Abstract
Colorectal cancer (CRC) has a high prevalence and mortality rate. Biomarkers for predicting the recurrence of CRC are not clinically available. This study investigated the role of circulating miR-15b in the prediction of CRC recurrence and the associated mechanism. miR-15b levels in plasma and tissues were measured by real-time PCR. Metastasis suppressor-1 (MTSS1) and Klotho protein expression were detected by Western blot and immunohistochemistry. Invasion and migration of CRC tumor cells were measured by transwell plates. Liver metastasis was established by intraspleen injection of HCT116 cells. Plasma miR-15b levels were significantly higher in CRC patients than in healthy controls, in CRC patients with metastasis than in CRC patients without metastasis, and in CRC patients with recurrence than in CRC patients without recurrence in the 5-year follow-up. miR-15b level in CRC tumors was significantly higher than that in peritumoral tissues. High plasma miR-15b level and negative MTSS1 and Klotho expression in tumor tissues significantly correlated with poor survival. Inhibition of miR-15b activity by adenovirus carrying antimiR-15b sequence significantly increased MTSS1 and Klotho protein expression and subsequently decreased colony formation ability, invasion, and migration of HCT116 cells in vitro and liver metastasis of HCT116 tumors in vivo. In conclusion, high abundance of circulating miR-15b correlated with tumor metastasis, recurrence, and poor patient prognosis through downregulation of MTSS1 and Klotho protein expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer with a median diagnosis age of 70 years [1]. In 2014, 136,830 individuals were newly diagnosed with CRC, and 50,310 CRC patients died in the USA [2]. Most CRC patients present with local or locally advanced disease, and surgical resection is the first option for these patients. Although preoperative radio (chemo) therapy and postoperative chemotherapy are used for CRC patients, a meta-analysis demonstrated that the use of postoperative chemotherapy in patients with rectal cancer receiving preoperative radio (chemo) therapy has no significant benefit for overall survival or disease-free survival [3]. The 5-year survival rate for all stages combined was 65.4 % for colon cancer and 67.7 % for rectal cancer [4]. Approximately 40 % of CRC patients experience local, regional, or systemic recurrence in 5 years after treatment [5, 6]. However, reliable biomarkers for predicting the recurrence of CRC are not yet available.
MicroRNAs (miRNAs) are non-coding, small RNA molecules consisting of about 18–25 nucleotides. In animals, miRNA functions in posttranscriptional regulation of gene expression through incompletely binding to the three prime untranslated region (3′-UTR) [7]. The network of miRNA regulation is rather complex. For example, one miRNA can regulate anywhere from one to thousands of genes and one gene can be regulated by up to hundreds of miRNAs. It is estimated that over 60 % of human protein-coding genes can be regulated by miRNA [8]. miRNAs have been widely revealed to influence numerous cancer-relevant processes such as tumor cell differentiation, proliferation, apoptosis, migration, and metabolism, as well as tumor stem cell activation, chemo, and radioresistance [9]. In cancer, miRNA functions as an oncogene or tumor-suppressive gene. Moreover, miRNAs have been demonstrated to be independent biomarkers for the progression and prognosis of a variety of tumors [10, 11]. Recently, circulating miRNAs have been widely recognized as important biomarkers for the early diagnosis and predicting recurrence and prognosis of cancers including colorectal cancer [12, 13]. miR-15b was proposed as an especially important miRNA in melanoma and is associated with poor prognosis and tumorigenesis [14]. miR-15b was found to target the 3′-UTR of MTSS1 (metastasis suppressor protein 1) in breast cancer and correlated with poor prognosis of patients [15]. Increased miR-15b was observed in colorectal cancer cells [16]. The bioinformatic analysis suggests that the tumor suppressor Klotho gene is a target of miR-15b. We hypothesize that miR-15b may be a biomarker for metastasis and recurrence in CRC.
