and histone modifications) [11,12,13] can also be influenced by both genetic and environmental factors and may further contribute to the pathogenesis of T1D. Defects in antigen presentation by thymic cells contribute to the escape of autoreactive T cells from negative selection [14]. Regulatory T cells, which are crucial for maintaining self-tolerance and suppressing autoimmune responses, may also be deficient or defective in individuals with T1D. Genetic and cellular studies in patients with T1D point to an imbalance between effector T cells and regulatory T cells as a potential driver of the disease [15,16,17,18,19,20]. However, the exact role of regulatory T cells in the progression of T1D remains uncertain [21, 22].
It is believed that environmental factors can also exacerbate the susceptibility to T1D. Epidemiological studies showing variations in the incidence of T1D both seasonally and geographically, support this hypothesis [23]. Viral triggers, for example, are known to cause the immune system to become overactive or misdirected, leading to an autoimmune attack on the beta cells [24,25,26,27]. Viral infections can also disrupt the thymic selection process. Other environmental factors, such as diet, early childhood nutrition, and exposure to toxins, can also influence the course of T1D. Diet and exposure to environmental toxins and pollutants may also contribute to the disruption of immune tolerance, further amplifying the autoimmune response.
The combination of genetic susceptibility, immune system dysregulation, and environmental triggers, results in a breakdown in self-tolerance, allowing autoreactive T cells to evade elimination. The resulting autoimmune destruction of pancreatic beta cells, is a defining characteristic of T1D.
The initiation of T1D
T1D is thought to start in a few isolated islets within the pancreas. Insulitis is the process of inflammation in which cells of the immune system invade the pancreas and accumulate in proximity to, and within, pancreatic islets [28]. In rodents, this process occurs intensively as the disease progresses until most islets display extensive infiltration. However, this feature of intense insulitis is largely absent in the human pancreas. Studies from pre-diabetic pancreata have shown that the damage is initially self-limiting and progresses in a relapsing/remitting pattern within a marginal number of islets, and therefore, does not initially affect glycemic control. However, the autoimmune response progresses swiftly within a given islet and the eventual destruction of beta cells (following the initial targeting of self-antigens), leads to the release of danger signals that amplify the immune response. A critical point is reached at an as yet unpredictable point of time, when the situation becomes uncontrollable and the majority of islets are affected, leading to clinical manifestation of the disease.
In T1D, the antigens which are targeted by the patient’s own immune system include proteins expressed by beta cells, including insulin, glutamic acid decarboxylase (GAD), islet-specific glucose 6 phosphatase catalytic subunit-related protein (IGRP), and insulinoma-associated protein 2 (IA-2). It is possible that the autoimmune response may be initiated by molecular mimicry, where external pathogens display antigens that closely resemble self-antigens, causing cross-reactivity. The immune system may react to these pathogen-derived antigens and inadvertently attack beta-cell antigens, which sets off an autoimmune inflammatory cascade [29]. Ordinarily, autoreactive cells are either eradicated or strictly regulated to prevent the development of autoimmunity. However, a failure in regulatory mechanisms occurs during T1D development, which leads to the activation of autoreactive T cells that mistakenly target beta-cell antigens [30]. The immune system then initiates insulitis, the immune system’s way of trying to eliminate the perceived threat. However, this process inadvertently causes collateral damage to healthy tissues within the pancreas. Autoreactive T cells (the majority of these being CD8+) recognize and attack beta cells in the pancreas leading to their destruction and subsequent insulin deficiency.
Immunopathology at different stages of T1D development
Overt hyperglycemia, metabolic imbalance, and clinical symptomatology form the basis of the T1D diagnosis. However, the critical immunological events leading to islet cell damage occur prior to diagnosis, which is one of the many difficulties of studying T1D pathology in humans. In addition, access to human pancreatic samples (especially samples pre-diagnosis) is difficult and researchers often have to study the immunopathology of human T1D using cells from peripheral blood rather than from within the pancreas. Sharing of postmortem pancreatic material from patients with recent-onset disease has given researchers a chance to increase our knowledge about the pathology of T1D in humans.
Earlier diagnosis of T1D in first- or second-degree relatives of individuals with T1D has also enabled further research into the underlying mechanisms of T1D pathology and led to the design of T1D prevention clinical trials, with enrollment criteria and end points based on specific disease stages [31,32,33]. Recent multinational studies have included both people at high risk of developing T1D and those with recent onset disease.
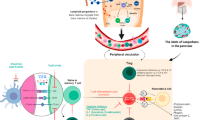
The results of these studies have shown that T1D progresses sequentially in a variable but predictable manner through distinct identifiable stages prior to the onset of symptoms (Table 1) [34]. The rate of progression from onset of beta cell autoimmunity to glucose intolerance and then to symptomatic disease can last from months to decades (Fig. 1) [35].
Inflammation in the pancreas at different stages of T1D. This human, formalin-fixed, paraffin-embedded section from a donor with T1D (#6247) from the Network for Pancreatic Organ Donors with Diabetes (nPOD) was stained with whole-slide immunofluorescence for insulin (green), CD45 (magenta) and Hoechst as the nuclei stain (blue). Insulin-deficient islets (IDI) were identified in a consecutive slide stained for glucagon, and a pixel classifier was trained to identify IDI based on the glucagon signal. The map with IDI was created and then transferred to the insulin-stained slide showed in the picture. Insulin-containing islets (ICI) were manually added based on the insulin signal. ICI were classified depending on the amount of CD45 + cell infiltration into: ≤ 3 cells (no infiltration, blue), > 3 but < 15 cells (low infiltration, yellow), ≥ 15 cells (high infiltration, red), and IDI (white). The images at the bottom of the Figure are a visual representation of the process of beta cell destruction at different stages of T1D development
Immunopathology during Stage 1 of T1D development (prediabetes)
Proteins and peptides such as insulin, proinsulin, GAD65, IA-2, and ZnT8 have been identified as target antigens in T1D and autoantibodies secreted by autoreactive B cells which are specific for these islet antigens can be detected in the serum of peripheral blood [36]. Children who develop ≥ 2 of these autoantibodies have an increased likelihood of developing clinical T1D later in life, compared with children who have fewer autoantibodies [37,38,39]. In this stage of T1D development, ≥ 2 autoantibodies directed towards these peptides can be detected but the beta cells are still able to produce insulin and control blood sugar levels.
Why do some people produce the autoantibodies that precede the development of T1D? It is very difficult to determine which of the factors that are present in this early stage of T1D are triggers or accelerators of the autoimmune process, and which factors are caused by the disease process itself. There is some evidence to suggest that abnormal lipid metabolism may precede seroconversion to autoantibody positivity [40, 41]. Furthermore, in the TRIGR study, Ludvigsson et al. showed that increased glucose concentration in peripheral blood was related to the initial production of islet cell autoantibody. This beta cell stress hypothesis [42] suggests that an increased amount of sugar in peripheral circulation will place a high demand on insulin secretion, leading to beta cell stress that contributes to a rapid progression to symptomatic T1D [43,44,45]. The progression rate of T1D at this very early stage of disease can be influenced by age at the time of seroconversion and high titers of insulin autoantibodies [46].
Pancreas volume declines early in the course of T1D progression [47,48,49,50,51,52,53,54,55]. The reason for the smaller pancreas is not known but it is thought that changes in the exocrine pancreas may be important in T1D pathogenesis and both reduced acinar cell number and evidence of fibrosis have been reported [55,56,57]. Although the mechanism is not known, it is possible that the process affecting the exocrine compartment ultimately leads to beta cell loss [58, 59]. Indeed, reduced pancreatic function (fecal elastase) and pancreatic exocrine insufficiency have been reported in some individuals with T1D [56, 60].
Immunopathology during Stage 2 of T1D development (prediabetes)
During this stage of T1D development some initial islet destruction is present along with signs of beta cell autoimmunity and some associated dysglycemia, but there are no other clinical symptoms of T1D.
