Abstract
We aimed to investigate the expression of FOXP1 in ovarian tumors and correlate it with clinicopathological parameters, chemotherapy resistance, and prognosis. FOXP1 messenger RNA (mRNA) expression was examined in fresh ovarian cancer tissues and normal ovarian tissues, and FOXP1 protein expression was determined in a total of 201 ovarian tissue samples, including 152 cases of primary epithelial ovarian cancer, 26 borderline ovarian tumors, 13 benign ovarian tumors, and 10 normal ovarian tissues. Complete chemotherapy and follow-up data were available in 92 of the 152 epithelial ovarian cancer patients. The relationship between FOXP1 protein expression and ovarian cancer pathological characteristics, chemotherapy resistance, and survival time was analyzed. FOXP1 mRNA expression was downregulated in ovarian cancer tissues compared with that in normal ovarian tissues. Decreased nuclear and increased cytoplasmic FOXP1 protein expression was correlated with increasing tumor grade. Nuclear FOXP1 expression was an independent risk factor associated with chemotherapy resistance and the prognosis of patients with ovarian cancer. FOXP1 expression is closely related to the degree of malignancy of epithelial ovarian cancer and may be a reliable index of the chemoresistance and prognosis of ovarian cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
FOXP1 is a transcription factor of the fork-head box (FOX) family with multiple physiological functions, including B cell development, cardiac valve formation, monocyte differentiation, and lung development [1–4]. FOXP1 plays an oncogenic role in lymphoma, and its upregulation is associated with poor prognosis [5, 6]. Interestingly, however, FOXP1 is located on chromosome 3p14.1 in a region reported to contain various tumor suppressor genes, such as Rassfia and Fhit [7, 8]. In 2001, Banham et al. [9] showed that FOXP1 messenger RNA (mRNA) and protein expression was significantly lower in tumor cells derived from the epithelium (such as colon cancer and renal cell carcinoma) than in the corresponding normal tissues, indicating that the transcription factor FOXP1 may behave as a tumor suppressor in epithelial tumors. The biological functions of FOXP1 were shown to differ among various tissues. Further research revealed loss or ectopic cytoplasmic expression of FOXP1 in several solid tumors, and its expression was closely related to the steroid hormone receptor signaling pathway. Nevertheless, there are no studies assessing the relationship between FOXP1 expression and the malignant biological behaviors of ovarian tumors [10, 11].
In our preliminary work, we performed whole genome screening in three ovarian cancer cell lines with different grades of malignancy. The results showed that FOXP1 mRNA levels decreased significantly with an increase in the malignancy grade. However, Kim et al. found that FOXP1 mRNA was upregulated in chemoresistant ovarian tissues [12]. Therefore, further studies are needed to clarify this issue. The objective of the present study was to determine the levels of FOXP1 expression (mRNA and protein) and the subcellular localization of FOXP1 in ovarian cancer tissues and cells, and to analyze its correlation with clinical pathological parameters, chemotherapy resistance, and the prognosis of ovarian cancer.
Materials and methods
Patient information
Ovarian tumors (malignant, borderline, and benign) and normal ovarian tissues (from the normal ovarian tissues excised during cervical cancer operations) were collected retrospectively from Shengjing Hospital of China Medical University between 2003 and 2009 (Approval Letter 20101115B, Shengjing Hospital of China Medical University). Clinical and pathological information, including age, FIGO, lymphatic node metastasis, differentiation, and residual tumor size, were collected from clinical records. There were 152 cases of primary epithelial ovarian cancer, 26 borderline ovarian tumors, 13 benign ovarian tumors, and 10 normal ovarian tissues (excised during cervical cancer operations), and none of the patients received chemoradiotherapy before surgery. The comparison of age is shown in supplemental Table 1, and the other detailed information of patients is shown in Table 2.
