Abstract
Multiple studies have shown that CC motif chemokine ligand 19 (CCL19) promotes cell proliferation in several human cancers. In this study, we investigated the clinical significance of CCL19 and its specific receptor CCR7 and its function in our large collection of prostate samples. Between August 2000 and December 2013, 108 patients with histologically confirmed prostate cancer (PCa) and 80 with benign prostate hyperplasia (BPH) were recruited into the study. Quantitative RT-PCR immunohistochemistry analyses were used to quantify CCL19 and CCR7 expression in PCa cell lines and clinical samples. The functional role of CCL19 in PCa cell lines was evaluated by small interfering RNA-mediated depletion of the protein followed by analyses of cell proliferation and invasion. The positive rate of CCL19 staining was 87.04 % (94/108) in 108 cases of prostatic carcinoma and 16.25 % (13/80) in 80 cases of BPH, and high expression of CCR7 was observed in 83.33 % (90/108) of the PCa tissues versus (17.50 %; 14/80) of the BPH tissues, the difference of CCL19 and CCR7 expression between two groups was statistically significant, respectively. The results were confirmed by quantitative real-time PCR. CCL19 and CCR7 were significantly elevated in all five PCa cell lines when compared to the RWPE-1 cells. Silencing of CCL19 inhibited the proliferation of DU-145 cells which have a relatively high level of CCL19 in a time- and concentration-dependent manner, and the invasion and migration of DU-145 cells were distinctly suppressed. Our data suggest that the pathogenesis of human PCa maybe mediated by the CCL19/CCR7 axis, and CCL19 inhibition treatment may provide a promising strategy for the anti-tumor therapy of PCa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Prostate cancer (PCa) is one of the leading causes of cancer-related deaths worldwide. There has been a trend towards increased incidence and morbidity of prostate cancer in Asia in recent years [1]. In recent years, novel diagnostic and therapeutic technologies have been advanced for early diagnosis and treatment of PCa, but the clinical outcome of PCa patients remains unsatisfactory. Although PSA has been the epoch-making marker for PCa screening and diagnosis, some newly diagnosed PCa patients already have metastatic disease [2]. Besides, approximately 30 % of patients experience recurrence after primary therapy [3]. The management of prostate cancer is complex, but androgen-deprivation therapy (ADT) or hormonal therapy remains a definitive treatment for both localized and metastatic PCa. Unfortunately, despite an initial response to ADT, most prostate cancers eventually develop metastatic castration-resistant disease (mCRPC) stage with increased proliferation and malignancy [4, 5]. The incomplete understanding of molecular features of PCa might be one of the reasons for this unsatisfied situation; therefore, it is important to investigate the molecular mechanisms underlying the progression of PCa to provide effective strategies for the prevention and therapy of PCa.
Chemokines and chemokine receptors are components of cancer-related inflammatory conditions, which can promote various aspects of tumor growth [6]. The chemokine superfamily is small, cytokine-like proteins that induce cytoskeleton rearrangement, firm adhesion to endothelial cells, and directional migration. The superfamily was classified into four subgroups (CC, CXC, CX3C, and C) on the basis of their N-terminal cysteine motifs. They are involved in all stages of tumor development, including initiation, growth, and progression [7]. Several previous published studies suggested that members of the chemokine family may serve as potential biomarker and novel molecular target in cancer therapy for PCa [8, 9].
The chemokines CCL19 (ELC/MIP-3β), released by lymphatic endothelial cells and T cells of the lymphnodes, mainly recruit and influence immunocytes, such as macrophages, lymphocytes, and dendritic cells [10]. CCL19 is strongly expressed in T cell zones of lymphoid organs and chemoattracts T, B, dendritic, macrophage progenitor, and NK (natural killer) cells and may be involved in the interactions of DCs and T cells in secondary lymphoid tissues. The chemokines CCL19 and its specific receptor CCR7 would seem to have a biological significance in the development and progression of various malignant diseases. The CCL19/CCR7 axis induces many events related to tumorigenesis, such as tumor cell proliferation, tumor cell surveillance, adhesion, migration, invasion, and angiogenesis [11, 12]. However, the expression and function of CCL19 in PCa remains yet to be established. Our aims of this study were to illustrate the possible mechanism of CCL19/CCR7 promoting proliferation and migration of tumor cells and investigate its clinicopathological significance in PCa.
