Abstract
The purpose of this study is to determine the expression of CCL19, CCL21, and CCR7 in samples of oral squamous cell carcinoma (OSCC) and their relationship with clinical and microscopic parameters. A comparative analysis was made of the mRNA expression of these chemokines and receptor in OSCC and normal oral mucosa. The immunoexpression of CCR7, CCL19, and CCL21 was also verified in OSCC and lymph nodes. Statistical significance was accepted at P < 0.05. Similar levels of CCR7, CCL19, and CCL21 mRNA in OSCC and normal oral mucosa were seen. A low expression of CCL19 and CCL21 in the intra- and peritumoral regions was observed. Scarce CCL19+ and CCL21+ cells were also noted in metastatic and non-metastatic lymph nodes. No association was found between the expression of these chemokines and clinical and microscopic parameters. Our findings would suggest that CCL19 and CCL21 may not be associated with cervical lymph node metastasis or other clinical and microscopic factors in OSCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemokines are small chemotactic cytokines which induce the migration and activation of leukocytes [1] and have recently been implicated in the regulation of tumor growth and the organ-specific spread of different tumor cells [2–5]. These molecules work through selective membrane-bound G protein-coupled receptors, whose two major families are CCR and CXCR [1].
The chemokines CCL19 (ELC/MIP-3β) and CCL21 (SLC/6Ckine), released by lymphatic endothelial cells and T cells of the lymph nodes, act as ligands of CCR7 [5]. In various malignant diseases, including breast, pancreatic, and head and neck cancers [6–8], the expression of CCR7 in cancer cells would seem to be related to tumor cell capacity to establish lymph node metastasis Moreover, it has also been suggested that the CCR7 receptor, activated by CCL19 and CCL21, is involved in other events related to tumorigenesis, such as tumor cell proliferation [9], tumor cell surveillance [10], adhesion [11], migration [12], invasion [13], and angiogenesis [14].
Oral squamous cell carcinoma (OSCC) is characterized by a substantial degree of local invasion and an elevated rate of metastasis to the cervical lymph nodes, which directly affects prognosis [15, 16]. In this respect, studies undertaken by our group have shown a positive association between cervical lymph node metastasis and the expression of chemokines and their specific receptors in OSCC [17–19]. One of our recent studies has shown a positive association between the stromal cell-derived factor-1 (CXCL12) and its specific receptor, CXCR4, and the spread of tumor cells to the cervical lymph nodes in patients with OSCC [18].
In head and neck cancer and in OSCC, certain studies have indicated an expression of CCR7 in tumor cells and a positive association between this receptor and cervical lymph node metastasis [8, 9, 11, 20]. In addition, the CCR7/CCL19/CCL21 axis may be involved in the advanced clinical stage of the disease, as well as tumor relapse and death, thus contributing to a more negative prognosis in patients with OSCC [9].
The chemokines CCL19 and CCL21 and their specific receptor CCR7 would seem to have a biological significance in the development and progression of OSCC [8, 9, 11, 20]. Some studies have demonstrated a positive association between the CCR7 expression and regional metastasis in patients with oral cancer [8, 9, 11, 20]. However, for this disease data on CCL19 and CCL21 are lacking, in particular on the association between these chemokines and clinical factors. So, against this background, this study set out to investigate the expression of CCR7, CCL19, and CCL21 and the relationship between these proteins and the clinicopathological factors of OSCC.
Materials and methods
This study was approved by the Institute’s Research Ethics Committee for human subjects.
Quantitative reverse transcription–polymerase chain reaction
Samples were taken from 20 patients with primary OSCC (12 males and 8 females, aged between 42 and 85 with a median age of 60.8 years) in the Goiás State Oral Disease Center at the Federal University of Goiás Dental School. Six samples of clinically healthy gingival mucosa, collected during third molar extraction, were used as the control group. The samples were divided into two equal parts. Half of each specimen was immersed in Trizol reagent (Life Technologies, Grand Island, NY, USA) and stored at −80 °C (MDF-C8V, Sanyo Scientific, USA). The other half was fixed in neutral buffered formalin, embedded by routine technique in paraffin wax, and cut into 5-μm sections for hematoxylin and eosin staining to confirm the diagnosis of OSCC.