In this study, we investigated miR-15b expression in plasma, tumor tissues, and peritumoral tissues of colorectal cancers and further analyzed its functions in predicting the prognosis and recurrence of CRC patients, as well as its functions in regulating tumor invasion and metastasis.
Materials and methods
Subjects
Two hundred twelve tumor tissues, 204 plasma samples, and 106 peritumoral tissues were collected from 212 colorectal cancer patients at Xiangya Hospital from January 2006 to December 2009. The colorectal cancer was diagnosed by immunohistochemical staining. The 212 colorectal cancer patients consisted of 122 males and 90 females with an average age of 65.2 years. No patient was given preoperative chemotherapy or radiotherapy. The survival information was collected by phone and letters for 5 years. Plasma was collected from colorectal cancer patients who survived for 5 years. Plasma samples were also collected from 156 age- and gender-matched healthy people (89 males, 67 females, average age = 64.7 years). This study was approved by the Ethics Committee for Human Research of Xiangya Hospital.
Real-time amplification of miR-15b
Ten milliliters of peripheral fasting blood was collected, and the plasma was collected by centrifugation at 1500 rpm for 5 min and kept at −80 °C until use. Total RNA was extracted from plasma, tumor tissues, and peritumoral tissues using Trizol reagent (Invitrogen, Carlsbad, CA, USA) by following the manufacturer’s instructions. Reverse transcription was performed using One Step PrimeScript® miRNA cDNA Synthesis Kit by following the manufacturer’s instructions. Real-time quantitative PCR was performed using SYBR® Premix Ex Taq™ II (RAKARA, Japan). The data were analyzed using the comparative CT method (ΔΔCT method) and the ΔCt value was compared between groups. The forward primers used for miR-15b amplification were the sequences of mature miR-15b and the reverse primers were provided by the kit.
Immunohistochemistry
The rabbit antihuman MTSS1 and Klotho antibodies were purchased from Abcam (Shanghai, China). MTSS1 and Klotho protein in tissues were immunohistologically stained using EnVision detection kit (Dako Laboratories, California, USA). Four-micrometer sections were cut from paraffin-embedded tissues. After being deparaffinized, the sections underwent antigen retrieval and were incubated with 3 % H2O2. Afterward, sections were incubated with MTSS1 or Klotho antibody for 1 h at room temperature (RT). After washing with PBS, HRP-conjugated secondary antibody (solution A in the kit) was applied to the sections for 30 min at RT, followed by incubation with color development solution and hematoxylin counterstain. Five hundred cells from 10 random fields were counted. A 25 % positive staining was set as a cutoff value. Cases with ≥25 % positive staining cells were designated as positive, and cases with <25 % of cells positive were designated as negative. Positive control biopsies were provided with the EnVision detection kit. A GFP antibody was used as negative control.
Preparation of adenovirus expressing antimiR-15b
The complimentary short hairpin RNA expressing miR-15b antisense mRNA (anti miR-15b) was synthesized and cloned into the pSilencer vector at Hind III and Bam HI sites (Life Techologies). The miRNA expression vector including the H1 promoter and antimiR-15b minigene was subcloned into the shuttle vector of an adenovirus packaging system, and the virus (Ad-antimiR-15b) was produced as previously described [17]. A sequence not targeting any known gene (AATTCTCCGAACGTGTCACGT) was used to replace the miR-15b antisense sequence to produce the control adenovirus (Ad-ST).
Cell culture
The HCT16 cell line was purchased from Cell Biology of Shanghai Institute (Shanghai, China). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA, USA), containing 100 U/ml penicillin, 100 μg/ml streptomycin, and 10 % fetal bovine serum (FBS; Invitrogen). Cells were cultured in a humidified atmosphere of 5 % CO2 at 37 °C.
