Abstract
Aim
The incidence of cardiovascular and renal events was investigated in patients with type 2 diabetes who were classified according to anemia and the components of dialysis-independent chronic kidney disease (CKD) in a prospective observational study.
Methods
A population of 778 Japanese patients with type 2 diabetes was prospectively analyzed for 4 years. The outcomes were the incidence of cardiovascular events and renal events.
Results
In all subjects, the incidence of cardiovascular and renal events was found to be 5% and 11%, respectively. Even after adjusting for a reduced estimated glomerular filtration rate (eGFR <60 mL/min/1.73 m2), the incidence of cardiovascular events was significantly higher (hazard ratio [HR]: 5.73) in patients with anemia and albuminuria than in those without anemia and albuminuria. The incidence of renal events was significantly higher in patients with no anemia and albuminuria (HR: 2.93) and further in those with anemia and albuminuria (HR: 7.56) than in those without anemia and albuminuria even after adjusting for a reduced eGFR.
Conclusion
Anemia combined with albuminuria is a risk factor for vascular events in patients with type 2 diabetes, regardless of the eGFR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anemia is closely related to chronic kidney disease (CKD), cardiovascular disease, and heart failure [1,2,3,4,5]. Because these pathological conditions affect each other, inducing the formation of a vicious cycle, the concept of cardio-renal anemia (CRA) syndrome has been proposed [6].
Cardiovascular disease and end-stage kidney disease (ESKD) are major complications in patients with diabetes mellitus. Anemia is reportedly found more frequently in patients with diabetes than in the general population or non-diabetic CKD patients [7,8,9,10,11,12,13]. We previously reported that the frequency of anemia was significantly higher in patients with type 2 diabetes than in subjects who had undergone a health checkup and that anemia was found in more than half of patients with advanced diabetic nephropathy [14, 15]. Furthermore, even mild anemia that was not indicated for treatment was independently associated with diabetic microangiopathies and macroangiopathies based on a cross-sectional study of 1189 patients with type 2 diabetes [15].
While anemia reportedly increases the risk of cardiovascular events and death, independent of the presence of CKD [16,17,18], cardiovascular events, mortality, and ESKD are generally increased in dialysis-independent diabetic patients when anemia and CKD are both present [2, 3, 9, 12,13,14]. However, no study has investigated the influence of anemia on diabetic vascular events from the perspective of its combination with albuminuria, an item considered in the diagnosis of CKD.
We, therefore, assessed the incidence of cardiovascular and renal events through a prospective observational study of dialysis-independent patients with type 2 diabetes classified according to anemia and the components of CKD. Our findings are expected to be useful as real-world data for assessing the risk of diabetic angiopathy in patients with albuminuria and a preserved estimated glomerular filtration rate (eGFR).
Materials and methods
Study population and outcomes
Initially, 788 dialysis-independent patients with type 2 diabetes who were registered for a previous cross-sectional study [19] were prospectively observed. All patients were evaluated for the eGFR, urinary albumin-to-creatinine ratio (uACR) in random spot urine samples, and diabetic macroangiopathies in the Department of Diabetes, Metabolism and Kidney Disease of Edogawa Hospital between August and November 2014.
Subjects with malignant diseases who were currently under treatment were excluded from the study. After additionally excluding the subjects receiving sodium-glucose cotransporter 2 (SGLT2) inhibitors (n=9) and glucagon-like peptide-1 (GLP-1) receptor agonists (n=1), which are considered to affect the cardiovascular and renal prognosis [20,21,22,23], we analyzed 778 patients who had been prospectively observed for the present cohort.
We continuously treated the study subjects for 4 years until the end of 2018 or death. The outcomes of the present study were the incidence of cardiovascular events (cardiovascular death, nonfatal myocardial infarction, heart failure or nonfatal stroke) and renal events (ESKD or a decline in the eGFR by ≥30% from the start of observation).
