Abstract
Type 1 diabetes is a multifactorial disease resulting from a complex interplay between host genetics, the immune system and the environment, that culminates in the destruction of insulin-producing beta cells. The incidence of type 1 diabetes is increasing at an alarming rate, especially in children under the age of 5 (Gepts in Diabetes 14(10):619-613, 1965; Foulis et al. in Lancet 29(5):267-274, 1986; Gamble, Taylor and Cumming in British Medical Journal 4(5887):260-262 1973). Genetic predisposition, although clearly important, cannot explain this rise, and so, it has been proposed that changes in the ‘environment’ and/or changes in ‘how we respond to our environment’ must contribute to this rising incidence. In order to gain an improved understanding of the factors influencing the disease process, it is important, firstly, to focus on the organ at the centre of the illness—the pancreas. This review summarises our knowledge of the pathology of the endocrine pancreas in human type 1 diabetes and, in particular, explores the progression of this understanding over the past 25 years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
What Was Known 25 Years Ago?
While there had been numerous studies of the autopsy pancreas in young persons with diabetes before, the foundations of this subject were laid by the seminal article of Willy Gepts in 1965 [1]. Gepts noted the presence of insulitis (a predominantly lymphocytic inflammatory infiltrate of islets) in 15 of 22 (68 %) autopsies of persons, under the age of 30, dying within 6 months of a diagnosis of diabetes [1]. These findings were later confirmed in a larger collection of UK samples, where 47 out of 60 (78 %) young patients (<20 years) with recent-onset type 1 diabetes were found to have evidence of insulitis [2]. Prior to the development of immunohistochemistry, Gepts also noted that the pancreas in persons who had had the disease for many years was characterised by an almost complete lack of insulin-secreting beta cells. From these observations, he raised two hypotheses: the islet inflammation could be secondary to a viral infection of beta cells or it could represent an immunologically driven process, akin to autoimmune thyroiditis.
Support for both these theories followed. Gamble, Taylor and Cumming [3] found that patients with recently diagnosed type 1 diabetes were more likely than controls to have antibodies to Coxsackie viruses, and Coxsackie B4 virus was cultured from the pancreas of a 10-year-old boy who had died at clinical presentation of type 1 diabetes [4]. This case may have been exceptional in that the virus was also cultured from the brain of the child, raising the possibility of an incidental viraemia. Many attempts have since been made to culture viruses from the pancreas of recent onset diabetic patients, largely without success.
In 1974, two separate groups reported the presence of islet cell autoantibodies in patients with type 1 diabetes [5–7], leading to the disease being later classified as an organ-specific autoimmune disease with the insulin-secreting beta cells as the targets.
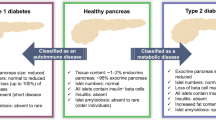
Following the advent of immunohistochemistry being applied to paraffin-embedded tissue, it was shown that within the pancreas of children dying at clinical presentation of their disease the majority of islets (70 %) were small and had no beta cells [8]. They had a normal complement of glucagon-secreting alpha cells, somatostatin-secreting delta cells and pancreatic polypeptide-secreting PP cells and were termed insulin-deficient islets (IDI). The remainder of the islets had residual beta cells and were called insulin-containing islets (ICI). Insulitis affected 18 % of ICI but only 1 % of IDI. This was the first evidence that insulitis affected ICI primarily and lent support to the idea that it represented an immunologically driven destruction of beta cells [8]. Importantly, the lobular distribution of the disease was also noted (Fig. 1), where a seemingly unaffected lobe, or one where ICI with insulitis could be observed, was frequently surrounded by lobes containing only IDI.
The lobular nature of type 1 diabetes. A photomicrograph of a pancreas from a type 1 diabetes patient stained via immunohistochemistry for the presence of glucagon (a) and a serial section stained for insulin (b). The lobe containing ICIs can be seen on the right (hashed line), whereas the neighbouring lobe contains only IDI
It also became clear that the pancreas at clinical presentation could be studied to look at the time course of events within the organ. Insulin-deficient islets were islets where beta cells had been destroyed in the past; ICI with insulitis were islets where beta cells were being destroyed at the time of the death of the patient, and ICI with no insulitis were islets where the beta cells were yet to be attacked and destroyed. Gepts [1] had suggested that the destruction of beta cells must happen over years, and support for this was the finding of insulitis affecting ICI up to 6 years after clinical onset of diabetes [2].
Prior to the development of antigen retrieval, many antigens could only be studied by immunohistochemistry on tissues that had not been fixed in formalin. Characterization of the inflammatory cell infiltrate in insulitis for many years relied on one report where fresh frozen pancreas had been collected at the time of death from a child with recent onset diabetes [9]. The majority of the inflammatory cells were T lymphocytes, and the majority of them expressed CD8, suggesting that they were likely to be cytotoxic T cells. Macrophages were not abundant.
Cytotoxic CD8 T cells can recognise an antigen when it is presented to them bound to cellular class I major histocompatibility complex (MHC) molecules. This presentation is enhanced if there is increased expression of class I MHC. Bottazzo et al. [9] noticed that endocrine cells in some islets in the single pancreas that they studied hyperexpressed class I MHC but they did not fully characterise this phenomenon and did not describe which cells were affected. This feature was later elucidated in a study of 23 pancreases from persons dying of recent onset type 1 diabetes [10]. Hyperexpression of class I MHC was found in all pancreases where there were residual beta cells (an example is shown in Fig. 2). It affected 92 % of ICI but only 1 % of IDI. The phenomenon was not seen in 95 control pancreases, which included normal and diseased pancreases (graft versus host disease, Coxsackie viral infection, type 2 diabetes, chronic pancreatitis, cystic fibrosis). It was thus as much a characteristic of type 1 diabetes as insulitis and insulin-deficient islets.
Within affected islets, all endocrine cells (alpha, beta, delta and PP) hyperexpressed class I MHC. Whilst islets complicated by insulitis uniformly hyperexpressed class I MHC if they had residual beta cells, many otherwise normal ICI, with no evidence of insulitis even on serial sectioning, also displayed this phenomenon [10]. This suggested that hyperexpression of class I MHC must precede insulitis in the disease process.
It was hypothesised that if alpha, delta and PP cells hyperexpressed class I MHC when they were adjacent to beta cells in ICI, but ceased to do this when they were physically divorced from beta cells in IDI, following the destruction of beta cells in the insulitis process, then perhaps, the islet beta cells were releasing some paracrine substance capable of causing hyperexpression of class I MHC on adjacent islet endocrine cells. Candidates for this role included type 1 interferons (interferon-alpha (IFNα) and beta) and interferon-gamma, as they had been shown to cause hyperexpression of class I MHC on islet endocrine cells in vitro [11]. Interferon-gamma is released only by T lymphocytes; so attention was focused on type 1 interferons.