Liver metastasis model
Thirty Balb/C nude mice (BALB/c, nu/nu) weighing 20–22 g were purchased from the animal center of Shanghai Biological Science Institution. Mice were housed in the animal facility of Central South University with food and water provided ad libitum. The animal protocol was preapproved by Central South University, and all experiments were performed in accordance to the animal care guidelines of the Chinese Council.
HCT116 cells were infected with Ad-ST or Ad-antimiR-15b (10 MOI) for 2 h and harvested before inoculation. Mice were divided into two groups receiving intraspleen injection of HCT116 cells infected with Ad-ST (n = 15) and Ad-antimiR-15b (n = 15), respectively. Mice were anesthetized with an intraperitoneal injection of 1 % pentobarbital (Trust Chemist Limited, Bengaluru, Karnataka) at a dose of 45 mg/kg. After shaving and disinfecting, a small incision was made through the skin over the spleen. The spleen was grasped and a small incision was made over the tip. HCT116 cell suspension (30 μl, 1 × 106 cells/ml) was injected through a 29-gauge needle into the parenchyma of the spleen. The spleen was removed 2 min later, and the incision in the skin was closed. Ten days later, mice were euthanized and the livers were excised.
Colony formation assay
HCT116 cells were incubated in 10-cm dishes and infected with or without Ad-ST or Ad-antimiR-15b virus (10 MOI) for 2 h. Single cell suspensions (2 × 104) were resuspended in a 1:1 mixture of Matrigel (BD Sciences, San Jose, CA, USA) to medium and plated on 6-well plates. Three replicate wells were plated for each treatment. Fifteen days post incubation, cells were incubated with 1 mg/ml of iodonitrotetrazolium chloride solution (Sigma-Aldrich, USA) overnight, and colonies were counted. Colony formation was assessed in four different experiments.
Migration and invasion assays
Cell migration and invasion assays were performed using Matrigel-coated (invasion assay) or uncoated (migration assay) 24-well transwell plates (8-μm pore size) (BD Biosciences, Franklin Lakes, NJ). Briefly, HCT116 cells were infected with or without Ad-ST or Ad-antimiR-15b for 2 h. Cells were then harvested and suspended in DMEM containing 1 % FCS. Cells (2 × 105) were seeded into the upper chamber of each well, and DMEM containing 20 % FCS was placed in the lower chamber. After 48 h of incubation, the membranes that separated the upper and lower chamber were fixed with methanol and stained with 1 % toluidine blue in 1 % borax and the cells on the lower surface of the membrane were counted with the use of a light microscope. Experiments were repeated for four times.
Western blot
Cells were lysed in 1× lysis buffer (50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 0.1 % SDS, and 1 % NP-40) supplemented with protease inhibitors. About 10 μg of total proteins were separated on SDS-PAGE gel and transferred onto polyvinylidene difluoride (PVDF) membranes (BioRad, VA, USA). Membranes were then incubated with rabbit antihuman NTSS1 and Klotho antibodies (Abcam) and subsequently with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling technology). The ECL kit (Amersham) was used to visualize protein bands via the ChemiDoc XRS imaging system (Bio-Rad). To control for loading, the membranes were stripped and reblotted with GAPDH antibody (Abcam). The bands were screened using Photoshop.
Cell viability assay
The cell viability was analyzed using MTT assay. Briefly, HCT116 cells at 50–60 % confluency were infected with or without Ad-ST or Ad-antimiR-15b (10 MOI) for 2 h. Cells were harvested, resuspended in DMEM containing 1 % FCS, and seeded in 96-well plates at 1 × 104 cells per well. Twenty-four hours later, live cells in the Petri dishes were analyzed by MTT assay.
Statistical analysis
Data were presented as mean ± standard error (SEM) and analyzed using SPSS v17.0 (Chicago, IL, USA). The relationships between MTSS1 and Klotho protein expression and histological or clinical factors were analyzed by χ 2 test or Fisher’s exact test. Univariate survival analysis was conducted with Kaplan–Meier method (log-rank test). Other data were analyzed using one-way ANOVA or two-tailed student’s t test. A p < 0.05 was considered statistically significant.