Confounding factors
Anemia was defined as a hemoglobin level <135 g/L in men and <120 g/L in women according to the guidelines established by the ERA-EDTA [24] and NKF [25]. Obese individuals were defined as those with a body mass index (BMI) ≥25 kg/m2 according to the criteria set by the Japan Society for the Study of Obesity [26]. A current drinker was defined as a person consuming >20 g ethanol equivalent/day. Hypertension was defined as a systolic blood pressure ≥140 mmHg and/or a diastolic blood pressure ≥90 mmHg. Participants currently using antihypertensive medications were also considered positive for hypertension. Hyper-low-density lipoprotein (LDL) cholesterolemia was defined as either a serum concentration of LDL-cholesterol ≥3.62 mmol/L (140 mg/dL) or the current use of statins or ezetimibe. Hypo-high-density lipoprotein (HDL) cholesterolemia was defined as a serum concentration of HDL-cholesterol <1.03 mmol/L (40 mg/dL). The eGFR was calculated using the formula which is recommended by the Japanese Society of Nephrology [27]. A reduced eGFR was defined as <60 mL/min/1.73 m2 in the present study. Albuminuria was defined as a uACR ≥30 mg/gCr in the spot urine. CKD was defined in cases with albuminuria or a reduced eGFR. Hyperuricemia was defined by a serum uric acid level >416 μmol/L (7.0 mg/dL) or when patients were using urate-lowering agents. Diabetic retinopathy was defined as simple, pre-proliferative or proliferative retinopathy based on the results of a funduscopic examination performed by expert ophthalmologists. Diabetic peripheral neuropathy was diagnosed by the presence of two or more components among clinical symptoms (bilateral spontaneous pain, hypoesthesia or paraesthesia of the legs), the absence of ankle tendon reflexes, and decreased vibration sensations using a C128 tuning fork. Cerebrovascular disease was diagnosed by physicians in cases with a history of ischemic stroke using brain computed tomography or magnetic resonance imaging. Coronary heart disease was diagnosed based on a history of myocardial infarction, angina pectoris or interventions after a coronary angiographic examination. Peripheral arterial disease was diagnosed by the absence of a pulse in the legs along with ischemic symptoms, obstructive findings on an ultrasonographic or angiographic examination of the lower extremities or an ankle brachial pressure index <0.9.
Statistical analyses
All data are presented as the mean±standard deviations. The χ2 test was used for between-group comparisons of categorical variables. None of the continuous variables (age, duration of diabetes, BMI, blood pressure, HbA1c, eGFR, serum lipid or uric acid concentrations) showed a normal distribution in the Shapiro–Wilk tests, so Wilcoxon’s rank-sum test was used to assess the significance of differences in the continuous variables. The Kaplan–Meier curves showed changes in the incidence of cardiovascular and renal events during the observation period. The log-rank test was used to assess between-group differences in the incidence of cardiovascular or renal events. The risk of cardiovascular or renal events was determined using the univariate and multivariate Cox proportional hazards models. P values of <0.05 (two tailed) were considered to indicate statistical significance. Data analyses were performed using the statistical software package JMP version 12.2.0 (SAS Institute, Cary, NC, USA) and EZR version 1.42 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics and clinical features associated with anemia
The clinical characteristics of the subjects at the baseline are shown in Table 1. A total of 39% of subjects showed anemia. The patients with anemia were older and had a longer duration of diabetes than those without anemia. While the rates of obesity and metformin use were lower, the rates of using insulin, no antidiabetics, renin–angiotensin system (RAS) inhibitors, antiplatelet agents, and urate-lowering agents as well as hypertension, a reduced eGFR, albuminuria and diabetic microangiopathies and macroangiopathies were higher in the group with anemia than in the group without anemia.
Outcomes
In all subjects, the cumulative incidence of cardiovascular and renal events during the observation period was 5% (n=33) and 11% (n=69), respectively. The incidence of cardiovascular events was significantly higher (P < 0.01, log-rank test) in the group with anemia (n=20, 8%) than in the group without anemia (n=13, 3%) (Fig. 1A). The incidence of renal events was also significantly higher (P <0.01, log-rank test) in the group with anemia (n=44, 18%) than in the group without anemia (n=25, 6%) (Fig. 1B). The sample sizes required to compare survival curves in the anemia and non-anemic groups calculated from these results were 269 and 417 for the cardiovascular events and 97 and 150 for the renal events, respectively.
The incidence of each event is shown in Supplementary Table S1. According to Kaplan–Meier analyses, the incidence of heart failure (Supplementary Fig. S1), ESKD, and ≥30% decline in eGFR (Supplementary Fig. S2) were significantly higher in the group with anemia than in the group without anemia.
When the patients with anemia were divided into two groups using hemoglobin level of 110 g/L (the threshold for starting treatment for renal anemia in adult dialysis-independent CKD patients [28]) as a cutoff value, the incidence of cardiovascular (Fig. 2A) and renal (Fig. 2B) events was higher in patients with severe anemia (<110 g/L) than in those with mild anemia (≥110 g/L). Among the components of cardiovascular and renal events, the degree of anemia was significantly associated with the incidence of heart failure (Supplementary Fig. S3), ESKD, and ≥30% decline in eGFR (Supplementary Fig. S4).