The possibility that the secretion of IFNα by islet beta cells plays a role in the induction of class I MHC hyperexpression in ICI was then assessed [12]. In a study of 34 patients with type 1 diabetes, insulin-containing beta cells were shown to exhibit IFNα in 93 % of islets which hyperexpressed class I MHC but in only 0.4 % of islets which did not show this phenomenon. Among 80 control pancreases with a variety of diseases, only in four neonates affected by Coxsackie B viral infection were beta cells found to express IFNα [12].
The following sequence of events leading to diabetes was then hypothesised. In a given individual, pancreatic beta cells might harbour a non-cytopathic chronic viral infection to which the body could react in a number of ways, perhaps partly determined by their genetic susceptibility to diabetes. If there was no immune response, then no disease might result. By contrast, if the infected cells expressed interferon-alpha (as part of an innate immune response to the double stranded RNA of the virus), this would result in hyperexpression of class I MHC by the endocrine cells of the islet. Such expression might provoke loss of immune tolerance and infiltration of affected islets by inflammatory cells. Even a very weak autoimmune response to beta cell antigens in the context of class I MHC hyperexpression would eventually (over the years) lead to their destruction and the development of clinical diabetes (Fig. 3; Foulis Oakley Lecture 1987 [13]).
Insulitis: 25 Years on….
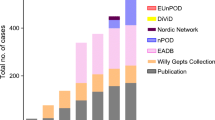
Since the seminal contributions of Gepts, the concept that type 1 diabetes arises from a process of immune-mediated destruction of pancreatic beta cells has continued to develop and expand. However, an important question still remains—how strong is the evidence that insulitis is a characteristic feature of human type 1 diabetes? Certainly, Gepts was persuaded by the concept when he noted in 1981 that insulitis is ‘a common finding in the pancreas of recent onset juvenile diabetic subjects’. Unfortunately, however, firm corroboration has been achieved only slowly, to the extent that In’t Veld, in reviewing the weight of evidence, has recently defined insulitis in the human pancreas as an ‘elusive lesion’ [14]. In particular, he noted that the number of recorded cases of insulitis worldwide has hardly increased over the last 25 years such that the total currently stands at only about 200. As a result, the essential features of the lesion are still debated.
An important issue arising from the study of insulitis is that the number of infiltrating cells in any given islet is relatively few [15]. This stands in marked contrast to the situation in the non-obese diabetic (NOD) mouse (a model of spontaneous autoimmune diabetes) where, typically, inflamed islets are surrounded by an army of infiltrating cells which initially adopt a peripheral configuration before migrating deep within the islet structure to achieve close contact with the endocrine cells [16]. In our experience, the equivalent situation is rare in human tissue. Rather, in the case of human islets, the inflammatory infiltrate often numbers only a few cells in any given islet cross-section and the majority of these reside around the islet perimeter (Fig. 4; peri-insulitis and/or focal insulitis). Very few immune cells actually cross the threshold to enter the margins of the islet structure and come into close contact with the endocrine cells. Thus, the process of beta cell destruction appears rather inefficient, and this may account for the relatively protracted time course preceding the onset of clinical symptoms in many patients.
This, in turn, prompts a further critical issue in that it becomes extremely important to establish a firm definition of ‘insulitis’ in human pancreas since it is probable that small numbers of immune cells are likely to be present within even ‘normal’ (non-inflamed) islets. Distinguishing the normal from the ‘inflamed’ situation then becomes a key priority. To help address this issue, a meeting was held in Florida in early 2013 at which a number of investigators who have studied human insulitis, were present. Collectively, these workers developed a consensus statement to define insulitis: ‘In a pancreas containing insulin-deficient islets, the lesion should only be confirmed if at least three individual islet sections contain a minimum of 15 lymphocytes (located either at the islet periphery and/or within the islet structure)’ [17]. This figure was chosen as it represents at least double the number of such cells found in the islets of control tissue (after extensive analysis of several thousand islets across multiple pancreases). This consensus definition represents the state of the art in 2013 and provides an important context in which the process can be studied [17].
Definition of the numbers of infiltrating immune cells within islets represents an important step forward in the study of insulitis but it is not, itself, a fully informative statistic. This is because the nature of the infiltrate will determine the cytokine profile within the islet milieu; therefore, a second important objective is to establish the phenotypes of the various immune cells that may be present. We undertook a comprehensive analysis of the immune cell phenotypes present within insulitic lesions in 29 separate individuals who had died shortly after being diagnosed with type 1 diabetes [15]. This revealed that both the absolute number and the profile of immune cells present in inflamed islets are variables. Indeed, immune cells appear to enter and leave islets as the process progresses, such that the profile of immune cell subsets differs according to the extent of beta cell loss. Despite this, some clear trends emerged; notably that CD8+ T cells comprise the majority of the population at all stages of insulitis. By contrast, CD4+ T cells are relatively underrepresented in the immune cell population [15].
It is self-evident that the dynamics of immune cell infiltration cannot be fully assessed in fixed tissue preparations taken at a single time point but, nevertheless, it is still possible to make reasonable inferences by studying islets at varying stages of beta cell destruction. When the data were processed in this way, a dynamic model emerged in which CD8+ T cells featured as the most prominent cells leading the islet attack and they were accompanied by reduced proportions of CD4+ T cells and CD68+ macrophages. Importantly, the CD8+ cells increased in number as beta cell destruction proceeded, implying that they play a primary role in mediating this demise. By contrast, CD4+ and CD68+ cell numbers remained much more constant. More surprisingly, a further lymphocyte subset was found to mirror the profile of CD8+ T cells, in that CD20+ B cells also increased in numbers markedly, to achieve a status as the second most prevalent cell type in many of the patients [15]. The functional activity of these cells remains to be determined but they do not appear to be antibody-secreting plasma cells. By analogy with the situation in NOD mouse (which, as indicated above, provides an exaggerated representation of human insulitis), it is possible that these B cells are somehow trophic for the CD8+ T cells, allowing them to reach their full cytotoxic potential [18]. However, it has also become clear that CD20+ cells are not absolutely required for CD8+ cells to promote beta cell loss since, in a more recent study reported in abstract form, a subset of patients was identified whose islets are not infiltrated by significant numbers of CD20+ cells but that, nevertheless, progressed to clinical type 1 diabetes [19]. Conceivably, then, the CD20+ cells may play a facilitating rather than a primary role in beta cell cytotoxicity.
Insulitic lesions in type 1 diabetes. Representative islets from nPOD cases immunostained for insulin (blue), the Pan lymphocyte marker CD45 (green) and TOPRO (red) demonstrating the proposed progression of the insulitic lesion. a Peri-insulitis. b Focal insulitis. c Progressive insulitis. d Invasive insulitis
In an earlier study using samples recovered from a different (and much smaller) group of patients, Dotta and colleagues had identified the presence of NK cells within some insulitic lesions [20]. This suggests that such cells might also play a role in mediating beta cell loss under some circumstances, although in our series of patients we were unable to detect significant infiltration of NK cells [15].