Results
The expression pattern of miR-15b and its targets MTSS1 and Klotho protein in plasma and tumor tissues of CRC patients
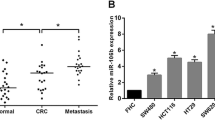
The expression of miR-15b in plasma and tissues was measured by real-time PCR. Results showed that plasma miR-15b level was significantly higher in CRC patients (n = 212) than in healthy controls (n = 156) (p < 0.001) (Fig. 1a). The plasma miR-15b level was significantly higher in CRC patients with lymph node metastasis (n = 88) than in CRC patients without lymph node metastasis at diagnosis (n = 124) (Fig. 1b). The plasma miR-15b levels were measured in CRC patients who survived for five or more years. The miR-15b level was significantly higher in CRC patients with distant metastasis (n = 19, p < 0.001) and local relapse (n = 5, p < 0.01) than in CRC patients without local relapse and distant metastasis (n = 51) (Fig. 1c). The miR-15b level was also significantly higher in tumor tissues (n = 212) than in peritumoral tissues (n = 106) (Fig. 1d, p < 0.001). We further measured MTSS1 (Fig. 1e) and Klotho (Fig. 1f) protein expression in tumor tissues by immunohistochemistry. Negative MTSS1 (54.2 % case) and Klotho (53.3 % case) expression was significantly associated with the poor differentiation (p = 0.001 and p < 0.001, respectively), lymph node metastasis (p = 0.004 and p = 0.001, respectively), invasion (p < 0.001 and p = 0.004, respectively), and high TNM stage (p = 0.001 and p = 0.002, respectively) (Table 1). No significant associations were observed between MTSS1 or Klotho protein expression and the age, gender, tumor location, and plasma CEA concentration (Table 1).
miR-15b mRNA, MTSS1, and Klotho protein expression. a Comparison of plasma miR-15b expression between CRC patients (CRC) and healthy controls (HC). **p < 0.001 vs. HC. N = 212 for CRC. N = 158 for HC. b Comparison of plasma miR-15b levels between CRC patients with lymph node metastasis (CRC-LMT, n = 88) and CRC patients without lymph node metastasis (CRC-no LMT, n = 124). **p < 0.001 vs. CRC no MT (metastasis). c Comparison of plasma miR-15b levels between CRC patients with local relapse (LR), distant metastasis (DMT), and without LR and DMT. *p < 0.01, **p < 0.001 vs. CRC-no LR/DMT; #p < 0.01 vs. CRC-LR. N = 51, 5, 19 for no LR/DMT, LR, and DMT, respectively. d Comparison of miR-15b levels between peritumoral tissues (PT, n = 106) and CRC tumor tissues (CRC, n = 212). **p < 0.001. e Representative positive and negative MTSS1 expression in CRC tumor tissues. f Representative positive and negative Klotho expression in CRC tumor tissues
The associations between plasma miR-15b mRNA, tumor tissue MTSS1 and Klotho protein expression, and survival of CRC patients
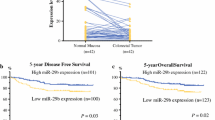
The CRC patients were followed up for 5 years. The information on local tumor relapse and distant metastasis in patients who survived for 5 years or more was also collected. The overall survival and disease-free survival (without relapse and distant metastasis) were analyzed. Seventy-five CRC patients survived longer than 5 years including 5 patients with local relapse and 19 patients with distant metastasis to the liver, lung, and brain. Low plasma miR-15b (Fig. 2a, b), positive MTSS1 (Fig. 2c, d), and Klotho (Fig. 2e, f) expression in tumor tissues significantly correlated with longer overall (Fig. 2a, c, e) and disease-free survival (excluding patients with local relapse and distant metastasis) (Fig. 2b, d, e) in CRC patients (p < 0.05 or p < 0.001).