Materials and methods
Patients and tissue samples
A total of 108 patients had undergone radical prostatectomy and bilateral lymphadenectomy at the Department of Urology, 456 Hospital of PLA, between August 2000 and December 2013 and for whom archival tissues were included in this study. No patient was managed preoperatively with either hormonal or radiation therapy, and no secondary cancers were observed. Eighty cases of benign prostatic hyperplasia (BPH) were obtained from men undergoing suprapubic prostatectomy or transurethral plasmakinetic enucleation of prostate. The stages of cancer for all patients were determined by the American Joint Committee on Cancer (AJCC) 2002 system. The specimens were examined by two staff pathologists who were blinded to the clinical outcome and follow-up data. The evaluation of the specimen was performed according to the guidelines of the College of American Pathologists. Paraffin-embedded pathological specimens from these patients with PCa were obtained from the archives. Besides, freshly frozen tissue samples were available. Samples were snap frozen in liquid nitrogen immediately after surgery and experiments were performed. This study was approved by the Ethics Committee of 456 Hospital of PLA. Patients’ offering samples for the study signed informed consent forms.
Cell lines and cell culture
Human prostate normal epithelial cell line RWPE-1 and prostate cancer cell lines LNCaP-AI, LNCaP-AD, DU145, PC3, and 22RV1 were obtained directly from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai Institute of Cell Biology, Chinese Academy of Sciences, China) for fewer than 6 months. LNCaP-AD, PC3, and DU145 were maintained in Roswell Park Memorial Institute (RPMI) medium 1640 (GIBCO, Carlsbad, CA, USA) supplemented with 10 % fetal bovine serum (FBS), 2 mmol/L l-glutamine, and 25 mmol/L HEPES. LNCaP-AI and 22RV1 cells were maintained in phenol red-free RPMI medium 1640 (GIBCO) supplemented with 10 % charcoal-stripped FBS, 300 mg/L l-glutamine, 2000 mg/L glucose, and 2000 mg/L NaHCO3. RWPE-1 cells were maintained in Keratinocyte-SFM (10724, GIBCO), supplemented with 5 mg/ml human recombinant epidermal growth factor (rEGF) and 0.05 mg/ml bovine pituitary extract l-glutamine. All cell lines were maintained in a humidified incubator at 5 % CO2 and 37 °C.
Immunohistochemical analysis
Specimens were fixed in 10 % neutral buffered formalin, embedded in paraffin, and cut into serial sections at a thickness of 3 μm. Paraffin-embedded tissues were dewaxed in xylene, rehydrated by serial concentrations of ethanol, and then rinsed in phosphate buffer solution (PBS) and treated with 3 % H2O2 to refrain endogenous peroxidase. After being heated in a microwave at 750 W for 15 min for antigen retrieval, the sections were incubated with 10 % goat serum at room temperature for 10 min to block nonspecific reactions. The following primary antibodies were used: polyclonal goat anti-human CCL19 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 1:100; monoclonal mouse anti-human CCR7 (Imgenex, San Diego, CA, USA) at 1:100, at 4 °C overnight in a humidified chamber. The slides were followed by a PBS wash and incubated by anti-rabbit EnVision™ kit (DAKO, USA) for 30 min at 37 °C. After a PBS wash, the sections were developed in diaminobenzidine (DAB) substrate. The sections were then counterstained in hematoxylin and then dehydrated in ethanol and xylene before being mounted. Sections were re-prepared by EnVision immunohistochemical staining. PBS instead of primary antibodies was used as negative control.
