Abstract
Background
Silibinin, a flavonolignan, is known to have a variety of pharmacological activities, including antioxidant activity, but its antioxidant mechanism in the eye is unclear.
Objective
This study aimed to evaluate whether silibinin could protect human retinal pigment epithelial ARPE-19 cells from oxidative injury.
Results
Silibinin attenuated cell viability reduction and DNA damage in ARPE-19 cells treated with hydrogen peroxide (H2O2), while inhibiting intracellular reactive oxygen species (ROS) production and preserving diminished glutathione (GSH). Silibinin also antagonized H2O2-induced inhibition of the expression and activity of antioxidant enzymes, such as GSH peroxidase and manganese superoxide dismutase, which was associated with inhibition of mitochondrial ROS production. Moreover, silibinin rescued ARPE-19 cells from H2O2-induced apoptosis by restoring the reduced Bcl-2/Bax ratio and reducing caspase-3 activation. In addition, silibinin suppressed the release of mitochondrial cytochrome c into the cytoplasm, which was achieved by interfering with mitochondrial membrane disruption.
Conclusion
These findings imply that silibinin has potent ROS scavenging activity with the potential to protect against oxidative stress-mediated ocular diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative stress due to impairment of antioxidant defense systems and/or excessive accumulation of reactive oxygen species (ROS) acts as a cause of damage to intracellular biomolecules and organelles. Cells exposed to continuous oxidative stress ultimately result in cell death accompanied by mitochondrial dysfunction and DNA damage (Chaudhary et al. 2023; Zia et al. 2022). Eyes, which undergo active oxidative metabolism to form vision, are constantly exposed to solar radiation and are vulnerable to oxidative stress. Accumulating evidence has shown that oxidative stress has been implicated in the pathogenesis of various eye disorders, including age-related macular degeneration (AMD) and dry eye (Dammak et al. 2023; Hsueh et al. 2022). In addition, it has been demonstrated that ROS act as important executors in the initiation of mitochondria-dependent apoptosis. Therefore, improving mitochondrial function while increasing antioxidant potential may be a promising strategy for protection against oxidative stress-mediated eye diseases (Zia et al. 2022; Demine et al. 2019). To this end, interest is growing in using natural products to discover ideal drugs that can overcome oxidative stress without side effects.
Silybum marianum (L.) Gaertn., also known as milk thistle, belongs to the Asteraceae family and has been used as a remedy for various ailments, especially for treating hepatic diseases such as cirrhosis and hepatitis and protecting the liver from toxic substances (Nawaz et al. 2023; Wang et al. 2020). These pharmacological effects are closely related to a complex of polyphenolic flavonoids called silymarin, first discovered in the achenes of this plant (Nawaz et al. 2023; Wadhwa et al. 2022). The potential of silymarin as an antioxidant comes from its ability to scavenge small molecule free radicals (Nawaz et al. 2023; Wang et al. 2020). Silibinin, a natural flavonolignan also called silybin, is one of the major and most active flavonoids identified in the silymarin complex (Nawaz et al. 2023; Islam et al. 2021). Accumulated research has proven that silibinin possesses a wide range of pharmacological activities, including antidiabetic, cardioprotective, antihepatotoxic, anti-inflammatory, neuroprotective, antiviral and anticarcinogenic effects (Singh et al. 2023; Křen and Valentová 2022; Takke and Shende 2019). In particular, the improvement of chronic diseases by silibinin is closely related to its antioxidant activity by eliminating ROS through the regulation of antioxidant signaling pathways. For example, alleviation of oxidative stress-mediated mitochondrial impairment and apoptosis by silibinin could be achieved by blocking ROS production through restoring activity of intracellular antioxidant enzymes (Li et al. 2023; Chen et al. 2020). Silibinin could also successfully reverse nephrotoxicity and hepatotoxicity by scavenging free radicals and increasing intracellular antioxidant defenses in cisplatin-induced acute kidney injury model and liver tissue of itraconazole-treated rats, respectively, thereby protecting renal and hepatic cells from apoptosis (Yang et al. 2022; Sozen et al. 2015). Mao et al. (2018) have reported that the anti-apoptotic effect of silibinin in advanced glycation end product (AGE)-treated osteoblasts was due to inhibition of mitochondrial ROS (mtROS) generation by blocking AGE receptor-dependent mitochondrial dysfunction. In addition, silibinin exhibited anti-aging effects by suppressing oxidative and inflammatory properties in hydrogen peroxide (H2O2)-treated embryonic fibroblast cells (Baeeri et al. 2018). Guo et al. (2020) also demonstrated that the protective capacity of silibinin against H2O2-induced apoptosis in trophoblast cells was related to the enhanced mitochondrial membrane potential (MMP). Similar to their results, the neuroprotective effect of silibinin against H2O2 in neuroblastoma cells was found to be associated with reduced mitochondrial damage and inhibition of ROS generation by preserving catalase and superoxide dismutase (SOD) levels (Tie et al. 2022).
Recently, Pooja et al. (2022) showed that silibinin was able to inhibit epithelial-mesenchymal transition and excessive proliferation of lens epithelial cells, which is the key process of posterior lens opacification. In addition, it has been shown that silibinin had a protective effect against acute retinal ganglion cell damage caused by blue light and prevented corneal damage caused by nitrogen mustard (Shen et al. 2019; Goswami et al. 2018). Additionally, silibinin had a preventive effect against endotoxin-induced uveitis in human retinal pigment epithelial (RPE) cells and counteracted retinal edema and angiogenesis in a hypoxia-induced AMD model using RPE cells (Chen et al. 2017; Lin et al. 2013). These studies indicate that silibinin has potent preventive and therapeutic effects against various eye diseases, but the underlying mechanism of silibinin for its antioxidant activity is not fully elucidated. Therefore, this study focused on the antioxidant properties of silibinin under conditions mimicking the oxidative environment induced by H2O2 exposure using human RPE ARPE-19 cells.