Survival analysis. a Kaplan–Meier plots of overall survival (OS) of patients with high plasma miR-15b and low plasma miR-15b levels. High plasma miR-15b level was defined as the case with a plasma miR-15b level higher than or equal to the mean miR-15b level of all CRC samples. b Kaplan–Meier plots of relapse free survival (RFS) of patients with high plasma miR-15b and low plasma miR-15b levels. c Kaplan–Meier plots of overall survival in patients with MTSS1 positive and negative expression. d Kaplan–Meier plots of relapse-free survival in patients with MTSS1 positive and negative expression. e Kaplan–Meier plots of overall survival in patients with Klotho positive and negative expression. f Kaplan–Meier plots of relapse-free survival in patients with Klotho positive and negative expression
Overexpression of miR-15b downregulated MTSS1 and Klotho protein expression and increased colony formation ability in colorectal cancer cells
A previous study has demonstrated that MTSS1 gene is a direct target of miR-15b [15]. The bioinformative analysis showed that the Klotho gene is a target of miR-15a/b/c. To further validate the regulative effect of miR-15b on MTSS1 and Klotho gene in CRC, HCT116 cells were infected with antimiR-15b expression vector. Ad-antimiR-15b significantly increased MTSS1 (Fig. 3a, b) and Klotho (Fig. 3a, c) protein expression in HCT116 cells. The increased MTSS1 and Klotho protein expression showed no significant effect on cell viability of the starved HCT116 cells (Fig. 3d). In contrast, Ad-antimiR-15b infection significantly decreased the colony formation ability (Fig. 3e, f), suggesting that overexpression of miR-15b inhibits MTSS1 and Klotho gene expression and subsequently increases cancer stem cell activity.
Anti-miR-15b regulates MTSS1 and Klotho protein expression, cell viability, and colony formation ability. a Representative Western blots of MTSS1, Klotho, and GAPDH protein expression. HCT-116 cells were infected with Ad-ST (Ad-ST) virus, Ad-antimiR-15b virus (Ad-antimiR-15b), or without virus infection (Ctl). b Semiquantitative analysis of MTSS1 protein expression in (a). c Semiquantitative analysis of Klotho protein expression in (a). d MTT assay of cell viability. e Representative images of Matrigel colony formation. f The number (No.) of colonies. *p < 0.01, **p < 0.001 vs. Ctl and Ad-ST groups, N = 4
Inhibition of miR-15b activity decreased invasion and migration of CRC cells in vitro and liver metastasis in vivo
The HCT116 cells infected with or without Ad-ST and Ad-antimiR-15b were subjected to cell migration and invasion assays using Matrigel-coated (invasion assay) or uncoated (migration assay) 24-well transwell plates. Results showed that Ad-antimiR-15b infection significantly decreased invasion (Fig. 4a) and migration (Fig. 4b) in HCT116 cells compared to cells without virus infection and infected with Ad-ST. The liver metastasis models of colorectal cancer were established in nude mice by intraspleen injection of human HCT116 cells. Inoculation of HCT116 cells infected with Ad-antimiR-15b significantly decreased the number and size of metastatic nodules as well as liver weights (Fig. 4c, d). These findings suggest that inhibition of miR-15b expression can decrease CRC metastasis.