The nonpathogenic autoantibodies are viewed as biomarkers of the autoimmune process, with the presence of multiple autoantibodies being predictive of the transition from Stage 2 to Stage 3 disease [37, 61, 62]. CD4+ and CD8+ T cells can be detected and isolated from islets and pancreatic lymph nodes of patients with T1D and these cells and their associated cloned cell lines have been shown to react to a range of islet antigens and post-translationally modified peptides or neoantigens [63,64,65].
Studies from autopsy pancreas samples, from patients who died soon after the diagnosis of T1D, have revealed that the principal cells involved in the destructive process in the islets are likely to be CD8+ cytotoxic T cells [66,67,68,69]. However, other immune cells such as macrophages and CD20+ B cells are also found to infiltrate the islets. At this stage the progression of T1D occurs in a lobular manner across the pancreas, which is indicative of a process that includes local as well as systemic drivers (Fig. 2) [70,71,72,73].
Immunopathology during Stage 3 of T1D development
By Stage 3, when T1D is often clinically diagnosed, autoimmune damage has occurred throughout the pancreas. Signs and symptoms of T1D, which can range from diabetic ketoacidosis to modest hyperglycemia, are usually present, and insulin therapy can be initiated. Although there will have been a loss of both beta cells and acinar cells, some beta cells are still present and functional. Beta cells from many patients with longstanding T1D still secrete small amounts of insulin [55]. However, by the time of clinical presentation most of the remaining islets are insulin-negative with only occasional rare islets present which contain functional beta cells. It is estimated that ~ 60–90% of beta cell mass has been lost by this time [74]. Immune cell infiltration can be seen both in the islets and the exocrine pancreas [69].
A “honeymoon phase” often occurs soon after diagnosis, during which patients with T1D experience a period of partial remission in which they have reduced insulin requirements [75,76,77,78,79,80]. A longer remission period is associated with better residual beta cell function and a reduced risk of acute and long-term T1D complications [75,76,77,78, 81] It is possible that novel treatments could be utilized to promote, extend and enhance the remission period by targeting the subset of immune cells which facilitate a naturally occurring mechanism of protection during this time [82]. Treatment during this therapeutic window may further protect the remaining beta cells and reduce long-term complications.
Factors involved in the progression of T1D
The conditions that drive the progression of T1D from autoantibody positivity to islet cell destruction are unclear but are likely to be multifactorial in nature. Metabolomics studies suggest that metabolic dysregulation precedes the clinical presentation of T1D [83,84,85]. In particular, specific sugar derivatives and microbial metabolites differ between rapid and slow progressors to T1D at this stage in disease development [44]. The Finnish T1D Prediction and Prevention study (DIPP) has given an insight into the mechanisms that may underlie progression to clinical disease. In this study, the participants, who had increased HLA susceptibility to T1D, were prospectively observed for various islet cell antibodies from the age of 3 months to 15 years of age or to the diagnosis of T1D [44, 86]. Increasing levels of sugar derivatives in the peripheral circulation were found to contribute to accelerated progression to T1D [44].
Studies have also shown that dysregulated microbial metabolites in the gut microbiota are associated with the development of T1D [87,88,89,90,91,92]. Microbiota-derived indole metabolites promote intestinal homeostasis and are known for their immunomodulatory signaling activity [88, 93, 94]. Microbiota-derived metabolite 3-indole-acetic acid was found to be upregulated in children who rapidly progressed to T1D following the appearance of autoantibodies [44]. An increase in such tryptophan catabolites suggests a shift from saccharolytic to proteolytic fermentation in the gut [44, 89]. This shift in microbial fermentation could dysregulate microbial tryptophan catabolism and exacerbate the development of T1D.
The growing prevalence of overweight and obese children with T1D has led to much interest in the coexistence of these conditions. Higher body mass index (BMI) in children is associated with preserved C-peptide at T1D onset [95,96,97], however, it does not protect from a rapid decrease in beta cell function in these patients. Among a pooled European cohort of teens with T1D over 1 year of follow-up [98], higher BMI at diagnosis was associated with a greater rate of fasting C-peptide decrease, thus more rapid disease progression. The significant decrease in residual beta cell function in children with the highest BMI may be the result of a negative impact of elevated levels of inflammatory cytokines on pancreatic beta cells in the course of obesity [95,96,97].
Persistent infection by pathogens that can evade or suppress the immune system and establish a long-term infection may also drive the progression of autoimmunity in T1D [99]. In addition to molecular mimicry, persistent infections can induce polyclonal B cell activation, leading to the production of autoantibodies [100]. Furthermore, persistent infections can lead to the activation of toll-like receptors which can trigger the production of pro-inflammatory cytokines and chemokines, leading to chronic inflammation and tissue damage [101].
Heterogeneity of autoimmunity in T1D and T2D
The extent and timing of beta cell loss varies significantly with age. Individuals in whom T1D develops early in life (before the age of 7 years) lose beta cells more quickly/more extensively than those who receive a diagnosis after the age of 7 years of age [69, 102, 103]. The existence of distinct profiles of insulitis, which are associated with very different rates of beta cell loss [68, 69], or intrinsic age-related differences in the function of the immune system may contribute to these age-related differences.
The American Diabetes Association classification of type 2 diabetes (T2D) is “a non-autoimmune progressive loss of adequate beta cell insulin secretion, frequently on the background of insulin resistance and metabolic syndrome” [104]. However, many patients who are initially diagnosed with T2D, may also have undiagnosed beta cell autoimmunity. The prevalence of T2D is increasing and if 10% of these patients are positive for islet autoantibodies then testing for them during the diagnostic assessment will be of benefit to many patients [105]. Such assessments may reveal patients who are likely to have a higher rate of progression to insulin requirement and, may therefore, help to personalize treatment plans for these patients [106].
Individuals with T1D are prone to develop diabetic ketoacidosis (DKA) which is a serious metabolic complication [107]. Certain individuals with T2D also develop DKA without precipitating factors, (referred to as ketosis-prone T2D [KPD]). Because KPD often presents with acute hyperglycemia and DKA, just like acute-onset T1D, it is very important to differentiate between these two types of diabetes at the onset. Unprovoked KPD is characterized by male predominance, onset at a young age, obesity, sudden onset of DKA without precipitating factors, and a transient decrease in insulin secretion capacity, which can be recovered with temporary intensive insulin therapy. It is possible that the mechanism of transiently impaired insulin secretion in these cases is associated with glucotoxicity or lipotoxicity.
The diagnostic criteria for slowly progressive T1D (slowly progressive insulin dependent diabetes mellitus; SPIDDM) have recently been revised by the Committee on T1D of the Japan Diabetes Society [108]. All of the following three criteria must be met: (1) presence of anti-islet autoantibodies at some point in time during the disease course; (2) absence of ketosis or ketoacidosis at diagnosis with no immediate requirement for insulin treatment; and (3) gradual decrease of insulin secretion over time. The phenotype of progressive beta cell dysfunction in patients with SPIDDM which occurs at > 30 years of age is similar to that of latent autoimmune diabetes of the adult (LADA), which is generally defined by diagnosis at > 30 years of age, presence of circulating islet autoantibodies, and lack of insulin requirement for 6 months after diagnosis. However LADA is a concept that includes both insulin-dependent and non-insulin-dependent states with anti-islet autoantibody positivity [109].
Patients with LADA need insulin earlier during disease progression and are likely to respond poorly to oral anti-diabetic mediation. However, these patients could respond favorably to immunomodulator therapy. Anti-inflammatory and immunomodulatory therapies have also proven effective in improving the metabolic profile of many patients with T2D, by disrupting autoimmune processes and inhibiting the decline of beta cell function [105, 110]. The presence of self-reactive T cells in autoantibody-negative patients with T2D also identifies an autoimmune process that is associated with metabolic dysregulation [111]. Further research is needed to understand the complex immune interactions relevant to T2D and specific immunomodulation strategies to treat these patients. However, in the meantime it is important to remember that lifestyle changes also repress inflammation and autoimmune reactions, and are therefore, a major tool to help decrease beta cell stress.