Materials and methods
Cell culture
ARPE-19 cells were cultured according to a previously described method (Park et al. 2023). Silibinin and H2O2 (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich) to prepare stock solutions, which were then diluted to appropriate concentrations in culture medium for treating cells. To trigger oxidative stress, ARPE-19 cells were cultured in medium containing H2O2. Silibinin, N-acetyl-L-cysteine (NAC) and Mito-TEMPO (ZnPP, Thermo Fisher Scientific, Waltham, MA, USA) were added at 1 h before H2O2 exposure. The final concentration of DMSO diluted in the culture medium did not exceed 0.05%, which did not show significant toxicity to ARPE-19 cells.
Assessment of cytotoxicity
To evaluate the cytotoxicity of ARPE-19 cells stimulated with H2O2 in the presence or absence of silibinin, cell viability was estimated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay following the method of Cao et al. (2023). After treatment, morphological changes of cells were observed using an optical microscope (Carl Zeiss, Oberkochen, Germany). In addition, the degree of lactate dehydrogenase (LDH) release into the culture medium for cells cultured under the same conditions was quantitatively compared using the LDH Assay kit (Abcam, Inc., Cambridge, UK).
Comet assay
To investigate the degree of DNA damage, a Comet Assay kit (Trevigen, Inc., Gaithersburg, MD, USA) was used. In brief, cells were mixed in a low melting-point agarose solution, spread evenly on comet slides according to the manufacturer's instructions. Electrophoresis was then performed and cells were immediately stained with the fluorescent dye provided in the kit, followed by visualization of fluorescent images with a fluorescence microscope (Carl Zeiss).
Immunostaining for analysis of phosphorylation of histone H2AX (γH2AX) expression
To assess the expression level of γH2AX, cells were exposed to H2O2 in the presence or absence of silibinin, fixed, and then permeabilized (Park et al. 2023). Cells were then probed with an anti-γH2AX antibody (Thermo Fisher Scientific) and reacted with Alexa Fluor 594-conjugated secondary antibody (Sigma-Aldrich). After staining the nuclei using 4',6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich), images were observed under a fluorescence microscope. γH2AX-stained nuclei are red-fluorescent, and DAPI-stained nuclei are blue-fluorescent.
8-Hydroxyguanosine (8-OHdG) assay
To analyze the degree of oxidative DNA damage, the level of intracellular 8-OHdG was evaluated using the 8-OHdG ELISA Kit (Abcam, Inc.) according to the manufacturer’s instructions. In brief, DNA isolated from H2O2-treated cells with or without silibinin was digested using DNA Digestion Mix and the level of 8-OHdG was measured with a competitive enzyme immunoassay. The optical density (OP) of each treatment group at 450 nm was recorded. Results are expressed as ng of 8-OHdG/mL.
Quantitative analysis of apoptosis
The frequency of apoptotic cells was determined by flow cytometry using the Annexin V-Fluorescein Isothiocyanate (FITC)/Propidium Iodide (PI) Apoptosis Staining/Detection kit (Abcam, Inc.). In brief, collected cells were double-stained with annexin V and PI in the dark according to the manufacturer's protocol. After staining, annexin V-positive cells were regarded as apoptosis-induced cells using a flow cytometer (BD Biosciences, San Jose, CA, USA).
Protein isolation and immunoblotting
To examine expression levels of proteins of interest using immunoblotting, total cellular proteins were isolated using RIPA lysis buffer (Cell Signaling Technology, Beverly, MA, USA) according to the manufacturer’s experimental protocol. Mitochondrial and cytoplasmic fractions were extracted using a Mitochondrial Fractionation Kit (Thermo Fisher Scientific). After protein quantification, immunoblotting was performed according to the method described previously (Mukherjee et al. 2022). Cytochrome c oxidase IV (COX IV) and β-actin were used as housekeeping proteins for mitochondrial and cytoplasmic fractions, respectively. Antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), Cell Signaling Technology, and Abcam, Inc. (Table 1).
Caspase-3 activity
Caspase-3 activity in cells exposed to H2O2 with or without silibinin was measured using the Caspase-3 Assay Kit (Thermo Fisher Scientific) based on the spectrophotometric detection of p-nitroaniline (p-NA) liberated from the substrate of caspase-3 (N-acetyl-Asp-Glu-Val-Asp- p-NA). According to the manufacturer's instructions, cell lysates were obtained using reagents provided in the kit. The OP of free p-NA was then quantified at 405 nm. Based on OP values, caspase-3 activity in each treatment group was determined relative to that of the untreated control group.
MMP assay
To determine mitochondrial activity, MMP was measured using 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) dye (Abcam, Inc.). The fluorescence intensity of JC-1 monomeric and aggregate forms was examined by flow cytometry according to the manufacturer's protocol. Percentages of JC-1 monomers were expressed to represent cells that lost MMP.
Intracellular ROS production analysis
To analyze the scavenging activity of silibinin on intracellular ROS levels induced by H2O2, cells treated with H2O2 for 1 h in the presence or absence of silibinin were stained with 2’,7’-dichlorofluorescein diacetate (DCF-DA, Sigma-Aldrich). According to the manufacturer's protocols, the levels of ROS were quantitatively evaluated by DCF-fluorescence representing the percentage of control cells through flow cytometry (Sukjamnong et al. 2022). Fluorescence images were also taken with a fluorescence microscope to visually detect the difference in emitted DCF fluorescence intensity.
