Abstract
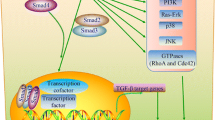
Transforming growth factor β (TGFβ) receptor signaling plays a paradoxical effect in the tumorigenesis of pancreatic ductal adenocarcinoma (PDAC), in which its tumor-inhibitory role at early stages turns into a tumor-promoting role at later stages. The underlying mechanism remains far from clear. Here we provide strong evidence that the activation of TGFβ receptor signaling in PDAC cells increased SMAD3 phosphorylation and nuclear translocation to inhibit cell growth. Meanwhile, it also activated SMAD7 to induce nuclear translocation and retention of β-catenin, which not only attenuated the inhibition of cell growth by nuclear SMAD3 but also activated vascular endothelial growth factor A (VEGF-A) to promote vascularization. Our data thus support a model involving crosstalk of the TGFβ and Wnt signaling pathways, for regulating the complicated effect of TGFβ signaling on the tumorigenesis of PDAC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pancreatic cancers originate from both the endocrine and exocrine pancreas. Pancreatic ductal adenocarcinoma (PDAC) is an exocrine tumor, which represents over 90 % of all pancreatic malignant tumors [1]. PDAC patients suffer from a very poor prognosis and the 5-year survival rate of PDAC is as low as 5 %, largely due to its local expansion and invasion, early metastasis, and potential resistance to conventional therapies [1]. Since PDAC is often asymptomatic or lacks specific symptoms in its early stage, it may have reached an advanced stage and may have also metastasized when the symptoms occur.
The transforming growth factor β (TGFβ) receptor signaling pathway plays critical and complicated roles in various biological events [2–7] and specifically has a paradoxical effect on the tumorigenesis of PDAC, in which its tumor-inhibitory role at early stages turns into a tumor-promoting role at later stages [8–10]. When a ligand binds to a type II TGFβ receptor, it catalyzes the phosphorylation of a type I TGFβ receptor, which triggers phosphorylation of two intracellular proteins, SMAD2 and SMAD3, to form heteromeric complexes with SMAD4. The activated SMAD complexes then translocate to the nucleus, where they regulate the transcription of target genes [2]. SMAD6 and SMAD7 have inhibitory roles on SMAD3 signaling. In the pancreas, only SMAD7 is expressed and is upregulated at the transcriptional level after binding of the ligands to a receptor [11–13]. SMAD7 can block R-SMAD phosphorylation [14], degrade type I receptors [15], and exert the inhibitory effect in the nucleus [16]. Although the critical role of the TGFβ signaling pathway during tumorigenesis of PDAC has been well shown, its exact molecular mechanism as well as its paradoxical role at different stages of the cancer remains unclear.
The vascular endothelial growth factor (VEGF) family is the most important signal protein in vasculogenesis and angiogenesis during development, tissue regeneration and repair, and tumorigenesis [17]. The VEGF family is composed of six secreted proteins: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placental growth factor (PLGF) [18], among which VEGF-A appears to be the most abundant and most potent angiogenic factor [19, 20]. By differential mRNA splicing, the human VEGF-A gene can give rise to three protein isoforms, VEGF121, VEGF165, and VEGF189. Whereas VEGF189 is heparin-binding and mainly associated with the cell surface and with the extracellular matrix, VEGF121 is freely diffusible due to the lack of exons 6 and 7 that encode heparan sulfate proteoglycan-binding domains. The predominant isoform, VEGF165, appears to have the highest bioavailability and biological potency and exhibits only partial binding to the cell surface and extracellular matrix. VEGF-A-mediated increase in angiogenesis of PDAC and other tumors has been shown to be critical for cancer growth and metastasis [21–25], while its regulation by the TGFβ receptor signaling pathway in PDAC has been rarely addressed before [26].
Here we aimed to figure out the precise role of the TGFβ receptor signaling pathway in the growth of PDAC and dissect the underlying mechanism. We provide strong evidence that the activation of TGFβ receptor signaling in PDAC cells increased SMAD3 phosphorylation and nuclear translocation to inhibit cell growth. Meanwhile, it also activated SMAD7 to induce nuclear translocation and retention of β-catenin, which not only attenuated the inhibition of cell growth by nuclear SMAD3 but also activated VEGF-A to promote vascularization. Our data thus support a model involving crosstalk of the TGFβ and Wnt signaling pathways, for regulating the complicated effect of TGFβ signaling on the tumorigenesis of PDAC.