Taken together, the weight of recent evidence supports the view that CD8+ T cells are likely to be primarily responsible for driving beta cell loss and this conclusion is supported by a more recent development which represents an important step forward. In particular, work by Coppieters and colleagues has attempted to define whether the CD8+ T cells present in inflamed human islets are antigen specific [21]. This critical goal has been achieved by exploiting the power of peptide-loaded, engineered, loaded, engineered, MHC Class I molecules (‘tetramers’) which allow the selective identification of T cells recognising the specific peptide in question. Using this approach, it was demonstrated that a proportion (currently undetermined) of the T cells present in inflamed islets in humans are directed against specific beta cell antigens [21]. Thus, these T cells may be homing directly to the islets, guided by the presentation of antigens within the islet milieu. As such, these cells may be responding to highly specific cues rather than simply to a generalised chemokine gradient existing within the vicinity of the islet.
In this context, chemokines are well known to play an important role in guiding immune cells to sites of injury or infection and it has been surmised that the generation of chemokine gradients may underlie the homing of immune cells to inflamed islets. In support of this, a number of workers have shown that chemokines can be elaborated from islet cells, including molecules such as CXCL10 which may be important for T cell recruitment [22, 23]. However, the evidence base supporting a chemokine-driven recruitment mechanism in inflamed islets in human type 1 diabetes remains relatively weak. Indeed, the morphology of inflamed islets in humans seems oddly inconsistent with such a mechanism. Thus, as discussed previously, the absolute numbers of immune cells that reach inflamed islets are relatively small, and it is difficult to understand why most immune cells appear to simply pass by if there are strongly chemotactic influences available to recruit them. While such observations do not exclude a role of chemokines, it must be acknowledged that important questions remain to be answered about immune cell targeting. The possibility of antigen-specific homing certainly represents a key step in this quest.
The Peri-Islet Capsule—a Brake on Beta Cell Destruction?
Questions surrounding the localisation and homing mechanisms employed by infiltrating immune cells in human islets are important, and it remains unclear why the majority are localised around the islet periphery. A reasonable hypothesis would be to suggest that immune cells enter the islet vasculature from the wider circulation and are then subjected to homing signals (be they chemokines or other factors) which encourage the typical rolling adhesion as the cells transit the islet. Ultimately, strong adhesion and subsequent extravasation probably occur in postcapillary venules where shear forces are minimised. The postcapillary venules of the islet are located outside the peri-islet capsule [24] suggesting that extravasated immune cells may exit the vasculature at a point outside the islet and may then need to negotiate the capsule before they can gain direct access to the beta cells themselves. Important new advances have been made recently in understanding the components of the peri-islet capsule, which consists of an islet basement membrane (BM) and subjacent interstitial matrix (IM). The islet BM and IM enclose the islet, separating the endocrine cells from the surrounding exocrine acinar cells. The function of the BM is to act as a barrier to soluble molecules and to cells, and the IM confers flexibility and elasticity [24–26]. The peri-islet capsule is made up of a complex mixture of extracellular matrix components [24–26]. Interestingly, it has been argued that immune cells cannot normally infiltrate the islet unless the structure of the capsule is destabilised. This may explain why the peri-insulitis observed frequently in the islets of type 1 diabetes patients (Figs. 3a and 5) tends to occur in those with a near normal complement of beta cells [15].
The islet BM can be digested by a variety of enzymes, such as heparanases [25] and cathepsins [26], which are probably released by peri-islet immune cells [25, 26]. One could therefore hypothesise, that as the number and type of recruited immune cells increase around the islets, the damage inflicted on the capsule is enhanced (Fig. 3, focal and progressive insulitis; Fig. 5) [17]. Once the peri-islet capsule has been sufficiently degraded, the immune cells can infiltrate and destroy the beta cells (Fig. 3, invasive insulitis; Fig. 5). Typically, islets with heavy infiltration are those in which the beta cells are most reduced in number. The need to penetrate the peri-islet BM could therefore be one reason why the destructive process takes a protracted period of time. Interestingly, following the complete destruction of the beta cells, some islets are able to reform the BM (Fig. 5), suggesting that it is not the beta cells themselves that facilitate the production of this protective layer [26]. This may have importance as a clinical target in the future. If the destruction of the islet BM can be inhibited, the natural barrier to immune cell infiltration may prolong the survival of the beta cells.
A Role for Enteroviruses in Type 1 Diabetes
As noted previously, early studies on the human type 1 diabetic pancreas provided indirect evidence that viral infection may play a role in disease pathogenesis. This, coupled with mounting epidemiological evidence, has led investigators to consider that direct infection of the beta cells could be driving the development of type 1 diabetes, at least in some patients. Many different viruses, including cytomegalovirus (CMV), rubella, Epstein–Barr virus (EBV), rotavirus and in particular enteroviruses have been nominated as candidates [27]. Among these, the most convincing evidence implicates enteroviruses (single-stranded, positive-sense RNA viruses that belong to the Picornaviridae family) [28]. Over the last 25 years, a plethora of epidemiological studies have considered whether enteroviral infections are associated with the development of islet autoimmunity or the onset of clinical type 1 diabetes. A recent meta-analysis of 26 such studies concluded that there is a statistically significant association between enteroviral infection and diabetes-related autoimmunity/clinical type 1 diabetes [28]. It was noted that the odds of having a detectable enterovirus infection in people with type 1 diabetes are almost ten times greater than in unaffected individuals and four times greater in non-diabetic individuals with diabetes-related autoantibodies [28]. Many of these studies were, however, performed on peripheral whole blood, preparations of PBMCs or serum samples, such that the authors also remarked that firm evidence of viral infection within the pancreas is still lacking [28]. This is in part due to the limited amount of material available for analysis. Despite this, the search for direct evidence of an infection within the pancreas (of those few cases available) began over 34 years ago.
Arguably, the most convincing early evidence that enteroviruses can specifically target cells within the pancreas and may play a role in the development of the diabetes came in the 1970s [4, 29]. In these case reports, viral infection was associated with islet inflammation and islet cell necrosis, indicative of an acute lytic infection [4, 29]. Since then, a range of studies have confirmed the tropism of enteroviruses for beta cells both in vitro [30–32] and in vivo (particularly in fulminant type 1 diabetes, described in more details below and in Tanaka et al. [33]). Importantly, this is not restricted to just one or two of the enterovirus family members, since Coxsackie virus B (CVB), Coxsackie virus A (CVA) and several of the echoviruses are capable of infecting isolated human islets [30–32]. The tropism of enteroviruses for the pancreas was further demonstrated in vivo in a series of neonates who had died from culture proven Coxsackie viral myocarditis. In situ hybridisation (ISH), using a CVB-specific probe [34, 35], confirmed the presence of CVB RNA in the heart of all cases, but interestingly also in the pancreas of five of the nine cases examined. The islets in particular were targeted while only occasional acinar cell positivity was observed [34, 36].