Another subset of T1D, fulminant T1D (FT1D), is characterized by extremely rapid pancreatic beta cell destruction with rapid progression of hyperglycemia and ketoacidosis. It was initially classified as idiopathic T1D due to the absence of autoimmune markers. However, subsequent studies showed evidence for the involvement of autoimmunity in the rapid beta cell loss in FT1D pathogenesis [112]. Since the introduction of immune checkpoint inhibitors for the treatment of cancer, several cases of FT1D have been reported. However, it is unclear if FT1D and immune checkpoint inhibitor-induced diabetes share the same pathology. In FT1D, beta cells are destroyed very rapidly and are almost absent at diagnosis, compared to the relatively slow destruction that takes place in conventional acute‐onset T1D, which can occur over several months/years. The speed of disease development in patients with immune checkpoint inhibitor‐related FT1D is placed between conventional acute‐onset T1D and FT1D in terms of timing of beta cell destruction, with destruction happening within the first few weeks. Regarding the trigger for disease initiation, in immune checkpoint inhibitor‐related FT1D blocking of the PD‐1/PD-1 ligand pathway is the known trigger. In contrast, viral infection has been suggested as a potential trigger in patients with FT1D, with common cold‐like symptoms frequently observed prior to symptom-onset and IGRP-specific CD8 + T cells are thought to significantly contribute to the pathogenesis [113]. It is currently unknown whether immune checkpoint inhibitor molecules are involved in the destruction of beta cells in other subsets of T1D [114, 115].
While the fundamental pathophysiological process remains consistent across different populations, there are notable variations in clinical presentation and genetic predisposition between Eastern and Western countries. Understanding these differences is crucial for tailoring effective management strategies and advancing our global approach to the treatment of T1D. The frequency of T1D in Japan and most East Asian countries is very low and typically less than one-tenth that seen in white populations of European descent. In contrast, most people with FT1D are from East Asian countries and only a limited number of cases have been reported in white European populations. [116,117,118,119,120]
Western populations: in Western populations, extensive research has established a strong association between T1D and specific HLA class II alleles. Notably, HLA-DR3-DQ2 and HLA-DR4-DQ8 haplotypes are critical risk factors. These alleles play a central role in immune recognition and activation, contributing to the autoimmune attack on pancreatic beta cells.
Eastern populations: contrastingly, Eastern populations exhibit distinct genetic profiles. A recent genome-wide association study conducted by the Japan Diabetes Society focused on Japanese patients with FT1D. The study not only confirmed the strong association with Class II HLA genes but also revealed a novel genetic locus: the CSAD/lnc-ITGB7-1 region on chromosome 12. Interestingly, these SNPs did not show any linkage to autoimmune T1D, suggesting a distinct pathogenesis in FT1D.
Despite genetic and environmental differences, the core pathophysiological process—autoimmune destruction of beta cells—remains consistent. Autoreactive T cells play a central role in both Eastern and Western T1D. Collaborative efforts between Eastern and Western researchers can enhance our understanding of T1D. Comparative studies may reveal additional genetic loci and provide insights into shared mechanisms.
Do beta cells play an active role in the development of T1D?
It had previously been thought that during the development of T1D, the beta cell was an “innocent victim” of an unprovoked “attack” by an overactive or under-regulated immune system. However, the idea that the beta cells themselves may influence their own destruction during the development of T1D is a notion that has recently been gaining acceptance in the field [121].
Beta cells are certainly the primary targets of the immune system in the context of T1D. However, the extent to which they contribute to their own targeting, either by facilitating or resisting it, is not fully understood. Pre-diabetes typically lasts several years before clinical manifestation and during this time, beta cells often display signs of stress and malfunction even before they become the direct targets of the immune response [122, 123]. It is now hypothesized that the progression towards T1D is partly due to the highly secretory nature of the beta cells and a well-vascularized environment, which make them more susceptible to changes in the cellular environment [70, 124,125,126]. Paradigm-shifting research has suggested that beta cells may actively “reveal” themselves by presenting their own antigens when they experience cellular stress [121]. There are likely multiple mechanisms at play including beta cell dysfunction; altered interactions between beta cells and immune cells; and increased immunogenicity. These mechanisms may also be amplified by internal factors such as endoplasmic reticulum stress or external stress from viral infections and inflammatory cytokines.
Heightened expression of HLA class I molecules on the surface of beta cells is largely attributed to inflammatory stress within the cellular environment [127, 128]. The consequence of this hyperexpression is believed to be an enhanced visibility of these beta cells to CD8+ T cells infiltrating the islets. These T cells identify and destroy beta cells that display surface class I molecules presenting autoantigenic epitopes [129] essentially “revealing” themselves as targets for the immune system. Islets from different regions of the pancreas are differentially affected, creating a multifocal pattern [71]. The cause of this lobularity remains unclear, although viral infections, the inflammatory milieu in the pancreas, innervation, and the intrinsic etiology are all thought to play a role.
In situations where the stress on beta cells is heightened and the demand for insulin throughout the body surpasses the ability of the cells to secrete it, the system responsible for processing proinsulin (an inactive precursor of insulin) can become overloaded. An increase in the expression of proinsulin relative to insulin has been observed in beta cells in the islets of donors with T1D (both early in the development of T1D and in long-standing T1D) [130, 131]. The accumulation of proinsulin within beta cells may be due to various factors, including endoplasmic reticulum stress and impaired processing.
These new insights into T1D pathology underscore the intricate relationship between beta cell dysfunction, immune inflammation, and the role of beta cells themselves in triggering the autoimmune response. The limited long-term efficacy of immune intervention therapies and the continued presence of beta cells even after diagnosis and progression of T1D, suggests that both beta cells and the immune system, play an active role in the development of T1D and that both will need to be targeted to provide an effective therapy. Therapeutic strategies that aim to prevent or treat T1D at its earliest stages by preserving beta cell function, are therefore, currently undergoing rigorous assessment.
Novel therapeutic approaches for the treatment of T1D
By the time that we detect T1D, it is normally too late to prevent it, so we need to learn to identify which individuals are at risk, watch out for very early signals, and treat the underlying immunological mechanisms. The complexity of the pathways that lead to inflammation in T1D and the number of mechanisms that are involved means that it has been difficult to find a suitable method to treat all individuals. Currently available therapies include daily insulin injections to maintain blood glucose levels, suppressive immunotherapy to decrease the symptoms associated with autoimmunity, and islet transplantation to replace destroyed beta cells and restore insulin production. Lifestyle modifications are also recommended to increase sensitivity to insulin, control blood sugar levels and lower the risk of heart disease and nerve damage. An immune-targeted therapy that has recently been shown to have efficacy for the treatment of T1D is the T cell-specific anti-CD3 antibody teplizumab, which delayed disease onset in participants at high risk for T1D in the TrialNet 10 study [5, 132, 133]. The mechanisms of action of other T1D therapies that are being studied in clinical trials are discussed in Table 2.
Several therapies currently in development aim to boost the regulatory T cell population in patients with T1D [145] and other potential future therapies include anti-IL21, conversion of alpha cells to beta cells, GLP-1 receptor agonists, calcium channel blockers, such as verapamil and low-dose anti-thymocyte globulin (ATG). The treatment strategy will differ depending on the stage/severity of disease and can include treatment before the development of autoimmunity to islet autoantigens (primary prevention); after the development of humoral or metabolic markers of high risk of progression to diabetes (secondary prevention); maintenance of residual beta‐cell function after the onset of diabetes (tertiary prevention), and/or islet cell transplantation (Table 3).