Materials and methods
Cell line culture
PANC-1 has been generated from a human carcinoma of the exocrine pancreas in 1975 [27] and was purchased from ATCC. PANC-1 was cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20 % fetal bovine serum (Invitrogen, Carlsbad, CA, USA). Recombinant TGFβ was purchased from Sigma (USA).
Plasmid transfection
TGFβ, with (by a 2A sequence to connect) or without small short hairpin interfering RNA (shRNA) for SMAD3 or SMAD7 construct, was sub-cloned into pcDNA3.1-EGFP. The small 2A peptide sequences, when cloned between genes, allow for efficient, stoichiometric production of discrete protein products within a single vector through a novel cleavage event within the 2A peptide sequence. Transfection was performed with Lipofectamine 2000 reagent (Invitrogen, USA), according to the instructions of the manufacturer. Stable transduced PANC-1 cells were purified by flow cytometry based on GFP.
Cell proliferation assay
For assay of cell proliferation, pretreated cells were seeded into a 96-well plate at 5000 cells per well and subjected to a cell proliferation kit (MTT, Roche, USA), according to the instruction from the manufacturer.
Western blot
Nuclear and cytoplasmic proteins were isolated from the cultured cells with Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific, USA). The primary antibodies were rabbit anti-SMAD3, anti-phosphorylated SMAD3, anti-β-catenin, anti-LaminB1, and anti-α-tubulin (all from Cell Signaling, USA). The secondary antibody is HRP-conjugated anti-rabbit (Jackson Labs, USA). Figure images were representative from five repeats. α-Tubulin (cytosol protein (CY)) and LaminB1 (nuclear protein (NU)) were used as protein loading controls.
RT-qPCR
Total RNA was extracted from cells with RNeasy Kit (Qiagen, Hilden, Germany). cDNA was then synthesized by reverse transcription (Qiagen). Quantitative real-time PCR (RT-qPCR) was performed in duplicate with QuantiTect SYBR Green PCR Kit (Qiagen). All primers were purchased from Qiagen. Values of genes were first normalized against α-tubulin and then compared to controls.
Statistical analysis
All statistical analyses were carried out using the SPSS 18.0 statistical software package. All values are depicted as mean ± standard deviation from five individuals and are considered significant if p < 0.05. All data were statistically analyzed using one-way ANOVA with a Bonferroni correction.
Results
Activation of TGFβ receptor signaling in PDAC cells increased nuclear translocation of SMAD3 and β-catenin and activated SMAD7 and VEGF-A
In order to understand the paradoxical role of TGFβ receptor signaling in the pathogenesis of PDAC, we used a human PDAC line, PANC-1, to study the interaction of TGFβ receptor signaling with other signaling pathways, which might be responsible for the complicated effects of TGFβ receptor signaling in PDAC. We gave PANC-1 cells with TGFβ1 to robustly activate the receptor. We found that activation of TGFβ receptor signaling by TGFβ1 not only significantly increased the transcript level of SMAD7, which is consistent with the established TGFβ receptor signaling pathway, but also significantly increased the transcript level of VEGF-A, suggesting an angiogenic effect (Fig. 1a).
Activation of TGFβ receptor signaling in PDAC cells increased nuclear translocation of SMAD3 and β-catenin and activated SMAD7 and VEGF-A. We used a human PDAC line, PANC-1, to study the TGFβ receptor signaling. We gave PANC-1 cells with TGFβ1 to robustly activate the receptor. a Activation of TGFβ receptor signaling by TGFβ1 increased the transcript levels of SMAD7 and VEGF-A. b Activation of TGFβ receptor signaling by TGFβ1 significantly increased phosphorylation and nuclear translocation of SMAD3 and β-catenin. LaminB1 was used as a loading control for nuclear protein (NU). α-Tubulin was used as a loading control for cytosol protein (CY). *p < 0.05
Moreover, activation of TGFβ receptor signaling by TGFβ1 in PANC-1 cells significantly increased phosphorylation and nuclear translocation of SMAD3 (Fig. 1b), a direct effector of the activated TGFβ receptor signaling, which is supposed to be responsible for inhibition of cell growth. Furthermore, we also detected a less potent but significant nuclear translocation of β-catenin (Fig. 1b), the key factor in the Wnt signaling pathway, which has an established role in promoting cell growth in many cell types. Thus, these data suggest that nuclear translocation of β-catenin may be induced by TGFβ1 stimulation on PANC-1 cells to attenuate the inhibition of cell growth by nuclear SMAD3.