Evaluation of GSH/GSH disulfide (GSSG) ratio
The difference in the ratio of reduced GSH to oxidized GSSG in cells exposed to H2O2 for 24 h with or without silibinin was quantified using the GSH/GSSG Assay kit (Abcam, Inc.). In brief, after reacting cells of each treatment group under conditions recommended by the manufacturer, levels of GSH and GSSG were determined based on standard curves of GSH and GSSG. GSH/GSSG ratio was presented as a relative value to the control group.
Measurement of the activities of GSH peroxidase (GPx) and manganese (MnSOD)
Colorimetric kits purchased from Abcam, Inc. were used to measure the activities of GPx and MnSOD. In brief, fractions of cells exposed to H2O2 for 24 h with or without silibinin were prepared according to the manufacturer's recommendations and the activity of each enzyme was presented as relative values to the control.
MitoTracker Red assay
To investigate the effect of silibinin on inhibition of mitochondrial activity by H2O2 treatment, MitoTracker™ Red (Molecular Probes, Eugene, OG, USA) staining was performed. According to the manufacturer’s recommendations, cells were stained with MitoTracker Red after pretreatment with silibinin for 1 h and then exposed to H2O2 for 3 h. After staining, the nuclei were stained with DAPI and the fluorescence images were acquired using a fluorescence microscope.
Measurement of mitochondrial superoxide levels
To examine the effect of silibinin on mitochondrial peroxide production by H2O2 using MitoSOX™ Red (Thermo Fisher Scientific), cells cultured for 1 h in medium with or without silibinin were treated with H2O2 for 1 h. Briefly, after staining cells with MitoSOX according to the method suggested by the manufacturer, the emitted fluorescence of MitoSOX was detected by flow cytometry. In addition, cells were stained with MitoSOX and then additionally stained with DAPI, and the fluorescence images were compared using a fluorescence microscope.
Statistical analysis
Data are expressed as mean ± standard deviation (SD). Statistical comparisons were performed using GraphPad Prism version 8.0 (GraphPad Inc., San Diego, CA, USA). Statistical significance was set at p < 0.05.
Results
Inhibition of H2O2-induced cytotoxicity by silibinin in ARPE-19 cells
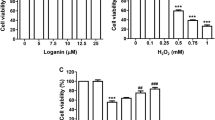
MTT assay was performed to investigate the inhibitory effect of silibinin on H2O2-induced cytotoxicity in ARPE-19 cells. MTT assay results showed that silibinin decreased cell viability in a concentration-dependent manner when the treatment concentration was above 30 μM, but there was no significant change compared to the control group when the treatment concentration was 20 μM or less (Fig. 1A). Therefore, the optimal pretreatment concentration to examine the inhibitory effect of silibinin on H2O2-induced cytotoxicity was selected as 20 μM or less. The treatment concentration of H2O2 to mimic oxidative damage was set at 0.5 mM, which showed a survival rate of about 60% compared to the control group, referring to previous studies (Park et al. 2023). As presented in Fig. 1B, silibinin pretreatment at concentrations of 10 μM and 20 μM increased the decrease in cell viability caused by H2O2 treatment to approximately 70% and 82%, respectively. To further investigate the cytotoxic protective effect of silibinin, LDH leakage levels were measured and results showed that silibinin pretreatment significantly attenuated the H2O2-induced increase in LDH leakage levels (Fig. 1C). In addition, cells cultured in normal medium were uniform in size and tightly arranged, but as the size after H2O2 treatment became more diverse, the shape became irregular and adhesion was lost. However, in the presence of silibinin, the morphology of cells treated with H2O2 was clearly improved as showed in Fig. 1D. These results clearly indicate that silibinin had a cytoprotective effect against oxidative stress in ARPE-19 cells.
Suppression of H2O2-induced cytotoxicity by silibinin in ARPE-19 cells. A and B Results of MTT assay after treating cells with various concentrations of silibinin for 24 h A or pre-treating cells with silibinin (10 μM and 20 μM) for 1 h and then treating them with 0.5 mM H2O2 for 24 h B. C LDH release from cells cultured under the indicated conditions was measured in the supernatant. ***p < 0.001 vs. control group; ##p < 0.01 and ### p < 0.001 vs. H2O2 treatment group. D Morphological images of cells cultured in H2O2-treated medium with or without silibinin
Protection of H2O2-induced DNA damage by silibinin in ARPE-19 cells
To investigate whether the blocking efficacy of silibinin against H2O2-induced cytotoxicity in ARPE-19 cells was related to attenuation of DNA damage, it was first evaluated the inhibitory effect of silibinin on H2O2-induced comet tail formation. Compared to the control group, H2O2 treatment induced DNA damage, as evidenced by an increase in tail length, indicating double-strand breaks in DNA, but this was clearly reduced in cells pretreated with silibinin (Fig. 2A). To provide additional evidence for the inhibition of H2O2-mediated DNA damage by silibinin, the expression of γH2AX, another indicator of double-strand breakage of DNA, and the level of 8-OHdG, a biomarker of oxidative guanine base damage, were further evaluated. As shown in Fig. 2B and C, silibinin pretreatment remarkably blocked the increase in γH2AX fluorescence intensity in the nucleus and 8-OHdG content caused by H2O2 treatment, demonstrating that silibinin was able to block DNA damage caused by oxidative stimulation.
