Abstract
Background
Cardiovascular simulations for patients with single ventricles undergoing the Fontan procedure can assess patient-specific hemodynamics, explore surgical advances, and develop personalized strategies for surgery and patient care. These simulations have not yet been broadly accepted as a routine clinical tool owing to a number of limitations. Numerous approaches have been explored to seek innovative solutions for improving methodologies and eliminating these limitations.
Purpose
This article first reviews the current state of cardiovascular simulations of Fontan hemodynamics. Then, it will discuss the technical progress of Fontan simulations with the emphasis of its clinical impact, noting that substantial improvements have been made in the considerations of patient-specific anatomy, flow, and blood rheology. The article concludes with insights into potential future directions involving clinical validation, uncertainty quantification, and computational efficiency. The advancements in these aspects could promote the clinical usage of Fontan simulations, facilitating its integration into routine clinical practice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Congenital heart diseases (CHDs) affect ~ 1% of newborns and are the number one leading cause of birth–death in the United States and worldwide. While single ventricular defects are rare CHDs, they are severe and can lead to financial and mental burdens for not only the affected families but also the entire medical system. Families of patients with CHDs are often overwhelmed by higher caregiving hours and diminished mental health.55 In addition, the medical care of single ventricular defects costs ~ $400 million per year in total, which comprises ~ 8% of the total cost of CHDs in the United States.80
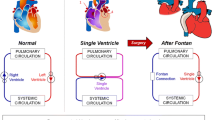
The Fontan procedure is the most common treatment for palliating single ventricular defects. This procedure usually has two to three stages: (1) Norwood surgery if needed, (2) Glenn (or hemi-Fontan) surgery, and (3) Fontan completion, as illustrated in Fig. 1. The final stage generates the total cavopulmonary connection (TCPC), rerouting systemic venous return to passively flow to the pulmonary circulation without the aid of a subpulmonary pumping chamber.

adapted from images: https://www.chop.edu/treatments/staged-reconstruction-heart-surgery.
Schematic representation of a common 3-stage Fontan procedure using hypoplastic left heart syndrome as an example, consisting of the formation of (1) Norwood, (2) Glenn, and (3) total cavopulmonary connection (TCPC). Schematics are
The primary purpose of the TCPC is to address the mixing of oxygenated and deoxygenate blood in the single ventricle patient. Since its advent, this procedure has saved numerous lives and generally provided favorable short-term outcomes. Nevertheless, Fontan circulation is physiologically altered from a normal biventricular one due to the lack of a subpulmonary pumping chamber. For this reason, long-term complications are broadly present, and the life expectancy of post-Fontan patients remains low. The formation and progression of Fontan-related complications are undoubtedly multifactorial in etiology. Many researchers have endeavored to delve into associations of these complications with Fontan hemodynamics. The focus was on hemodynamics within the TCPC because the construction of TCPC is the crucial step in the Fontan procedure. In other words, TCPC hemodynamics is a modifiable risk factor and thus has great potential for improvements. Examples of some associations include:
-
1.
Reduced exercise capacity, which universally exists in all Fontan patients, was found to be correlated with elevated TCPC power loss (PL).37, 86
-
2.
Heart failure of Fontan patients could attribute to suboptimal TCPC energetics.71, 99
-
3.
The quality of life of Fontan patients is negatively correlated with TCPC PL.49
-
4.
Fontan-related liver diseases, a rising concern for Fontan patients, are suspected to be linked to elevated central venous pressure.14, 71 Recent studies also demonstrated that inefficient TCPC design with high TCPC resistance might contribute to the progression of liver fibrosis.93, 94
-
5.
Pulmonary arteriovenous malformations (PAVMs) have been associated with the deprivation of a mysterious nutrient from the liver or “hepatic factor,” as referred to by previous literature.59, 81 Therefore, a suboptimal hepatic flow distribution (HFD) may lead to PAVMs, and correction of HFD has been approved to be effective in treating these diseases.26, 82
These findings have suggested that personalized treatment paradigms for Fontan surgeries would be useful, including pre-procedural planning that optimizes the patient-specific TCPC design before the surgery and post-procedural follow-up analysis for improving the prognosis of Fontan-related complications.26 Patient-specific Fontan simulations play a vitally important role in both pre-procedural planning and post-procedural follow-up analyses. Rooted in fundamental engineering concepts and techniques, Fontan simulations combine advanced medical imaging with computational modeling to assess complex, patient-specific flow fields inside TCPCs and evaluate hemodynamic metrics.8, 11, 17, 26, 52, 92 The translational value of these simulations has been demonstrated by their use in exploring novel surgical techniques, such as the Y-graft,34, 95, 116 and in examining the effectiveness of (novel) medical devices.88, 91
Even though the basic workflow remains nearly the same in the past decade, techniques involved in the Fontan simulation have substantially advanced. Therefore, this article will review the important considerations in technical advancements and discuss their impacts on clinical practice.
Workflow of Fontan Simulation
Image Acquisition
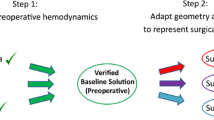
In general, the patient-specific Fontan simulation commences with image acquisition for the patient, as indicated in Fig. 2. Cardiovascular magnetic resonance (CMR) can obtain both high-resolution anatomy and time/spatially resolved blood flow without ionizing radiation; therefore, it is commonly used to assess Fontan patients. Computed Tomography (CT) is another option when CMR is not readily available or expects significant artifacts; however, blood flow hemodynamics must utilize echocardiography or cardiac catheterization with their limitations which is less than optimal. Blood flow data from CMR is acquired using the phase-contrast MRI technique (PC-MRI) or Doppler echocardiography if CT is needed. The former technique is favored because of a) higher accuracy, b) higher reproducibility, c) more reflective of true physiology as it is averaged over many heartbeats and d) capability of resolving three-dimensional flow fields. Detailed reviews of medical imaging techniques used in Fontan simulation can be found in Refs. 1, 26.
Image Processing
The next step is image processing (Fig. 2). This step involves reconstructing the patient’s anatomy and flow data from the medical images. The technology for reconstructing patient-specific anatomy has been steadily advancing in the past decade. Many commercial and open-source software facilitate manual segmentation of blood vessels from a stack of medical images, interpolate between consecutive layers of medical images, and export a surface or volumetric mesh for the simulation. Examples of these image processing packages include Materialise Mimics (Materialise NV, Leuven, Belgium), 3D Slicer (http://www.slicer.org/), and VMTK (http://www.vmtk.org/). Since manual segmentation can be time-intensive, current efforts have been made to automate the process of segmenting the vessels for anatomical reconstructions. Thus far, the substantial inter-patient variability of Fontan anatomy has delayed the progress of these efforts, although endeavors in artificial intelligence hold promise for future automation.
It is relatively easier to segment patient-specific PC-MRI images. Open-source packages can adequately track the vessel automatically and obtain the flow over a cardiac cycle. An example is Mediviso Segment (http://medviso.com/segment/). Doppler echocardiography often provides plots with blood velocity waveforms in two dimensions. Therefore, plot digitizers (e.g., https://apps.automeris.io/wpd/) can be a useful tool to reproduce these waveforms.
Computational Modeling
Computational modeling techniques, including computational fluid dynamics (CFD) and fluid–structure interaction (FSI), have extensively been used for the assessment of patient-specific hemodynamics inside the TCPC. Adequate CFD solvers include commercial software such as ANSYS Fluent (ANSYS Inc, Canonsburg, PA) and Simcenter Star-CCM + (Siemens PLM, Plano, TX). Companion structural modeling programs, such as ANSYS Mechanical (ANSYS Inc, Canonsburg, PA) and Abaqus FEA (Dassault Systèmes, Vélizy-Villacoublay, France), can be coupled with explicit numerical schemes for FSI modeling. Open-source scientific packages, like SimVascular,98 CRIMSON (http://www.crimson.software/), FEBio (https://febio.org/), and LifeV,4 are also widely used. In particular, implicit numerical schemes for fluid–structure coupling in these open-source packages are advantageous for resolving detailed interactions between blood flow and vessel structures.