Evaluation of immunohistochemical results
Immunohistochemical analysis demonstrates positive for CCL19 and CCR7 if purple-brown granules located diffusely in the cytoplasm of the tumor cells. Lack of any obvious purple-brown or brown-red pigmentation in the cytoplasm of tumor cell was considered negative. Relative CCL19 and CCR7 expression was divided into three groups based on the intensity of CCL19 and CCR7 staining and the number of positive cells (staining intensity—negative = 0, weak = 1, moderate = 2, strong = 3; and the percentage of cells stained—0 = 0–10 %, 1 = 11–50 %, 2 = 51–80 %, 3 = 81–100 %). The staining index score of 4 or above was used to define tumors as high expression and the score of 3 or less as low expression of CCL 19 or CCR7. The results were scored by two independent pathologists who were blinded to the subtype of the tumors.
Quantitative reverse transcription-polymerase chain reaction
Total RNA was isolated using TRIzol (Invitrogen, USA) according to the manufacturer’s instructions. Quantitative RT-PCR was performed using SYBR Master Mix (Takara) on an ABI Prism 7900 HT (Applied Biosystems). PCR primers were as follows: CCL19 sense primer: 5′-GTGACCTGCATTAACTCTTTACTTGC-3′; antisense primer: 5′-TATGGCTCTGGCTCTACTGGTTG-3′. CCR7 sense primer: 5′-AGATGAGGTCACGGACGATTACA-3′; antisense primer: 5′-ATGATAGGGAGGAACCAGGCTTT-3′. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) sense primer: 5′-GGACCTGACCTGCCGTCTAG-3′; antisense primer: 5′-GTAGCCCAGGATGCCCTFGA-3′. A human GAPDH gene was used as an endogenous control for sample normalization. Results were presented as the fold expression relative to that of GAPDH.
Transfection and si-CCL19 RNA screening
Three siRNA oligos segments (si#1, si#2, si#3) as well as the scrambled siRNA were designed and synthesized by Genepharma (Shanghai, China). The PCa cells were seeded onto the 6-well plates at 1 × 105 cells per well before transfection. Cells were cultured for 24 h until cell density was around 50 % and then siRNA to CCL19 was transfected into the cells mediated by Lipofectamine TM 2000 reagent (Invitrogen). About 48 h after transfection, total RNA was extracted with the TRIzol reagent and then added it to a reverse transcription reaction to generate cDNA. Cell protein was extracted after 72 h of transfection; qRT-PCR was applied to detect the inhibitory efficiency of siRNA.
PCa cells were seeded in six-well plates uniformly and divided into four groups: group 1 stimulated by 100 ng/mL rhCCL19 (R&D, Minneapolis, MN, USA), group 2 stimulated by 10 ng/mL rhCCL19, group 3 added the same amount of PBS (negative control group). The three groups mentioned above were also added scrambled siRNA TM with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Group 4: CCL19 of PCa cells were effectively downregulated by siRNA with Lipofectamine 2000TM.
Cell survival assay
The effects of CCL19 on PCa cells survival were determined by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide) assay. Four groups of cells were seeded into 96-well plates (5 × 103 cells/well) and cultured for 120 h. After treatments, cells were incubated with MTT (Sigma-Aldrich, St. Louis, MO, 20 μl/well) at 37 °C for 4 h and then 200 μl DMSO was added into each well. Cells were subjected to absorbance reading at 570 nm using a 96-well microplate reader. Percentage of residual cell viability was determined as [(OD of experiment group − OD of blank group) / (OD of negative group − OD of blank group)] × 100%. Assays were performed three times.
Cell migration and invasion assays
For the migration assay, 5 × 104 cells were trypsinized, washed, resuspended in serum-free DMEM, and placed in the top portion of the chamber (Corning, Corning, NY, USA). The lower compartment of the chamber contained 5 % FBS as a chemoattractant. The chambers were incubated at 37 °C, 5 % CO2 for 24 h. The cells on the membrane were washed with PBS, fixed in 100 % methanol, stained with hematoxylin, photographed, and counted. For the invasive ability assay, precooled serum-free DMEM was mixed with 1:3 diluted Matrigel (BD Biosciences, USA). The upper compartments were filled with 100 μl of the mixture, and the Matrigel was allowed to solidify at 37 °C for 4 h. The chambers were incubated for 24 h.