Protection of H2O2-induced DNA damage by silibinin in ARPE-19 cells. Before treating cells with 0.5 mM H2O2 for 24 h, they were incubated in the presence or absence of 20 μM silibinin for 1 h. After treatment, the inhibitory effect of silibinin on H2O2-induced DNA damage was determined. A Representative images of comet assay were presented. B Representative immunofluorescence images stained with γH2AX (red) were taken under a fluorescence microscope. Nuclei were stained with DAPI (blue). C The amounts of 8-OHdG in culture supernatants were measured using an 8-OHdG assay kit. *** p < 0.001 vs. control group; ###p < 0.01 vs. H2O2 treatment group
Inhibition of H2O2-induced apoptosis by silibinin in ARPE-19 cells
To determine whether silibinin inhibited H2O2-induced apoptosis of ARPE-19 cells, flow cytometry analysis was performed after staining with Annexin V/PI. As shown in Fig. 3A and B, apoptosis was greatly increased in H2O2-treated cells compared to the control group. However, it was largely abolished by silibinin pretreatment. Immunoblotting was then applied to assess the effect of silibinin on the expression changes of key apoptosis regulatory factors in H2O2-treated cells. As depicted in Fig. 3C, H2O2 treatment markedly reduced the expression level of Bcl-2, which promotes cell survival, compared to untreated cells, but slightly increased the protein level of Bax, which promotes apoptosis. However, the effect of H2O2 treatment on Bcl-2 family members was mitigated in the presence of silibinin. And as shown in Fig. 3C and D, as the expression level of pro-caspase-3 protein was greatly reduced by H2O2 administration, its activity increased significantly and cleavage of poly(ADP-ribose) polymerase (PARP), a substrate protein of caspase-3, was induced. However, these changes caused by H2O2 treatment were also effectively attenuated when silibinin was applied simultaneously.
Inhibition of apoptosis by silibinin in H2O2-treated ARPE-19 cells. Cells were treated with silibinin for 1 h before treatment with H2O2 for 24 h. A and B Annexin V/PI staining was followed by flow cytometry. Representative histogram (A) and quantitative analysis results (B) were shown. C Changes in expression of the indicated proteins were obtained through immunoblotting using antibodies corresponding to each protein. D Differences in caspase-3 activity in each treatment group were presented. ***p < 0.001 vs. control group; ###p < 0.01 vs.H2O2 treatment group
Reduction of H2O2-induced mitochondrial damage by silibinin in ARPE-19 cells
To analyze whether the inhibitory effect of silibinin against H2O2-induced apoptosis was associated with its inhibitory ability against mitochondrial dysfunction, MMP, an indicator of mitochondrial function, were monitored using the fluorescent dye JC-1, which is sensitive to mitochondrial polarity. Flow cytometry results showed that the proportion of JC-1 aggregates was down-regulated in H2O2-treated cells, whereas the frequency of JC-1 monomers, indicating the frequency of cells lacking MMP, was significantly up-regulated as showed in Fig. 4A andB. In good agreement with these results, the fluorescence intensity of MitoTracker Red probe, which binds to mitochondria with an active membrane potential, was noticeably reduced in H2O2-treated cells (Fig. 4C). In addition, cytochrome c expression was promoted in the cytosolic fraction by H2O2 insult with the parallel decrease into mitochondrial fraction (Fig. 4D and E), demonstrating that cytochrome c present in mitochondria was released into the cytoplasm by H2O2 treatment due to disruption of mitochondrial membrane stability. However, these changes caused by H2O2 were all clearly abolished in cells pretreated with silibinin, indicating that functional damage to mitochondria induced by H2O2 was alleviated by silibinin.
Attenuation of H2O2-induced mitochondrial impairment by silibinin in ARPE-19 cells. Cells cultured for 1 h in medium with or without silibinin were treated with H2O2 for 24 h. (A and B) Representative histograms of flow cytometry using JC-1 staining in each treatment group (A) and quantitative results of JC-1 monomer ratios (B) were shown. *** p < 0.001 vs. control group; ###p < 0.01 vs. H2O2 treatment group. C Mitochondria and nuclei were stained with MitoTracker Red (red) and DAPI (blue), respectively, and representative fluorescence images were presented. D and E Mitochondrial and cytoplasmic fractions were separated and the expression pattern of cytochrome c was investigated by immunoblotting
Suppression of ROS generation and restoration of GSH/GSSG ratio by silibinin in H2O2-treated ARPE-19 cells
To examine the antioxidant activity of silibinin, DCF-DA, a cell-permeant indicator for ROS, was used to evaluate the intracellular total ROS levels produced by H2O2. As shown in Fig. 5A andB, the frequency of oxidized DCF-positive cells, an indicator of intracellular ROS production, was significantly higher than that of untreated cells. However, it was dramatically abrogated in silibinin-pretreated cells, and treatment with silibinin alone had no significant effect on ROS production. As a result of reconfirming the ROS scavenging ability of silibinin through fluorescence microscopy, a significant increase in DCF fluorescence intensity (green) was observed in cells treated with H2O2 as seen in the micrograph shown in Fig. 5C, and the increase was greatly reduced in the presence of silibinin. Because GSH is oxidized to GSSH under oxidative stress conditions, GSH depletion is widely used as an indicator of oxidative stress, so the effect of silibinin on the change of GSH/GSSG ratio by H2O2 treatment was further evaluated. As shown in Fig. 5D, the reduced GSH/GSSG ratio upon the administration of H2O2 was significantly restored by silibinin pretreatment. These results suggest that depletion of intracellular GSH might be responsible for enhanced ROS generation with H2O2 treatment, as shown in the results of pretreatment with NAC, a ROS scavenger, and silibinin could lower the intracellular oxidative stress caused by H2O2.