These studies suggested that detection of viral RNA in the pancreas might be a viable means to verify whether enteroviral infection is associated with type 1 diabetes. However, there are a number of important issues relating to the detection of viral RNA in the pancreas which must be considered. Firstly, the pancreas is a particularly noxious environment for RNA; it is rich in RNAses, meaning that free RNA is susceptible to rapid degradation. Secondly, the majority of tissue studied to date have undergone fixation with formalin, a process which has a dramatic impact on RNA stability and integrity. Thirdly, the majority of samples tested so far are from autopsies meaning that the pancreas may have been recovered many hours after death. Therefore, despite the initial optimism arising from the demonstration of positive ISH signals in Coxsackie-proven infections, the subsequent relative failure to reproduce this [34, 37] in the pancreases of type 1 diabetes patients should be interpreted with caution.
As RNA stability is an issue when examining pancreatic tissue, researchers have also taken an alternative approach by attempting to detect viral proteins in this tissue, since these are likely to be better preserved in autopsy pancreas. In the first such study [36], two rabbit polyclonal antibodies raised either against a fusion protein containing sequences from CVB3 capsid proteins (VP4, VP2 and VP3) or against the CVB3 VP1 structural protein were employed [38]. Both detected the presence of enterovirus in the heart and pancreas of confirmed Coxsackie-infected neonates, suggesting that they were suitable for detection of widespread, systemic, enteroviral infection in formalin-fixed autopsy tissues [36]. In addition, these antibodies were shown to identify various CVBs (CVB2, CVB4 and CVB5) despite being raised against CVB3 [36]. However, when applied to sections of autopsy pancreases from 88 patients who had died at, or shortly after, clinical presentation of type 1 diabetes, no positive signals were obtained [36]. This might be because viruses were not present in the tissue, or alternatively, it might also be because these antisera failed to bind to proteins expressed by the serotype of virus present. A third possibility is that the sensitivity of the antisera was insufficient to detect the very modest levels of protein expression achieved during a sublytic infection. Fourthly, because antigen retrieval was not in use at the time of this work, other technical issues might also have militated against virus detection.
Why is it Easier to Find the Culprit in Coxsackie-Infected Neonates and Fulminant Type 1 Diabetes?
As hinted above, direct evidence of an enteroviral infection has been relatively easy to find in the pancreases of individuals presenting with an acute Coxsackie infection or with fulminant type 1 diabetes. The latter is characterised by the acute onset of clinical symptoms and differs pathologically ‘from classical’ type 1 diabetes in that there is evidence of lysis of both beta and alpha cells [39, 40]. Fulminant diabetes accounts for approximately 25 % of type 1 diabetes cases in Japan, but is much rarer in European populations. By contrast with fulminant diabetes, in typical autoimmune type 1 diabetes, there is no evidence of cell lysis and the cell loss is selective for beta cells. Islet inflammation is present in both forms of diabetes, but the complement of immune cells in fulminant disease differs from that of observed in typical type 1 diabetes (reviewed in [41]). This has led some to suggest that fulminant type 1 diabetes represents a non-autoimmune form of the disease [42], characterised by the absence of diabetes-related autoantibodies, normal expression of class I MHC, lymphocytic infiltration of the exocrine and endocrine tissue, elevated serum pancreatic enzyme levels and a remarkably aggressive disease progression [42]. It appears therefore that the ease of identification of enterovirus in these pancreas samples may be due, in part, to the development of an acute lytic infection where abundant amounts of viral protein are present in multiple cell types. In contrast, evidence of viral protein production has been much harder to find in the pancreases of patients with autoimmune type 1 diabetes. Why is this?
In autoimmune type 1 diabetes, diabetes-related autoantibodies appear in most individuals years before clinical onset of the disease and the appearance of these islet specific autoantibodies is believed to be an indicator of a beta cell stress. Accumulating evidence from birth cohort studies has shown that the appearance of the first autoantibody correlates with evidence of an enteroviral infection in the preceding 6 months [43, 44]. This had led some to hypothesise that the initial insult to the beta cell is a viral infection. This might lead to secretion of interferon-alpha by beta cells and hyperexpression of class I MHC in infected islets, leading to the activation of autoreactive cells in genetically susceptible individuals [13].
Increasing the Sensitivity of Detection of Viral Proteins in the Pancreas
The development of antigen retrieval techniques (often termed ‘heat-induced epitope retrieval’ (HIER)) in the 1990s was game changing in the field of pathology. This technique allowed certain antigens that had previously been inaccessible to antibodies in formalin-fixed paraffin-embedded tissues to be unmasked. HIER also allows the use of reduced primary antibody incubation times (and lower dilutions) such that staining is revealed in formalin-fixed tissues that fail to stain by conventional methods [45]. In summary, the application of this technique increases the sensitivity of antigen detection by individual antisera. A second major development in the enterovirus field was the production of more sensitive, broad spectrum antisera directed against enteroviral capsid proteins. One such antibody (clone 5D8/1) is marketed commercially by Dako. The antibody is monoclonal in origin, was raised against the VP1 protein of Coxsackievirus B5 and has provided particularly sensitive and specific detection opportunities such that it has now been used extensively to detect enterovirus in formalin-fixed tissues [46–52]. In combination, therefore, the use of HIER and the availability of new antisera have enabled an increase in both the spectrum of enteroviral serotypes which can be detected in fixed tissues and an improvement in the sensitivity of their detection [46–54] (and unpublished results, Richardson et al.).
These important methodological advances have provided better tools to use in the search for enteroviral proteins in tissue from recent-onset type 1 diabetes patients. Accordingly, in a landmark paper published in 2007, Dotta et al. reported that enteroviral VP1 protein was present in the islets of two of five recent-onset type 1 diabetes cases and in a whole pancreas graft recovered from a 26-year-old recipient [20]. Importantly, this work revealed the presence of small numbers of intensely stained endocrine cells, shown to be beta cells, within the islets. We now term these ‘Dotta’ cells to recognise the importance of this contribution. The immunohistochemical evidence was supported by electron microscopic studies revealing the presence of virus particles within islet cells and by the isolation of a serotype of CVB4 (now referred to as the ‘Tuscany’ isolate) from one of the cases [20]. Subsequent to this, we conducted a more comprehensive analysis of a cohort of recent-onset type 1 diabetes cases collected within the UK and were able to demonstrate the presence of VP1+ Dotta cells, again proven to be beta cells, in 44 of the 72 (61 %) cases examined. In contrast, only four Dotta cells were identified in three islets from the 50 (7.7 %) neonatal and paediatric control cases examined [55]. We further demonstrated that HIER allowed the antiserum used in the original study that had failed to detect viral proteins [36], to now reveal staining in specific islets. Strikingly, these islets were also stained positively by clone 5D8/1 in serial sections. A further pan-enterovirus antibody (clone 9D5; Millipore) was also found to label individual endocrine cells in the islets of patients with type 1 diabetes [55]. A comparison of the staining patterns achieved with these three antisera in heart tissue from CVB-infected neonates revealed that the Dako 5D8/1 clone was both more sensitive and produced more consistent staining in the face of a variety of different fixatives when compared to the other two [54, 55] (and unpublished results Richardson et al.). Hence, when considered together, these studies provided the first firm evidence that enteroviral infection, confined to beta cells, can be detected in the islet cells of a significant number of people with a recent diagnosis of type 1 diabetes.