SMAD3 phosphorylation and nuclear translocation inhibited PDAC cell growth
Since both SMAD3 and SMAD7 are two direct targets of an activated TGFβ receptor signaling and both were activated by TGFβ1 in PANC-1 cells (Fig. 1a, b), we thus tried to determine whether the SMAD3 or SMAD7 signal transduction pathway was responsible for the nuclear translocation of β-catenin and activation of the Wnt signaling pathway. Then we overexpressed TGFβ1 in PANC-1 cells, with or without a shRNA for SMAD3 or SMAD7 (PDAC-TGFβ1, PDAC-TGFβ1-shSMAD3, PDAC-TGFβ1-shSMAD7), for specifically inhibiting one downstream signal cascade. Metformin increased the phosphorylation of β-catenin and decreased β-catenin protein levels leading to suppression of Wnt/β-catenin signaling [28]. We thus gave metformin to TGFβ1-overexpressing PNAC-1 cells (PDAC-TGFβ1//metformin) to strongly promote phosphorylation of β-catenin and prevent its nuclear translocation for inhibiting Wnt signaling. PANC-1 cells transduced with a null plasmid were used as a control (PDAC-null).
Overexpression of TGFβ1 in PDAC-TGFβ1, PDAC-TGFβ1-shSMAD3, and PDAC-TGFβ1-shSMAD7 cells, as well as inhibition of SMAD7 in PDAC-TGFβ1-shSMAD7 cells, was all confirmed by RT-qPCR (Fig. 2a). Inhibition of nuclear SMAD3 in PDAC-TGFβ1-shSMAD3 cells and inhibition of nuclear β-catenin in PDAC-TGFβ1//metformin cells were assured by Western blot (Fig. 2b).
Effects of TGFβ1, SMAD3, and SMAD7 modulation on protein nuclear translocation in PDAC cells. We overexpressed TGFβ1 in PANC-1 cells, with or without shRNA for SMAD3 or SMAD7 (PDAC-TGFβ1, PDAC-TGFβ1-shSMAD3, PDAC-TGFβ1-shSMAD7). We also gave metformin to TGFβ1-overexpressing PNAC-1 cells (PDAC-TGFβ1//metformin) to strongly promote phosphorylation of β-catenin and prevent its nuclear translocation for inhibiting Wnt signaling. PANC-1 cells transduced with a null plasmid were used as a control (PDAC-null). a RT-qPCR for gene transcripts. b Western blot for nuclear and cytosol proteins. LaminB1 was used as a loading control for nuclear protein (NU). α-Tubulin was used as a loading control for cytosol protein (CY). *p < 0.05. NS non-significant
TGFβ1 stimulation resulted in significant inhibition in cell growth, which was abolished by SMAD3 knockout, or was augmented by SMAD7 knockout (Fig. 3), suggesting that the inhibitory effect of TGFβ1 stimulation on PDAC cells was mediated by SMAD3 phosphorylation and nuclear translocation, which was inhibited by SMAD7.
β-Catenin nuclear translocation attenuated cell growth inhibitory effect by nuclear SMAD3 in PDAC cells
Cell growth inhibition by TGFβ1 stimulation was further augmented by metformin-induced inhibition of β-catenin nuclear translocation (Fig. 3), suggesting that β-catenin nuclear translocation may attenuate the inhibitory effect of TGFβ1 stimulation on the growth of PDAC cells.
β-Catenin nuclear translocation was induced by SMAD7 in PDAC cells
SMAD7 knockout in PDAC-TGFβ1-shSMAD7 cells completely inhibited TGFβ1-induced β-catenin nuclear translocation (Fig. 2b), resulting in a further decrease in cell growth. However, SMAD3 knockout in PDAC-TGFβ1-shSMAD3 cells did not affect TGFβ1-induced β-catenin nuclear translocation (Fig. 2b). These data suggest that β-catenin nuclear translocation may be induced by SMAD7 activation, but not SMAD3 modulation, in PDAC cells.
β-Catenin nuclear translocation activated VEGF-A
Interestingly, similar to what we had found in TGFβ1-treated PANC-1 cells, here we detected that TGFβ1-overexpressing PANC-1 cells (PDAC-TGFβ1) also upregulated VEGF-A (Fig. 2a). Moreover, this upregulation in VEGF-A was abolished by inhibition of SMAD7 (PDAC-TGFβ1-shSMAD7), but not by inhibition of SMAD3 (PDAC-TGFβ1-shSMAD3) (Fig. 2a). Furthermore, metformin also inhibited VEGF-A activation in TGFβ1-overexpressing PANC-1 cells (PDAC-TGFβ1//metformin) (Fig. 2a), suggesting that VEGF-A may be activated through SMAD7-induced β-catenin nuclear translocation, in response to TGFβ1 stimulation. VEGF-A upregulation may improve cancer vascularization and subsequently facilitate its growth and invasion, as a possible contributor to the cancer-promoting role of TGFβ receptor signaling in the later stages of PDAC, in contrast to its growth-inhibitory role at the early stages.