Mesh generation is a critical step in computational modeling. Dedicated work must be performed for a good quality mesh; otherwise, the simulated results could be highly inaccurate and unreliable. Most commercial software, fortunately, has embedded meshing modules with advanced mesh generation capabilities. Open-source mesh generation packages are also convenient, including Gmsh (https://gmsh.info/) and Netgen (https://ngsolve.org/). Though the most tedious meshing work has been encapsulated, the users have full control over the mesh size (i.e., the most critical parameter to quantify the mesh quality). A smaller mesh size, in general, leads to a better simulation result at the cost of increased computational time and power. Therefore, mesh independence studies are essential for identifying an adequate mesh size, resulting in acceptable discrepancies in the simulation compared with a further refined mesh. It is worth mentioning that the “adequacy” of mesh size may change regarding different variables from the simulation. A relatively coarse mesh is acceptable to obtain mesh-independent primitive variables, i.e., velocity and pressure, while a much finer mesh (~ 15 times smaller in mesh cell volume) is necessary for achieving mesh independency for metrics involving derivatives of the primitive variables, such as shear stress.12, 108 Lastly, the choice of time step size should suffice for the Courant-Friederichs-Lewy (CFL) condition for numerical stability. Solvers with explicit time-marching schemes typically require a CFL condition = ~ 1; whereas implicit solvers tolerate a looser requirement in the CFL condition.
Hemodynamic Metrics
Computational modeling can generate a wide range of fluid and structure dynamics data. Most Fontan studies concentrated on clinically relevant metrics associated with Fontan-related complications, and the primary metrics are HFD and PL. Additionally, the pre-procedural planning of Fontan surgeries (one of the primary clinical applications of Fontan simulation) usually attempts to optimize these two metrics to provide a personalized TCPC design for a given Fontan patient.
HFD is a measurement of the amount of hepatic factor being transported through the TCPC to both lungs. This metric, by definition, is quantified by weighing the percentage of hepatic flow to one side of the lung. The calculation of HFD can be based on either Eulerian or Lagrangian methods. The Eulerian method treated the hepatic factor as a scalar that flows with the blood from the liver, while the Lagrangian method treats hepatic factors as particles. Both methods are acceptable for the calculation of HFD. The Lagrangian method could quantify and elucidate other important features of complicated flow fields inside the TCPC, such as particle residence time; therefore, it is more frequently used.78, 85
The expression of PL is
where p, u, n, ρ, A, and CS are the static pressure, velocity vector, normal unit vector, the blood density, and a control surface (original vessel ends and the TCPC wall in Fontan simulations). To eliminate inter-patient variations, PL is usually indexed using clinical metrics, including the venous return, Qs, and body surface area, BSA, leading to a unitless measure:
Other approaches have also been proposed for indexing the power loss, such as using the dimension of vessels associated with the TCPC.41 Index power loss (iPL) provides only a single, time-averaged value characterizing TCPC energetics without delving into detailed flow characteristics that lead to energy transformation. Recently, another hemodynamic metric, the indexed viscous dissipation rate (iVDR), was proposed.108 In the scope of Fontan hemodynamics, iVDR is theoretically equivalent to iPL and has time-resolved, spatial distribution contours, which may enable additional clinical and engineering insights that iPL may not provide.
Other important metrics include particle residence time and wall shear stress. Both particle residence time and wall shear stress are hypothesized to be associated with the thrombosis risks in Fontan patients,85, 107, 115, 116 but no direct correlations have yet been observed. A good review of approaches of computing particle residence time can be found in Ref. 70.
Patient-Specific Aspects of Fontan Simulation
The patient-specific nature of the Fontan simulation relies heavily on being detailed regarding the individual anatomy and blood flow data. Both, technically, are boundary conditions for simulations and play critical roles in deriving simulated Fontan hemodynamics. The anatomy is the wall boundary that enforces “no-slip” conditions and prevents blood flow from penetrating through. The blood flow data regulates flow behavior at the corresponding locations, e.g., the vessel ends, in the TCPC.
Patient-Specific Anatomy
Early Fontan studies used idealized TCPC anatomies, which simplified the patient’s TCPC anatomy to four tubes with uniform cross-sectional areas. Idealized anatomies reasonably reproduce key features of Fontan hemodynamics, including the collision between the Fontan pathway (FP) and SVC flow streams (FP-SVC or IVC-SVC flow collision).16, 33, 58, 79 Therefore, studies with variations of idealized TCPCs provide valuable insights to understand patient-specific hemodynamics in Fontan patients. For example, it was demonstrated that a larger FP-SVC offset (i.e., the separation between both vessels as they connect to the pulmonary artery) could reduce the power loss in the TCPC by avoiding the FP-SVC flow collision; however, this offset may deteriorate the balance of HFD to pulmonary arteries.16, 73 Population studies with patient-specific data later confirmed this compounding effect of the FP-SVC offset.54, 84 Nevertheless, the idealized TCPC failed to represent many aspects of the specifics of individual patients, including varying vessel diameters and changing angles between the vessels. These anatomical features of a patient’s TCPC could cause a 150% discrepancy in simulated hemodynamics when comparing with using idealized TCPCs56, 73! Therefore, the usage of patient-specific TCPC anatomies has been advocated in recent studies.15, 18
Another critical piece of an individual patient’s TCPC anatomy is vessel compliance or material properties. It is not trivial to obtain the patient-specific compliance for a TCPC as the TCPC contains tissues from venae cavae and pulmonary arteries as well as artificial materials.62 One workaround is to reproduce the patient-specific wall motion acquired from medical images, which takes the patient-specific compliance into account, by utilizing one-way FSI modeling.57 If unavoidably required, e.g., in two-way FSI modeling, the patient-specific compliance can be estimated by either (1) the use of cohort-averaged compliance for vessels that composes the TCPC,48 or (2) treating the TCPC as a single bulk material and obtaining the patient-specific bulk compliance.83, 89, 90 Despite the different approaches of considering compliance, previous studies have concluded that under resting conditions, the clinically relevant hemodynamic metrics (such as PL and HFD) are relatively unaffected by the effect of vessel compliance while local metrics, e.g., wall shear stress, could be affected.48, 57 Nevertheless, FSI modeling is plagued with poor computational efficiency, and therefore, it is as yet impractical to employ this numerical technique for large-cohort Fontan investigations or pre-procedural planning for Fontan surgeries.
Despite errors introduced by idealized TCPCs and non-physiological vessel compliance, the medical image is also a primary source of error for anatomical reconstructions, especially in retrospective studies. Advanced CMR techniques such as 3D SPACE, which yields high-resolution dark blood images, the MUSIC sequence,60, 117 and inversion recovery gradient echo imaging using ferumoxytol, could be employed for enhanced image resolution, thereby reducing the error from medical images. However, it remains a substantial challenge to accurately predict patient-specific anatomies in prospective investigations. A good example is the pre-procedural planning for Fontan patients, which extrapolates the post-operative anatomy based on the pre-operative state,75, 92 while surgeons exercise their discretion in the operating room at the time of Fontan reconstruction. Additionally, Fontan cohorts consist of children where growth is inevitable and could substantially alter TCPC anatomy and blood flow over time.69 The absence of consensus is evident in previous literature; for example, the diameter change of TCPC-associated vessels over time in different cohorts was found to be proportional,7, 29 not to somatic growth.61, 63, 68, 87, 103 Therefore, no reliable model has yet been developed for predicting the temporal variation of patient-specific TCPC anatomies due to patient growth.