We also collected information on clinical chemotherapeutic treatments received and follow-up of 92 cases among a total of 152 patients with malignant ovarian cancer. These 92 cases underwent treatment for ovarian cancer that included cytoreductive surgery followed by six to eight postoperative cycles of paclitaxel (Taxol®) plus carboplatin (TC) conventional chemotherapy and were followed up for a minimum of 3 years after completion of chemotherapy, respectively. In accordance with the National Comprehensive Cancer Network guidelines (NCCN 2011 guidelines), the 92 patients were divided into two groups: a chemotherapy-resistant group (n = 34) and a chemotherapy-sensitive group (n = 58, including six cases partially sensitive to chemotherapy). The clinicopathological parameters of 92 patients are shown in supplemental Table 2. The NCCN guidelines state that (1) the chemotherapy-resistant group includes patients who had a clinical response to the initial TC chemotherapy program but experienced subsequent relapse, either in the late stage of chemotherapy or within 6 months after the completion of chemotherapy, (2) the partially chemotherapy-sensitive group includes patients who experienced ovarian cancer relapse within 6–12 months after the completion of chemotherapy, and (3) the chemotherapy-sensitive group includes patients who maintained a clinical response for ≥12 months. The guidelines associated with ovarian cancer relapse included continuously increased CA125 levels, solid lesions identified by gynecological examination, tumors identified by images, and signs of ascites.
After obtaining written informed consent from all patients included in the study, samples of primary epithelial ovarian cancer and normal ovarian tissues (excised during cervical cancer operations) were snap-frozen in liquid nitrogen and stored at −80 °C. The analysis of patient tissues was approved by the Institutional Review Board of Shengjing Hospital of China Medical University.
Tissue analysis
Quantitative real-time polymerase chain reaction and statistical analysis
Total RNA from ovarian cancer tissues and normal ovary tissues was isolated by gentle homogenization using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The generations of cDNA and RT-qPCR were performed as previously described [13]. Primers for FOXP1 were as follows: forward primer 5′-CAGTGGTAACCCTTCCCTT-3′, reverse primer 5′-CGTTCAGCTCTTCCCGTA-3′. Primers for GAPDH were as follows: forward primer 5′-CCTTCATTGACCTCCACTAC-3′, reverse primer 5′-GTTGTCATACTTCTCATGGTTC-3′. Real-time PCR analysis was performed on an Applied TAKARA 7500 Sequence Detection System (Takara Co, Japan). The relative quantification of the data obtained was performed according to the user’s manual. The fold change for gene expression, between the cancer and normal samples, was calculated using the threshold cycle (CT): fold change = 2-−ΔΔCT , method, where ΔΔCT = [(CT gene of interest − CT of GAPDH)cancer sample − (CT gene of interest − CT of GAPDH)normal sample. The fold change was log2-transformed for analyzing differential expression.
Immunohistochemistry
Each tumor was cut at intervals of 5 μm. Tissues were dewaxed, and antigen retrieval was performed using a low pH buffer (pH = 6.0) for 20 min at 100 °C. FOXP1 staining was performed using JC12 (isotype lgG2a) mouse anti-human monoclonal antibodies (diluted 1:40, JC12 was kindly provided by Alison H. Banham, University of Oxford, UK) using the Envision detection kit (Maixin-Bio, Fuzhou, China). Positive myoepithelial cell staining and negative stromal cell staining in breast carcinoma were used as internal positive and negative controls, respectively. FOXP1 nuclear expression was scored using the following system: negative = 0, weak/focal = 1, strong focal/widespread moderate staining = 2, or strong/widespread staining = 3. Tumors that scored 2 or 3 were considered nuclear positive for FOXP1. FOXP1 cytoplasmic expression was scored as follows: low-power images of sections were scored based on the staining intensity—no staining, light yellow staining, light brown staining, and brown staining were scored as 0, 1, 2, and 3, respectively. Subsequently, a total of five high-power fields in series were selected from each slice for the scoring of individual cells. The mean percentages of positively stained cells were then calculated for each field. Fields with less than 5 % positive cells were scored as 0; 5 to 25 % as 1; 26 to 50 % as 2; 51 to 75 % as 3; and greater than 75 % as 4. These two scores were then multiplied to yield the final score. A final score of 0 to 2 was considered (−); 3 to 4, (+); 5 to 8, (++); and 9 to 12, (+++). Tumors with scores ≥3 were considered positive for cytoplasmic FOXP1.
Logistic multivariate analysis and COX survival analysis
Among 152 cases with ovarian cancer, complete information on clinical chemotherapeutic treatments and follow-up was available in 92 patients. In the 92 cases, binary logistic regression analysis was performed using age, differentiation, FIGO stage, presence of lymphatic metastasis, residual tumor size (<1 cm, optimal cytoreduction, or >1 cm, suboptimal cytoreduction), and FOXP1 nuclear expression as dependent variables, and chemotherapy resistance as the independent variable; COX survival analysis was performed using the above variables as dependent variables and overall survival as the independent variable. We defined the overall survival time of the patients as the time from the date of surgery to the date of death or the last follow-up. The follow-up ended in November 2012, 7 years and 6 months from the inclusion of the first patient, 3 years and 5 months from the inclusion of the final patient. A total of 30 out of 34 patients in the resistant group and 26 out of 58 patients in the sensitive group died. Kaplan–Meier analysis was performed between groups, and the log-rank test was used to produce survival curves.