Statistical analysis
Data analyses were performed using SPSS statistical package 15.0 (SPSS Inc, USA). Patient characteristics are shown as the mean ± SD for continuous variables, and as the count and percent for discrete variables. Phenotypic differences in quantitative traits were assessed by genotype using the t test or ANOVA. Differences in the distribution of qualitative traits by genotype were assessed by standard chi-square analysis and Fisher’s exact test. A P value less than 0.05 was considered significant.
Results
CCL19 was highly expressed in PCa tissues compared with the BPH tissues
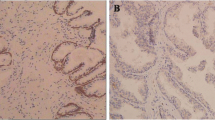
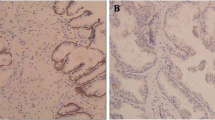
The expression of CCL19 and CCR7 was measured in a series of tissue samples from 108 human PCa tissues and 80 BPH tissues by immunohistochemistry and quantitative real-time PCR. The results showed that the positive rate of CCL19 staining was 87.04 % (94/108) in 108 cases of prostatic carcinoma and 16.25 % (13/80) in 80 cases of BPH, the difference of CCL19 expression between PCa and BPH was statistically significant (P < 0.001). High expression of CCR7 was observed in 83.33 % (90/108) of the PCa tissues versus (17.50 %; 14/80) of the BPH tissues, the difference of CCR7 expression between two groups was statistically significant (P < 0.001). Representative images of CCL19 and CCR7 in PCa and BPH tissues are shown in Fig. 1.
Furthermore, we took 30 cases of PCa tissues and BPH tissues to detect CCL19 and CCR7 mRNA by quantitative real-time PCR (Fig. 2). The expression level of CCL19 and CCR7 mRNA was significantly increased in PCa tissues compared with that in BPH tissues (P < 0.01). The expression of CCL19 and CCR7 mRNA also exhibited different expression patterns in terms of localization depending on pathological category of PCa and metastasis (Fig. 3).
High expression of CCL19 and CCR7 in PCa cell lines
Quantitative RT-PCR was used to determine the levels of CCL19 and CCR7 mRNA in five PCa cell lines and the prostate normal epithelial cell line RWPE-1. CCL19 and CCR7 were significantly elevated in all five PCa cell lines when compared to the RWPE-1 cells at the mRNA levels (Fig. 4). CCL19/CCR7 was highly expressed in DU-145 cells, so we chose this cell line in our research.
Effects of CCL19 depletion on cell proliferation in PCa
QRT-PCR analysis of PCa cells demonstrated that the expression of CCL19 was significantly decreased after transfection with three siRNA oligos segments to varying degrees. And the CCL19-si#1 (5′-GGUACAUCGUGAGGAACUUTTAAGUUCCUCACGAUGUACCTT-3′) is the most efficient one to downregulate CCL19.
We next studied the impact of CCL19 silencing on cell proliferation in PCa. The results of the MTT assay showed that the proliferation rate of DU-145 cells in siCCL19-treated group was significantly reduced than the negative control group and cells in rhCCL19-treated groups grew more fastly than the negative control group (Fig. 5). Differences between these groups were significant (P < 0.01), and the data presented dose dependency.
Inhibition of migration and invasiveness of PCa cells by CCL19
We used the Transwell assay to verify the effect of CCL19 deletion on migration and invasion of PCa cells in vitro. The results of DU-145 cells showed that both in invasion assay and migration assay, the number of DU-145 cells that penetrated through the membrane in the si-CCL19-treated group passed out less cells than the negative control group (P < 0.01), while the number of DU-145 cells that penetrated through the membrane in the rhCCL19-treated groups was significantly more than the negative control group (P < 0.001) (Fig. 6).
Discussion
Inflammation has been reported to play a major role in prostate carcinogenesis; however, until now, the precise molecular and cellular mechanisms linking inflammation to carcinogenesis have remained unclear. Chemokines and chemokine receptors are components of cancer-related inflammatory conditions, which can promote various aspects of tumor growth. Recent studies have suggested chemokine/chemokine receptor interactions play key roles in cancer progression. Chemokines contribute to leukocyte infiltration in tumors, and some chemokines, such as IL-8, CXCL13, and CXCL5, also have potential effects on tumor growth, immunity, invasion, and metastasis [13].