Improvement of H2O2-induced intracellular ROS generation and GSH/GSSG ratio reduction in ARPE-19 cells by silibinin. Cells were treated with H2O2 for 1 h (A–C) or 24 h (D) following a 1 h pre-incubation with silibinin or NAC. (A and B) Flow cytometry was performed after DCF-DA staining. Representative flow cytometry results of each treatment group (A) and frequencies of DCF-positive cells (B) were shown. C Representative images obtained under a fluorescence microscope after DCF-DA staining. D The change in the ratio of GSH and GSSG content in each treatment group was measured using a colorimetric kit. *** p < 0.001 vs. control group; ###p < 0.01 vs. H2O2 treatment group (D)
Inhibition of H2O2 -induced reduction of GPx and MnSOD activities and increase in mtROS production by silibinin in ARPE-19 cells
To further investigate the antioxidant activity of silibinin, we evaluated the role of GPx, which catalyzes the reduction of H2O2 to oxygen and water, and MnSOD, a key enzyme that detoxifies oxygen superoxide, a major byproduct of mitochondrial respiration. As expected, H2O2 treatment greatly decreased the expression and activity of GPx and MnSOD (Fig. 6A–C), indicating that activation of MnSOD and GPx were negatively correlated with ROS levels. However, silibinin pretreatment counteracted their expression and enzymatic activity reduced by H2O2, indicating that silibinin could protect ARPE-19 cells against oxidative environments by attenuating excessive generation of intracellular and mtROS. Next, we used MitoSOX, a specific fluorescent probe for detecting mitochondrial superoxide production, to evaluate whether the protective effect of silibinin on the regulation of mitochondrial homeostasis was related to the protection of mtROS production. As can be seen from the flow cytometry results shown in Fig. 6D and E, H2O2 markedly promoted mitochondrial superoxide generation. However, such effect was greatly mitigated by silibinin pretreatment. In parallel with these results, the red fluorescence of oxidized MitoSOX was found to be enhanced in cells treated with H2O2 but decreased in the presence of silibinin (Fig. 6F), suggesting that the source of ROS may be mitochondria. In particular, Mito-TEMPO, a potent mitochondria-targeted antioxidant, more obviously prevented H2O2 induced mitochondrial superoxide production (Fig. 6D–F). Overall, the current data demonstrated that H2O2-induced cytotoxicity in ARPE-19 cells was mediated at least through the mitochondrial damage pathway by the generation of mtROS.
Elimination of H2O2-induced mtROS production by silibinin in ARPE-19 cells. Before treating cells with H2O2 for 24 h (A–C) or 1 h (D–F), cells were cultured in a medium with or without silibinin or Mito-TEMPO for 1 h. A Changes in expression of PGx1 and MnSOD proteins were obtained through immunoblotting using antibodies corresponding to each protein. B and C PGx and MnSOD activities of cells were estimated compared to the control group using the corresponding assay kit. D and E To quantitatively analyze mitochondrial superoxide levels, flow cytometry was performed after staining with MitoSOX. Representative histogram (D) and quantitative analysis results (E) were shown. (F) After staining the cells using MitoSOX (red) and DAPI (blue), the stained cells were visualized by fluorescence microscopy and representative fluorescence images were shown
Discussion
Several previous studies have reported the applicability of silibinin to block ocular diseases (Pooja et al. 2022; Shen et al. 2019; Goswami et al. 2018; Chen et al. 2017; Lin et al. 2013), but its efficacy against oxidative damage to RPE cells has not yet been properly evaluated. Therefore, in the current study, it was investigated whether silibinin could reduce oxidative damage elicited by H2O2 in RPE ARPE-19 cells. According to the results of this study, oxidative stress caused mitochondrial damage, leading to DNA damage and apoptosis in ARPE-19 cells, but silibinin maintained cell stability by inhibiting mtROS generation (Fig. 7).
There is growing evidence that DNA and mitochondrial damage induced by oxidative stimuli in RPE cells is closely associated with the induction of ROS-dependent apoptosis. Indeed, many previous results have shown that the cytotoxic effects of H2O2, known as the most stable ROS, on RPE cells are mostly related to mitochondrial dysfunction and apoptosis associated with damage to intracellular macromolecules including DNA (Park et al. 2023; Clementi et al. 2022; Hernandez et al. 2021). According to results of the present study, silibinin could attenuate the process of H2O2 damaging the viability and cell morphology of ARPE-19 cells. And the cytotoxic inhibitory ability of silibinin against H2O2 was also clearly shown in the analysis of LDH release, an indicator of damage to cell membrane integrity. However, in cells treated with silibinin alone, there was no significant difference in cell viability, cell shape, LDH release, etc. compared to the control group. Marazita et al. (2016) have shown that oxidative damage in RPE cells is critically involved in the initiation of AMD by causing DNA damage and promoting premature cellular senescence. In addition, H2O2 -induced apoptosis of ARPE-19 cells was accompanied by DNA damage, and silibinin was able to significantly block this, as evidenced by suppression of DNA tail formation and γH2AX expression, which are hallmarks of DNA double-strand breaks (Cordelli et al. 2021; Kopp et al. 2019). Silibinin also normalized the level of 8-OHdG, a widely used biomarker for oxidative stress in nucleic acids (Hahm et al. 2022), increased by H2O2 treatment. Therefore, the current results document that silibinin abolished oxidative stimuli-mediated DNA damage in RPE cells as well as in other cells.