The associations between baseline characteristics and the incidence of cardiovascular or renal events determined by Cox proportional hazard models are shown in Table 2. Although anemia was significantly associated with the incidence of cardiovascular events according to the univariate analysis, a reduced eGFR, albuminuria, and hyperuricemia were significant risk factors after a multivariate analysis. Anemia, a reduced eGFR, albuminuria, HbA1c, and the use of calcium channel blockers were significantly associated with the incidence of renal events according to a multivariate Cox proportional hazard model.
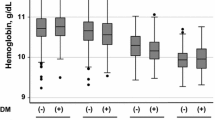
Figure 3 shows the Kaplan–Meier analyses of the cardiovascular events in the group classified by anemia and the components of CKD. Although the incidence of cardiovascular events was not significantly higher in the anemia (+)/CKD (–) group than in the anemia (–)/CKD (–) group, this incidence was significantly increased in the anemia (–)/CKD (+) group, and further increased in the anemia (+)/CKD (+) group (Fig. 3A). The incidence of cardiovascular events was not also elevated in the anemia (+)/reduced eGFR (–) group compared with the anemia (–)/reduced eGFR (–) group (Fig. 3B). This incidence was significantly higher in the groups with a reduced eGFR than in the anemia (–)/reduced eGFR (–) group. A similar tendency with regard to the incidence of cardiovascular events was observed when the study subjects were classified by the presence or absence of anemia and albuminuria (Fig. 3C). The clinical characteristics of the subjects at the baseline in the subgroups corresponding to Fig. 3A–C are shown in Supplementary Tables S2, S3, and S4, respectively.
Kaplan–Meier analyses of cardiovascular events in the group classified by anemia and CKD (A), eGFR (B), or uACR (C). The hazard ratio to the group without anemia and CKD, reduced eGFR or albuminuria was calculated using Cox proportional hazard models (Table 3)
Figure 4 shows the Kaplan–Meier analyses of the renal events in the group classified by anemia and the components of CKD. The incidence of renal events was not significantly higher in any of the groups of anemia (+)/CKD (–) (Fig. 4A), anemia (+)/reduced eGFR (–) (Fig. 4B) or anemia (+)/albuminuria (–) (Fig. 4C) than in the reference group. The incidence of renal events was significantly increased in the groups with CKD, a reduced eGFR or albuminuria compared with the groups of anemia (–)/CKD (–), anemia (–)/reduced eGFR (–), and anemia (–)/albuminuria (–), respectively. When anemia coexisted with these components, the incidence of renal events was several times higher than in the corresponding groups with CKD, a reduced eGFR and albuminuria without anemia.
Kaplan–Meier analyses of renal events in the groups classified by anemia and CKD (A), eGFR (B), or uACR (C). The hazard ratio to the group without anemia and CKD, reduced eGFR or albuminuria was calculated using Cox proportional hazard models (Table 3)
Hazard ratios (HRs) of the incidence of cardiovascular or renal events to the reference groups according to the univariate and multivariate Cox hazard models are shown in Table 3. Anemia was significantly associated with cardiovascular events when combined with CKD, a reduced eGFR or albuminuria. Even after adjusting for hyperuricemia and a reduced eGFR, this incidence was significantly higher (HR: 5.59, 95% CI 1.77–24.80) in the anemia (+)/albuminuria (+) group and tended to be higher in the anemia (+)/albuminuria (–) group (HR: 3.34, 95% CI 0.87–15.95, P = 0.08) than in the anemia (–)/albuminuria (–) group. After adjusting for calcium channel blocker use and HbA1c, the incidence of renal events was significantly higher in the anemia (+)/CKD (+) group than in the anemia (–)/CKD (–) (HR: 5.73, 95% CI 2.83–12.83) and anemia (–) /CKD (+) groups (HR: 3.05, 95% CI 1.68–5.77, P <0.01). Although the incidence of renal events did not increase in the anemia (–)/reduced eGFR (+) group compared to the anemia (–)/reduced eGFR (–) group, this incidence was significantly higher in the anemia (+)/reduced eGFR (+) group than in the anemia (–)/reduced eGFR (–) (HR: 3.88, 95% CI 2.07–7.42) and anemia (–) /reduced eGFR (+) groups (HR: 2.89, 95% CI 1.31–7.30, P <0.01). The incidence of renal events was significantly increased in the anemia (–)/albuminuria (+) group (HR: 2.93, 95% CI 1.30–7.02) and further increased in the anemia (+)/albuminuria (+) group (HR: 7.56, 95% CI 3.64–17.33) compared to the anemia (–)/albuminuria (+) group after adjusting for calcium channel blocker use, HbA1c, and a reduced eGFR. This incidence was also significantly higher in the anemia (+)/albuminuria (+) group than in the anemia (–)/albuminuria (+) group (HR: 2.58, 95% CI 1.38–4.99, P <0.01).