Total RNA was extracted from the samples using a Trizol reagent, in accordance with the manufacturer’s protocol (Invitrogen Corp.). The quantity and purity of total RNA were determined on a BioPhotometer (Eppendorf, Hamburg, Germany) by evaluating absorbance at 260 nm and the 260:280 nm ratio, respectively. Complementary DNA was synthesized by reverse transcription of 400 ng of total RNA using oligo (dT) as primers (High Capacity cDNA synthesis kit, Applied Biosystems, Warrington, UK). A real-time polymerase chain reaction (qPCR) was performed using a StepOne thermocycler (Applied Biosystems). The reaction included 1 μL of the RT reaction product in a 20-μL total volume PCR reaction mix which included 8 μL of nuclease-free water, 10 μL of TaqMan qPCR master mix, and 1 μL of TaqMan gene expression assays, including forward and reverse primers, as well as the fluorophore-conjugated probe (Applied Biosystems) for human genes (Table 1). The optimized thermal cycling conditions were: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. For each sample, analyses of gene expression were performed in duplicate. The experiments were performed with three different samples in each experimental group. For all genes, primers and probes spanning exon boundaries were selected to avoid amplification of contaminating genomic DNA. To determine the relative levels of gene expression, the relative standard curve method (User Bulletin #2, Applied Biosystems) was used and normalized to the housekeeping gene β-actin. The relative mRNA expression was arbitrarily set to 100 % for gingival mucosa.
Immunohistochemistry
Samples from 54 patients with primary OSCC were obtained from the files of the Anatomopathology and Cytopathology Division of the Araújo Jorge Hospital, Goiás Combat Cancer Association, Goiânia, Brazil. Samples of lymph nodes (n = 30) were also taken from the patients with oral tumors but without cervical lymph node metastasis (negative). Samples were obtained of both the lymph node with metastasis (positive) and the lymph node without metastasis (negative) from the patients with cervical lymph node metastasis. All the patients with oral tumors had been admitted for surgical treatment, and none had undergone radiotherapy, chemotherapy, or any other treatment prior to surgery. Clinical data (gender, age, ethnicity, tobacco and alcohol consumption, tumor location, extension, T and N stages) and follow-up information (clinical outcome and survival time) were obtained from medical records. The inclusion criteria stipulated for this study were: patients with oral cavity SCC, of either gender, over 35 years of age, T2/T3 primary tumor size, and a minimum follow-up of 48 months. The exclusion criteria were patients with SCC at other sites, people without any clinical history, and those who had undergone radiotherapy, chemotherapy, or any other treatment prior to surgery.
All specimens were fixed in 10 % buffered formalin (pH 7.4) and paraffin embedded. The microscopic features were evaluated from the analysis of one 5-μm section of each sample, stained routinely with hematoxylin and eosin. All of the SCC sections were graded according to WHO tumor classification [21]. All the sections were examined by light microscopy to confirm the presence or absence of lymph node metastasis and to characterize the OSCC.
Paraffin-embedded tissues were sectioned (3 μm) and collected in serial sections on glass slides coated with 2 % 3-aminopropyltriethylsilane (Sigma–Aldrich, St. Louis, MO). The immunohistochemistry reaction was conducted as previously described [18]. The following primary antibodies were used: polyclonal goat anti-human CCL19 (N-18; sc-9777, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 1:100; polyclonal goat anti-human CCL21 (C-15; sc-5808, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 1:100; monoclonal mouse anti-human CCR7 (IMG-71209, Imgenex, San Diego, CA, USA) at 1:100, monoclonal mouse anti-human Ki-67 (MM1; Novocastra, Newcastle, UK) at 1:100; monoclonal mouse anti-human bcl-2 (clone 124, DAKO, Glostrup, Denmark) at 1:500; and polyclonal rabbit anti-human Bax (clone A3533, DAKO, Glostrup, Denmark) at 1:500, at 4 °C overnight in a humidified chamber.
After washing in TBS, the sections were treated using a labeled streptavidin–biotin kit (K0690, DAKO, Carpinteria, CA). The subsequent steps were also performed as previously described [18]. Negative controls were obtained by omitting primary antibodies, which were substituted by 1 % PBS-BSA and by non-immune rabbit (X0902, Dako) or mouse (X501-1, Dako) serum. The external positive control for CCR7, CCL19, and CCL21 was lymphocytes of archived amygdala samples.
Cell counting and statistical analysis
A quantitative analysis was performed to assay the immunoexpression of CCR7 by neoplastic and stromal cells. The percentage of CCL19+ and CCL21+ cells in the intratumoral and peritumoral regions was determined. In the lymph node samples, the numbers of CCL19+ and CCL21+ nodal cells were calculated per square millimeter. All counts were performed in ten alternate microscopic high-power fields (×400) using an integration graticule (4740680000000-Netzmikrometer 12.5x, Carl Zeiss, Göttingen, Germany).