miR-15b-regulated invasion, migration, and liver metastasis of HCT-116 cells. a Invasion of HCT-116 cells through Matrigel-coated transwells. Results represent mean number of Ad-antimiR-15b and Ad-ST virus-infected cells that passed through the Matrigel-coated membranes of the transwell relative to control cells without virus infection (Ctl). b Migration assay. Plotted was the mean number of Ad-antimiR-15b and Ad-ST virus-infected HCT-116 cells that migrated through transwell membranes not coated with Matrigel relative to control cells (Ctl). *p < 0.01 vs. other groups. N = 4. c Representative livers showing the metastatic nodules. d Comparison of metastatic nodules on the surface of livers. *p < 0.01 vs. Ad-ST group. N = 15. e Liver weights. Infection with Ad-anti-miR-15b significantly decreased the liver weight. *p < 0.05 vs. Ad-ST group, N = 15
Discussion
Although progress has been made in identifying circulating miRNA as biomarkers for the recurrence of colorectal cancer [18], the circulating miRNAs which can be used as biomarkers for predicting recurrence after treatments are not widely available in the clinic. This study found high plasma miR-15b level in CRC patients, which was associated with local occurrence, distant metastasis, and poor prognosis. We further found that miR-15b functions as an enhancer of metastasis through downregulating the gene expression of metastasis suppressor protein 1 (MTSS1) and Klotho and subsequent inhibition of invasion and migration of CRC tumor cells.
MTSS1 (metastasis suppressor-1) was first identified as a metastasis suppressor in metastatic bladder carcinoma cells [19]. The following studies found that MTSS1 is a multifunctional protein and plays critical roles in cancer carcinogenesis, development, and metastasis [19]. For example, MTSS1 downregulation was proposed a stratifying marker for adjuvant therapy in early-stage squamous cell carcinoma of the lung [20], a novel diagnostic biomarker or therapeutic target in cervical cancer patients [21], and a prognosis biomarker in glioblastoma [22] and pancreatic cancer [23]. miR-15b was found to target the 3′-UTR of MTSS1 in breast cancer and correlated with a poor prognosis of patients [15]. This study showed that transduction of the antisense sequence of miR-15b increased MTSS1 protein expression in HCT116 colorectal cancer cells, which correlated with the decreases in invasion and migration and liver metastasis in an intraspleen metastasis animal model. The lack of MTSS1 expression in human tumor tissues significantly correlated with the poor prognosis of CRC patients. Thus, as a target of miR-15b, downregulation of MTSS1 expression may be responsible for the role of miR-15b in enhancing the metastasis of CRC.
The Klotho gene was first identified as an anti-aging gene in mice, but most following studies found that Klotho functions as a tumor suppressor gene through regulating insulin/IGF1, p53/p21, ROS, and Wnt signaling [24]. The roles of Klotho in tumor metastasis have not been intensively investigated, and only a few studies investigated the associations between Klotho expression and tumor cell invasion and metastasis. For example, Klotho was found to decrease the melanoma cell-invasive potential through the inhibition of calpain [25]. The administration of Klotho protein suppressed metastasis of human renal cancer xenografts in mice [26]. This study first showed that inhibition of miR-15b activity by an adenovirus carrying a miR-15b antisense sequence increased Klotho protein expression in colorectal cancer cells, which correlated with decreases in invasion and migration of HCT116 cells and liver metastasis. Although inhibition of miR-15b activity also inhibited MTSS1 expression, the loss of Klotho expression in CRC tumors significantly correlated with the poor prognosis of CRC patients. Thus, target inhibition of Klotho is also responsible for the role of miR-15b in enhancing the metastasis of CRC.
Dysregulation of miR-15b expression was observed in a variety of cancers. The role of miR-15b as a tumor suppressor or oncogene is tissue type-dependent. For example, MiR-15b was found to reduce glioma cell invasion and angiogenesis by directly targeting NRP-2 and MMP-3 expression [27] and reduce metastasis by directly targeting MTSS1 in breast cancer [15]. Zhao et al.’s study revealed that miR-15b directly targeted phosphatidylethanolamine-binding protein 4 (PEBP4) and increased cisplatin resistance and metastasis in human lung adenocarcinoma cells [28]. miR-15b was also found to modulate multidrug resistance by targeting BCL2 in gastric cancer cells [29] and regulate cell cycle progression by targeting cyclins in glioma cells [30]. This study first reported that miR-15b targeted MTSS1 in colorectal cancer cells. We also first identified Klotho as a target of miR-15b. Interestingly, we found that plasma miR-15b was higher not only in CRC patients with metastasis compared to patients without metastasis but also in patients with local and distant recurrence. These findings suggest that circulating miR-15b can be a diagnosis marker of CRC and a marker for predicting the metastasis in CRC. In this study, the ΔCt value of real-time PCR for the plasma miR-15b in healthy controls was less than 2. Therefore, a ΔCt value larger than 2 could be an index to diagnose CRC, a ΔCt value larger than 5 could be an index to diagnose relapse, and a ΔCt value larger than 6 could be an index to diagnose metastasis of CRC. This study also demonstrated that miR-15b is a predictive marker for poor prognosis of patients with CRC.