Kaplan–Meier curves for the combination of anemia and albuminuria with or without a reduced eGFR are shown in Supplementary Figs. S5 and S6. When the patients were grouped according to the presence or absence of a reduced eGFR, the incidence of cardiovascular events was significantly increased in the group without a reduced eGFR (Supplementary Fig. S5), while the incidence of renal events was significantly increased in both groups (Supplementary Fig. S6).
Discussion
The combination of anemia and dialysis-independent CKD was a strong risk factor for cardiovascular and renal events in patients with type 2 diabetes in the current study, whereas only anemia was not significantly associated with these incidences in the patients without CKD or components of CKD. It was previously reported that the incidence of cardiovascular disease was increased in diabetic patients with anemia and CKD [2] and that the incidence of ESKD increased with the progression of anemia in patients with overt diabetic nephropathy [3]. Yamamoto et al. also reported that the incidence of cardiovascular disease and ESKD increased with the deterioration of the GFR in anemic patients with CKD [4]. However, there have been no reports investigating whether or not complication of anemia and albuminuria is associated with vascular events in patients with diabetes. To our knowledge, the present study is the first showing that anemia coexisting with albuminuria increases the risk of cardiovascular and renal events, regardless of the eGFR. In particular, the findings that the incidence of renal events was higher in the patients with anemia and albuminuria than in those with no anemia and albuminuria, regardless of the GFR, should be noted. Although the GFR is an important predictor of the renal prognosis in patients with type 2 diabetes, its impact is inferior to that of albuminuria [29, 30].
A reduced eGFR without anemia at the baseline was not significantly associated with renal events, and only the coexistence of anemia and a reduced eGFR was a risk factor for renal events in the present study. Most of the anemia in this study was likely to have been due to renal anemia related to renal dysfunction. It is reported that pathological changes caused by nephrosclerosis or tubulointerstitial injury are frequently observed in patients with renal dysfunction and no albuminuria and that the renal prognosis is comparatively favorable in such patients [31,32,33]. We also previously reported that subsequent decline in eGFR was unlikely to occur in type 2 diabetic patients with a reduced eGFR without albuminuria according to another prospective observation study [34]. In actual clinical practice, salt restriction guidance, strict control of blood pressure, and treatment of serum lipid and urate concentrations as well as hyperglycemia are performed, particularly in diabetic patients who show a reduced eGFR. The renal function in such patients can be preserved, in the current era, though the above-mentioned multidisciplinary treatment for type 2 diabetes. As a result, there may have been a discrepancy between the renal prognosis and the presence of anemia. Although our results did not provide direct evidence that correcting anemia will improve the prognosis, therapeutic interventions for hemoglobin levels may reduce the incidence of renal events further when anemia is associated with albuminuria, even when the eGFR value is preserved. Whether the pharmacological treatment of anemia improves the cardiovascular or renal prognosis is currently unclear, partly because target hemoglobin levels have not been established. Several studies reported that correction of anemia using an iron supplement and/or erythropoiesis-stimulating agent (ESA) improved the disturbed quality of life (QOL), the left ventricular hypertrophy [35, 36], and renal prognosis [37,38,39] in patients with dialysis-independent CKD. Recently, it was reported that the oral administration of daprodustat, a hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitor, remained comparable to darbepoetin alfa with regard to the elevation of the hemoglobin levels, the incidence of cardiovascular events, and the progression of CKD in patients with dialysis-independent CKD [40]. Therefore, the further accumulation of clinical trials, including the target hemoglobin level, is considered to be necessary for the evaluation of pharmacological treatment of renal anemia on the cardiovascular and renal prognoses.
Anemia was found in 39% of the subjects in the present study. Although, in this study, there were no control subjects with whom to compare the frequency of anemia, anemia is more frequent in patients with diabetes than in non-diabetic subjects according to previous studies [7,8,9,10,11,12,13]. It is well known that the frequency of anemia increases with the progression of renal impairment in patients with CKD. The patients with type 2 diabetes in our previous study [14, 15] developed anemia more frequently at all eGFR stages than the Japanese dialysis-independent CKD patients registered in a nationwide clinical database [41].
Several mechanisms have been proposed to explain the high frequency of anemia in patients with diabetes, including erythropoietin deficiency due to sympathetic nerve dysfunction of the kidneys as a result of diabetic neuropathy, functional iron deficiency causing secondary to subclinical inflammation through increased hepcidin levels, persistent proteinuria resulting in transferrin and erythropoietin loss, a reduced erythrocyte lifespan because of advanced glycation end products and the frequent use of RAS inhibitors through the inhibition of physiologic erythropoiesis of angiotensin II [12, 13]. Although the cause of anemia was not evaluated in the present study, the active use of SGLT2 inhibitors showing the elevation of hemoglobin levels [22, 42] might be more useful for relieving anemia than ESAs or HIF-PH inhibitors, which are limited to the treatment of renal anemia, anemic patients with albuminuria and no reduced eGFR.