Theoretically, errors derived from the boundary conditions decay exponentially with the distance away from the boundary.100 The Fontan simulation is intrinsically an internal flow problem. With small vessel diameters (10-20 mm), any errors from TCPC anatomies could considerably affect the hemodynamics throughout the TCPC. Trusty et al. also confirmed that enhanced anatomical prediction could greatly improve the prediction of Fontan hemodynamics in pre-procedural planning.96 Therefore, approaches for minimizing errors involved in the patient’s anatomy are warranted for future investigation and improvements.
Patient-Specific Flow Data and Boundary Conditions
Typical Fontan simulations directly use patient-specific flow data derived from medical images (either mass flow rate or velocity) as inflow boundary conditions. Outflow boundary conditions can also be patient-specific flow data, especially for applications with rigid vessel wall assumptions.104, 118 When considering patient-specific vessel compliance, it is vitally important to obtain a physiological level of pressure. Therefore, other choices of outflow boundary conditions should be considered, such as lumped parameter models. A typical open-loop lumped parameter model is the Windkessel model, which lumps the resistive, elastic, and inertial properties of blood flow in vessels downstream of TCPC as resistors, capacitors, and impedances. The parameters of a Windkessel model can be calculated by analytical solutions for fluid dynamics, e.g., the Hagen-Poiseuille equation, based on patient-specific in vivo measurements.51, 66 Close-loop lumped parameter models usually involve cardiovascular lumped parameter networks (LPNs). The advantage and challenges related to LPNs will be discussed in Patient-specific Circulatory System. Nevertheless, since using lumped-parameter models applies pressure to outflow boundaries, it often faces numerical instability because of backflow divergence. Recent progress on backflow stabilization has greatly improved the numerical stability without much extra computational cost.21, 53, 113
Though the blood flow is pulsatile, time-averaged flow data with spatially parabolic velocity profiles were historically used in Fontan simulations.20, 73 While the parabolic profile is acceptable when compared with the real, patient-specific spatial profiles,106 remarkable differences have been observed between simulations using pulsatile and time-averaged flow data,36, 109 especially when the flow pulsatility is high. Flow pulsatility in TCPC is by respiration as well as the cardiac cycle.27, 32, 35, 110 Low blood flow pulsatility may occur when the patient is under breath-hold (BH) conditions, while high pulsatility (> 100%) may occur under free-breathing (FB) and exercise conditions.
The flow data under FB conditions contains respiratory cycles that can only be captured by real-time measurements such as real-time PC-MRI.32, 110 Unfortunately, real-time acquisitions under FB conditions may have disadvantages, including relatively low spatial and temporal resolution (40 ms), artifacts related to vessel motion, and flow errors related to eddy currents. Therefore, cardiac-gated BH acquisitions are widely used for routine scans unless the patient cannot follow the breath-holding protocols. While exercise is a critical component in the overall health of Fontan patients and has been the subject of much research, flow acquisitions are generally much easier under resting conditions. With the capability of capturing exercise flow with both respiratory and cardiac components, real-time PC-MR measurement disadvantages may be amplified in exercise.
Several methods have been proposed to estimate “patient-specific” exercise flows. Many simulation studies have utilized patient-specific flows under exercise conditions obtained by the cardiac-gated PC-MRI.6, 37, 86 These studies have advocated that the respiration effect on blood flow could be ignored under exercise conditions because of the strong effect of the cardiac cycle under these conditions. Another option is the use of LPNs. Kung et al. presented a cardiovascular LPN that adapts the patient-specific flow data under resting conditions to exercise conditions by implementing cohort-averaged physiological changes during exercise for Fontan patients.42, 43 This method has been validated against clinical data and obtained acceptable agreement in time-averaged measurements for most cases (“larger discrepancies do occur in a few cases”).42 Therefore, cardiovascular LPNs could be a cost-effective tool to extrapolate patient-specific exercise data when direct measurements are absent. Lastly, scaling the patient-specific flow rates under resting conditions by a factor could be used to approximate the exercise flows,97, 112 considering many physiological studies have repeatedly confirmed these factors.31, 32, 77 However, the factor scaling method may be less accurate than other methods.
Like TCPC anatomical reconstructions, patient-specific flow data can usually be obtained for retrospective studies while challenging to be predicted prospectively. Utilizing cardiovascular LPNs with physiological changes over time for Fontan patients could be a solution; however, no large-cohort validation has yet been conducted. Nevertheless, the effort of enhancing flow predictions may be less effective than the improvement of the anatomy described in the previous section.96 The primary reason is that the TCPC vessels usually are long (~ 100 mm) to diminish the errors originated from flow boundary conditions, while they are relatively narrow (~ 20 mm) to dissipate errors from anatomy.
Patient-Specific Rheology
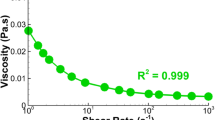
Blood is well-known as a non-Newtonian fluid (nNF), but many previous studies have utilized Newtonian fluid (NF) models for Fontan simulation. This assumption is generally acceptable for cardiovascular simulations involving large vessels, which are usually associated with high flow velocities. While TCPCs consist of large vessels, they have a unique fluid mechanic feature - the colliding flow streams from the upper and lower body, as mentioned in the section of Patient-specific Anatomy. This flow collision creates a recirculating region, thereby leading to low flow regions and manifesting the non-Newtonian effect. Recently, Cheng et al. confirmed elevated low-shear blood viscosity in Fontan patients, associated with increased pulmonary vascular resistance and decreased pulmonary blood flow presented in their patient cohort.10 As demonstrated by both in vitro experiments and computational simulations, using a nNF model could considerably affect clinically relevant hemodynamics metrics, especially wall shear stress,9, 105, 107 for certain patients. At the population-level, no clinically significant difference was observed between using NF and nNF models regarding iPL, iVDR, and HFD.107 Nevertheless, nNF models were suggested for Fontan simulation, primarily because these models could (1) provide more physiological results and (2) save computational cost. The higher viscosity while using nNF models may diminish the flow unsteadiness in TCPCs, thereby leading to better numerical convergence and reduced computational time.50, 107 It is worth noting that previous studies used cohort-averaged blood rheological data for their nNF models, while inter-patient variations in hematocrit could lead to unique nNF models for each patient. Therefore, the implementation of patient-specific nNF models is warranted for future investigations.
Patient-Specific Circulatory System
TCPC is just one piece of the compromised Fontan circulation. To capture the interaction between the TCPC hemodynamics and the rest of circulatory system, closed-loop multiscale simulations have been developed to integrate 3D TCPC simulation into cardiovascular LPNs.2, 38, 39, 51 The cardiovascular LPNs are usually zero-dimensional for simplicity and computational efficiency. The LPNs are valuable to study (1) the pathophysiological responses to exercise in Fontan patients,43 (2) hemodynamic differences between the univentricular circulation in Fontan patients and normal biventricular circulations,47 (3) the effectiveness of ventricular assist devices to the Fontan circulation,24, 64 and so on. One dimensional LPNs have also been developed and used in Fontan investigations when pulse wave propagation is concerned.25, 65 It is worth noting that, though cardiovascular LPNs (0D or 1D) demand much less computational power than 3D simulations, the construction of a patient-specific cardiovascular LPN is still time-consuming. Poignant lack of clinical measurements leaves researchers no choice but to estimate parameters in LPNs based on patient-cohort values or automated approaches.76 The estimation of parameters, in any case, diminishes the patient’s characteristics from the cardiovascular LPNs.
Pathway Towards Routine Clinical Practice
Cardiovascular simulation has been widely used to investigate Fontan hemodynamics and assist clinicians in optimizing real-world surgeries. Despite its potential benefits and the power to revolutionize current healthcare delivery, patient-specific Fontan simulation has not yet been integrated into routine clinical practice because of various limitations. This section attempts to discuss the current challenges which delay clinical integration and potential directions for methodology improvements.