In conclusion, the present study suggests that circulating miR-15b can be used as a biomarker for predicting the metastasis, poor prognosis, and recurrence of CRC. miR-15b enhances invasion and migration of tumor cells and metastasis of CRC tumor through downregulating the expression of MTSS1 and Klotho genes.
References
Jorgensen ML, Young JM, Solomon MJ. Optimal delivery of colorectal cancer follow-up care: improving patient outcomes. Patient Relat Outcome Meas. 2015;6:127–38.
Siegel R, Ma J, Zhaohui Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29.
Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–17.
Bujko K, Glimelius B, Valentini V, Michalski W, Spalek M. Postoperative chemotherapy in patients with rectal cancer receiving preoperative radio(chemo)therapy: a meta-analysis of randomized trials comparing surgery ± a fluoropyrimidine and surgery + a fluoropyrimidine ± oxaliplatin. Eur J Surg Oncol. 2015;41:713–23.
Buie WD, Attard JA. Follow-up recommendations for colon cancer. Clin Colon Rectal Surg. 2005;18:232–43.
Meyerhardt JA, Mangu PB, Flynn PJ, Korde L, Loprinzi CL, Minsky BD, et al. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2013;31:4465–70.
Barrett LW, Sue FS, Wilton SD. Regulation of eukaryotic gene expression by the untranslated gene regions and other non-coding elements. Cell Mol Life Sci. 2012;69:3613–34.
Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105.
Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol. 2012;6:590–610.
Bertoli G, Cava C, Castiglioni I. MicroRNAs: new biomarkers for diagnosis, prognosis, therapy prediction and therapeutic tools for breast cancer. Theranostics. 2015;5:1122–43.
Hollis M, Nair K, Vyas A, Chaturvedi LS, Gambhir S, Vyas D. MicroRNAs potential utility in colon cancer: early detection, prognosis, and chemosensitivity. World J Gastroenterol. 2015;21:8284–92.
Mostert B, Sieuwerts AM, Martens JW, Sleijfer S. Diagnostic applications of cell-free and circulating tumor cell-associated miRNAs in cancer patients. Expert Rev Mol Diagn. 2011;11:259–75.
Fesler A, Jiang J, Zhai H, Ju J. Circulating microRNA testing for the early diagnosis and follow-up of colorectal cancer patients. Mol Diagn Ther. 2014;18:303–8.
Satzger I, Mattern A, Kuettler U, Weinspach D, Voelker B, Kapp A, et al. MicroRNA-15b represents an independent prognostic parameter and is correlated with tumor cell proliferation and apoptosis in malignant melanoma. Int J Cancer. 2010;126:2553–62.
Kedmi M, Ben-Chetrit N, Körner C, Mancini M, Ben-Moshe NB, Lauriola M, et al. EGF induces microRNAs that target suppressors of cell migration: miR-15b targets MTSS1 in breast cancer. Sci Signal. 2015;8:ra29.
Ota T, Doi K, Fujimoto T, Tanaka Y, Ogawa M, Matsuzaki H, et al. KRAS up-regulates the expression of miR-181a, miR-200c and miR-210 in a three-dimensional-specific manner in DLD-1 colorectal cancer cells. Anticancer Res. 2012;32:2271–5.