Conclusion
Understanding why people get T1D and how it progresses has advanced our knowledge of the disease and led to the discovery of specific targets that can be manipulated to change the lives of the millions of people who are living with T1D worldwide. Recent advances in the treatment of T1D include immunotherapies targeting T cells and the development of innovative technologies to enable islet cell transfer to be an effective and well-tolerated treatment. Hopefully, future research will lead to strategies to not only treat T1D, but also to prevent and reverse it.
References
Jeyagaran A, Lu CE, Zbinden A, Birkenfeld AL, Brucker SY, Layland SL. Type 1 diabetes and engineering enhanced islet transplantation. Adv Drug Deliv Rev. 2022;189: 114481.
Chiarelli F, Giannini C, Primavera M. Prediction and prevention of type 1 diabetes in children. Clin Pediatr Endocrinol. 2019;28:43–57.
Janzen KM, Steuber TD, Nisly SA. GLP-1 agonists in Type 1 diabetes mellitus. Ann Pharmacother. 2016;50:656–65.
Burganova G, Bridges C, Thorn P, Landsman L. The role of vascular cells in pancreatic beta-cell function. Front Endocrinol (Lausanne). 2021;12: 667170.
Iacobucci G. Type 1 diabetes: US approves drug that can delay onset. BMJ. 2022;379: o2798.
Hampton RF, Jimenez-Gonzalez M, Stanley SA. Unravelling innervation of pancreatic islets. Diabetologia. 2022;65:1069–84.
Leete P. Type 1 diabetes in the pancreas: a histological perspective. Diabet Med. 2023;40: e15228.
Kissler S. Genetic modifiers of thymic selection and central tolerance in type 1 diabetes. Front Immunol. 2022;13: 889856.
Benkahla MA, Sabouri S, Kiosses WB, Rajendran S, Quesada-Masachs E, von Herrath MG. HLA class I hyper-expression unmasks beta cells but not alpha cells to the immune system in pre-diabetes. J Autoimmun. 2021;119: 102628.
Richardson SJ, Rodriguez-Calvo T, Gerling IC, Mathews CE, Kaddis JS, Russell MA, Zeissler M, Leete P, Krogvold L, Dahl-Jorgensen K, von Herrath M, Pugliese A, Atkinson MA, Morgan NG. Islet cell hyperexpression of HLA class I antigens: a defining feature in type 1 diabetes. Diabetologia. 2016;59:2448–58.
Dang MN, Buzzetti R, Pozzilli P. Epigenetics in autoimmune diseases with focus on type 1 diabetes. Diabetes Metab Res Rev. 2013;29:8–18.
Rakyan VK, Beyan H, Down TA, Hawa MI, Maslau S, Aden D, Daunay A, Busato F, Mein CA, Manfras B, Dias KR, Bell CG, Tost J, Boehm BO, Beck S, Leslie RD. Identification of type 1 diabetes-associated DNA methylation variable positions that precede disease diagnosis. PLoS Genet. 2011;7: e1002300.
Stefan M, Zhang W, Concepcion E, Yi Z, Tomer Y. DNA methylation profiles in type 1 diabetes twins point to strong epigenetic effects on etiology. J Autoimmun. 2014;50:33–7.
Creusot RJ, Postigo-Fernandez J, Teteloshvili N. Altered function of antigen-presenting cells in type 1 diabetes: a challenge for antigen-specific immunotherapy? Diabetes. 2018;67:1481–94.
Glisic-Milosavljevic S, Wang T, Koppen M, Kramer J, Ehlenbach S, Waukau J, Jailwala P, Jana S, Alemzadeh R, Ghosh S. Dynamic changes in CD4+ CD25+(high) T cell apoptosis after the diagnosis of type 1 diabetes. Clin Exp Immunol. 2007;150:75–82.
Viisanen T, Gazali AM, Ihantola EL, Ekman I, Nanto-Salonen K, Veijola R, Toppari J, Knip M, Ilonen J, Kinnunen T. FOXP3+ regulatory T cell compartment is altered in children with newly diagnosed type 1 diabetes but not in autoantibody-positive at-risk children. Front Immunol. 2019;10:19.
Luczynski W, Stasiak-Barmuta A, Wawrusiewicz-Kurylonek N, Kowalczuk O, Ilendo E, Glowinska-Olszewska B, Urban R, Szczepanski W, Urban M, Kretowski A, Chyczewski L. Disturbances in some gene expression in T regulatory cells separated from children with metabolic syndrome. Scand J Immunol. 2010;71:115–22.
Cabello-Kindelan C, Mackey S, Sands A, Rodriguez J, Vazquez C, Pugliese A, Bayer AL. Immunomodulation followed by antigen-specific T(reg) infusion controls islet autoimmunity. Diabetes. 2020;69:215–27.
Hope CM, Welch J, Mohandas A, Pederson S, Hill D, Gundsambuu B, Eastaff-Leung N, Grosse R, Bresatz S, Ang G, Papademetrios M, Zola H, Duhen T, Campbell D, Brown CY, Krumbiegel D, Sadlon T, Couper JJ, Barry SC. Peptidase inhibitor 16 identifies a human regulatory T-cell subset with reduced FOXP3 expression over the first year of recent onset type 1 diabetes. Eur J Immunol. 2019;49:1235–50.
Haseda F, Imagawa A, Murase-Mishiba Y, Terasaki J, Hanafusa T. CD4(+) CD45RA(-) FoxP3high activated regulatory T cells are functionally impaired and related to residual insulin-secreting capacity in patients with type 1 diabetes. Clin Exp Immunol. 2013;173:207–16.
Putnam AL, Vendrame F, Dotta F, Gottlieb PA. CD4+CD25high regulatory T cells in human autoimmune diabetes. J Autoimmun. 2005;24:55–62.
Ben-Skowronek I, Sieniawska J, Pach E, Wrobel W, Skowronek A, Tomczyk Z, Rosolowska I. Potential therapeutic application of regulatory T cells in diabetes mellitus type 1. Int J Mol Sci. 2021;23:390.
Tuomilehto J, Ogle GD, Lund-Blix NA, Stene LC. Update on worldwide trends in occurrence of childhood type 1 diabetes in 2020. Pediatr Endocrinol Rev. 2020;17:198–209.
Aarnisalo J, Veijola R, Vainionpaa R, Simell O, Knip M, Ilonen J. Cytomegalovirus infection in early infancy: risk of induction and progression of autoimmunity associated with type 1 diabetes. Diabetologia. 2008;51:769–72.
Oikarinen S, Tauriainen S, Hober D, Lucas B, Vazeou A, Sioofy-Khojine A, Bozas E, Muir P, Honkanen H, Ilonen J, Knip M, Keskinen P, Saha MT, Huhtala H, Stanway G, Bartsocas C, Ludvigsson J, Taylor K, Hyoty H, VirDiab Study G. Virus antibody survey in different European populations indicates risk association between coxsackievirus B1 and type 1 diabetes. Diabetes. 2014;63:655–62.
Alnek K, Talja I, Laht B, Metskula K, Mandel M, Reppo I, Lubi M, Uibo R. IgA-type enterovirus antibodies are increased among adults and children with recently diagnosed type 1 diabetes. Biomed Res Int. 2022;2022:7603062.
Lemos JRN, Hirani K, von Herrath M. Immunological and virological triggers of type 1 diabetes: insights and implications. Front Immunol. 2023;14:1326711.
Morgan NG. Insulitis in human type 1 diabetes: lessons from an enigmatic lesion. Eur J Endocrinol. 2024;190:R1–9.
Coppieters KT, Wiberg A, von Herrath MG. Viral infections and molecular mimicry in type 1 diabetes. APMIS. 2012;120:941–9.