Results
Expression of FOXP1 mRNA in different ovarian tissues
In our previous research, whole genome screening of three pairs of ovarian cancer cell lines (results in the GEO database, GSE34396) indicated that transcription factor FOXP1 expression was lower in all high-grade malignant cells than in the corresponding parent cells (unpublished data). To confirm these findings, we used gene microarray in ovarian cancer tissues and normal ovarian tissues by real-time PCR, and the results indicated that the expression of FOXP1 mRNA in ovarian cancer tissues and normal ovarian tissues was 0.2893 ± 0.2352 and 1.0752 ± 0.4323, respectively (P < 0.001; Fig. 1)
The expression of FOXP1 protein in different ovarian tissues
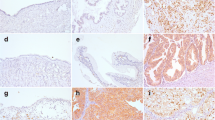
With respect to the FOXP1 protein expression in the different ovarian tissues, nuclear staining decreased and cytoplasmic staining increased along with an increasing degree of malignancy. The percentages of positive nuclear FOXP1 staining in normal ovarian tissues, borderline ovarian tumors, benign ovarian tumors, and malignant ovarian tissues were 71.43, 50.00, 53.80, and 36.2 %, respectively, indicating a significant difference between the normal and malignant ovarian tumor groups (P = 0.0097; Table 1). Furthermore, FOXP1 protein expression was detected in different locations and at different levels among the 152 ovarian cancer tissue samples. Thirteen cases were negative; 31 cases were positive in the cytoplasm; 65 cases were positive in both the cytoplasm and nucleus; and 43 cases were mainly positive in the nucleus (Fig. 2).
Immunohistochemical detection of the expression of FOXP1 in ovarian tissues. a Normal ovarian tissue, b benign, c borderline, d malignant (note: (1) four forms of FOXP1 expression in ovarian malignant tissue; (2) pathology number 0619187 (D1) is mucoid tumor, and 0772078 (D2); 0810700 (D3); 0902376 (D4) are serous tumors)
Correlation between FOXP1 expression and pathological parameters of ovarian cancer
A correlation was observed between FOXP1 nuclear/cytoplasmic expression and the pathological parameters of 152 cases of ovarian cancer (Table 2). Nuclear FOXP1 expression decreased and cytoplasmic expression increased in advanced ovarian cancer (stages III–IV) compared with early stages (stages I–II); (P = 0.036, P = 0.023, respectively).
Correlation between FOXP1 expression and ovarian cancer drug resistance and prognosis
In 58 drug-sensitive samples and 34 drug-resistant samples, positive nuclear FOXP1 protein expression rates were 43.1 and 20.6 %, respectively, showing a significant decrease of FOXP1 expression in the resistant group (P = 0.028). Positive cytoplasmic FOXP1 protein expression rates were 65.9 and 65.4 %, respectively (Table 3, Fig. 3), and there was no statistically significant difference between the two groups. Multivariable logistic regression analysis showed that pathological stage, residual tumor size, and nuclear FOXP1 expression were independent risk factors associated with chemotherapy resistance of ovarian cancer (Table 4). COX survival analysis indicated that residual tumor size and nuclear FOXP1 expression were independent risk factors that strongly correlated with prognosis of ovarian cancer (Table 5); however, other clinical pathological parameters and cytoplasmic FOXP1 expression did not correlate with prognosis. Taking residual tumor size (>1 vs. <1 cm) or taking nuclear FOXP1 expression (0 ~ 1 vs. 2 ~ 3) as the factor, Kaplan–Meier survival analysis was applied (the log-rank test, P = 0.064, P = 0.002, respectively; Fig. 4).
Discussion
Ovarian cancer has the highest mortality rate of all gynecologic cancers, and 75 % of patients diagnosed with ovarian cancer are already at an advanced stage. Chemotherapy is important in treating and preventing the recurrence of ovarian cancer; however, resistance is an obstacle that must be overcome, and finding new treatment strategies has become increasingly valuable. This study is the first to analyze large amounts of samples to determine FOXP1 expression at the gene and protein levels and its subcellular localization in ovarian cancer to examine the association between FOXP1 expression and chemotherapy resistance and the prognosis of ovarian cancer.