Tumor proliferation (Ki-67+ neoplastic cells) and regulatory apoptotic proteins (bcl-2+ and Bax+ neoplastic cells) were assessed by calculating the proportion of positive cells to the total neoplastic cell population at the OSCC invasion front. The comparative analyses between experimental groups were performed using the nonparametric Kruskal–Wallis, followed by the Dunn test, and/or the Mann–Whitney test. A comparative analysis of the number of CCL19+ and CCL21+ nodal cells per square millimeter between metastatic and non-metastatic lymph nodes in the same patients was performed using the parametric Wilcoxon signed-rank test.
In addition, comparative analyses between the percentages of CCL19+ and CCL21+ neoplastic and stromal cells with clinical (cervical lymph node metastasis) and microscopic characteristics (proliferation index, measured by the proportion of neoplastic cells with Ki-67 antibodies; and apoptosis index, measured by the proportion of bcl-2+ and Bax+ neoplastic cells) were calculated by the nonparametric Mann–Whitney test, and the values were dichotomized by the median value.
The influence of tumor-associated CCL19 and CCL21 on the prognosis of OSCC patients was evaluated by the Kaplan–Meier test. Survival time was calculated from the moment of surgical resection to a patient’s last follow-up appointment or death. CCL19 and CCL21 were dichotomized by their median values, and differences in survival between the groups were evaluated by the log rank test. Significance was set at 0.05.
Results
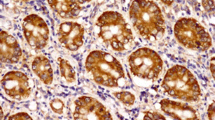
Assessment of the mRNA expression revealed similar levels of CCR7, CCL19, and CCL21 in both the OSCC (n = 20) and control groups (n = 6; P > 0.05 for all genes analyzed; Fig. 1). In relation to CCR7, CCL19, and CCL21 protein expression, in all the OSCC samples, neoplastic cells displayed a high expression of CCR7 (>90 %; Fig. 2). However, only a few CCR7+ inflammatory cells were observed in the peritumoral region (Fig. 2). Our results also presented a low expression of CCL19 and CCL21 in the intra- and peritumoral regions of primary tumors. Furthermore, similar percentages of CCL19+ and CCL21+ neoplastic and stromal cells were found for the groups of primary OSCC with and without lymph node metastasis (P > 0.05; Table 2).
Expressions of CCR7, CCL19, and CCL21 in clinically healthy gingival mucosa (Control; white bars) and oral squamous cell carcinoma (gray bars). Quantitative reverse transcription–polymerase chain reaction showed no differences in gene expression between control and OSCC samples. mRNA expression was normalized against the expression of the housekeeping gene β-actin. The bars represent mean and the vertical lines the standard deviation of the mean of the samples in each experimental group
When evaluating the lymph node tissues, a small number of CCL19+ and CCL21+ lymph nodal cells in metastatic and non-metastatic lymph nodes was observed (Fig. 3). However, the density of CCL19+ lymph nodal cells was significantly higher in non-metastatic than in the metastatic lymph nodes of different patients (P = 0.02).
The main clinical and microscopic features of our series of 54 OSCC patients are summarized in Table 3. No association was found between the percentages of CCL19+ and CCL21+ neoplastic and stromal cells and other microscopic features, such as proliferation index, proportion of bcl2+ neoplastic cells, and proportion of Bax+ neoplastic cells (P > 0.05 for all evaluations).
As regards the last follow-up appointment, mean survival time was 45.28 months (95 % CI = 23.50–67.06). A log-rank test showed no difference in survival time between the CCL19 and CC21 groups (P = 0.90/P = 0.57 and P = 0.65/P = 0.72, respectively, for intra-/peritumoral regions).
Discussion
Malignant cells that can metastasize to a specific organ may have various properties supporting their tissue invasion or growth, such as enhanced adherence to microvascular cells, higher responsiveness to chemotactic signals released from the target organs, and increased affinity of the specific receptor to soluble or tissue-associated growth signals in the target organ [1, 3, 5]. There is some evidence that the chemokine receptor CCR7 and its ligands CCL19 and CCL21 are involved in the directional migration of neoplastic cells to regional lymph nodes in many types of cancers [6, 7, 10, 12, 13]. Recent studies observed a positive relationship between CCR7 expression in neoplastic cells and cervical metastasis in OSCC [9, 11]. High levels of CCL19 and CCL21 have been demonstrated in cervical lymph nodes that present metastatic CCR7+ neoplastic cells in head and neck cancer [20] and OSCC [11].