The UK cohort used in our work represents the largest single collection of recent-onset type 1 diabetes pancreases in the world and has proved invaluable as a means to study the underlying pathology of this illness. However, despite this, there are several limitations (notably the historical nature of the collection; its specific geographical location and the non-uniform fixation methods employed). To address these, we have recently had the opportunity to examine a second cohort of pancreases from patients with type 1 diabetes, collected under the auspices of the Juvenile Diabetes Research Foundation’s network of Pancreatic Organ Donors with Diabetes (nPOD) programme (http://www.jdrfnpod.org/index.php) [56]. This important and growing resource promises much, since it should facilitate future collaborative studies to better understand the pathogenesis of type 1 diabetes. However, the number of recent-onset cases (<1 year) in this collection is still very small. Indeed, initially, we were only able to access one case of short clinical duration among ten cases which retained insulin-containing islets [57]. We also examined a further seven cases in which only insulin-deficient islets were present. Importantly, the mean time since diagnosis in the 17 type 1 diabetes cases studied was 11.9 ± 2.3 years, which compared to only 8.2 ± 4.1 months (i.e. 0.68 years) in the UK cohort. However, despite this, we noted that many of the pathological features highlighted in the UK cohort could still be observed in the American cases. We were therefore intrigued to investigate whether islet enteroviral infection could be detected in this cohort.
In short, the firm answer to this question is ‘yes’. In eight of the ten cases where residual beta cells were present, intensely stained VP1+ (Dotta) cells were seen [57]. By contrast, no evidence of staining was observed in the seven cases with only insulin-deficient islets. Serial sections of a representative islet from an nPOD case are shown in Fig. 2 demonstrating VP1 expression in an insulin-containing islet with hyperexpression of class I MHC. Importantly, VP1+ cells were seen in only 1 of 12 age-matched non-diabetic controls [57]. Within this (and the UK) cohort, it is importance to emphasise the absolute numbers of VP1+ cells within any given patient is vanishingly small. Indeed, even in those cases with the highest proportion of Dotta cells, we calculate that less than 0.005–0.01 % of the cells within the entire pancreas section stain positively for VP1, suggesting that viral infection of beta cells does not proceed with a typical acute lytic course.
One further consideration is important since it has been proposed that clone 5D8/1 can, under certain conditions, cross-react with two additional proteins in the pancreas, creatine kinase B (CKB) and ATP5B [58]. To address this issue, we have examined this cross-reactivity in greater detail and show that, under optimised conditions, the immunostaining achieved with clone 5D8/1 in formalin-fixed paraffin-embedded tissue or cells retains its specificity for VP1 [59].
Additional support for the hypothesis that the VP1 staining of the beta cells in the islets of type 1 diabetes patients represents a bona fide infection is the finding that the pathogen recognition receptor, protein kinase R (PKR), is selectively upregulated in VP1+ islet cells. This has been confirmed in both the UK and nPOD cohorts [57]. It has been shown by others that PKR is both induced and activated following an enteroviral infection and that this leads to phosphorylation of an eukaryotic initiation factor, eIF2alpha, involved in protein synthesis [60, 61]. This causes translational arrest and, as such, will result in the selective depletion of the more labile proteins. Therefore, we took advantage of this situation to study the expression of a labile, anti-apoptotic protein, Mcl-1, in VP1+ (PKR+) islet cells. Mcl-1 is constitutively expressed at high levels in most beta cells [57], and, from in vitro studies, the protein is understood to be a critical determinant of beta cell fate in response to stressors (such as pro-inflammatory cytokines and viral infection) [62]. Importantly, we observed that Mcl-1 was selectively depleted from beta cells expressing VP1 and PKR, suggesting that these individual cells might then be rendered more sensitive to the detrimental effects of the pro-inflammatory milieu existing within inflamed islets [57].
Is Islet VP1 Immunopositivity the Tip of an Iceberg?
The presence of interferon-alpha within most residual beta cells in patients at clinical onset of type 1 diabetes contrasts with the relative scarcity of Dotta cells expressing enteroviral VP1 protein. How can this be explained? In an acute lytic enteroviral infection, the positive strand RNA of the virus is transcribed within the infected cell to produce a negative strand RNA template. This in turn is transcribed to produce hundreds of copies of positive strand RNA viral molecules which are then translated, with synthesis of complete viral particles. Upon reaching a critical mass of these particles, the cells burst and release virus. By contrast, in a chronic non-lytic enteroviral infection, there are equal numbers of positive and negative viral RNA molecules and there is a little synthesis of complete viral particles expressing viral capsid proteins [63–65]. Thus, the Dotta cells seen in type 1 diabetes may represent the ‘tip of the iceberg’ of viral infection within the islet. The beta cell-synthesizing interferon-alpha may will be chronically infected by enterovirus existing, not as complete viral particles, but as double stranded RNA, a well-recognised stimulant of interferon synthesis (reviewed in [66]). In our study [55], we found that among young children, enteroviral infection of beta cells was much more frequent in patients with type 1 diabetes than those without. However, we suspect that it is not just the presence or absence of virus per se within a beta cell which is of most significance for the onset of diabetes. Rather, it may be the response of the cell to an ongoing viral infection that matters. Conceivably, it is those children who respond most vigorously to an early beta cell enteroviral infection, by mounting a strong interferon response, who are at the greatest risk of triggering islet autoimmunity. Interferon secretion will cause hyperexpression of class I MHC within the islet endocrine cells, which in turn probably initiates insulitis. Those children who respond minimally (or not at all) to an enteroviral infection of beta cells may be relatively protected from developing islet autoimmunity.
Are the Beta Cells Trying to Fight Back?