The following data supported the above conclusion: (1) whole genome screening indicated that FOXP1 mRNA expression level is significantly decreased in all high grade malignant cells compared to that in the corresponding parental cells, and in clinical ovarian cancer tissues compared to normal ovarian tissues as determined by real-time PCR; (2) immunohistochemistry indicated that increased tumor grade is correlated with decreased nuclear FOXP1 protein expression and increased cytoplasmic expression, with a significant differences between the normal and malignant groups (P = 0.0097); (3) furthermore, immunohistochemical staining from follow-up ovarian cancer patients indicated that nuclear FOXP1 protein expression in the chemotherapy resistance group was significantly lower compared to the sensitivity group, although the differences of cytoplasmic FOXP1 protein expression were not significant between the groups. Multivariable logistic regression analysis and COX survival analysis proved that the nuclear FOXP1 protein is an independent risk factor that correlates with chemotherapy resistance and the prognosis of ovarian cancer. Using nuclear FOXP1 expression as a factor, Kaplan–Meier analysis and the log-rank test showed that significant differences with P < 0.05. The above results indicate that nuclear FOXP1 expression is closely related to the grade of malignancy in ovarian cancer and may be a reliable index for the resistance and prognosis of ovarian cancer.
The decrease of FOXP1 expression in breast cancer [14, 15], endometrial cancer [16], and clear cell renal cell carcinoma [17] is similar to our results, and these provide more justification for the study of FOXP1 functions in suppressing tumors of epithelial origin. However, there are still divergent opinions on the correlation between FOXP1 and cancer resistance or prognosis.
In one study of diffuse large B cell lymphoma (DLBCL), in which FOXP1 staining of >30 % of the tumoral nuclei was considered FOXP1-positive, the median overall survival in the FOXP1-positive group was 1.6 years, compared to 12.2 years in the FOXP1-negative group [6]. Barrans et al. confirmed and extended this observation by correlating the effect of FOXP1 expression with the overexpression of BCL-2 and MUM1/IRF4. Yet, Hans et al. found a correlation between FOXP1 expression and prognosis in GC and non-GC groups of DLBCL [18]. In research on tumors of epithelial origin, the correlation between FOXP1 and prognosis is still controversial. For example, in breast cancer, Rayoo’s research indicated that patients with a high expression of FOXP1 protein have a good prognosis [19]; yet, Giatromanolaki et al. have shown that in low-risk, early-stage endometrial cancers, there was no correlation between FOXP1 and overall survival. Kim et al. reported that FOXP1 mRNA decreases in stage III serous ovarian carcinoma; yet, the prognosis of patients with chemoresistance was predicted to be very poor when genes such as RNses K, FOXP1, and LAMB2 were upregulated [12]. The present study is the first to analyze a large sample of chemotherapy-resistant tissues from follow-up patients, and our findings indicate that the downregulation of FOXP1 protein expression is associated with chemotherapy resistance and poor prognosis of ovarian cancer patients. We further demonstrated the correlation between FOXP1 protein expression and the prognosis of tumors with an epithelial origin. This research thus indicates that the targeted reactivation of FOXP1 protein expression may reduce chemotherapy resistance and improve prognosis.
In the present study, we observed increased cytoplasmic FOXP1 protein expression in ovarian cancer tissues, and a stronger correlation between nuclear FOXP1 protein and ERβ expression (to be published). Although research has shown that cytoplasmic FOXO3a have different functions and that serine phosphorylation determines its localization, we still do not know the eukaryotic pattern or the role of ectopic cytoplasmic FOXP1 expression in increasing subtype expression and different functions. In conclusion, our results have shown that FOXP1 plays an important role in ovarian cancer development, but the mechanism is not clear, and further research is needed.
References
Hu H, Wang B, Borde M, Nardone J, Maika S, Allred L, et al. Foxp1 is an essential transcriptional regulator of B cell development. Nat Immunol. 2006;7:819–26.
Wang B, Weidenfeld J, Lu MM, Maika S, Kuziel WA, Morrisey EE, et al. Foxp1 regulates cardiac outflow tract, endocardial cushion morphogenesis and myocyte proliferation and maturation. Development. 2004;131:4477–87.
Shi C, Sakuma M, Mooroka T, Liscoe A, Gao H, Croce KJ, et al. Down-regulation of the forkhead transcription factor Foxp1 is required for monocyte differentiation and macrophage function. Blood. 2008;112:6699–711.