Discussion
PDAC is the most common type of pancreatic tumor, with an extremely high lethality. The low 5-year survival rate of PDAC largely results from its lack of specific symptoms in its early stages, leading to poor early prognosis. Therefore, the understanding of the mechanism regulating the outgrowth of PDAC appears to be critical for its early prognosis and therapy [1]. The TGFβ receptor signaling pathway plays critical and complicated roles in various biological events [2–7] and specifically has a paradoxical effect on the tumorigenesis of PDAC, in which its tumor-inhibitory role at early stages turns into a tumor-promoting role at later stages [8–10]. Since TGFβ receptor signaling mainly transduces from phosphorylation and subsequent nuclear translocation of SMAD2 or SMAD3, which is attenuated by SMAD7 as a negative feedback, we thus examined SMAD3 and SMAD7 pathways. SMAD3 is directed bound by SMAD7. However, this is at the protein level. So far, there are no reports on the regulation of SMAD3 transcription by SMAD7 [15].
We used a human PDAC line, PANC-1, to study the transduction cascades of TGFβ receptor signaling. We found that activation of TGFβ receptor signaling by TGFβ1 not only significantly induced nuclear translocation of SMAD3 and increased the transcript level of SMAD7 but also significantly increased the transcript level of VEGF-A and induced nuclear translocation of β-catenin. Thus, we hypothesized that neovascularization and the Wnt signaling pathway may be also regulated by TGFβ receptor signaling, besides cell growth.
To further understand the underlying mechanism, we thus overexpressed TGFβ1 in PANC-1 cells, with or without a shRNA for SMAD3 or SMAD7. We also gave metformin to TGFβ1-overexpressing PANC-1 cells to strongly promote phosphorylation of β-catenin and prevent its nuclear translocation for inhibiting Wnt signaling. TGFβ1 stimulation resulted in significant inhibition of cell growth, which was abolished by SMAD3 knockout, or was augmented by SMAD7 knockout or inhibition of β-catenin nuclear translocation, suggesting that the inhibitory effect of TGFβ1 stimulation on PDAC cells was mediated by SMAD3 phosphorylation and nuclear translocation, which was inhibited by SMAD7 or β-catenin nuclear translocation. To determine the causal link between SMAD7 and β-catenin nuclear translocation, we examined β-catenin nuclear translocation in SMAD7-knockout PDAC-TGFβ1-shSMAD7 and SMAD3-knockout PDAC-TGFβ1-shSMAD3 cells. Our data strongly support that β-catenin nuclear translocation is induced by SMAD7 activation, but not SMAD3 modulation, in PDAC cells. We also looked at the effect on VEGF-A by TGFβ1 stimulation. The upregulation in VEGF-A by TGFβ1 stimulation was abolished by inhibition of SMAD7, but not by inhibition of SMAD3. Further, metformin also inhibited VEGF-A activation in TGFβ1-overexpressing PANC-1 cells, suggesting that VEGF-A may be activated through SMAD7-induced β-catenin nuclear translocation, in response to TGFβ1 stimulation. Since VEGF-A upregulation may improve vascularization of PDAC and subsequently facilitate its growth and invasion, it may positively contribute to the long-term survival and progress of PDAC. Thus, although TGFβ receptor signaling may inhibit cancer cell growth in the early stages of PDAC, its promoting role in angiogenesis may improve the long-term survival and progress of PDAC, and it appears to be tumorigenesis-enhancing at the later stages. Our study thus provides a novel model to explain the paradoxical role of TGFβ receptor signaling in the development of PDAC (Fig. 4).
References
Han H, Von Hoff DD. Snapshot: pancreatic cancer. Cancer Cell. 2013;23:424–424 e421.
Massague J. TGFbeta in cancer. Cell. 2008;134:215–30.
Xiao X, Gaffar I, Guo P, Wiersch J, Fischbach S, Peirish L, et al. M2 macrophages promote beta-cell proliferation by up-regulation of SMAD7. Proc Natl Acad Sci U S A. 2014;111:E1211–1220.
Yi JJ, Barnes AP, Hand R, Polleux F, Ehlers MD. TGF-beta signaling specifies axons during brain development. Cell. 2010;142:144–57.
Ewen ME, Sluss HK, Whitehouse LL, Livingston DM. TGF beta inhibition of Cdk4 synthesis is linked to cell cycle arrest. Cell. 1993;74:1009–20.