In this study, on the other hand, although an elevated percentage of CCR7+ neoplastic cells was seen in all the OSCC samples (irrespective of whether the patient developed cervical metastasis or not), a low expression of its ligands CCL19 and CCL21 in the microenvironment of the primary tumor and lymph nodes was noted. Additionally, similar mRNA levels of CCR7, CCL19, and CCL21 in both primary OSCC and control tissues were found. Thus, considering that CCR7+ SCC cells migrate toward CCL21 and CCL19 in a dose-dependent manner [8, 11], it can be suggested that in our OSCC cases the chemotactic gradient in the local microenvironment and lymph node tissues was not sufficient to stimulate cancer cell migration.
These divergent findings can be explained by differences in disease outcome, tumor location and parameters analyzed. Also, despite the fact that all previous studies have used immunohistochemistry there are differences in methods of cell labeling and quantification. In this context, it is important to note that this study was the first to investigate CCL19 and CCL21 expressions separately in the parenchyma and stroma of primary tumors, and evaluate the relationship between the CCL21 and CCL19 immunoexpression and regional metastasis, patient survival, proliferation/apoptotic index, and other clinical and microscopic factors in oral cavity SCC.
There is some evidence supporting the relationship between CCR7 and tumor size in OSCC [9, 11] since this receptor may be involved in tumor cell proliferation [9] and neoplastic cell surveillance through apoptosis inhibition [8]. Wang et al. demonstrated the influence of the CCL19 and CCL21 expressions on tumor growth in head and neck SCC since CCL19 exercised an antiapoptotic effect and CCL21 induced tumor cell growth in a paracrine manner [8]. However, in this study, no association was found between the expression of CCL19/CCL21 and the regulatory apoptotic proteins and proliferation index. Thus, considering that the secretion of both CCL19 and CCL21 by SCC cells and by paracrine sources can combine to promote CCR7 activation in neoplastic cells [8], we suggest that the chemokine expression was insufficient to stimulate this receptor in this study. Additionally, no association was found between the percentages of CCL19+ and CCL21+ neoplastic and stromal cells and other clinical features, such as the TNM stage and survival rate.
Although our results showed few CCL19+ and CCL21+ in lymph nodes (irrespective of whether metastatic or not), the density of the CCL19+ lymph nodal cells was higher in non-metastatic lymph nodes than in metastatic lymph nodes of different patients. Interestingly, it has been demonstrated by our group that the same non-metastatic lymph nodes present a higher density of activated cytotoxic T lymphocytes (CD8+/Perforin+) than metastatic lymph nodes (data not shown). Based on this fact, it could be suggested that the chemokine CCL19 may be contributing to the host defense against cancer progression since T CD8+ lymphocytes express CCR7 receptor [5] and are probably being attracted to these lymph node areas. However, further studies would be needed to confirm this hypothesis.
In this study, although we had observed an elevated percentage of CCR7+ neoplastic cells, there was a low expression of CCR7+ ligands in the lymph nodes. It could be concluded from this that the chemokines CCL19 and CCL21 might not be involved in the establishment of cervical lymph node metastasis in OSCC. On the other hand, earlier results of our group demonstrated strong evidence that the CXCR4/CXCL12 axis is related to cervical metastasis and neoplastic cell proliferation in OSCC [18]. Thus, taking into account our previous results and these present findings, we postulate that the CXCR4/CXCL12 axis, but not the CCR7/CCL21/CCL19, is an activation pathway involved in the establishment of cervical lymph node metastasis in OSCC.
References
Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi:10.1146/annurev.immunol.18.1.217.
Arya M, Patel HR, Williamson M. Chemokines: key players in cancer. Curr Med Res Opin. 2003;19:557–64. doi:10.1185/030079903125002216.
Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–50. doi:10.1038/nrc1388.
Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–65. doi:10.1016/j.canlet.2007.05.013.
Zlotnik A. Chemokines in neoplastic progression. Sem Cancer Biol. 2004;14:181–5. doi:10.1016/j.semcancer.2003.10.004.
Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi:10.1038/35065016.
Nakata B, Fukunaga S, Noda E, Amano R, Yamada N, Hirakawa K. Chemokine receptor CCR7 expression correlates with lymph node metastasis in pancreatic cancer. Oncology. 2008;74:69–75. doi:10.1159/000139126.
Wang J, Seethala RR, Zhang Q, Gooding W, van Waes C, Hasegawa H, et al. Autocrine and paracrine chemokine receptor 7 activation in head and neck cancer: implications for therapy. J Natl Cancer Inst. 2008;100:502–12. doi:10.1093/jnci/djn059.