Clinical Validation
Many Fontan studies have validated their simulations against in vitro experiments in the past decade and obtained good comparisons.72, 104, 118 These validation studies served as an excellent first step for developing the credibility of Fontan simulations in assessing patient-specific hemodynamics. Recent clinical validation studies have also emerged with encouraging results. Haggerty et al. compared Fontan simulation with PC-MRI and demonstrated acceptable agreement in the flow field and HFD (mean, [min, max] = 11.8%, [3%, 16%]).30 Yang et al. reported reasonable discrepancies in HFD (mean ± std = 4.3 ± 10%) between simulated HFD and in vivo lung perfusion data.115 General consistency has also been achieved in validating oxygen consumption, although ~ 50% inconsistency remains in a few cases.13, 40 However, the sample size involved in the above studies is limited. Therefore, further validations against clinical measurements in larger patient cohorts are necessary to promote the clinical value of Fontan simulations and to drive advancements in the simulation workflow.
Uncertainty Quantification
Cardiovascular simulations can be contaminated by errors introduced by a myriad of sources, thereby causing differences from in vivo Fontan hemodynamics. Common sources of errors can originate from CFD/FSI solvers and their inputs derived from medical images, including the patient-specific anatomy (with compliance) and flow data. Uncertainties, because of these errors, could remarkably affect the simulation and diminish trust in the simulated results. For example, Restrepo et al.67 demonstrated that small anatomical variations could lead to over 100% change in iPL and HFD. Therefore, progress has been made to quantify uncertainties for Fontan simulations. However, because of the complexity of Fontan anatomy, previous studies have mostly focused on uncertainties derived from flow acquisitions and data processing.74, 76, 96
Additionally, for Fontan patients, the problem of accounting for patient growth is inevitable. So far, it is very challenging to estimate the uncertainty derived from deviation in the patient’s anatomy and flow data because of the patient’s growth. In other words, cardiovascular simulations technically can only assess the patient-specific hemodynamics when the medical images were acquired, and its predictive value is limited at this time. This drawback has considerably postponed the application of cardiovascular simulations in the prognosis and management of Fontan-related complications. Big data analysis could provide a solution to this problem. The first step could be the establishment of population-based registries of Fontan patients, for example, the Australian and New Zealand Fontan Registry (https://www.fontanregistry.com/), the PREpArE-Fontan registry in the United Kingdom (https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/prepare-fontan-registry/), the Georgia Tech-Children’s Hospital of Philadelphia Fontan database,84, 106 and the recently organized FORCE registry (https://fontanregistry.squarespace.com/).
Computational Efficiency
Limited computational power (or interminable computational time) is always one of the primary bottlenecks for achieving accurate Fontan simulations and comprehensive uncertainty quantifications. In the past decades, numerical algorithms for Fontan simulations have progressed, including in the field of
- 1.
- 2.
-
3.
preconditioners and time-adaptive solvers to accelerate the solution of cardiovascular simulations23, 28, 101;
-
4.
numerical methods, such as immersed boundary methods that have inherent advantages for handling complex deformation.111, 114
-
5.
Turbulence models,5, 19, 113 especially for applications of blood pumps in Fontan patients.
Nevertheless, these improvements are still far from resolving the lack of computational efficiency in capturing realistic in vivo Fontan hemodynamics. Moreover, the clinical usage of Fontan simulation, i.e., in pre-procedural planning, is hindered by the low computational efficiency of current methods. Fontan pre-procedural planning usually has a short time-frame and needs to assess the hemodynamics of multiple surgical options relatively quickly. Heretofore, a pre-procedural planning case usually proposes 2-3 options and conducts very minimal uncertainty quantifications even with super-computing clusters.92 Therefore, clinicians are generally skeptical of making clinical decisions solely relying on the observations provided by Fontan simulations.
As computer technology is evolving at an unprecedented rate nowadays, it may not be long before the next generation of supercomputers, such as quantum computers, can altogether remove the concerns of computational efficiency. Nevertheless, even before that happens, computational experts have already attempted to enhance computational efficiency by using contemporary, cutting-edge techniques, including machine learning (ML). For example, Liang et al. has demonstrated the feasibility of utilizing ML to directly estimate the pressure and flow fields inside the thoracic aorta.46 The ML is ~ 1000 times faster than a full CFD simulation, while the discrepancies between the ML-estimation and the full simulation is ~ 2%.46 Therefore, it is reasonable to assume that ML could offer a potential solution to simulating patient-specific TCPC hemodynamics in a real-time fashion given adequate data for training. This could significantly encourage the clinical application of Fontan simulations.
Summary
In the past few decades, cardiovascular simulations for Fontan patients have made substantial progress forward. In its current state, the cardiovascular simulation is a promising tool to assess patient-specific Fontan hemodynamics and provide clinical insights for better surgical outcomes, patient management, and prognosis of Fontan-related complications. However, clinical validation studies in large patient cohorts are a necessary step in the pathway toward integrating Fontan simulations into routine clinical practice. Finally, efforts to reduce simulation uncertainties and enhance computational efficiency could significantly promote the routine application of these simulations.
References
Babu-Narayan, S. V., G. Giannakoulas, A. M. Valente, W. Li, and M. A. Gatzoulis. Imaging of congenital heart disease in adults. Eur. Heart J. 37(15):1182–1195, 2016. https://doi.org/10.1093/eurheartj/ehv519.
Baretta, A., C. Corsini, W. Yang, I. E. Vignon-Clementel, A. L. Marsden, J. A. Feinstein, et al. Virtual surgeries in patients with congenital heart disease: a multi-scale modelling test case. Philos. Trans. A 2011(369):4316–4330, 1954. https://doi.org/10.1098/rsta.2011.0130.
Bazilevs, Y., M. C. Hsu, D. J. Benson, S. Sankaran, and A. L. Marsden. Computational fluid-structure interaction: methods and application to a total cavopulmonary connection. Comput. Mech. 45(1):77–89, 2009. https://doi.org/10.1007/s00466-009-0419-y.
Bertagna, L., S. Deparis, L. Formaggia, D. Forti, A. Veneziani. The LifeV library: engineering mathematics beyond the proof of concept. 2017.
Bertagna, L., A. Quaini, and A. Veneziani. Deconvolution-based nonlinear filtering for incompressible flows at moderately large Reynolds numbers. Int. J. Numer. Meth. Fl. 81(8):463–488, 2016. https://doi.org/10.1002/fld.4192.
Bossers, S. S., M. Cibis, F. J. Gijsen, M. Schokking, J. L. Strengers, R. F. Verhaart, et al. Computational fluid dynamics in Fontan patients to evaluate power loss during simulated exercise. Heart 100(9):696–701, 2014. https://doi.org/10.1136/heartjnl-2013-304969.
Bossers, S. S., M. Cibis, L. Kapusta, W. V. Potters, M. M. Snoeren, J. J. Wentzel, et al. Long-term serial follow-up of pulmonary artery size and wall shear stress in Fontan patients. Pediatr. Cardiol. 37(4):637–645, 2016. https://doi.org/10.1007/s00246-015-1326-y.
Capelli, C., E. Sauvage, G. Giusti, G. M. Bosi, H. Ntsinjana, M. Carminati, et al. Patient-specific simulations for planning treatment in congenital heart disease. Interface Focus. 8(1):20170021, 2018. https://doi.org/10.1098/rsfs.2017.0021.
Cheng, A. L., N. M. Pahlevan, D. G. Rinderknecht, J. C. Wood, and M. Gharib. Experimental investigation of the effect of non-newtonian behavior of blood flow in the Fontan circulation. Eur. J. Mech. B 68:184–192, 2018. https://doi.org/10.1016/j.euromechflu.2017.12.009.
Cheng, A. L., C. M. Takao, R. B. Wenby, H. J. Meiselman, J. C. Wood, and J. A. Detterich. Elevated low-shear blood viscosity is associated with decreased pulmonary blood flow in children with univentricular heart defects. Pediatr. Cardiol. 37(4):789–801, 2016. https://doi.org/10.1007/s00246-016-1352-4.