Rojas M, Restrepo-Jiménez P, Monsalve DM, Pacheco Y, Acosta-Ampudia Y, Ramírez-Santana C, Leung PSC, Ansari AA, Gershwin ME, Anaya JM. Molecular mimicry and autoimmunity. J Autoimmun. 2018;95:100–23.
Hagopian WA, Lernmark A, Rewers MJ, Simell OG, She JX, Ziegler AG, Krischer JP, Akolkar B. TEDDY–the environmental determinants of diabetes in the young: an observational clinical trial. Ann N Y Acad Sci. 2006;1079:320–6.
Diabetes Prevention Trial-Type 1 Diabetes Study G. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685–91.
Skyler JS, Greenbaum CJ, Lachin JM, Leschek E, Rafkin-Mervis L, Savage P, Spain L, Type 1 Diabetes TrialNet Study G. Type 1 diabetes trialnet–an international collaborative clinical trials network. Ann N Y Acad Sci. 2008;1150:14–24.
Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, Greenbaum CJ, Herold KC, Krischer JP, Lernmark A, Ratner RE, Rewers MJ, Schatz DA, Skyler JS, Sosenko JM, Ziegler AG. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the endocrine society, and the American diabetes association. Diabetes Care. 2015;38:1964–74.
Achenbach P, Hummel M, Thumer L, Boerschmann H, Hofelmann D, Ziegler AG. Characteristics of rapid vs slow progression to type 1 diabetes in multiple islet autoantibody-positive children. Diabetologia. 2013;56:1615–22.
Smith MJ, Simmons KM, Cambier JC. B cells in type 1 diabetes mellitus and diabetic kidney disease. Nat Rev Nephrol. 2017;13:712–20.
Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, Winkler C, Ilonen J, Veijola R, Knip M, Bonifacio E, Eisenbarth GS. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–9.
Jia X, Gu Y, High H, Yu L. Islet autoantibodies in disease prediction and pathogenesis. Diabetol Int. 2020;11:6–10.
Anand V, Li Y, Liu B, Ghalwash M, Koski E, Ng K, Dunne JL, Jonsson J, Winkler C, Knip M, Toppari J, Ilonen J, Killian MB, Frohnert BI, Lundgren M, Ziegler AG, Hagopian W, Veijola R, Rewers M, Group TDS. Islet autoimmunity and HLA markers of presymptomatic and clinical type 1 diabetes: joint analyses of prospective cohort studies in finland, Germany, Sweden, and the US. Diabetes Care. 2021;44:2269–76.
Balzano-Nogueira L, Ramirez R, Zamkovaya T, Dailey J, Ardissone AN, Chamala S, Serrano-Quilez J, Rubio T, Haller MJ, Concannon P, Atkinson MA, Schatz DA, Triplett EW, Conesa A. Integrative analyses of TEDDY omics data reveal lipid metabolism abnormalities, increased intracellular ROS and heightened inflammation prior to autoimmunity for type 1 diabetes. Genome Biol. 2021;22:39.
Holm LJ, Krogvold L, Hasselby JP, Kaur S, Claessens LA, Russell MA, Mathews CE, Hanssen KF, Morgan NG, Koeleman BPC, Roep BO, Gerling IC, Pociot F, Dahl-Jorgensen K, Buschard K. Abnormal islet sphingolipid metabolism in type 1 diabetes. Diabetologia. 2018;61:1650–61.
Ludvigsson J, Cuthbertson D, Becker DJ, Kordonouri O, Aschemeier B, Pacaud D, Clarson C, Krischer JP, Knip M, Investigators T. Increasing plasma glucose before the development of type 1 diabetes-the TRIGR study. Pediatr Diabetes. 2021;22:974–81.
Ludvigsson J. Why diabetes incidence increases–a unifying theory. Ann N Y Acad Sci. 2006;1079:374–82.
Lamichhane S, Sen P, Dickens AM, Krakstrom M, Ilonen J, Lempainen J, Hyoty H, Lahesmaa R, Veijola R, Toppari J, Hyotylainen T, Knip M, Oresic M. Circulating metabolic signatures of rapid and slow progression to type 1 diabetes in islet autoantibody-positive children. Front Endocrinol (Lausanne). 2023;14:1211015.
Helminen O, Aspholm S, Pokka T, Ilonen J, Simell O, Veijola R, Knip M. OGTT and random plasma glucose in the prediction of type 1 diabetes and time to diagnosis. Diabetologia. 2015;58:1787–96.
Steck AK, Johnson K, Barriga KJ, Miao D, Yu L, Hutton JC, Eisenbarth GS, Rewers MJ. Age of islet autoantibody appearance and mean levels of insulin, but not GAD or IA-2 autoantibodies, predict age of diagnosis of type 1 diabetes: diabetes autoimmunity study in the young. Diabetes Care. 2011;34:1397–9.
Campbell-Thompson M, Wasserfall C, Montgomery EL, Atkinson MA, Kaddis JS. Pancreas organ weight in individuals with disease-associated autoantibodies at risk for type 1 diabetes. JAMA. 2012;308:2337–9.
Virostko J, Williams J, Hilmes M, Bowman C, Wright JJ, Du L, Kang H, Russell WE, Powers AC, Moore DJ. Pancreas volume declines during the first year after diagnosis of type 1 diabetes and exhibits altered diffusion at disease onset. Diabetes Care. 2019;42:248–57.
Campbell-Thompson ML, Filipp SL, Grajo JR, Nambam B, Beegle R, Middlebrooks EH, Gurka MJ, Atkinson MA, Schatz DA, Haller MJ. Relative pancreas volume is reduced in first-degree relatives of patients with type 1 diabetes. Diabetes Care. 2019;42:281–7.
Chiarelli F, Verrotti A, Altobelli E, Blasetti A, Morgese G. Size of the pancreas in type I diabetic children and adolescents. Diabetes Care. 1995;18:1505–6.
Fonseca V, Berger LA, Beckett AG, Dandona P. Size of pancreas in diabetes mellitus: a study based on ultrasound. Br Med J (Clin Res Ed). 1985;291:1240–1.
Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14:619–33.
Gaglia JL, Guimaraes AR, Harisinghani M, Turvey SE, Jackson R, Benoist C, Mathis D, Weissleder R. Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J Clin Invest. 2011;121:442–5.
Williams AJ, Thrower SL, Sequeiros IM, Ward A, Bickerton AS, Triay JM, Callaway MP, Dayan CM. Pancreatic volume is reduced in adult patients with recently diagnosed type 1 diabetes. J Clin Endocrinol Metab. 2012;97:E2109–13.
Powers AC. Type 1 diabetes mellitus: much progress, many opportunities. J Clin Invest. 2021. https://doi.org/10.1172/JCI142242.
Alexandre-Heymann L, Mallone R, Boitard C, Scharfmann R, Larger E. Structure and function of the exocrine pancreas in patients with type 1 diabetes. Rev Endocr Metab Disord. 2019;20:129–49.
Wright JJ, Saunders DC, Dai C, Poffenberger G, Cairns B, Serreze DV, Harlan DM, Bottino R, Brissova M, Powers AC. Decreased pancreatic acinar cell number in type 1 diabetes. Diabetologia. 2020;63:1418–23.
Skog O, Korsgren S, Melhus A, Korsgren O. Revisiting the notion of type 1 diabetes being a T-cell-mediated autoimmune disease. Curr Opin Endocrinol Diabetes Obes. 2013;20:118–23.
Korsgren S, Molin Y, Salmela K, Lundgren T, Melhus A, Korsgren O. On the etiology of type 1 diabetes: a new animal model signifying a decisive role for bacteria eliciting an adverse innate immunity response. Am J Pathol. 2012;181:1735–48.
Foster TP, Bruggeman B, Campbell-Thompson M, Atkinson MA, Haller MJ, Schatz DA. Exocrine pancreas dysfunction in type 1 diabetes. Endocr Pract. 2020;26:1505–13.