Tsuzuki H, Takahashi N, Kojima A, Narita N, Sunaga H, Takabayashi T, et al. Oral and oropharyngeal squamous cell carcinomas expressing CCR7 have poor prognoses. Auris Nasus Larynx. 2006;33:37–42. doi:10.1016\j.anl.2005.07.019.
Kochetkova M, Kumar S, McColl SR. Chemokine receptors CXCR4 and CCR7 promote metastasis by preventing anoikis in cancer cells. Cell Death Differ. 2009;16:664–73. doi:10.1038/cdd.2008.190.
Shang ZJ, Liu K, Shao Z. Expression of chemokine receptor CCR7 is associated with cervical lymph node metastasis of oral squamous cell carcinoma. Oral Oncol. 2009;45:480–5. doi:10.1016\j.oraloncology.2008.06.005.
Ding Y, Shimada Y, Maeda M, Kawabe A, Kaganoi J, Komoto I, et al. (2003) Association of CC chemokine receptor 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clin Cancer Res 9:3406–3412. http://clincancerres.aacrjournals.org/content/9/9/3406
Wagner PL, Moo TA, Arora N, Liu YF, Zarnegar R, Scognamiglio T, et al. The chemokine receptors CXCR4 and CCR7 are associated with tumor size and pathologic indicators of tumor aggressiveness in papillary thyroid carcinoma. Ann Surg Oncol. 2008;15:2833–41. doi:10.1245/s10434-008-0064-2.
Brühl H, Mack M, Niedermeier M, Lochbaum D, Schölmerich J, Straub RH. Functional expression of the chemokine receptor CCR7 on fibroblast-like synoviocytes. Rheumatology (Oxford). 2008;47:1771–4. doi:10.1093/rheumatology/ken383.
Almofti A, Uchida D, Begum NM, Tomizuka Y, Iga H, Yoshida H, et al. The clinicopathological significance of the expression of CXCR4 protein in oral squamous cell carcinoma. Int J Oncol. 2004;25:65–71.
Uchida D, Begum NM, Almofti A, Nakashiro K, Kawamata H, Tateishi Y, et al. Possible role of stromal-cell-derived factor-1/CXCR4 signaling on lymph node metastasis of oral squamous cell carcinoma. Exp Cell Res. 2003;290:289–302. doi:10.1016\S0014-4827(03)00344-6.
Silva TA, Ribeiro FLL, Oliveira-Neto HH, Watanabe S, Alencar RCG, Fukada S, et al. Dual role of CCL3/CCR1 in oral squamous cell carcinoma: implications in tumour metastasis and local host defense. Oncol Rep. 2007;18:1107–13.
Oliveira-Neto HH, Silva ET, Leles CR, Mendonça EF, Alencar RC, Silva TA, et al. Involvement of CXCL12 and CXCR4 in lymph node metastases and development of oral squamous cell carcinomas. Tumor Biol. 2008;29:262–71. doi:10.1159/000152944.
Ferreira FO, Ribeiro FLL, Batista AC, Leles CR, Alencar RCG, Silva TA. Association of CCL2 with lymph node metastasis and macrophage infiltration in oral cavity and lip squamous cell carcinoma. Tumor Biol. 2008;29:114–21. doi:10.1159/000137669.
Muller A, Sonkoly E, Eulert C, Gerber PA, Kubitza R, Schirlau K, et al. Chemokine receptors in head and neck cancer: association with metastatic spread and regulation during chemotherapy. Int J Cancer. 2006;118:2147–57. doi:10.1002/ijc.21514.
Pindborg JJ, et al. Histological typing of cancer and precancer of the oral mucosa. World Health Organization Classification of Tumours. 2nd ed. Berlin: Springer; 1997. p. 21–31.
Acknowledgments
The authors wish to thank the Araújo Jorge Hospital of the Goiás Combat Cancer Association, Goiânia and the Department of Physiology and Pathology, Dental School, São Paulo State University, Araraquara, São Paulo, Brazil. This work was supported by grants from the National Council for Scientific and Technological Development (CNPq) (grants 470951/2009-5 and 554445/2009-3). Drs. Batista, Silva, and Mendonça are CNPq Research Fellows.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oliveira-Neto, H.H., de Souza, P.P.C., da Silva, M.R.B. et al. The expression of chemokines CCL19, CCL21 and their receptor CCR7 in oral squamous cell carcinoma and its relevance to cervical lymph node metastasis. Tumor Biol. 34, 65–70 (2013). https://doi.org/10.1007/s13277-012-0511-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-012-0511-3