Studies in the UK recent-onset paediatric cohort of type 1 diabetes patients have provided evidence that endocrine cells (both beta and alpha) display a tenfold increase in proliferation when compared to controls [67]. This was strongly associated with the presence of insulitis suggesting that factors released from the immune cells could be driving an attempt by the beta cells to increase their numbers in the face of the attack [67]. The presence of proliferating endocrine cells was also frequently observed in islets with VP1 staining, implying that a viral infection could be inducing the recruitment of the immune cells, which in turn release factors that promote endocrine cell proliferation [68]. The finding that endocrine cell proliferation was increased in some islet autoantibody positive organ donors with evidence of insulitis [69] and that proliferation was not observed in adult, longer duration type 1 diabetes patients [70] who rarely have evidence of insulitis, lends support to this hypothesis.
Proposed Sequence of Events—2014
In individuals at risk of developing type 1 diabetes, a non-cytopathic chronic viral infection of pancreatic beta cells is sensed by host pathogen recognition receptors. Activation of these receptors induces the expression of interferon-alpha (as part of an innate immune response to the double-stranded RNA of the virus) which results in hyperexpression of class I MHC by the endocrine cells of the islet and release of factors that promote the recruitment of immune cells. However, the islet has a protective basement membrane that can prevent direct contact between the immune cells and the beta cells. As the recruitment of more immune cells to the affected islets occurs, a slow progression of insulitic destruction may occur (demonstrated by peri-insulitis, focal insulitis, progressive insulitis and finally invasive insulitis), invasive insulitis occurring once the basement membrane has been sufficiently degraded by enzymes released by the infiltrating cells. At this point, the invasive immune cells actively target and kill the beta cells leading to the formation of insulin-deficient islets, where under certain circumstances the basement membrane can reform. This progressive destruction of islets (over the years) leads to the development of clinical diabetes (Fig. 5).
Summary
We have come a long way in 25 years—but we still have a very long way to go. Many questions remain unanswered, and the limited amount and type of material available in which to address these questions restricts the speed at which they can be answered. However, the establishment of large international collaborative networks such as nPOD and EU Framework 7 funded PEVNET (persistent virus infection as a cause of pathogenic inflammation in type 1 diabetes—an innovative research programme of biobanks and expertise) are enabling expert researchers to combine their skills, question hypotheses and push forward our knowledge in this area. In particular, the nPOD (organ donor pancreases) and the Norwegian DiViD (distal pancreatectomy specimens in patients with recent-onset type 1 diabetes) collections [71] are likely to prove critical to finally answering the virus question as these tissues are optimally preserved, can be used to perform islet dissection and are suitable for next-generation sequencing analysis. We are sure that the next 25 years will prove to be even more fruitful than the last, as this collective effort really starts to help us understand the changes that beset the pancreas of type 1 diabetes patients.
Twenty-five years ago, findings in the diabetic pancreas suggested that both hypotheses raised by Gepts might be right: viral infection of islet beta cells might lead to a destructive autoimmune response directed against them. Twenty-five years further, on we still think he is right!
References
Gepts W (1965) Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 14 (10):619–633
Foulis AK, Liddle CN, Farquharson MA, Richmond JA, Weir RS (1986) The histopathology of the pancreas in type 1 (insulin-dependent) diabetes mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. Diabetologia 29 (5):267–274
Gamble DR, Taylor KW, Cumming H (1973) Coxsackie viruses and diabetes mellitus. British medical journal 4 (5887):260–262
Yoon JW, Austin M, Onodera T, Notkins AL (1979) Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. The New England journal of medicine 300 (21):1173–1179
Bottazzo GF, Florin-Christensen A, Doniach D (1974) Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet 2 (7892):1279–1283
MacCuish AC, Irvine WJ, Barnes EW, Duncan LJ (1974) Antibodies to pancreatic islet cells in insulin-dependent diabetics with coexistent autoimmune disease. Lancet 2 (7896):1529–1531
MacCuish AC, Jordan J, Campbell CJ, Duncan LJ, Irvine WJ (1974) Cell-mediated immunity to human pancreas in diabetes mellitus. Diabetes 23 (8):693–697
Foulis AK, Stewart JA (1984) The pancreas in recent-onset type 1 (insulin-dependent) diabetes mellitus: insulin content of islets, insulitis and associated changes in the exocrine acinar tissue. Diabetologia 26 (6):456–461
Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR (1985) In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. The New England journal of medicine 313 (6):353–360
Foulis AK, Farquharson MA, Hardman R (1987) Aberrant expression of class II major histocompatibility complex molecules by B cells and hyperexpression of class I major histocompatibility complex molecules by insulin containing islets in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 30 (5):333–343
Pujol-Borrell R, Todd I, Doshi M, Gray D, Feldmann M, Bottazzo GF (1986) Differential expression and regulation of MHC products in the endocrine and exocrine cells of the human pancreas. Clin Exp Immunol 65 (1):128–139
Foulis AK, Farquharson MA, Meager A (1987) Immunoreactive alpha-interferon in insulin-secreting beta cells in type 1 diabetes mellitus. Lancet 2 (8573):1423–1427
Foulis AK (1987) C. L. Oakley lecture (1987). The pathogenesis of beta cell destruction in type I (insulin-dependent) diabetes mellitus. J Pathol 152 (3):141–148
In’t Veld P (2011) Insulitis in human type 1 diabetes: The quest for an elusive lesion. Islets 3 (4):131–138.
Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG (2009) Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol 155 (2):173–181
Carrero JA, Calderon B, Towfic F, Artyomov MN, Unanue ER (2013) Defining the transcriptional and cellular landscape of type 1 diabetes in the NOD mouse. PLoS One 8 (3):e59701.
Campbell-Thompson ML, Atkinson MA, Butler AE, Chapman NM, Frisk G, Gianani R, Giepmans BN, von Herrath MG, Hyoty H, Kay TW, Korsgren O, Morgan NG, Powers AC, Pugliese A, Richardson SJ, Rowe PA, Tracy S, In’t Veld PA (2013) The diagnosis of insulitis in human type 1 diabetes. Diabetologia 56 (11):2541–2543.
Silveira PA, Grey ST (2006) B cells in the spotlight: innocent bystanders or major players in the pathogenesis of type 1 diabetes. Trends in endocrinology and metabolism: TEM 17 (4):128–135.
Leete P, Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG (2013) Identification of two distinct patterns of immune cell infiltration in the insulitic lesions in human type 1 diabetes. Diabetologia 56 (Suppl1):S63
Dotta F, Censini S, van Halteren AG, Marselli L, Masini M, Dionisi S, Mosca F, Boggi U, Muda AO, Prato SD, Elliott JF, Covacci A, Rappuoli R, Roep BO, Marchetti P (2007) Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proceedings of the National Academy of Sciences of the United States of America 104 (12):5115–5120
Coppieters KT, Dotta F, Amirian N, Campbell PD, Kay TW, Atkinson MA, Roep BO, von Herrath MG (2012) Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. The Journal of Experimental Medicine 209 (1):51–60.