Naka K, Hoshii T, Muraguchi T, Tadokoro Y, Ooshio T, Kondo Y, et al. TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature. 2010;463:676–80.
Xiao X, Wiersch J, El-Gohary Y, Guo P, Prasadan K, Paredes J, et al. TGFbeta receptor signaling is essential for inflammation-induced but not beta-cell workload-induced beta-cell proliferation. Diabetes. 2013;62:1217–26.
Truty MJ, Urrutia R. Basics of TGF-beta and pancreatic cancer. Pancreatology. 2007;7:423–35.
Kleeff J, Ishiwata T, Maruyama H, Friess H, Truong P, Buchler MW, et al. The TGF-beta signaling inhibitor Smad7 enhances tumorigenicity in pancreatic cancer. Oncogene. 1999;18:5363–72.
Villanueva A, Garcia C, Paules AB, Vicente M, Megias M, Reyes G, et al. Disruption of the antiproliferative TGF-beta signaling pathways in human pancreatic cancer cells. Oncogene. 1998;17:1969–78.
Afrakhte M, Moren A, Jossan S, Itoh S, Sampath K, Westermark B, et al. Induction of inhibitory Smad6 and Smad7 mRNA by TGF-beta family members. Biochem Biophys Res Commun. 1998;249:505–11.
Yan X, Chen YG. Smad7: not only a regulator, but also a cross-talk mediator of TGF-beta signalling. Biochem J. 2011;434:1–10.
Itoh S, ten Dijke P. Negative regulation of TGF-beta receptor/Smad signal transduction. Curr Opin Cell Biol. 2007;19:176–84.
Hayashi H, Abdollah S, Qiu Y, Cai J, Xu YY, Grinnell BW, et al. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell. 1997;89:1165–73.
Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700.
Zhang S, Fei T, Zhang L, Zhang R, Chen F, Ning Y, et al. Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol Cell Biol. 2007;27:4488–99.
Ferrara N. Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2009;29:789–91.
Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76.
Xiao X, Prasadan K, Guo P, El-Gohary Y, Fischbach S, Wiersch J, et al. Pancreatic duct cells as a source of VEGF in mice. Diabetologia. 2014;57:991–1000.
Xiao X, Guo P, Chen Z, El-Gohary Y, Wiersch J, Gaffar I, et al. Hypoglycemia reduces vascular endothelial growth factor a production by pancreatic beta cells as a regulator of beta cell mass. J Biol Chem. 2013;288:8636–46.
Ma J, Sawai H, Ochi N, Matsuo Y, Xu D, Yasuda A, et al. PTEN regulates angiogenesis through PI3K/Akt/VEGF signaling pathway in human pancreatic cancer cells. Mol Cell Biochem. 2009;331:161–71.
Cabebe E, Fisher GA. Clinical trials of VEGF receptor tyrosine kinase inhibitors in pancreatic cancer. Expert Opin Investig Drugs. 2007;16:467–76.
Hotz HG, Hines OJ, Masood R, Hotz B, Foitzik T, Buhr HJ, et al. VEGF antisense therapy inhibits tumor growth and improves survival in experimental pancreatic cancer. Surgery. 2005;137:192–9.
Mao D, Zhang Y, Lu H, Zhang H. Molecular basis underlying inhibition of metastasis of gastric cancer by anti-VEGFa treatment. Tumour Biol. 2014;35:8217–23.
Zhou X, Qi Y. PLGF inhibition impairs metastasis of larynx carcinoma through MMP3 downregulation. Tumour Biol. 2014;35:9381–6.
Teraoka H, Sawada T, Nishihara T, Yashiro M, Ohira M, Ishikawa T, et al. Enhanced VEGF production and decreased immunogenicity induced by TGF-beta 1 promote liver metastasis of pancreatic cancer. Br J Cancer. 2001;85:612–7.
Lieber M, Mazzetta J, Nelson-Rees W, Kaplan M, Todaro G. Establishment of a continuous tumor-cell line (PANC-1) from a human carcinoma of the exocrine pancreas. Int J Cancer. 1975;15:741–7.
Takatani T, Minagawa M, Takatani R, Kinoshita K, Kohno Y. AMP-activated protein kinase attenuates Wnt/beta-catenin signaling in human osteoblastic Saos-2 cells. Mol Cell Endocrinol. 2011;339:114–9.
Acknowledgments
This study was supported by the National Science Foundation of China (grant no. 81273955).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, H., Liu, C., Kong, Y. et al. TGFβ signaling in pancreatic ductal adenocarcinoma. Tumor Biol. 36, 1613–1618 (2015). https://doi.org/10.1007/s13277-014-2757-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2757-4