Chung, B., and J. R. Cebral. CFD for evaluation and treatment planning of aneurysms: review of proposed clinical uses and their challenges. Ann. Biomed. Eng. 43(1):122–138, 2015. https://doi.org/10.1007/s10439-014-1093-6.
Cibis, M., K. Jarvis, M. Markl, M. Rose, C. Rigsby, A. J. Barker, et al. The effect of resolution on viscous dissipation measured with 4D flow MRI in patients with Fontan circulation: evaluation using computational fluid dynamics. J. Biomech. 48(12):2984–2989, 2015. https://doi.org/10.1016/j.jbiomech.2015.07.039.
Conover, T., A. M. Hlavacek, F. Migliavacca, E. Kung, A. Dorfman, R. S. Figliola, et al. An interactive simulation tool for patient-specific clinical decision support in single-ventricle physiology. J. Thorac. Cardiovasc. Surg. 155(2):712–721, 2018. https://doi.org/10.1016/j.jtcvs.2017.09.046.
Daniels, C. J., E. A. Bradley, M. J. Landzberg, J. Aboulhosn, R. H. Beekman, 3rd, W. Book, et al. Fontan-associated liver disease: proceedings from the american college of cardiology stakeholders meeting, october 1 to 2, 2015, Washington DC. J. Am. Coll. Cardiol. 70(25):3173–3194, 2017. https://doi.org/10.1016/j.jacc.2017.10.045.
Dasi, L. P., R. Krishnankuttyrema, H. D. Kitajima, K. Pekkan, K. S. Sundareswaran, M. Fogel, et al. Fontan hemodynamics: importance of pulmonary artery diameter. J. Thorac. Cardiovasc. Surg. 137(3):560–564, 2009. https://doi.org/10.1016/j.jtcvs.2008.04.036.
de Leval, M. R., G. Dubini, F. Migliavacca, H. Jalali, G. Camporini, A. Redington, et al. Use of computational fluid dynamics in the design of surgical procedures: application to the study of competitive flows in cavo-pulmonary connections. J. Thorac. Cardiovasc. Surg. 1996;111(3):502-13.
de Zelicourt, D. A., and V. Kurtcuoglu. Patient-specific surgical planning, where do we stand? The example of the Fontan procedure. Ann. Biomed. Eng. 44(1):174–186, 2016. https://doi.org/10.1007/s10439-015-1381-9.
de Zelicourt, D. A., A. Marsden, M. A. Fogel, and A. P. Yoganathan. Imaging and patient-specific simulations for the Fontan surgery: current methodologies and clinical applications. Prog. Pediatr. Cardiol. 30(1–2):31–44, 2010. https://doi.org/10.1016/j.ppedcard.2010.09.005.
Delorme, Y. T., M. D. Rodefeld, and S. H. Frankel. Multiblock high order Large Eddy Simulation of powered Fontan hemodynamics: towards computational surgery. Comput. Fluids 143:16–31, 2017. https://doi.org/10.1016/j.compfluid.2016.10.032.
Dubini, G., M. R. de Leval, R. Pietrabissa, F. M. Montevecchi, R. Fumero. A numerical fluid mechanical study of repaired congenital heart defects. Application to the total cavopulmonary connection. J. Biomech. 1996;29(1):111-21. https://doi.org/10.1016/0021-9290(95)00021-6.
Esmaily Moghadam, M., Y. Bazilevs, T.-Y. Hsia, I. E. Vignon-Clementel, and A. L. Marsden. A comparison of outlet boundary treatments for prevention of backflow divergence with relevance to blood flow simulations. Comput. Mech. 48(3):277–291, 2011. https://doi.org/10.1007/s00466-011-0599-0.
Esmaily Moghadam, M., I. E. Vignon-Clementel, R. Figliola, and A. L. Marsden. A modular numerical method for implicit 0D/3D coupling in cardiovascular finite element simulations. J. Comput. Phys. 244:63–79, 2013. https://doi.org/10.1016/j.jcp.2012.07.035.
Esmaily-Moghadam, M., Y. Bazilevs, and A. L. Marsden. A new preconditioning technique for implicitly coupled multidomain simulations with applications to hemodynamics. Comput. Mech. 52(5):1141–1152, 2013. https://doi.org/10.1007/s00466-013-0868-1.
Farahmand, M., M. N. Kavarana, P. M. Trusty, and E. O. Kung. Target flow-pressure operating range for designing a failing Fontan cavopulmonary support device. IEEE Trans. Biomed. Eng. 2020. https://doi.org/10.1109/TBME.2020.2974098.
Flores, J., J. Alastruey, and Poire E. Corvera. A novel analytical approach to pulsatile blood flow in the arterial network. Ann. Biomed. Eng. 44(10):3047–3068, 2016. https://doi.org/10.1007/s10439-016-1625-3.
Fogel, M. A., R. H. Khiabani, and A. Yoganathan. Imaging for preintervention planning: pre- and post-Fontan procedures. Circ. Cardiovasc. Imaging 6(6):1092–1101, 2013. https://doi.org/10.1161/CIRCIMAGING.113.000335.
Fogel, M. A., P. M. Weinberg, A. Hoydu, A. Hubbard, J. Rychik, M. Jacobs, et al. The nature of flow in the systemic venous pathway measured by magnetic resonance blood tagging in patients having the fontan operation. J. Thorac. Cardiovasc. Surg. 114(6):1032–1041, 1997. https://doi.org/10.1016/s0022-5223(97)70017-5.
Gauthier, A., F. Saleri, and A. Veneziani. A fast preconditioner for the incompressible Navier Stokes Equations. Comput. Vis. Sci. 6(2–3):105–112, 2004. https://doi.org/10.1007/s00791-003-0114-z.
Gupta, A., C. Gillett, P. Gerard, M. M. H. Cheung, J. P. Mynard, and E. Kung. Predictive models for pulmonary artery size in Fontan patients. J. Cardiovasc. Transl. Res. 2020. https://doi.org/10.1007/s12265-020-09993-4.
Haggerty, C. M., M. Restrepo, E. Tang, D. A. de Zelicourt, K. S. Sundareswaran, L. Mirabella, et al. Fontan hemodynamics from 100 patient-specific cardiac magnetic resonance studies: a computational fluid dynamics analysis. J. Thorac. Cardiovasc. Surg. 148(4):1481–1489, 2014. https://doi.org/10.1016/j.jtcvs.2013.11.060.
Hjortdal, V. E., T. D. Christensen, S. H. Larsen, K. Emmertsen, and E. M. Pedersen. Caval blood flow during supine exercise in normal and Fontan patients. Ann. Thorac. Surg. 85(2):599–603, 2008. https://doi.org/10.1016/j.athoracsur.2007.08.062.
Hjortdal, V. E., K. Emmertsen, E. Stenbog, T. Frund, M. R. Schmidt, O. Kromann, et al. Effects of exercise and respiration on blood flow in total cavopulmonary connection: a real-time magnetic resonance flow study. Circulation 108(10):1227–1231, 2003. https://doi.org/10.1161/01.CIR.0000087406.27922.6B.
Honda, T., K. Itatani, M. Takanashi, E. Mineo, A. Kitagawa, H. Ando, et al. Quantitative evaluation of hemodynamics in the Fontan circulation: a cross-sectional study measuring energy loss in vivo. Pediatr. Cardiol. 35(2):361–367, 2014. https://doi.org/10.1007/s00246-013-0783-4.
Hsia, T. Y. Taming the Fontan with the Y-graft: a nod and a wink to the great Yu. J. Thorac. Cardiovasc. Surg. 151(6):1537–1539, 2016. https://doi.org/10.1016/j.jtcvs.2016.03.027.
Hsia, T. Y., S. Khambadkone, A. N. Redington, F. Migliavacca, J. E. Deanfield, M. R. de Leval. Effects of respiration and gravity on infradiaphragmatic venous flow in normal and Fontan patients. Circulation. 2000;102(19 Suppl 3):III148-53. https://doi.org/10.1161/01.cir.102.suppl_3.iii-148.