Because metformin use reduced the risk of diabetes-related endpoints in overweight patients [43], this drug has been the first-choice drug for the treatment of type 2 diabetes [44]. Metformin is also effective for improving hyperglycemia, even in Japanese subjects, irrespective of obesity [45]. Approximately one-third of the study subjects were using metformin at the baseline of the current study. This frequency of use may seem low in comparison to Western countries. When this study started, there were many limitations on the usage of metformin in the recommendations issued by the Japan Diabetes Society and the drug package inserts by pharmaceutical companies. For example, the prescription of metformin was not recommended for patients with renal impairment (eGFR <45 mL/min/1.73 m2) or elderly people, especially subjects of ≥75 years of age. Subjects with an eGFR of <45 mL/min/1.73 m2, elderly people of ≥65 years of age, and those of ≥75 years of age accounted for 12%, 58%, and 27% of our study population, respectively. Therefore, the prescription rate of metformin among type 2 diabetes patients in Japan remained around 30% [46, 47]. Although use of metformin appears to be protective against cardiovascular and renal events according to univariate Cox proportional hazard models, it did not remain a significant predictor in the multivariate analyses of the present study. It seems to be quite obviously related to the fact that subjects utilizing metformin should generally be younger, with a better state of health and with a better kidney function at baseline.
Several limitations associated with the present study warrant mention. First, the present study was unable to investigate the changes in the hemoglobin level or uACR during the observation period. Of note, the current study demonstrated only the prognosis of the groups classified by the hemoglobin, eGFR, and uACR values at the baseline, although the clinical course differed among individuals depending on the treatment method and treatment response. Second, the measurement of uACR was based on a single spot urine sample obtained when the patients visited our department. Variability should be considered when confirming the CKD group with staging by uACR as it has been recommended that evaluations be performed in two or more samples. Third, the cause of anemia is unknown in this study although subjects with malignant diseases under treatment were excluded from this study. Because serum levels of iron, ferritin, and erythropoietin were not evaluated, the anemia in our subjects was not necessarily renal anemia. Fourth, the Kaplan–Meier analyses of the cardiovascular and renal events in the group classified by anemia and the components of CKD did not take into account the baseline difference in patient characteristics between the groups with and without anemia. However, the similar results were obtained in multivariate Cox proportional hazard models adjusted for patient background factors; thus, it is worthwhile to take anemia combined with CKD into consideration.
Despite these limitations, however, we believe that this cohort study is useful for managing patients presenting with anemia among a type 2 diabetes population. It is considered necessary to conduct further studies to investigate whether anemia combined with albuminuria is a predictor of cardiovascular or renal events over the kidney function because the evidence obtained from our study alone is not sufficiently convincing.
Conclusion
Complication with anemia and albuminuria is a risk factor for cardiovascular and renal events in patients with type 2 diabetes, regardless of the eGFR.
References
Anand I, McMurray JJ, Whitmore J, Warren M, Pham A, McCamish MA, et al. Anemia and its relationship to clinical outcome in heart failure. Circulation. 2004;110:149–54. https://doi.org/10.1161/01.CIR.0000134279.79571.73.
Vlagopoulos PT, Tighiouart H, Weiner DE, Griffith J, Pettitt D, Salem DN, et al. Anemia as a risk factor for cardiovascular disease and all-cause mortality in diabetes: the impact of chronic kidney disease. J Am Soc Nephrol. 2005;16:3403–10. https://doi.org/10.1681/ASN.2005030226.
Mohanram A, Zhang Z, Shahinfar S, Keane WF, Brenner BM, Toto RD. Anemia and end-stage renal disease in patients with type 2 diabetes and nephropathy. Kidney Int. 2004;66:1131–8. https://doi.org/10.1111/j.1523-1755.2004.00863.x.
Yamamoto T, Miyazaki M, Nakayama M, Yamada G, Matsushima M, Sato M, et al. Impact of hemoglobin levels on renal and non-renal clinical outcomes differs by chronic kidney disease stages: the Gonryo study. Clin Exp Nephrol. 2016;20:595–602. https://doi.org/10.1007/s10157-015-1190-3.
Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113:2713–23. https://doi.org/10.1161/CIRCULATIONAHA.105.577577.
Silverberg D, Wexler D, Blum M, Wollman Y, Iaina A. The cardio-renal anaemia syndrome: does it exist? Nephrol Dial Transplant. 2003;18(Suppl 8):viii7-12. https://doi.org/10.1093/ndt/gfg1084.