Sarkar SA, Lee CE, Victorino F, Nguyen TT, Walters JA, Burrack A, Eberlein J, Hildemann SK, Homann D (2012) Expression and regulation of chemokines in murine and human type 1 diabetes. Diabetes 61 (2):436–446.
Roep BO, Kleijwegt FS, van Halteren AG, Bonato V, Boggi U, Vendrame F, Marchetti P, Dotta F (2010) Islet inflammation and CXCL10 in recent-onset type 1 diabetes. Clin Exp Immunol 159:338–343
Virtanen I, Banerjee M, Palgi J, Korsgren O, Lukinius A, Thornell LE, Kikkawa Y, Sekiguchi K, Hukkanen M, Konttinen YT, Otonkoski T (2008) Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia 51 (7):1181–1191.
Irving-Rodgers HF, Ziolkowski AF, Parish CR, Sado Y, Ninomiya Y, Simeonovic CJ, Rodgers RJ (2008) Molecular composition of the peri-islet basement membrane in NOD mice: a barrier against destructive insulitis. Diabetologia 51 (9):1680–1688.
Korpos E, Kadri N, Kappelhoff R, Wegner J, Overall CM, Weber E, Holmberg D, Cardell S, Sorokin L (2013) The peri-islet basement membrane, a barrier to infiltrating leukocytes in type 1 diabetes in mouse and human. Diabetes 62 (2):531–542.
Craig ME, Nair S, Stein H, Rawlinson WD (2013) Viruses and type 1 diabetes: a new look at an old story. Pediatr Diabetes 14 (3):149–158.
Yeung WC, Rawlinson WD, Craig ME (2011) Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ (Clinical research ed 342:d35.)
Gladisch R, Hofmann W, Waldherr R (1976) [Myocarditis and insulitis following coxsackie virus infection]. Z Kardiol 65 (10):837–849
Roivainen M, Ylipaasto P, Savolainen C, Galama J, Hovi T, Otonkoski T (2002) Functional impairment and killing of human beta cells by enteroviruses: the capacity is shared by a wide range of serotypes, but the extent is a characteristic of individual virus strains. Diabetologia 45 (5):693–702
Elshebani A, Olsson A, Westman J, Tuvemo T, Korsgren O, Frisk G (2007) Effects on isolated human pancreatic islet cells after infection with strains of enterovirus isolated at clinical presentation of type 1 diabetes. Virus Res 124 (1–2):193–203
Paananen A, Ylipaasto P, Rieder E, Hovi T, Galama J, Roivainen M (2003) Molecular and biological analysis of echovirus 9 strain isolated from a diabetic child. J Med Virol 69 (4):529–537
Tanaka S, Nishida Y, Aida K, Maruyama T, Shimada A, Suzuki M, Shimura H, Takizawa S, Takahashi M, Akiyama D, Arai-Yamashita S, Furuya F, Kawaguchi A, Kaneshige M, Katoh R, Endo T, Kobayashi T (2009) Enterovirus infection, CXC chemokine ligand 10 (CXCL10), and CXCR3 circuit: a mechanism of accelerated beta-cell failure in fulminant type 1 diabetes. Diabetes 58 (10):2285–2291.
Foulis AK, McGill M, Farquharson MA, Hilton DA (1997) A search for evidence of viral infection in pancreases of newly diagnosed patients with IDDM. Diabetologia 40 (1):53–61
Hilton DA, Variend S, Pringle JH (1993) Demonstration of Coxsackie virus RNA in formalin-fixed tissue sections from childhood myocarditis cases by in situ hybridization and the polymerase chain reaction. J Pathol 170 (1):45–51.
Foulis AK, Farquharson MA, Cameron SO, McGill M, Schonke H, Kandolf R (1990) A search for the presence of the enteroviral capsid protein VP1 in pancreases of patients with type 1 (insulin-dependent) diabetes and pancreases and hearts of infants who died of coxsackieviral myocarditis. Diabetologia 33 (5):290–298
Ylipaasto P, Klingel K, Lindberg AM, Otonkoski T, Kandolf R, Hovi T, Roivainen M (2004) Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 47 (2):225–239
Werner S, Klump WM, Schonke H, Hofschneider PH, Kandolf R (1988) Expression of coxsackievirus B3 capsid proteins in Escherichia coli and generation of virus-specific antisera. DNA 7 (5):307–316
Foulis AK, Francis ND, Farquharson MA, Boylston A (1988) Massive synchronous B-cell necrosis causing type 1 (insulin-dependent) diabetes—a unique histopathological case report. Diabetologia 31 (1):46–50
Sayama K, Imagawa A, Okita K, Uno S, Moriwaki M, Kozawa J, Iwahashi H, Yamagata K, Tamura S, Matsuzawa Y, Hanafusa T, Miyagawa J, Shimomura I (2005) Pancreatic beta and alpha cells are both decreased in patients with fulminant type 1 diabetes: a morphometrical assessment. Diabetologia 48 (8):1560–1564
Richardson SJ, Willcox A, Bone AJ, Morgan NG, Foulis AK (2013) Viruses in the Human Pancreas. In: Taylor KW, Hyoty H, Toniolo A, Zuckerman AJ (eds) Diabetes and Viruses. Springer, New York pp 167–176.
Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y (2000) A proposal of three distinct subtypes of type 1 diabetes mellitus based on clinical and pathological evidence. Annals of medicine 32 (8):539–543
Oikarinen S, Martiskainen M, Tauriainen S, Huhtala H, Ilonen J, Veijola R, Simell O, Knip M, Hyoty H (2011) Enterovirus RNA in blood is linked to the development of type 1 diabetes. Diabetes 60 (1):276–279.
Laitinen OH, Honkanen H, Pakkanen O, Oikarinen S, Hankaniemi MM, Huhtala H, Ruokoranta T, Lecouturier V, Andre P, Harju R, Virtanen SM, Lehtonen J, Almond JW, Simell T, Simell O, Ilonen J, Veijola R, Knip M, Hyoty H (2013) Coxsackievirus B1 Is Associated With Induction of beta-Cell Autoimmunity That Portends Type 1 Diabetes. Diabetes 63(2):446–55. doi:10.2337/db13-0619
Shi SR, Key ME, Kalra KL (1991) Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 39 (6):741–748
Chia AY, Chia JK (2009) Intestinal intussusception in adults due to acute enterovirus infection. Journal of clinical pathology 62 (11):1026–1028
Chia JK, Chia AY (2008) Chronic fatigue syndrome is associated with chronic enterovirus infection of the stomach. Journal of clinical pathology 61 (1):43–48.
Chia JK, Chia AY (2010) Acute gastritis associated with enterovirus infection. Arch Pathol Lab Med 134 (1):16–17
Hammerstad SS, Tauriainen S, Hyoty H, Paulsen T, Norheim I, Dahl-Jorgensen K (2013) Detection of enterovirus in the thyroid tissue of patients with graves’ disease. J Med Virol 85 (3):512–518.