Khiabani, R. H., M. Restrepo, E. Tang, D. De Zelicourt, F. Sotiropoulos, M. Fogel, et al. Effect of flow pulsatility on modeling the hemodynamics in the total cavopulmonary connection. J. Biomech. 45(14):2376–2381, 2012. https://doi.org/10.1016/j.jbiomech.2012.07.010.
Khiabani, R. H., K. K. Whitehead, D. Han, M. Restrepo, E. Tang, J. Bethel, et al. Exercise capacity in single-ventricle patients after Fontan correlates with haemodynamic energy loss in TCPC. Heart 101(2):139–143, 2015. https://doi.org/10.1136/heartjnl-2014-306337.
Kung, E., A. Baretta, C. Baker, G. Arbia, G. Biglino, C. Corsini, et al. Predictive modeling of the virtual Hemi-Fontan operation for second stage single ventricle palliation: two patient-specific cases. J. Biomech. 46(2):423–429, 2013. https://doi.org/10.1016/j.jbiomech.2012.10.023.
Kung, E., C. Corsini, A. Marsden, I. Vignon-Clementel, G. Pennati, R. Figliola, et al. Multiscale modeling of superior cavopulmonary circulation: hemi-Fontan and bidirectional Glenn are equivalent. Semin. Thorac. Cardiovasc. Surg. 32(4):883–892, 2020. https://doi.org/10.1053/j.semtcvs.2019.09.007.
Kung, E., G. Pennati, F. Migliavacca, T. Y. Hsia, R. Figliola, A. Marsden, et al. A simulation protocol for exercise physiology in Fontan patients using a closed loop lumped-parameter model. J. Biomech. Eng. 2014;136(8). https://doi.org/10.1115/1.4027271.
Kung, E., A. Marsden, C. Baker, A. Giardini, R. Figliola, and T. Y. Hsia. Does TCPC power loss really affect exercise capacity? Heart 101(7):575, 2015. https://doi.org/10.1136/heartjnl-2014-307379.
Kung, E., G. Pennati, F. Migliavacca, T. Y. Hsia, R. Figliola, A. Marsden, et al. A simulation protocol for exercise physiology in Fontan patients using a closed loop lumped-parameter model. J. Biomech. Eng. T ASME 136(8):1–13, 2014. https://doi.org/10.1115/1.4027271.
Kung, E., J. C. Perry, C. Davis, F. Migliavacca, G. Pennati, A. Giardini, et al. Computational modeling of pathophysiologic responses to exercise in Fontan patients. Ann. Biomed. Eng. 43(6):1335–1347, 2015. https://doi.org/10.1007/s10439-014-1131-4.
Lagana, K., R. Balossino, F. Migliavacca, G. Pennati, E. L. Bove, M. R. de Leval, et al. Multiscale modeling of the cardiovascular system: application to the study of pulmonary and coronary perfusions in the univentricular circulation. J. Biomech. 38(5):1129–1141, 2005. https://doi.org/10.1016/j.jbiomech.2004.05.027.
Lagana, K., G. Dubini, F. Migliavacca, R. Pietrabissa, G. Pennati, A. Veneziani, et al. Multiscale modelling as a tool to prescribe realistic boundary conditions for the study of surgical procedures. Biorheology 39(3–4):359–364, 2002.
Liang, L., W. Mao, and W. Sun. A feasibility study of deep learning for predicting hemodynamics of human thoracic aorta. J. Biomech. 99:2020. https://doi.org/10.1016/j.jbiomech.2019.109544.
Liang, F., H. Senzaki, C. Kurishima, K. Sughimoto, R. Inuzuka, and H. Liu. Hemodynamic performance of the Fontan circulation compared with a normal biventricular circulation: a computational model study. Am. J. Physiol. Heart Circ. Physiol. 307(7):H1056–H1072, 2014. https://doi.org/10.1152/ajpheart.00245.2014.
Long, C. C., M. C. Hsu, Y. Bazilevs, J. A. Feinstein, and A. L. Marsden. Fluid-structure interaction simulations of the Fontan procedure using variable wall properties. Int. J. Numer. Methods Biol. 28(5):513–527, 2012. https://doi.org/10.1002/cnm.1485.
Marino, B. S., M. Fogel, L. M. R. Mercer-Rosa, Z. A. W. Wei, P. M. Trusty, M. Tree, et al. Poor Fontan geometry, hemodynamics, and computational fluid dynamics are associated with worse quality of life. Circulation; 2017-11-14 00:00:002017. p. A18082-A.
Marrero, V. L., J. A. Tichy, O. Sahni, and K. E. Jansen. Numerical study of purely viscous non-Newtonian flow in an abdominal aortic aneurysm. J. Biomech. Eng. Trans. ASME. 136(10):2014. https://doi.org/10.1115/1.4027488.
Marsden, A.L. Multi-scale Modeling of Cardiovascular Flows. 2015.
Marsden, A. L. Simulation based planning of surgical interventions in pediatric cardiology. Phys Fluids (1994). 2013;25(10):101303. https://doi.org/10.1063/1.4825031.
Marsden, A. L., and M. Esmaily-Moghadam. Multiscale modeling of cardiovascular flows for clinical decision support. Appl. Mech. Rev. 67(3):1–11, 2015. https://doi.org/10.1115/1.4029909.
Marsden, A. L., V. M. Reddy, S. C. Shadden, F. P. Chan, C. A. Taylor, and J. A. Feinstein. A new multiparameter approach to computational simulation for Fontan assessment and redesign. Congenit. Heart. Dis. 5(2):104–117, 2010. https://doi.org/10.1111/j.1747-0803.2010.00383.x.
McClung, N., J. Glidewell, and S. L. Farr. Financial burdens and mental health needs in families of children with congenital heart disease. Congenit. Heart Dis. 13(4):554–562, 2018. https://doi.org/10.1111/chd.12605.
Migliavacca, F., G. Dubini, E. L. Bove, and M. R. de Leval. Computational fluid dynamics simulations in realistic 3-D geometries of the total cavopulmonary anastomosis: the influence of the inferior caval anastomosis. J Biomech Eng-T ASME. 125(6):805–813, 2003. https://doi.org/10.1115/1.1632523.
Mirabella, L., C. M. Haggerty, T. Passerini, M. Piccinelli, A. J. Powell, P. J. Del Nido, et al. Treatment planning for a TCPC test case: a numerical investigation under rigid and moving wall assumptions. Int. J. Numer. Method Biomed. Eng. 29(2):197–216, 2013. https://doi.org/10.1002/cnm.2517.
Murakami, H., N. Yoshimura, J. Kitahara, S. Otaka, F. Ichida, and T. Misaki. Collision of the caval flows caused early failure of the Fontan circulation. J. Thorac. Cardiovasc. Surg. 132(5):1235–1236, 2006. https://doi.org/10.1016/j.jtcvs.2006.08.006.
Nakamura, Y., T. Yagihara, K. Kagisaki, I. Hagino, and J. Kobayashi. Pulmonary arteriovenous malformations after a Fontan operation in the left isomerism and absent inferior vena cava. Eur. J. Cardiothorac. Surg. 36(1):69–76, 2009. https://doi.org/10.1016/j.ejcts.2009.02.046.
Nguyen, K. L., F. Han, Z. Zhou, D. Z. Brunengraber, I. Ayad, D. S. Levi, et al. 4D MUSIC CMR: value-based imaging of neonates and infants with congenital heart disease. J. Cardiovasc. Magn. Reson. 19(1):40, 2017. https://doi.org/10.1186/s12968-017-0352-8.