Jacobsen LM, Bocchino L, Evans-Molina C, DiMeglio L, Goland R, Wilson DM, Atkinson MA, Aye T, Russell WE, Wentworth JM, Boulware D, Geyer S, Sosenko JM. The risk of progression to type 1 diabetes is highly variable in individuals with multiple autoantibodies following screening. Diabetologia. 2020;63:588–96.
Krischer JP, Lynch KF, Schatz DA, Ilonen J, Lernmark A, Hagopian WA, Rewers MJ, She JX, Simell OG, Toppari J, Ziegler AG, Akolkar B, Bonifacio E, Group TS. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58:980–7.
Delong T, Wiles TA, Baker RL, Bradley B, Barbour G, Reisdorph R, Armstrong M, Powell RL, Reisdorph N, Kumar N, Elso CM, DeNicola M, Bottino R, Powers AC, Harlan DM, Kent SC, Mannering SI, Haskins K. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science. 2016;351:711–4.
Babon JA, DeNicola ME, Blodgett DM, Crevecoeur I, Buttrick TS, Maehr R, Bottino R, Naji A, Kaddis J, Elyaman W, James EA, Haliyur R, Brissova M, Overbergh L, Mathieu C, Delong T, Haskins K, Pugliese A, Campbell-Thompson M, Mathews C, Atkinson MA, Powers AC, Harlan DM, Kent SC. Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat Med. 2016;22:1482–7.
Mitchell AM, Alkanani AA, McDaniel KA, Pyle L, Waugh K, Steck AK, Nakayama M, Yu L, Gottlieb PA, Rewers MJ, Michels AW. T-cell responses to hybrid insulin peptides prior to type 1 diabetes development. Proc Natl Acad Sci U S A. 2021;118(6):e2019129118.
Morgan NG, Leete P, Foulis AK, Richardson SJ. Islet inflammation in human type 1 diabetes mellitus. IUBMB Life. 2014;66:723–34.
Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155:173–81.
Arif S, Leete P, Nguyen V, Marks K, Nor NM, Estorninho M, Kronenberg-Versteeg D, Bingley PJ, Todd JA, Guy C, Dunger DB, Powrie J, Willcox A, Foulis AK, Richardson SJ, de Rinaldis E, Morgan NG, Lorenc A, Peakman M. Blood and islet phenotypes indicate immunological heterogeneity in type 1 diabetes. Diabetes. 2014;63:3835–45.
Leete P, Willcox A, Krogvold L, Dahl-Jorgensen K, Foulis AK, Richardson SJ, Morgan NG. Differential insulitic profiles determine the extent of beta-cell destruction and the age at onset of type 1 diabetes. Diabetes. 2016;65:1362–9.
Atkinson MA, von Herrath M, Powers AC, Clare-Salzler M. Current concepts on the pathogenesis of type 1 diabetes–considerations for attempts to prevent and reverse the disease. Diabetes Care. 2015;38:979–88.
Rodriguez-Calvo T, Suwandi JS, Amirian N, Zapardiel-Gonzalo J, Anquetil F, Sabouri S, von Herrath MG. Heterogeneity and lobularity of pancreatic pathology in type 1 diabetes during the prediabetic phase. J Histochem Cytochem. 2015;63:626–36.
Richardson SJ, Willcox A, Bone AJ, Morgan NG, Foulis AK. Immunopathology of the human pancreas in type-I diabetes. Semin Immunopathol. 2011;33:9–21.
Richardson SJ, Morgan NG, Foulis AK. Pancreatic pathology in type 1 diabetes mellitus. Endocr Pathol. 2014;25:80–92.
Sherry NA, Tsai EB, Herold KC. Natural history of beta-cell function in type 1 diabetes. Diabetes. 2005;54(Suppl 2):S32–9.
Keymeulen B, Somers G. Immunointervention in type 1 (insulin-dependent) diabetes mellitus. Acta Clin Belg. 1993;48:86–95.
Muhammad BJ, Swift PG, Raymond NT, Botha JL. Partial remission phase of diabetes in children younger than age 10 years. Arch Dis Child. 1999;80:367–9.
Bober E, Dundar B, Buyukgebiz A. Partial remission phase and metabolic control in type 1 diabetes mellitus in children and adolescents. J Pediatr Endocrinol Metab. 2001;14:435–41.
Buyukgebiz A, Cemeroglu AP, Bober E, Mohn A, Chiarelli F. Factors influencing remission phase in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2001;14:1585–96.
Lombardo F, Valenzise M, Wasniewska M, Messina MF, Ruggeri C, Arrigo T, De Luca F. Two-year prospective evaluation of the factors affecting honeymoon frequency and duration in children with insulin dependent diabetes mellitus: the key-role of age at diagnosis. Diabetes Nutr Metab. 2002;15:246–51.
Abdul-Rasoul M, Habib H, Al-Khouly M. ‘The honeymoon phase’ in children with type 1 diabetes mellitus: frequency, duration, and influential factors. Pediatr Diabetes. 2006;7:101–7.
Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832–6.
Narsale A, Almanza F, Tran T, Lam B, Seo D, Vu A, Long SA, Cooney L, Serti E, Davies JD. Th2 cell clonal expansion at diagnosis in human type 1 diabetes. Clin Immunol. 2023;257:109829.
Lamichhane S, Kemppainen E, Trost K, Siljander H, Hyoty H, Ilonen J, Toppari J, Veijola R, Hyotylainen T, Knip M, Oresic M. Circulating metabolites in progression to islet autoimmunity and type 1 diabetes. Diabetologia. 2019;62:2287–97.
Oresic M, Simell S, Sysi-Aho M, Nanto-Salonen K, Seppanen-Laakso T, Parikka V, Katajamaa M, Hekkala A, Mattila I, Keskinen P, Yetukuri L, Reinikainen A, Lahde J, Suortti T, Hakalax J, Simell T, Hyoty H, Veijola R, Ilonen J, Lahesmaa R, Knip M, Simell O. Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. J Exp Med. 2008;205:2975–84.
Lamichhane S, Ahonen L, Dyrlund TS, Kemppainen E, Siljander H, Hyoty H, Ilonen J, Toppari J, Veijola R, Hyotylainen T, Knip M, Oresic M. Dynamics of plasma lipidome in progression to islet autoimmunity and type 1 diabetes - type 1 diabetes prediction and prevention study (DIPP). Sci Rep. 2018;8:10635.
Kupila A, Muona P, Simell T, Arvilommi P, Savolainen H, Hamalainen AM, Korhonen S, Kimpimaki T, Sjoroos M, Ilonen J, Knip M, Simell O, Juvenile Diabetes Research Foundation Centre for the Prevention of Type IDiF. Feasibility of genetic and immunological prediction of type I diabetes in a population-based birth cohort. Diabetologia. 2001;44:290–7.
Galderisi A, Pirillo P, Moret V, Stocchero M, Gucciardi A, Perilongo G, Moretti C, Monciotti C, Giordano G, Baraldi E. Metabolomics reveals new metabolic perturbations in children with type 1 diabetes. Pediatr Diabetes. 2018;19:59–67.
Balderas C, Ruperez FJ, Ibanez E, Senorans J, Guerrero-Fernandez J, Casado IG, Gracia-Bouthelier R, Garcia A, Barbas C. Plasma and urine metabolic fingerprinting of type 1 diabetic children. Electrophoresis. 2013;34:2882–90.
Vatanen T, Franzosa EA, Schwager R, Tripathi S, Arthur TD, Vehik K, Lernmark A, Hagopian WA, Rewers MJ, She JX, Toppari J, Ziegler AG, Akolkar B, Krischer JP, Stewart CJ, Ajami NJ, Petrosino JF, Gevers D, Lahdesmaki H, Vlamakis H, Huttenhower C, Xavier RJ. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature. 2018;562:589–94.