Thomas MC, MacIsaac RJ, Tsalamandris C, Power D, Jerums G. Unrecognized anemia in patients with diabetes: a cross-sectional survey. Diabetes Care. 2003;26:1164–9. https://doi.org/10.2337/diacare.26.4.1164.
Akizawa T, Makino H, Matsuo S, Watanabe T, Imai E, Nitta K, et al. Management of anemia in chronic kidney disease patients: baseline findings from Chronic Kidney Disease Japan Cohort Study. Clin Exp Nephrol. 2011;15:248–57. https://doi.org/10.1007/s10157-010-0396-7.
Joss N, Patel R, Paterson K, Simpson K, Perry C, Stirling C. Anaemia is common and predicts mortality in diabetic nephropathy. QJM. 2007;100:641–7. https://doi.org/10.1093/qjmed/hcm080.
Loutradis C, Skodra A, Georgianos P, Tolika P, Alexandrou D, Avdelidou A, et al. Diabetes mellitus increases the prevalence of anemia in patients with chronic kidney disease: a nested case-control study. World J Nephrol. 2016;5:358–66. https://doi.org/10.5527/wjn.v5.i4.358.
Palaka E, Grandy S, van Haalen H, McEwan P, Darlington O. The impact of CKD anaemia on patients: incidence, risk factors, and clinical outcomes—A systematic literature review. Int J Nephrol. 2020;2020:7692376. https://doi.org/10.1155/2020/7692376.
Deray G, Heurtier A, Grimaldi A, Launay Vacher V, Isnard BC. Anemia and diabetes. Am J Nephrol. 2004;24:522–6. https://doi.org/10.1159/000081058.
Mehdi U, Toto RD. Anemia, diabetes, and chronic kidney disease. Diabetes Care. 2009;32:1320–6. https://doi.org/10.2337/dc08-0779.
Ito H, Antoku S, Furusho M, Shinozaki M, Abe M, Mifune M, et al. The prevalence of the risk factors for atherosclerosis among type 2 diabetic patients is greater in the progressive stages of chronic kidney disease. Nephron Extra. 2013;3:66–72. https://doi.org/10.1159/000353592.
Ito H, Takeuchi Y, Ishida H, Otawa A, Shibayama A, Antoku S, et al. Mild anemia is frequent and associated with micro- and macroangiopathies in patients with type 2 diabetes mellitus. J Diabetes Investig. 2010;1:273–8. https://doi.org/10.1111/j.2040-1124.2010.00060.x.
Tong PC, Kong AP, So WY, Ng MH, Yang X, Ng MC, et al. Hematocrit, independent of chronic kidney disease, predicts adverse cardiovascular outcomes in Chinese patients with type 2 diabetes. Diabetes Care. 2006;29:2439–44. https://doi.org/10.2337/dc06-0887.
Zoppini G, Targher G, Chonchol M, Negri C, Stoico V, Pichiri I, et al. Anaemia, independent of chronic kidney disease, predicts all-cause and cardiovascular mortality in type 2 diabetic patients. Atherosclerosis. 2010;210:575–80. https://doi.org/10.1016/j.atherosclerosis.2009.12.008.
Levin A, Djurdjev O, Duncan J, Rosenbaum D, Werb R. Haemoglobin at time of referral prior to dialysis predicts survival: an association of haemoglobin with long-term outcomes. Nephrol Dial Transplant. 2006;21:370–7. https://doi.org/10.1093/ndt/gfi209.
Ito H, Yamashita H, Nakashima M, Takaki A, Yukawa C, Matsumoto S, et al. Current metabolic status affects urinary liver-type fatty-acid binding protein in normoalbuminuric patients with type 2 diabetes. J Clin Med Res. 2017;9:366–73. https://doi.org/10.14740/jocmr2934w.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. https://doi.org/10.1056/NEJMoa1504720.
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34. https://doi.org/10.1056/NEJMoa1515920.
Ito H, Matsumoto S, Izutsu T, Kusano E, Kondo J, Inoue H, et al. Different renoprotective effects of luseogliflozin depend on the renal function at the baseline in patients with type 2 diabetes: a retrospective study during 12 months before and after initiation. PLoS One. 2021;16:e0248577. https://doi.org/10.1371/journal.pone.0248577.
Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. https://doi.org/10.1056/NEJMoa1603827.
Locatelli F, Covic A, Eckardt KU, Wiecek A, Vanholder R, ERA-EDTA ERBP Advisory Board. Anaemia management in patients with chronic kidney disease: a position statement by the Anaemia Working Group of European Renal Best Practice (ERBP). Nephrol Dial Transplant. 2009;24:348–54. https://doi.org/10.1093/ndt/gfn653.