Ochiai, Y., Y. Imoto, M. Sakamoto, A. Sese, M. Tsukuda, M. Watanabe, et al. Longitudinal growth of the autologous vessels above and below the Gore-Tex graft after the extracardiac conduit Fontan procedure. Eur. J. Cardiothorac. Surg. 37(5):996–1001, 2010. https://doi.org/10.1016/j.ejcts.2009.12.010.
Ochiai, Y., Y. Imoto,M. Sakamoto, T. Kajiwara, A. Sese, M. Watanabe, et al. Mid-term follow-up of the status of Gore-Tex graft after extracardiac conduit Fontan procedure. Eur. J. Cardiothorac. Surg. 2009;36(1):63-7; discussion 7-8. https://doi.org/10.1016/j.ejcts.2009.02.013.
Ovroutski, S., P. Ewert, V. Alexi-Meskishvili, K. Holscher, O. Miera, B. Peters, et al. Absence of pulmonary artery growth after Fontan operation and its possible impact on late outcome. Ann. Thorac. Surg. 87(3):826–832, 2009. https://doi.org/10.1016/j.athoracsur.2008.10.075.
Pekkan, K., D. Frakes, D. De Zelicourt, C. W. Lucas, W. J. Parks, and A. P. Yoganathan. Coupling pediatric ventricle assist devices to the Fontan circulation: simulations with a lumped-parameter model. ASAIO J. 51(5):618–628, 2005. https://doi.org/10.1097/01.mat.0000176169.73987.0d.
Puelz, C., S. Acosta, B. Riviere, D. J. Penny, K. M. Brady, and C. G. Rusin. A computational study of the Fontan circulation with fenestration or hepatic vein exclusion. Comput. Biol. Med. 89:405–418, 2017. https://doi.org/10.1016/j.compbiomed.2017.08.024.
Quarteroni, A. C. A., and C. Vergara. Geometric multiscale modeling of the cardiovascular system, between theory and practice. Comput. Method Appl. M. 302:193–252, 2016. https://doi.org/10.1016/j.cma.2016.01.007.
Restrepo, M., M. Luffel, J. Sebring, K. Kanter, P. Del Nido, A. Veneziani, et al. Surgical planning of the total cavopulmonary connection: robustness analysis. Ann. Biomed. Eng. 43(6):1321–1334, 2015. https://doi.org/10.1007/s10439-014-1149-7.
Restrepo, M., L. Mirabella, E. Tang, C. M. Haggerty, R. H. Khiabani, F. Fynn-Thompson, et al. Fontan pathway growth: a quantitative evaluation of lateral tunnel and extracardiac cavopulmonary connections using serial cardiac magnetic resonance. Ann. Thorac. Surg. 97(3):916–922, 2014. https://doi.org/10.1016/j.athoracsur.2013.11.015.
Restrepo, M., E. Tang, C. M. Haggerty, R. H. Khiabani, L. Mirabella, J. Bethel, et al. Energetic implications of vessel growth and flow changes over time in Fontan patients. Ann. Thorac. Surg. 99(1):163–170, 2015. https://doi.org/10.1016/j.athoracsur.2014.08.046.
Reza, M. M. S., and A. Arzani. A critical comparison of different residence time measures in aneurysms. J. Biomech. 88:122–129, 2019. https://doi.org/10.1016/j.jbiomech.2019.03.028.
Rijnberg, F. M., M. G. Hazekamp, J. J. Wentzel, P. J. H. de Koning, J. J. M. Westenberg, M. R. M. Jongbloed, et al. Energetics of blood flow in cardiovascular disease: concept and clinical implications of adverse energetics in patients with a fontan circulation. Circulation 137(22):2393–2407, 2018. https://doi.org/10.1161/CIRCULATIONAHA.117.033359.
Roldan-Alzate, A., S. Garcia-Rodriguez, P. V. Anagnostopoulos, S. Srinivasan, O. Wieben, and C. J. Francois. Hemodynamic study of TCPC using in vivo and in vitro 4D Flow MRI and numerical simulation. J. Biomech. 48(7):1325–1330, 2015. https://doi.org/10.1016/j.jbiomech.2015.03.009.
Ryu, K., T. M. Healy, A. E. Ensley, S. Sharma, C. Lucas, and A. P. Yoganathan. Importance of accurate geometry in the study of the total cavopulmonary connection: computational simulations and in vitro experiments. Ann. Biomed. Eng. 29(10):844–853, 2001. https://doi.org/10.1114/1.1408930.
Sankaran, S., and A. L. Marsden. A stochastic collocation method for uncertainty quantification and propagation in cardiovascular simulations. J. Biomech. Eng. Trans. ASME. 133(3):2011. https://doi.org/10.1115/1.4003259.
Schiavazzi, D. E., G. Arbia, C. Baker, A. M. Hlavacek, T. Y. Hsia, A. L. Marsden, et al. Uncertainty quantification in virtual surgery hemodynamics predictions for single ventricle palliation. Int. J. Numer. Methods Biomed. Eng. 32(3):2016. https://doi.org/10.1002/cnm.2737.
Schiavazzi, D. E., A. Baretta, G. Pennati, T. Y. Hsia, and A. L. Marsden. Patient-specific parameter estimation in single-ventricle lumped circulation models under uncertainty. Int. J. Numer. Method Biomed. Eng. 33(3):1–34, 2017. https://doi.org/10.1002/cnm.2799.
Shachar, G. B., B. P. Fuhrman, Y. Wang, R. V. Lucas, Jr, and J. E. Lock. Rest and exercise hemodynamics after the Fontan procedure. Circulation 65(6):1043–1048, 1982. https://doi.org/10.1161/01.cir.65.6.1043.
Shadden, S. C., and A. Arzani. Lagrangian postprocessing of computational hemodynamics. Ann. Biomed. Eng. 43(1):41–58, 2015. https://doi.org/10.1007/s10439-014-1070-0.
Sharma, S., S. Goudy, P. Walker, S. Panchal, A. Ensley, K. Kanter, et al. In vitro flow experiments for determination of optimal geometry of total cavopulmonary connection for surgical repair of children with functional single ventricle. J. Am. Coll. Cardiol. 27(5):1264–1269, 1996. https://doi.org/10.1016/0735-1097(95)00598-6.
Simeone, R. M., M. E. Oster, C. H. Cassell, B. S. Armour, D. T. Gray, and M. A. Honein. Pediatric inpatient hospital resource use for congenital heart defects. Birth Defects Res. A 100(12):934–943, 2014. https://doi.org/10.1002/bdra.23262.
Srivastava, D., T. Preminger, J. E. Lock, V. Mandell, J. F. Keane, J. E. Mayer, Jr, et al. Hepatic venous blood and the development of pulmonary arteriovenous malformations in congenital heart disease. Circulation 92(5):1217–1222, 1995. https://doi.org/10.1161/01.cir.92.5.1217.
Sundareswaran, K. S., D. de Zelicourt, S. Sharma, K. R. Kanter, T. L. Spray, J. Rossignac, et al. Correction of pulmonary arteriovenous malformation using image-based surgical planning. JACC Cardiovasc. Imaging. 2(8):1024–1030, 2009. https://doi.org/10.1016/j.jcmg.2009.03.019.
Tang, E., Z. Wei, M. A. Fogel, A. Veneziani, A. P. Yoganathan. Fluid-structure interaction simulation of an intra-atrial Fontan connection. Biology. 2020;9(12). https://doi.org/10.3390/biology9120412.
Tang, E., M. Restrepo, C. M. Haggerty, L. Mirabella, J. Bethel, K. K. Whitehead, et al. Geometric characterization of patient-specific total cavopulmonary connections and its relationship to hemodynamics. JACC Cardiovasc. Imaging. 7(3):215–224, 2014. https://doi.org/10.1016/j.jcmg.2013.12.010.
Tang, E., Z. A. Wei, P. M. Trusty, K. K. Whitehead, L. Mirabella, A. Veneziani, et al. The effect of respiration-driven flow waveforms on hemodynamic metrics used in Fontan surgical planning. J. Biomech. 82:87–95, 2019. https://doi.org/10.1016/j.jbiomech.2018.10.013.