Li Y, Bourlet T, Andreoletti L, Mosnier JF, Peng T, Yang Y, Archard LC, Pozzetto B, Zhang H (2000) Enteroviral capsid protein VP1 is present in myocardial tissues from some patients with myocarditis or dilated cardiomyopathy. Circulation 101 (3):231–234
Li Y, Pan Z, Ji Y, Peng T, Archard LC, Zhang H (2002) Enterovirus replication in valvular tissue from patients with chronic rheumatic heart disease. European heart journal 23 (7):567–573
Chia J, Chia A, Voeller M, Lee T, Chang R (2010) Acute enterovirus infection followed by myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and viral persistence. Journal of clinical pathology 63 (2):165–168.
Oikarinen M, Tauriainen S, Penttila P, Keim J, Rantala I, Honkanen T, Hyoty H (2010) Evaluation of immunohistochemistry and in situ hybridization methods for the detection of enteroviruses using infected cell culture samples. J Clin Virol 47 (3):224–228.
Richardson SJ, Willcox A, Hilton DA, Tauriainen S, Hyoty H, Bone AJ, Foulis AK, Morgan NG (2010) Use of antisera directed against dsRNA to detect viral infections in formalin-fixed paraffin-embedded tissue. J Clin Virol 49 (3):180–185.
Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG (2009) The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 52 (6):1143–1151
Campbell-Thompson M, Wasserfall C, Kaddis J, Albanese-O’Neill A, Staeva T, Nierras C, Moraski J, Rowe P, Gianani R, Eisenbarth G, Crawford J, Schatz D, Pugliese A, Atkinson M (2012) Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev 28(7):608–617.
Richardson SJ, Leete P, Bone AJ, Foulis AK, Morgan NG (2013) Expression of the enteroviral capsid protein VP1 in the islet cells of patients with type 1 diabetes is associated with induction of protein kinase R and downregulation of Mcl-1. Diabetologia 56 (1):185–193.
Hansson SF, Korsgren S, Ponten F, Korsgren O (2013) Enteroviruses and the pathogenesis of type 1 diabetes revisited: cross-reactivity of enterovirus capsid protein (VP1) antibodies with human mitochondrial proteins. J Pathol 229 (5):719–728.
Richardson SJ, Leete P, Dhayal S, Russell MA, Oikarinen M, Laiho JE, Svedin E, Lind K, Rosenling T, Chapman N, Bone AJ, nPOD-V Consortium, Foulis AK, Frisk G, Flodstrom-Tullberg M, Hober D, Hyoty H, Morgan NG (2013) Evaluation of the fidelity of immunolabelling obtained with clone 5D8/1, a monoclonal antibody directed against the enteroviral capsid protein, VP1, in human pancreas. Diabetologia 57(2):392–401. doi:10.1007/s00125-013-3094-7
Flodstrom-Tullberg M, Hultcrantz M, Stotland A, Maday A, Tsai D, Fine C, Williams B, Silverman R, Sarvetnick N (2005) RNase L and double-stranded RNA-dependent protein kinase exert complementary roles in islet cell defense during coxsackievirus infection. J Immunol 174 (3):1171–1177
Garcia MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, Esteban M (2006) Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol Mol Biol Rev 70 (4):1032–1060
Colli ML, Nogueira TC, Allagnat F, Cunha DA, Gurzov EN, Cardozo AK, Roivainen M, Op de Beeck A, Eizirik DL (2011) Exposure to the viral by-product dsRNA or Coxsackievirus B5 triggers pancreatic beta cell apoptosis via a Bim/Mcl-1 imbalance. PLoS Pathogens 7 (9):e1002267.
Cunningham L, Bowles NE, Lane RJ, Dubowitz V, Archard LC (1990) Persistence of enteroviral RNA in chronic fatigue syndrome is associated with the abnormal production of equal amounts of positive and negative strands of enteroviral RNA. J Gen Virol 71 ( Pt 6):1399–1402
Klingel K, Hohenadl C, Canu A, Albrecht M, Seemann M, Mall G, Kandolf R (1992) Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage, and inflammation. Proceedings of the National Academy of Sciences of the United States of America 89 (1):314–318
Tam PE, Messner RP (1999) Molecular mechanisms of coxsackievirus persistence in chronic inflammatory myopathy: viral RNA persists through formation of a double-stranded complex without associated genomic mutations or evolution. J Virol 73 (12):10113–10121
Richardson SJ, Willcox A, Bone AJ, Morgan NG, Foulis AK (2011) Immunopathology of the human pancreas in type-I diabetes. Semin Immunopathol 33 (1):9–21.
Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG (2010) Evidence of increased islet cell proliferation in patients with recent-onset type 1 diabetes. Diabetologia 53 (9):2020–2028.
Willcox A, Richardson SJ, Bone AJ, Foulis A, Morgan NG (2011) Immunohistochemical analysis of the relationship between islet cell proliferation and the production of the enteroviral capsid protein, VP1, in the islets of patients with recent-onset type 1 diabetes. Diabetologia 54 (9):2417–2420
In’t Veld P, Lievens D, De Grijse J, Ling Z, Van der Auwera B, Pipeleers-Marichal M, Gorus F, Pipeleers D (2007) Screening for insulitis in adult autoantibody-positive organ donors. Diabetes 56 (9):2400–2404.
Butler AE, Galasso R, Meier JJ, Basu R, Rizza RA, Butler PC (2007) Modestly increased beta cell apoptosis but no increased beta cell replication in recent-onset type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia 50 (11):2323–2331.
Krogvold L, Edwin B, Buanes T, Ludvigsson J, Korsgren O, Hyoty H, Frisk G, Hanssen KF, Dahl-Jorgensen K. (2014) Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: experiences from the DiViD study. Diabetologia. doi:10.1007/s00125-013-3155-y.
Acknowledgments
We thank Pia Leete for preparation of figures.
Funding
The work was supported by funding from the European Union’s Seventh Framework Programme PEVNET [FP7/2007–2013] under grant agreement number 261441. Additional support was from Diabetes Research Wellness Foundation Non-Clinical Research Fellowship to S.J.R. The research was also performed with the support of the Network for Pancreatic Organ Donors with Diabetes (nPOD), a collaborative type 1 diabetes research project sponsored by the Juvenile Diabetes Research Foundation International (JDRF) and with a JDRF research grant awarded to the nPOD-V consortium. Organ Procurement Organizations (OPO) partnering with nPOD to provide research resources are listed at www.jdrfnpod.org/our-partners.php.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Richardson, S.J., Morgan, N.G. & Foulis, A.K. Pancreatic Pathology in Type 1 Diabetes Mellitus. Endocr Pathol 25, 80–92 (2014). https://doi.org/10.1007/s12022-014-9297-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-014-9297-8