KDOQI, National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006;47(5 Suppl 3):S11-145. https://doi.org/10.1053/j.ajkd.2006.03.010.
Examination Committee of Criteria for ‘Obesity Disease’ in Japan, Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ J. 2002;66:987–92. https://doi.org/10.1253/circj.66.987.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92. https://doi.org/10.1053/j.ajkd.2008.12.034.
Yamamoto H, Nishi S, Tomo T, Masakane I, Saito K, Nangaku M, et al. 2015 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Ren Replace Ther. 2017;3:36. https://doi.org/10.1186/s41100-017-0114-y.
Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Advance Collaborative Group, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–21. https://doi.org/10.1681/ASN.2008121270.
Tanaka N, Babazono T, Takagi M, Yoshida N, Toya K, Nyumura I, et al. Albuminuria and reduced glomerular filtration rate for predicting the renal outcomes in type 2 diabetic patients. Nephrology (Carlton). 2015;20:531–8. https://doi.org/10.1111/nep.12446.
Moriya T, Suzuki Y, Inomata S, Iwano M, Kanauchi M, Haneda M. Renal histological heterogeneity and functional progress in normoalbuminuric and microalbuminuric Japanese patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2014;2:e000029. https://doi.org/10.1136/bmjdrc-2014-000029.
Shimizu M, Furuichi K, Toyama T, Kitajima S, Hara A, Kitagawa K, Kanazawa Study Group for Renal Diseases and Hypertension, et al. Long-term outcomes of Japanese type 2 diabetic patients with biopsy-proven diabetic nephropathy. Diabetes Care. 2013;36:3655–62. https://doi.org/10.2337/dc13-0298.
Yamanouchi M, Furuichi K, Hoshino J, Toyama T, Hara A, Shimizu M, Research Group of Diabetic Nephropathy, the Ministry of Health, Labour and Welfare, the Japan Agency for Medical Research and Development, et al. Nonproteinuric versus proteinuric phenotypes in diabetic kidney disease: a propensity score-matched analysis of a nationwide, biopsy-based cohort study. Diabetes Care. 2019;42:891–902. https://doi.org/10.2337/dc13-0298.
Ito H, Antoku S, Izutsu T, Kusano E, Matsumoto S, Yamasaki T, et al. The prognosis of subjects showing a reduced estimated glomerular filtration rate without albuminuria in Japanese patients with type 2 diabetes: a cohort study for diabetic kidney disease. Clin Exp Nephrol. 2020;24:1033–43. https://doi.org/10.1007/s10157-020-01935-3.
Akizawa T, Gejyo F, Nishi S, Iino Y, Watanabe Y, Suzuki M, et al. Positive outcomes of high hemoglobin target in patients with chronic kidney disease not on dialysis: a randomized controlled study. Ther Apher Dial. 2011;15:431–40. https://doi.org/10.1111/j.1744-9987.2011.00931.x.
Hayashi T, Suzuki A, Shoji T, Togawa M, Okada N, Tsubakihara Y, et al. Cardiovascular effect of normalizing the hematocrit level during erythropoietin therapy in predialysis patients with chronic renal failure. Am J Kidney Dis. 2000;35:250–6. https://doi.org/10.1016/s0272-6386(00)70334-9.
Kuriyama S, Tomonari H, Yoshida H, Hashimoto T, Kawaguchi Y, Sakai O. Reversal of anemia by erythropoietin therapy retards the progression of chronic renal failure, especially in nondiabetic patients. Nephron. 1997;77:176–85. https://doi.org/10.1159/000190270.
Gouva C, Nikolopoulos P, Ioannidis JP, Siamopoulos KC. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney Int. 2004;66:753–60. https://doi.org/10.1111/j.1523-1755.2004.00797.x.
Tsubakihara Y, Gejyo F, Nishi S, Iino Y, Watanabe Y, Suzuki M, et al. High target hemoglobin with erythropoiesis-stimulating agents has advantages in the renal function of non-dialysis chronic kidney disease patients. Ther Apher Dial. 2012;16:529–40. https://doi.org/10.1111/j.1744-9987.2012.01082.x.
Singh AK, Carroll K, McMurray JJV, Solomon S, Jha V, Johansen KL, et al. Daprodustat for the treatment of anemia in patients not undergoing dialysis. N Engl J Med. 2021;385:2313–24. https://doi.org/10.1056/NEJMoa2113380.
Sofue T, Nakagawa N, Kanda E, Nagasu H, Matsushita K, Nangaku M, et al. Prevalence of anemia in patients with chronic kidney disease in Japan: a nationwide, cross-sectional cohort study using data from the Japan Chronic Kidney Disease Database (J-CKD-DB). PLoS One. 2020;15:e0236132. https://doi.org/10.1371/journal.pone.0236132.