CCL19 is strongly expressed in T cell zones of lymphoid organs and chemoattracts T, B, dendritic, macrophage progenitor, and NK (natural killer) cells and may be involved in the interactions of DCs and T cells in secondary lymphoid tissues. CCL19 played an anti-tumor role in some solid tumors in vivo and in vitro [14, 15]. The chemokines CCL19 and its specific receptor CCR7 would seem to have a biological significance in the development and progression of various tumors. The CCL19/CCR7 axis promotes migration, invasion, and chemotaxis of some cells, including T, B, natural killer (NK), mature dendritic (DCs), and some tumor cells [16].
Previous study has demonstrated that overexpression of CCL19 and CCR7 has been observed in a large number of malignant tumors, which was confirmed to be associated with tumor growth, invasion, and metastasis [14, 15]. However, the expression and function of CCL19/CCR7 axis in PCa remains yet to be established. In this study, we evaluated the expression and clinical relevance of CCL19 and CCR7 in a large set of prostate samples, including BPH, PIN, localized PCa, and metastatic PCa.
We first examined CCL19 and CCR7 expression in tissues from PCa and BPH patients, and our results showed that the positive rate of CCL19 staining was 87.04 % (94/108) in 108 cases of prostatic carcinoma and 16.25 % (13/80) in 80 cases of BPH, the difference of CCL19 expression between PCa and BPH was statistically significant (P < 0.001). High expression of CCR7 was observed in 83.33 % (90/108) of the PCa tissues versus (17.50 %; 14/80) of the BPH tissues, the difference of CCR7 expression between two groups was statistically significant (P < 0.001). Furthermore, CCL19 and CCR7 staining were stronger in prostatic carcinoma with metastasis than in prostatic carcinoma without metastasis. The results of qRT-PCR analysis showed that CCL19 and CCR7 mRNA level in PCa tissues revealed more than twofold increases compared with that in the BPH tissues. It suggested that the CCL19/CCR7 axis might play a role in the tumorigenesis of PCa. Additionally, the expression of CCL19 and CCR7 in five PCa cell lines and the prostate normal epithelial cell line RWPE-1 was detected by quantitative RT-PCR. Results show that CCL19 and CCR7 were significantly elevated in all five PCa cell lines when compared to the RWPE-1 cells at the mRNA levels.
To extend our clinical studies and investigate its biological function, we employed siRNA to knockdown CCL19 expression in PCa cell line DU-145. The DU-145 cell line was chosen because of its high abundance of CCL19/CCR7. Depletion of CCL19 attenuated proliferation of DU-145 cells in vitro, MTT cell proliferation assay showed that CIP2A siRNA significantly reduced the proliferation rate of DU-145 cells compared with the negative control, and the data presented dose dependency. Inhibition of CCL19 suppressed the proliferation of PCa cells, suggesting that CCL19 expression can significantly suppressed cell proliferation, migration and invasion in PCa.
Furthermore, we used the Transwell assay to verify the effect of CCL19 on migration and invasion of PCa cells in vitro. The results showed that DU-145 cells in the si-CCL19-treated group penetrated less cells through the polycarbonate membrane than the negative control group and the rhCCL19-treated group passed out more cells than the negative control group, the data also presented dose dependency. Depletion of CCL19 could inhibit cell migration and invasion in vitro, suggesting that CCL19 expression significantly promoted cell proliferation, migration, and invasion in PCa. Collectively, these data strongly suggest that CCLL10 served as a potential therapeutic target in PCa and the CCL19/CCR7 axis is a promising target for rational cancer therapy.
In conclusion, our study identified that both CCL19 and CCR7 mRNA and protein were obviously expressed in a higher degree in PCa tissues than BPH tissues. CCL19 knockdown inhibited PCa cells proliferation, invasion, and migration in a time- and dose-dependent manner. Our research indicates that the CCL19/CCR7 axis may play a significant role in the regulation of aggressiveness in human PCa. Though this mechanism is not completely clear, our study tries to reveal it in our further research. Moreover, we will also verify whether CCL19 can promote PCa tumorigenesis in vivo.