Li S, Wang Y, Zhang Y, Lu MM, DeMayo FJ, Dekker JD, et al. Foxp1/4 control epithelial cell fate during lung development and regeneration through regulation of anterior gradient 2. Development. 2012;139:2500–9.
Sagaert X, de Paepe P, Libbrecht L, Vanhentenrijk V, Verhoef G, Thomas J, et al. Forkhead box protein P1 expression in mucosa-associated lymphoid tissue lymphomas predicts poor prognosis and transformation to diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:2490–7.
Banham AH, Connors JM, Browen PJ, Cordell JL, Ott G, Sreenivasan G, et al. Expression of the FOXP1 transcription factor is strongly associated with inferior survival in patients with diffuse large B-cell lymphoma. Clin Cancer Res. 2005;11:1065–72.
Pronina IV, Loginov VI, Kholdyrev DS, Kazubskaia TP, Braga EA. Alterations of expression level of RASSFIA gene in primary epithelia tumors of various locations. Mol Biol (Mosk). 2012;46:260–8.
Palumbo E, Tosoni E, Matricardi L, Russo A. Genetic instability of the tumor suppressor gene FHIT in normal human cells. Genes Chromosomes Cancer. 2013;52:832–44.
Banham AH, Beasley N, Campo E, Fernandez PL, Fidler C, Gatter K, et al. The FOXP1 winged helix transcription factor is a novel candidate tumor suppressor gene on chromosome 3p. Cancer Res. 2001;61:8820–9.
Shigekawa T, Ijichi N, Ikeda K, Horie-Inoue K, Shimizu C, Saji S, et al. FOXP1, an eatrogen-inducible transcription factor, modulates cell proliferation in breast cancer cells and 5-year recurrence-free survival of patients with tamoxifen-treated breast cancer. Horm Cancer. 2011;2:286–97.
Takayama K, Horie-Inoue K, Ikeda K, Urano T, Murakami K, Hayashizaki Y, et al. FOXP1 is an androgen-responsive transcription factor that negatively regulates androgen receptor signaling in prostate cancer cells. Biochem Biophys Res Commun. 2008;374:388–93.
Kim YS, Hwan JD, Bae S, Bae DH, Shick WA. Identification of differentially expressed genes using an annealing control primer system in stage III serous ovarian carcinoma. BMC Cancer. 2010;10:576.
Chien WH, Gau SS, Chen CH, Tsai WC, Wu YY, Chen PH, et al. Increased gene expression of FOXP1 in patients with autism spectrum disorders. Mol Autism. 2013;4:23.
Fox SB, Brown P, Han C, Ashe S, Leek RD, Harris AL, et al. Expression of the forkhead transcription factor FOXP1 is associated with estrogen receptor alpha and improved survival in primary human breast carcinoma. Clin Cancer Res. 2004;10:3521–7.
Bates GJ, Fox SB, Han C, Launchbury R, Leek RD, Harris AL, et al. Expression of the forkhead transcription factor FOXP1 is associated with that of estrogen receptor-beta in primary invasive breast carcinomas. Breast Cancer Res Treat. 2008;111:453–9.
Giatromanolaki A, Koukourakis MI, Sivridis E, Gatter KC, Harris AL, Banham AH. Loss of expression and nuclear/cytoplasmic localization of the FOXP1 foxkhead transcription factor are common events in early endometrial cancer: relationship with estrogen receptors and HIF-1a expression. Mod Pathol. 2006;19:9–16.
Toma MI, Grosser M, Herr A, Aust DE, Meye A, Hoefling C, et al. Loss of heterozygosity and copy number abnormality in clear cell renal cell carcinoma discovered by high-density affymetrix 10K single nucleotide polymorphism mapping array. Neoplasia. 2008;10:634–42.
Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–82.
Rayoo M, Yan M, Takano EA, Bates GJ, Brown PJ, Banham AH, et al. Expression of the forkhead box transcription factor FOXP1 is associated with oestrogen receptor alpha, oestrogen receptor beta and improved survival in familial breast cancers. J Clin Pathol. 2009;62:896–902.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplement table 1
(DOC 30 kb)
Supplement table 2
(DOC 54 kb)
Rights and permissions
About this article
Cite this article
Hu, Z., Zhu, L., Gao, J. et al. Expression of FOXP1 in epithelial ovarian cancer (EOC) and its correlation with chemotherapy resistance and prognosis. Tumor Biol. 36, 7269–7275 (2015). https://doi.org/10.1007/s13277-015-3383-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3383-5