Many previous studies have already proven that oxidative stress has a pro-apoptotic effect on RPE cells through mitochondrial dysfunction, which is a major cause closely related to the degeneration of RPE cells (Zhang et al. 2023; Tong et al. 2022). As is well known, excessive accumulation of ROS caused by oxidative stress contributes to mitochondrial membrane depolarization, resulting in MMP loss, indicative of mitochondrial damage. Mitochondrial depolarization can trigger cytoplasmic release of cytochrome c, which in turn stimulates the activation of the caspase cascade, the central mediating pathway of apoptosis, and leads to initiation of mitochondria-mediated apoptotic pathway, ultimately resulting in degradation of target proteins of effector caspases such as PARP for the induction of apoptosis (Bock and Tait 2020; Kiraz et al. 2016). Consistent with several previous findings (Zou et al. 2023; Park et al. 2023; Clementi et al. 2022), H2O2 exposure significantly induced apoptosis in ARPE-19 cells, accompanied by accumulation of intracellular total ROS, as assessed using annexin V/PI and DCF-DA assays. In addition, H2O2 administration decreased the intensity of MitoTracker Red, consistent with the loss of MMP according to the JC-1 assay, indicating disruption of mitochondrial membrane stability. Moreover, in ARPE-19 cells treated with H2O2, an increase of the Bax/Bcl-2 protein expression ratio, cytosolic translocation of cytochrome c, and cleavage of PARP by activation of caspase-3, a key contributor to the apoptosis process, were observed. In the mitochondria-mediated apoptotic pathway, pro-apoptotic proteins including Bax are involved in mitochondrial pore formation, which disrupts mitochondrial membrane stability, while anti-apoptotic proteins such as Bcl-2 play the opposite role (Lalier et al. 2022; Kiraz et al. 2016). Therefore, when the Bax/Bcl-2 ratio increases, mitochondrial membrane permeability is increased and cytochrome c release from mitochondria to the cytosol is enhanced. However, all of these effects due to H2O2 were apparently eliminated in the presence of silibinin. These findings may be causally linked to the blockade of the caspase-3-dependent apoptotic pathway by oxidative stress following repair of H2O2-mediated MMP loss by silibinin.
The level of ROS within cells is tightly regulated by a series of oxidative defense systems. Among them, GSH, which acts as a cofactor for intracellular antioxidant enzymes, removes ROS and electrophiles, so the ratio of GSH to GSSG can be used as a measure of the redox state of cells (Lou 2022; Enns and Cowan 2017). Indeed, even in RPE cells, accumulating evidence have shown that increasing the ratio of GSH/GSSG can reduce H2O2-induced cytotoxicity, whereas lowering this ratio can increase H2O2-induced apoptosis. According to results of this study, the GSH/GSSG ratio was reduced in ARPE-19 cells cultured in medium supplemented with H2O2. However, as observed in several previous studies (Tie et al. 2022; Guo et al. 2020), such altered GSH/GSSG ratio was apparently restored by silibinin, which was related to the quenching of ROS production. Silibinin also increased the expression and activity of GPx involved in H2O2 detoxification, as shown in previous results (Aamani et al. 2022; Kalemci et al. 2015), suggesting that the preventive effect of silibinin against oxidative damage was mediated by attenuation of reactive oxygen intermediates. Mitochondria are organelles vulnerable to ROS and are also main sources of ROS such as H2O2 and superoxide in eukaryotic cells (Zia et al. 2022; Demine et al. 2019). Moreover, H2O2-induced mtROS release mediates mitochondrial depolarization (Anupama et al. 2023; Park and Choi 2012), contributing to the activation of the mitochondria-mediated apoptotic pathway. Among SODs, MnSOD, which is localized in the mitochondrial matrix, acts as an enzyme that removes mitochondrial superoxide to protect cells from oxidative damage (Islam et al. 2022; Palma et al. 2020). The antioxidant activity of MnSOD comes from its ability to decompose two molecules of superoxide anion (O2−) into oxygen (O2) and H2O2 (Liu et al. 2022; Palma et al. 2020). Although the possibility that MnSOD is involved in the antioxidant activity of silibinin has been suggested (Zappavigna et al. 2019), it is not yet clear whether it is related to mitochondrial function and accumulation of intracellular ROS. According to the present results, the expression and activity of MnSOD were reduced in ARPE-19 cells treated with H2O2, but silibinin clearly reversed this. In addition, the results of flow cytometric analysis using MitoSOX showed that the level of mitochondrial superoxide was significantly weakened in cells pretreated with silibinin before treatment with H2O2, and this was further confirmed through fluorescence microscopy. Since superoxide is converted to membrane permeable H2O2, they can readily diffuse into cells (Yan et al. 2020; Munro and Treberg 2017), these results indicate that the ROS scavenging activity of silibinin is due to the reduction of mitochondrial superoxide generation by preserving MnSOD activity. These data therefore suggest that mtROS generation is upstream of mitochondrial dysfunction. This notion may be further supported by the results showing that NAC or Mito-TEMPO abrogated H2O2-induced intracellular ROS generation and mitochondrial superoxide, respectively. Although the molecular mechanisms for the correlation of activities of GSH, GPx and MnSOD require further investigation, these results indicate that silibinin can protect ARPE-19 cells from genotoxicity under conditions of oxidative stress while exerting mtROS scavenging activity. However, it is imperative to investigate whether silibinin can modulate oxidative damage-mediated ocular diseases in vivo in further studies.