Vatanen T, Kostic AD, d’Hennezel E, Siljander H, Franzosa EA, Yassour M, Kolde R, Vlamakis H, Arthur TD, Hamalainen AM, Peet A, Tillmann V, Uibo R, Mokurov S, Dorshakova N, Ilonen J, Virtanen SM, Szabo SJ, Porter JA, Lahdesmaki H, Huttenhower C, Gevers D, Cullen TW, Knip M, Xavier RJ, Group DS. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 2016;165:1551.
Knip M, Siljander H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat Rev Endocrinol. 2016;12:154–67.
Zheng P, Li Z, Zhou Z. Gut microbiome in type 1 diabetes: a comprehensive review. Diabetes Metab Res Rev. 2018;34: e3043.
Hubbard TD, Murray IA, Bisson WH, Lahoti TS, Gowda K, Amin SG, Patterson AD, Perdew GH. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci Rep. 2015;5:12689.
Alexeev EE, Lanis JM, Kao DJ, Campbell EL, Kelly CJ, Battista KD, Gerich ME, Jenkins BR, Walk ST, Kominsky DJ, Colgan SP. Microbiota-derived indole metabolites promote human and murine intestinal homeostasis through regulation of interleukin-10 receptor. Am J Pathol. 2018;188:1183–94.
Kurpiewska E, Ciezki S, Jamiolkowska-Sztabkowska M, Polkowska A, Starosz A, Grubczak K, Moniuszko M, Bossowski A, Glowinska-Olszewska B. Excessive BMI is associated with higher C-peptide level at recognition but also with its greater loss in two years clinical observation in children with new onset type 1 diabetes. Front Immunol. 2023;14:1176403.
Szypowska A, Groele L, Wysocka-Mincewicz M, Mazur A, Lisowicz L, Ben-Skowronek I, Sieniawska J, Klonowska B, Charemska D, Nawrotek J, Jalowiec I, Bossowski A, Noiszewska K, Pyrzak B, Rogozinska I, Szalecki M. Factors associated with preservation of C-peptide levels at the diagnosis of type 1 diabetes. J Diabetes Complications. 2018;32:570–4.
Redondo MJ, Rodriguez LM, Escalante M, O’Brian Smith E, Balasubramanyam A, Haymond MW. Beta cell function and BMI in ethnically diverse children with newly diagnosed autoimmune type 1 diabetes. Pediatr Diabetes. 2012;13:564–71.
Lauria A, Barker A, Schloot N, Hosszufalusi N, Ludvigsson J, Mathieu C, Mauricio D, Nordwall M, Van der Schueren B, Mandrup-Poulsen T, Scherbaum WA, Weets I, Gorus FK, Wareham N, Leslie RD, Pozzilli P. BMI is an important driver of beta-cell loss in type 1 diabetes upon diagnosis in 10–18 year-old children. Eur J Endocrinol. 2015;172:107–13.
Op de Beeck A, Eizirik DL. Viral infections in type 1 diabetes mellitus–why the beta cells? Nat Rev Endocrinol. 2016;12:263–73.
Jog NR, James JA. Epstein barr virus and autoimmune responses in systemic lupus erythematosus. Front Immunol. 2020;11: 623944.
Vijay K. Toll-like receptors in immunity and inflammatory diseases: past, present, and future. Int Immunopharmacol. 2018;59:391–412.
Pipeleers D, Ling Z. Pancreatic beta cells in insulin-dependent diabetes. Diabetes Metab Rev. 1992;8:209–27.
Lernmark A, Kloppel G, Stenger D, Vathanaprida C, Falt K, Landin-Olsson M, Baskin DG, Palmer JP, Gown AM, Petersen JS, et al. Heterogeneity of islet pathology in two infants with recent onset diabetes mellitus. Virchows Arch. 1995;425:631–40.
American Diabetes Association Professional Practice C. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care 2024;47:S20-S42.
Itariu BK, Stulnig TM. Autoimmune aspects of type 2 diabetes mellitus - a mini-review. Gerontology. 2014;60:189–96.
Fourlanos S, Perry C, Stein MS, Stankovich J, Harrison LC, Colman PG. A clinical screening tool identifies autoimmune diabetes in adults. Diabetes Care. 2006;29:970–5.
Satomura A, Oikawa Y, Haisa A, Suzuki S, Nakanishi S, Katsuki T, Shimada A. Clinical significance of Insulin peptide-specific interferon-gamma-related immune responses in ketosis-prone type 2 diabetes. J Clin Endocrinol Metab. 2022;107:e2124–32.
Correction to New diagnostic criteria. For slowly progressive type 1 diabetes (SPIDDM): report from committee on type 1 diabetes of the Japan diabetes society (english version). J Diabetes Investig. 2023;2024(15):643.
Redondo MJ, Morgan NG. Heterogeneity and endotypes in type 1 diabetes mellitus. Nat Rev Endocrinol. 2023;19:542–54.
Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107.
Tuomi T, Carlsson A, Li H, Isomaa B, Miettinen A, Nilsson A, Nissen M, Ehrnstrom BO, Forsen B, Snickars B, Lahti K, Forsblom C, Saloranta C, Taskinen MR, Groop LC. Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes. 1999;48:150–7.
Luo S, Ma X, Li X, Xie Z, Zhou Z. Fulminant type 1 diabetes: a comprehensive review of an autoimmune condition. Diabetes Metab Res Rev. 2020;36: e3317.
Chujo D, Kawabe A, Matsushita M, Tsutsumi C, Haseda F, Imagawa A, Hanafusa T, Ueki K, Kajio H, Yagi K, Tobe K, Shimoda M. Fulminant type 1 diabetes patients display high frequencies of IGRP-specific type 1 CD8(+) T cells. Clin Immunol. 2021;233: 108893.
Imagawa A. Two types of fulminant type 1 diabetes mellitus: Immune checkpoint inhibitor-related and conventional. J Diabetes Investig. 2021;12:917–9.
Cho YK, Jung CH. Immune-checkpoint inhibitors-induced type 1 diabetes mellitus: from its molecular mechanisms to clinical practice. Diabetes Metab J. 2023;47:757–66.
Hanafusa T, Imagawa A. Fulminant type 1 diabetes: a novel clinical entity requiring special attention by all medical practitioners. Nat Clin Pract Endocrinol Metab. 2007;3(1):36–45.
Imagawa A, Hanafusa T. Fulminant type 1 diabetes–an important subtype in East Asia. Diabetes Metab Res Rev. 2011;27:959–64.
Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide diabetes mondiale (DiaMond). Diabetes Care. 2000;23(10):1516–26.
Moreau C, Drui D, Arnault-Ouary G, Charbonnel B, Chaillous L, Cariou B. Fulminant type 1 diabetes in caucasians: a report of three cases. Diabetes Metab. 2008;34:529–32.
Kawabata Y, Nishida N, Awata T, Kawasaki E, Imagawa A, Shimada A, Osawa H, Tanaka S, Takahashi K, Nagata M, Yasuda H, Uchigata Y, Kajio H, Makino H, Yasuda K, Kobayashi T, Hanafusa T, Tokunaga K, Ikegami H. Genome-wide association study confirming a strong effect of HLA and identifying variants in CSAD/lnc-ITGB7–1 on chromosome 12q13.13 associated with susceptibility to fulminant type 1 diabetes. Diabetes. 2019;68:665–75.
Toren E, Burnette KS, Banerjee RR, Hunter CS, Tse HM. Partners in crime: beta-cells and autoimmune responses complicit in type 1 diabetes pathogenesis. Front Immunol. 2021;12: 756548.
Battaglia M, Anderson MS, Buckner JH, Geyer SM, Gottlieb PA, Kay TWH, Lernmark Å, Muller S, Pugliese A, Roep BO, Greenbaum CJ, Peakman M. Understanding and preventing type 1 diabetes through the unique working model of TrialNet. Diabetologia. 2017;60:2139–47.
Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, Greenbaum CJ, Herold KC, Krischer JP, Lernmark Å, Ratner RE, Rewers MJ, Schatz DA, Skyler JS, Sosenko JM, Ziegler AG. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the endocrine society, and the American diabetes association. Diabetes Care. 2015;38:1964–74.
Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82.
Atkinson MA, Bluestone JA, Eisenbarth GS, Hebrok M, Herold KC, Accili D, Pietropaolo M, Arvan PR, Von Herrath M, Markel DS, Rhodes CJ. How does type 1 diabetes develop?: the notion of homicide or beta-cell suicide revisited. Diabetes. 2011;60:1370–9.
Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A. Type 1 diabetes mellitus as a disease of the beta-cell (do not blame the immune system?). Nat Rev Endocrinol. 2021;17:150–61.
Azoury ME, Tarayrah M, Afonso G, Pais A, Colli ML, Maillard C, Lavaud C, Alexandre-Heymann L, Gonzalez-Duque S, Verdier Y, Vinh J, Pinto S, Buus S, Dubois-Laforgue D, Larger E, Beressi JP, Bruno G, Eizirik DL, You S, Mallone R. Peptides derived from insulin granule proteins are targeted by CD8(+) T cells across MHC class I restrictions in humans and NOD mice. Diabetes. 2020;69:2678–90.
Gonzalez-Duque S, Azoury ME, Colli ML, Afonso G, Turatsinze JV, Nigi L, Lalanne AI, Sebastiani G, Carré A, Pinto S, Culina S, Corcos N, Bugliani M, Marchetti P, Armanet M, Diedisheim M, Kyewski B, Steinmetz LM, Buus S, You S, Dubois-Laforgue D, Larger E, Beressi JP, Bruno G, Dotta F, Scharfmann R, Eizirik DL, Verdier Y, Vinh J, Mallone R. Conventional and neo-antigenic peptides presented by β cells are targeted by circulating naïve CD8+ T cells in type 1 diabetic and healthy donors. Cell Metab. 2018;28:946–960.e6.
Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat Rev Endocrinol. 2021;17:150–61.
Sims EK, Bahnson HT, Nyalwidhe J, Haataja L, Davis AK, Speake C, DiMeglio LA, Blum J, Morris MA, Mirmira RG, Nadler J, Mastracci TL, Marcovina S, Qian WJ, Yi L, Swensen AC, Yip-Schneider M, Schmidt CM, Considine RV, Arvan P, Greenbaum CJ, Evans-Molina C. Proinsulin secretion is a persistent feature of type 1 diabetes. Diabetes Care. 2019;42:258–64.
Wasserfall C, Nick HS, Campbell-Thompson M, Beachy D, Haataja L, Kusmartseva I, Posgai A, Beery M, Rhodes C, Bonifacio E, Arvan P, Atkinson M. Persistence of pancreatic insulin mRNA expression and proinsulin protein in type 1 diabetes pancreata. Cell Metab. 2017;26:568–575.e3.
Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, Gitelman SE, Gottlieb PA, Krischer JP, Linsley PS, Marks JB, Moore W, Moran A, Rodriguez H, Russell WE, Schatz D, Skyler JS, Tsalikian E, Wherrett DK, Ziegler AG, Greenbaum CJ, Type 1 Diabetes TrialNet Study G. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med. 2019;381:603–13.
Kamrul-Hasan ABM, Mondal S, Nagendra L, Yadav A, Aalpona FTZ, Dutta D. Role of teplizumab, a humanized anti-CD3 monoclonal antibody, in managing newly diagnosed type 1 Diabetes: an updated systematic review and meta-analysis. Endocr Pract. 2024;30:431–40.
Calderon Martinez E, Castillo JL, Zachariah Saji S, Stein D, Khan TJ, Guardado Williams RF, Munguia ID, Arruarana VS, Velasquez K. Insulin pump therapy vs multiple daily insulin injections for glycemic control in children with type 1 diabetes: a systematic review and meta-analysis. Cureus. 2024;16: e52054.
Wake AD. Protective effects of physical activity against health risks associated with type 1 diabetes: “health benefits outweigh the risks.” World J Diabetes. 2022;13:161–84.
Mullard A. FDA approves first cell therapy for type 1 diabetes. Nat Rev Drug Discov. 2023;22:611.
Quattrin T, Haller MJ, Steck AK, Felner EI, Li Y, Xia Y, Leu JH, Zoka R, Hedrick JA, Rigby MR, Vercruysse F, Investigators TGS. Golimumab and beta-cell function in youth with new-onset type 1 diabetes. N Engl J Med. 2020;383:2007–17.
Waibel M, Wentworth JM, So M, Couper JJ, Cameron FJ, MacIsaac RJ, Atlas G, Gorelik A, Litwak S, Sanz-Villanueva L, Trivedi P, Ahmed S, Martin FJ, Doyle ME, Harbison JE, Hall C, Krishnamurthy B, Colman PG, Harrison LC, Thomas HE, Kay TWH, Group BS. Baricitinib and beta-cell function in patients with new-onset type 1 diabetes. N Engl J Med. 2023;389:2140–50.
Sanofi (2024) FrexalimAB in Preservation of Endogenous insULIN Secretion Compared to Placebo in adUlts and Adolescents on Top of inSulin Therapy (FABULINUS) (FABULINUS). https://clinicaltrials.gov/study/NCT06111586?cond=Type%201%20Diabetes&intr=Frexalimab&rank=1 Accessed 24 May
von Herrath M, Bain SC, Bode B, Clausen JO, Coppieters K, Gaysina L, Gumprecht J, Hansen TK, Mathieu C, Morales C, Mosenzon O, Segel S, Tsoukas G, Pieber TR, Anti ILlSGi, contributors. Anti-interleukin-21 antibody and liraglutide for the preservation of beta-cell function in adults with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2021;9:212–24.
Saleh M, Gittes GK, Prasadan K. Alpha-to-beta cell trans-differentiation for treatment of diabetes. Biochem Soc Trans. 2021;49:2539–48.
Yesildag B, Mir-Coll J, Neelakandhan A, Gibson CB, Perdue NR, Rufer C, Karsai M, Biernath A, Forschler F, Jin PW, Misun PM, Title A, Hierlemann A, Kreiner FF, Wesley JD, von Herrath MG. Liraglutide protects beta-cells in novel human islet spheroid models of type 1 diabetes. Clin Immunol. 2022;244: 109118.
Dutta D, Nagendra L, Raizada N, Bhattacharya S, Sharma M. Verapamil improves one-year C-peptide levels in recent onset type-1 diabetes: a meta-analysis. Indian J Endocrinol Metab. 2023;27:192–200.
Foster TP, Jacobsen LM, Bruggeman B, Salmon C, Hosford J, Chen A, Cintron M, Mathews CE, Wasserfall C, Brusko MA, Brusko TM, Atkinson MA, Schatz DA, Haller MJ. Low-dose antithymocyte globulin: a pragmatic approach to treating stage 2 type 1 diabetes. Diabetes Care. 2024;47:285–9.
Dwyer CJ, Ward NC, Pugliese A, Malek TR. Promoting immune regulation in type 1 diabetes using low-dose interleukin-2. Curr Diab Rep. 2016;16:46.
Acknowledgements
This review is a summary of my presentation in the Special Lecture at the 66th annual meeting of the Japan Diabetes Society, Kagoshima, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MvH is employed by Novo Nordisk A/S. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Human or animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Ling, E.M., Lemos, J.R.N., Hirani, K. et al. Type 1 diabetes: immune pathology and novel therapeutic approaches. Diabetol Int (2024). https://doi.org/10.1007/s13340-024-00748-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13340-024-00748-z