Zhang X, Kon T, Wang H, Li F, Huang Q, Rabbani ZN, et al. Enhancement of hypoxia-induced tumor cell death in vitro and radiation therapy in vivo by use of small interfering RNA targeted to hypoxia-inducible factor-1alpha. Cancer Res. 2004;64:8139–42.
Shivapurkar N, Weiner LM, Marshall JL, Madhavan S, Deslattes Mays A, Juhl H, et al. Recurrence of early stage colon cancer predicted by expression pattern of circulating microRNAs. PLoS One. 2014;9:e84686.
Xie F, Ye L, Ta M, Zhang L, Jiang WG. MTSS1: a multifunctional protein and its role in cancer invasion and metastasis. Front Biosci (Schol Ed). 2011;3:621–31.
Kayser G, Csanadi A, Kakanou S, Prasse A, Kassem A, Stickeler E, et al. Downregulation of MTSS1 expression is an independent prognosticator in squamous cell carcinoma of the lung. Br J Cancer. 2015;112:866–73.
Zhang J, Tong Y, Ren L, Li CD. Expression of metastasis suppressor 1 in cervical carcinoma and the clinical significance. Oncol Lett. 2014;8:2145–9.
Zhang S, Qi Q. MTSS1 suppresses cell migration and invasion by targeting CTTN in glioblastoma. J Neurooncol. 2015;121:425–31.
Zhou L, Li J, Shao QQ, Guo JC, Liang ZY, Zhou WX et al. Expression and significances of MTSS1 in pancreatic cancer. Pathol Oncol Res 2015 Jul 22. [Epub ahead of print]
Xie B, Chen J, Liu B, Zhan J. Klotho acts as a tumor suppressor in cancers. Pathol Oncol Res. 2013;19:611–7.
Camilli TC, Xu M, O'Connell MP, Chien B, Frank BP, Subaran S, et al. Loss of Klotho during melanoma progression leads to increased filamin cleavage, increased Wnt5A expression, and enhanced melanoma cell motility. Pigment Cell Melanoma Res. 2011;24:175–86.
Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286:8655–65.
Zheng X, Chopp M, Lu Y, Buller B, Jiang F. MiR-15b and miR-152 reduce glioma cell invasion and angiogenesis via NRP-2 and MMP-3. Cancer Lett. 2013;329:146–54.
Zhao Z, Zhang L, Yao Q, Tao Z. miR-15b regulates cisplatin resistance and metastasis by targeting PEBP4 in human lung adenocarcinoma cells. Cancer Gene Ther. 2015;22:108–14.
Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, et al. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123:372–9.
Xia H, Qi Y, Ng SS, Chen X, Chen S, Fang M, et al. MicroRNA-15b regulates cell cycle progression by targeting cyclins in glioma cells. Biochem Biophys Res Commun. 2009;380:205–10.
Acknowledgments
This study was supported by the National 863 Hi-tech Project of China (2007AA021803, 2007AA021901, 2007AA021809, 2007AA021811), the National Natural Science Foundation of China (81272972), National Basic Research Program of China (2010CB833605), Hunan Provincial Science and Technology Department (2010FJ4030), Incubation Program for National Natural Science Funds for Distinguished Young Scholar of Central South University (2010QYZD006), and Open-End Fund for the Valuable and Precision Instruments of Central South University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This study was approved by the Ethics Committee for Human Research of Xiangya Hospital. The animal protocol was preapproved by Central South University, and all experiments were performed in accordance to the animal care guidelines of the Chinese Council.
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Li, J., Chen, Y., Guo, X. et al. Inhibition of miR-15b decreases cell migration and metastasis in colorectal cancer. Tumor Biol. 37, 8765–8773 (2016). https://doi.org/10.1007/s13277-015-4396-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4396-9