Conclusion
The present study showed that silibinin could eliminate H2O2-induced genotoxicity and apoptosis while alleviating mitochondrial damage in ARPE-19 cells. Moreover, silibinin blocked H2O2-induced intracellular ROS production while preserving GHS content and PGx activity, and inhibition of mitochondrial superoxide generation was associated with restoration of MnSOD activity. These results demonstrate that blocking mtROS generation by silibinin may act as an upstream event in the induction of DNA damage and mitochondria-mediated apoptosis (Fig. 7). This is the first report showing that silibinin plays a critical role in the inhibition of oxidative stress-induced RPE cell demise. Collectively, these findings support the preventive potential of silibinin, acting as a mtROS scavenger, in oxidative damage-related ocular disease.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Aamani N, Bagheri A, Masoumi Qajari N, Malekzadeh Shafaroudi M, Khonakdar-Tarsi A (2022) JNK and p38 gene and protein expression during liver ischemia-reperfusion in a rat model treated with silibinin. Iran J Basic Med Sci 25:1373–1381
Anupama C, Shettar A, Ranganath SH, Srinivas SP (2023) Experimental oxidative stress breaks down the barrier function of the corneal endothelium. J Ocul Pharmacol Ther 39:70–79
Baeeri M et al (2018) Molecular and biochemical evidence on the protective role of ellagic acid and silybin against oxidative stress-induced cellular aging. Mol Cell Biochem 441:21–33
Bock FJ, Tait SWG (2020) Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol 21:85–100
Cao M, Fan B, Zhen T, Das A, Wang J (2023) Ruthenium biochanin-a complex ameliorates lung carcinoma through the downregulation of the TGF-β/PPARγ/PI3K/TNF-α pathway in association with caspase-3-mediated apoptosis. Toxicol Res 39:455–475
Chaudhary MR et al (2023) Aging, oxidative stress and degenerative diseases: mechanisms, complications and emerging therapeutic strategies. Biogerontology 24:609–662
Chen CL, Chen JT, Liang CM, Tai MC, Lu DW, Chen YH (2017) Silibinin treatment prevents endotoxin-induced uveitis in rats in vivo and in vitro. PLoS ONE 12:e0174971
Chen YH, Lin H, Wang Q, Hou JW, Mao ZJ, Li YG (2020) Protective role of silibinin against myocardial ischemia/reperfusion injury-induced cardiac dysfunction. Int J Biol Sci 16:1972–1988
Clementi ME, Pizzoferrato M, Bianchetti G, Brancato A, Sampaolese B, Maulucci G, Tringali G (2022) Cytoprotective effect of idebenone through modulation of the intrinsic mitochondrial pathway of apoptosis in human retinal pigment epithelial cells exposed to oxidative stress induced by hydrogen peroxide. Biomedicines 10:503
Cordelli E, Bignami M, Pacchierotti F (2021) Comet assay: A versatile but complex tool in genotoxicity testing. Toxicol Res (camb) 10:68–78
Dammak A et al (2023) Oxidative stress in the anterior ocular diseases: diagnostic and treatment. Biomedicines 11:292
Demine S, Renard P, Arnould T (2019) Mitochondrial uncoupling: a key controller of biological processes in physiology and diseases. Cells 8:795
Enns GM, Cowan TM (2017) Glutathione as a redox biomarker in mitochondrial disease-implications for therapy. J Clin Med 6:50
Goswami DG, Kant R, Tewari-Singh N, Agarwal R (2018) Efficacy of anti-inflammatory, antibiotic and pleiotropic agents in reversing nitrogen mustard-induced injury in ex vivo cultured rabbit cornea. Toxicol Lett 293:127–132
Guo H, Wang Y, Liu D (2020) Silibinin ameliorate H2O2-induced apoptosis and oxidative stress response by activating Nrf2 signaling in trophoblast cells. Acta Histochem 122:151620
Hahm JY, Park J, Jang ES, Chi SW (2022) 8-Oxoguanine: from oxidative damage to epigenetic and epitranscriptional modification. Exp Mol Med 54:1626–1642
Hernandez M et al (2021) Anti-inflammatory and anti-oxidative synergistic effect of vitamin D and nutritional complex on retinal pigment epithelial and endothelial cell lines against age-related macular degeneration. Nutrients 13:1423
Hsueh YJ, Chen YN, Tsao YT, Cheng CM, Wu WC, Chen HC (2022) The pathomechanism, antioxidant biomarkers, and treatment of oxidative stress-related eye diseases. Int J Mol Sci 23:1255
Islam A, Mishra A, Siddiqui MA, Siddiquie S (2021) Recapitulation of evidence of phytochemical, pharmacokinetic and biomedical application of silybin. Drug Res (stuttg) 71:489–503
Kalemci S et al (2015) Silibinin attenuates methotrexate-induced pulmonary injury by targeting oxidative stress. Exp Ther Med 10:503–507
Kiraz Y, Adan A, Kartal Yandim M, Baran Y (2016) Major apoptotic mechanisms and genes involved in apoptosis. Tumor Biol 37:8471–8486
Kopp B, Khoury L, Audebert M (2019) Validation of the γH2AX biomarker for genotoxicity assessment: a review. Arch Toxicol 93:2103–2114
Křen V, Valentová K (2022) Silybin and its congeners: From traditional medicine to molecular effects. Nat Prod Rep 39:1264–1281
Lalier L, Vallette F, Manon S (2022) Bcl-2 family members and the mitochondrial import machineries: the roads to death. Biomolecules 12:162
Li W et al (2023) Silibinin exerts neuroprotective effects against cerebral hypoxia/reoxygenation injury by activating the GAS6/Axl pathway. Toxicology 495:153598