Ito H, Matsumoto S, Izutsu T, Kusano E, Nishio S, Antoku S, et al. Comparison of the changes in the factors associated with the renal prognosis of non-elderly and elderly subjects treated with empagliflozin- a retrospective observation study in Japanese patients with type 2 diabetes. Diabetes Metab Syndr Obes. 2019;12:1783–94. https://doi.org/10.2147/DMSO.S221655.
UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–65. https://doi.org/10.1016/S0140-6736(98)07037-8.
Collins BS, Hilliard ME, Isaacs D, Johnson EL, Kahan S, Khunti K, Leon J, Lyons SK, Perry ML, Prahalad P, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(Suppl 1):S140-57. https://doi.org/10.2337/dc23-S009.
Ito H, Ishida H, Takeuchi Y, Antoku S, Abe M, Mifune M, et al. Long-term effect of metformin on blood glucose control in non-obese patients with type 2 diabetes mellitus. Nutr Metab (Lond). 2010;7:83. https://doi.org/10.1186/1743-7075-7-83.
Kohro T, Yamazaki T, Sato H, Harada K, Ohe K, Komuro I, et al. Trends in antidiabetic prescription patterns in Japan from 2005 to 2011. Int Heart J. 2013;54:93–7. https://doi.org/10.1536/ihj.54.93.
Ito H, Shinozaki M, Nishio S, Abe M. SGLT2 inhibitors in the pipeline for the treatment of diabetes mellitus in Japan. Expert Opin Pharmacother. 2016;17:2073–84. https://doi.org/10.1080/14656566.2016.1232395.
Acknowledgements
The authors thank Tomoko Koyanagi in the secretarial section of Edogawa Hospital for her valuable help with data collection.
Funding
The authors received no financial support for this study.
Author information
Authors and Affiliations
Contributions
HI contributed to the conception, design, analysis, interpretation, writing first draft, editing, and final approval. SM contributed to reviewing drafts, editing, and final approval. HI, TI, EK, SA, TY, TM, and MT contributed to data collection and final approval. All of the authors are in agreement with the content of the manuscript and have approved this submission.
Corresponding author
Ethics declarations
Conflict of interest
H Ito has received lecture fees from Eli Lilly Japan KK, Novo Nordisk Pharma Ltd., Sumitomo Pharma Co., Ltd. and Boehringer Ingelheim. S Matsumoto, H Inoue, T Izutsu, E Kusano, S Antoku, T Yamasaki, T Mori, and M Togane have no conflicts of interest.
Ethical approval
The study was conducted in accordance with the principles expressed in the 2008 Declaration of Helsinki. The Ethics Committee of Edogawa Hospital approved the study protocol (approved numbers: 2015-18 and 2022-1, approved date: July 6, 2015 and November 24, 2021, respectively) and waived the need for written informed consent because the data were analyzed anonymously for this analysis based on information stored in the hospital. The trial is retrospectively registered on UMIN-CTR, identifier UMIN000047632 (1 May, 2022).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13340_2023_637_MOESM1_ESM.pptx
Supplementary file1 (PPTX 115 KB) Supplementary Fig. 1. Kaplan-Meier analyses of the components of cardiovascular events in the groups with and without anemia. (A) Cardiovascular death, (B) nonfatal myocardial infarction, (C) heart failure, and (D) nonfatal stroke. Supplementary Fig. S2. Kaplan-Meier analyses of the components of renal events in the groups with and without anemia. (A) End-stage kidney disease, (B) decline in eGFR by ≥30%. Supplementary Fig. S3. Kaplan-Meier analyses of the components of cardiovascular events in the groups with anemia with a hemoglobin level of <110 g/L, with anemia with a hemoglobin level of ≥110 g/L, and without anemia. Supplementary Fig. S4. Kaplan-Meier analyses of the components of renal events in the groups with anemia with a hemoglobin level of <110 g/L and anemia with a hemoglobin level of ≥110 g/L and without anemia. Supplementary Fig. S5. Kaplan-Meier analyses of cardiovascular events in the groups classified by anemia and uACR with (A) and without (B) a reduced eGFR. Supplementary Fig. S5. Kaplan-Meier analyses of renal events in the groups classified by anemia and uACR with (A) and without (B) a reduced eGFR
About this article
Cite this article
Ito, H., Matsumoto, S., Inoue, H. et al. Anemia combined with albuminuria increases the risk of cardiovascular and renal events, regardless of a reduced glomerular filtration rate, in patients with type 2 diabetes: a prospective observational study. Diabetol Int 14, 344–355 (2023). https://doi.org/10.1007/s13340-023-00637-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-023-00637-x