References
Ren SC, Chen R, Sun YH. Prostate cancer research in China. Asian J Androl. 2013;15:350–3.
Saad F, Pantel K. The current role of circulating tumor cells in the diagnosis and management of bone metastases in advanced prostate cancer. Future Oncol. 2012;8:321–31.
Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localized disease. Eur Urol. 2011;59(1):61–71.
Lassi K, Dawson NA. Update on castrate-resistant prostate cancer: 2010. Curr Opin Oncol. 2010;22:263–7.
Wang T, Liu Z, Guo S, Wu L, Li M, Yang J, et al. The tumor suppressive role of CAMK2N1 in castration-resistant prostate cancer. Oncotarget. 2014;5(11):3611–21.
Roussos ET, Condeelis JS, Patsialou A. Chemotaxis in cancer. Nat Rev Cancer. 2011;11:573–87.
Gao Q, Zhao YJ, Wang XY, Qiu SJ, Shi YH, Sun J, et al. CXCR6 upregulation contributes to a proinflammatory tumor microenvironment that drives metastasis and poor patient outcomes in hepatocellular carcinoma. Cancer Res. 2012;72(14):3546–56.
Maxwell PJ, Neisen J, Messenger J, Waugh DJ. Tumor-derived CXCL8 signaling augments stroma-derived CCL2-promoted proliferation and CXCL12-mediated invasion of PTEN-deficient prostate cancer cells. Oncotarget. 2014;5(13):4895–908.
Qin L, Gong C, Chen AM, Guo FJ, Xu F, Ren Y, et al. Peroxisome proliferator-activated receptor γ agonist rosiglitazone inhibits migration and invasion of prostate cancer cells through inhibition of the CXCR4/CXCL12 axis. Mol Med Rep. 2014;10(2):695–700.
Cao M, Deng HX, Zhao J, Fan LY, Jiang Y, Wen YJ, et al. Antitumour activity of cationic-liposome-conjugated adenovirus containing the CCL19 [chemokine (C-C motif) ligand 19] gene. Biotechnol Appl Biochem. 2007;48(2):109–16.
Lu J, Zhao J, Feng H, Wang P, Zhang Z, Zong Y, et al. Antitumor efficacy of CC motif chemokine ligand 19 in colorectal cancer. Dig Dis Sci. 2014 Apr 5. [Epub ahead of print]
Oliveira-Neto HH, de Souza PP, da Silva MR, Mendonça EF, Silva TA, Batista AC. The expression of chemokines CCL19, CCL21 and their receptor CCR7 in oral squamous cell carcinoma and its relevance to cervical lymph node metastasis. Tumour Biol. 2013;34(1):65–70.
Johrer K, Pleyer L, Olivier A, Maizner E, Zelle-Rieser C, Greil R. Tumour-immune cell interactions modulated by chemokines. Expert Opin Biol Ther. 2008;8:269–90.
Hamanishi J, Mandai M, Matsumura N, et al. Activated local immunity by CC chemokine ligand 19-transduced embryonic endothelial progenitor cells suppresses metastasis of murine ovarian cancer. Stem Cells. 2010;28:164–73.
Wong JL, Muthuswamy R, Bartlett DL, Kalinski P. IL-18-based combinatorial adjuvants promote the intranodal production of CCL19 by NK cells and dendritic cells of cancer patients. Oncoimmunol. 2013;2(9):e26245.
Kochetkova M, Kumar S, McColl SR. Chemokine receptors CXCR4 and CCR7 promote metastasis by preventing anoikis in cancer cells. Cell Death Differ. 2009;16:664–73.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
The Publisher and Editor retract this article in accordance with the recommendations of the Committee on Publication Ethics (COPE). After a thorough investigation we have strong reason to believe that the peer review process was compromised.
An erratum to this article is available at http://dx.doi.org/10.1007/s13277-017-5487-6.
About this article
Cite this article
Peng, C., Zhou, K., An, S. et al. RETRACTED ARTICLE: The effect of CCL19/CCR7 on the proliferation and migration of cell in prostate cancer. Tumor Biol. 36, 329–335 (2015). https://doi.org/10.1007/s13277-014-2642-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2642-1