Lin CH et al (2013) Silibinin inhibits VEGF secretion and age-related macular degeneration in a hypoxia-dependent manner through the PI-3 kinase/Akt/mTOR pathway. Br J Pharmacol 168:920–931
Liu M, Sun X, Chen B, Dai R, Xi Z, Xu H (2022) Insights into manganese superoxide dismutase and human diseases. Int J Mol Sci 23:15893
Lou MF (2022) Glutathione and glutaredoxin in redox regulation and cell signaling of the lens. Antioxidants (basel) 11:1973
Mao YX et al (2018) RAGE-dependent mitochondria pathway: A novel target of silibinin against apoptosis of osteoblastic cells induced by advanced glycation end products. Cell Death Dis 9:674
Marazita MC, Dugour A, Marquioni-Ramella MD, Figueroa JM, Suburo AM (2016) Oxidative stress-induced premature senescence dysregulates VEGF and CFH expression in retinal pigment epithelial cells: Implications for age-related macular degeneration. Redox Biol 7:78–87
Mukherjee S, Park JP, Yun JW (2022) Carboxylesterase3 (Ces3) Interacts with bone morphogenetic protein 11 and promotes differentiation of osteoblasts via Smad1/5/9 pathway. Biotechnol Bioprocess Eng 27:1–16
Munro D, Treberg JR (2017) A radical shift in perspective: mitochondria as regulators of reactive oxygen species. J Exp Biol 220:1170–1180
Nawaz A, Zaib S, Khan I, Ahmed A, Shahzadi K, Riaz H (2023) Silybum marianum: An overview of its phytochemistry and pharmacological activities with emphasis on potential anticancer properties. Anticancer Agents Med Chem 23:1519–1534
Palma FR et al (2020) Mitochondrial superoxide dismutase: What the established, the intriguing, and the novel reveal about a key cellular redox switch. Antioxid Redox Signal 32:701–714
Park J, Choi C (2012) Contribution of mitochondrial network dynamics to intracellular ROS signaling. Commun Integr Biol 5:81–83
Park C et al (2023) Nrf2-mediated activation of HO-1 is required in the blocking effect of compound K, a ginseng saponin metabolite, against oxidative stress damage in ARPE-19 human retinal pigment epithelial cells. J Ginseng Res 47:311–318
Singh M et al (2023) A systematic review of the protective effects of silymarin/silibinin against doxorubicin-induced cardiotoxicity. Cancer Cell Int 23:88
Sozen H, Celik OI, Cetin ES, Yilmaz N, Aksozek A, Topal Y, Cigerci IH, Beydilli H (2015) Evaluation of the protective effect of silibinin in rats with liver damage caused by itraconazole. Cell Biochem Biophys 71:1215–1223
Sukjamnong S, Chen H, Saad S, Santiyanont R (2022) Fimbristylis ovata and Artemisia vulgaris extracts inhibited AGE-mediated RAGE expression, ROS generation, and inflammation in THP-1 cells. Toxicol Res 38:331–343
Takke A, Shende P (2019) Nanotherapeutic silibinin: an insight of phytomedicine in healthcare reformation. Nanomedicine 21:102057
Tie F, Fu Y, Hu N, Wang H (2022) Silibinin protects against H2O2-induced oxidative damage in SH-SY5Y cells by improving mitochondrial function. Antioxidants (basel) 11:1101
Tong Y, Zhang Z, Wang S (2022) Role of mitochondria in retinal pigment epithelial aging and degeneration. Front Aging 3:926627
Wadhwa K, Pahwa R, Kumar M, Kumar S, Sharma PC, Singh G, Verma R, Mittal V, Singh I, Kaushik D, Jeandet P (2022) Mechanistic insights into the pharmacological significance of silymarin. Molecules 27:5327
Wang X, Zhang Z, Wu SC (2020) Health benefits of Silybum marianum: phytochemistry, pharmacology, and applications. J Agric Food Chem 68:11644–11664
Yan J, Jiang J, He L, Chen L (2020) Mitochondrial superoxide/hydrogen peroxide: An emerging therapeutic target for metabolic diseases. Free Radic Biol Med 152:33–42
Yang F, Jia M, Deng C, Xiao B, Dai R, Xiang Y (2022) Silibinin ameliorates cisplatin-induced acute kidney injury via activating Nfe2l1-mediated antioxidative response to suppress the ROS/MAPK signaling pathway. J Mol Histol 53:729–740
Zappavigna S et al (2019) Silybin-induced apoptosis occurs in parallel to the increase of ceramides synthesis and miRNAs secretion in human hepatocarcinoma cells. Int J Mol Sci 20:2190
Zhang SM, Fan B, Li YL, Zuo ZY, Li GY (2023) Oxidative stress-involved mitophagy of retinal pigment epithelium and retinal degenerative diseases. Cell Mol Neurobiol 43:3265–3276
Zia A, Farkhondeh T, Pourbagher-Shahri AM, Samarghandian S (2022) The roles of mitochondrial dysfunction and reactive oxygen species in aging and senescence. Curr Mol Med 22:37–49
Zou GP et al (2023) Lactate protects against oxidative stress-induced retinal degeneration by activating autophagy. Free Radic Biol Med 194:209–219
Acknowledgement
Not applicable.
Funding
No relevant funding received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yung Hyun Choi declares that he has no conflict of interest.
Ethical approval
The article does not contain any studies with human and animal and this study was performed following institutional and national guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choi, Y.H. Silibinin alleviates DNA damage, mitochondrial dysfunction, and apoptosis caused by oxidative stress in human retinal pigment epithelial cells. Mol. Cell. Toxicol. 20, 709–721 (2024). https://doi.org/10.1007/s13273-023-00412-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-023-00412-8