Tang, E., Z. A. Wei, K. K. Whitehead, R. H. Khiabani, M. Restrepo, L. Mirabella, et al. Effect of Fontan geometry on exercise haemodynamics and its potential implications. Heart 103(22):1806–1812, 2017. https://doi.org/10.1136/heartjnl-2016-310855.
Tatum, G. H., G. Sigfusson, J. A. Ettedgui, J. L. Myers, S. E. Cyran, H. S. Weber, et al. Pulmonary artery growth fails to match the increase in body surface area after the Fontan operation. Heart 92(4):511–514, 2006. https://doi.org/10.1136/hrt.2005.070243.
Throckmorton, A. L., S. Lopez-Isaza, and W. Moskowitz. Dual-pump support in the inferior and superior vena cavae of a patient-specific fontan physiology. Artif. Organs 37(6):513–522, 2013. https://doi.org/10.1111/aor.12039.
Tree, M., Z. A. Wei, B. Munz, K. Maher, S. Deshpande, T. Slesnick, et al. A method for in vitro TCPC compliance verification. J. Biomech. Eng. Trans. ASME. 2017;139(6):064502-. https://doi.org/10.1115/1.4036474.
Tree, M., Z. A. Wei, P. M. Trusty, V. Raghav, M. Fogel, K. Maher, et al. Using a novel in vitro fontan model and condition-specific real-time MRI data to examine hemodynamic effects of respiration and exercise. Ann. Biomed. Eng. 46(1):135–147, 2018. https://doi.org/10.1007/s10439-017-1943-0.
Trusty, P. M., Z. Alan Wei, M. A. Fogel, K. Maher, S. R. Deshpande, and A. P. Yoganathan. Computational modeling of a right-sided Fontan assist device: effectiveness across patient anatomies and cannulations. J. Biomech. 109:2020. https://doi.org/10.1016/j.jbiomech.2020.109917.
Trusty, P. M., T. C. Slesnick, Z. A. Wei, J. Rossignac, K. R. Kanter, M. A. Fogel, et al. Fontan surgical planning: previous accomplishments, current challenges, and future directions. J. Cardiovasc. Transl. Res. 11(2):133–144, 2018. https://doi.org/10.1007/s12265-018-9786-0.
Trusty, P. M., Z. A. Wei, J. Rychik, A. Graham, P. A. Russo, L. F. Surrey, et al. Cardiac magnetic resonance-derived metrics are predictive of liver fibrosis in fontan patients. Ann. Thorac. Surg. 109(6):1904–1911, 2020. https://doi.org/10.1016/j.athoracsur.2019.09.070.
Trusty, P. M., Z. Wei, J. Rychik, P. A. Russo, L. F. Surrey, D. J. Goldberg, et al. Impact of hemodynamics and fluid energetics on liver fibrosis after Fontan operation. J Thorac Cardiov Sur. 156(1):267–275, 2018. https://doi.org/10.1016/j.jtcvs.2018.02.078.
Trusty, P. M., Z. Wei, M. Sales, K. R. Kanter, M. A. Fogel, A. P. Yoganathan, et al. Y-graft modification to the Fontan procedure: Increasingly balanced flow over time. J. Thorac. Cardiovasc. Surg. 159(2):652–661, 2020. https://doi.org/10.1016/j.jtcvs.2019.06.063.
Trusty, P. M., Z. A. Wei, T. C. Slesnick, K. R. Kanter, T. L. Spray, M. A. Fogel, et al. The first cohort of prospective Fontan surgical planning patients with follow-up data: how accurate is surgical planning? J. Thorac. Cardiovasc. Surg. 157(3):1146–1155, 2019. https://doi.org/10.1016/j.jtcvs.2018.11.102.
Trusty, P. M., Z. Wei, M. Tree, K. R. Kanter, M. A. Fogel, A. P. Yoganathan, et al. Local hemodynamic differences between commercially available Y-grafts and traditional Fontan baffles under simulated exercise conditions: implications for exercise tolerance. Cardiovasc. Eng. Technol. 8(3):390–399, 2017. https://doi.org/10.1007/s13239-017-0310-5.
Updegrove, A., N. M. Wilson, J. Merkow, H. Lan, A. L. Marsden, and S. C. Shadden. SimVascular: an open source pipeline for cardiovascular simulation. Ann. Biomed. Eng. 45(3):525–541, 2017. https://doi.org/10.1007/s10439-016-1762-8.
van der Ven, J. P. G, E. van den Bosch, A. Bogers, W. A. Helbing. State of the art of the Fontan strategy for treatment of univentricular heart disease. F1000Res. 2018;7. https://doi.org/10.12688/f1000research.13792.1.
Veneziani, A., and C. Vergara. An approximate method for solving incompressible Navier-Stokes problems with flow rate conditions. Comput. Method Appl. M 196(9–12):1685–1700, 2007. https://doi.org/10.1016/j.cma.2006.09.011.
Veneziani, A., and U. Villa. ALADINS: an algebraic splitting time adaptive solver for the Incompressible Navier-Stokes equations. J. Comput. Phys. 238:359–375, 2013. https://doi.org/10.1016/j.jcp.2012.11.049.
Vignon-Clementel, I. E., C. A. Figueroa, K. E. Jansen, and C. A. Taylor. Outflow boundary conditions for three-dimensional finite element modeling of blood flow and pressure in arteries. Comput Method Appl M. 195(29–32):3776–3796, 2006. https://doi.org/10.1016/j.cma.2005.04.014.
Voges, I., M. Jerosch-Herold, C. Hart, J. Scheewe, D. D. Gabbert, E. Pardun, et al. Anatomical and functional assessment of the intra-atrial lateral tunnel in the Fontan circulation. Eur. J. Cardiothorac. Surg. 44(3):462–467, 2013. https://doi.org/10.1093/ejcts/ezt066.
Wang, C., K. Pekkan, D. de Zelicourt, M. Horner, A. Parihar, A. Kulkarni, et al. Progress in the CFD modeling of flow instabilities in anatomical total cavopulmonary connections. Ann. Biomed. Eng. 35(11):1840–1856, 2007. https://doi.org/10.1007/s10439-007-9356-0.
Wei, H., A. L. Cheng, and N. M. Pahlevan. On the significance of blood flow shear-rate-dependency in modeling of Fontan hemodynamics. Eur. J. Mech. B. Fluids 84:1–14, 2020. https://doi.org/10.1016/j.euromechflu.2020.05.011.
Wei, Z. A., C. Huddleston, P. M. Trusty, S. Singh-Gryzbon, M. A. Fogel, A. Veneziani, et al. Analysis of inlet velocity profiles in numerical assessment of Fontan hemodynamics. Ann. Biomed. Eng. 47(11):2258–2270, 2019. https://doi.org/10.1007/s10439-019-02307-z.
Wei, Z., S. Singh-Gryzbon, P. M. Trusty, C. Huddleston, Y. Zhang, M. A. Fogel, et al. Non-Newtonian effects on patient-specific modeling of Fontan hemodynamics. Ann. Biomed. Eng. 48(8):2204–2217, 2020. https://doi.org/10.1007/s10439-020-02527-8.
Wei, Z. A., M. Tree, P. M. Trusty, W. Wu, S. Singh-Gryzbon, and A. Yoganathan. The advantages of viscous dissipation rate over simplified power loss as a fontan hemodynamic metric. Ann. Biomed. Eng. 46(3):404–416, 2018. https://doi.org/10.1007/s10439-017-1950-1.
Wei, Z. A., P. M. Trusty, M. Tree, C. M. Haggerty, E. Tang, M. Fogel, et al. Can time-averaged flow boundary conditions be used to meet the clinical timeline for Fontan surgical planning? J. Biomech. 50:172–179, 2017. https://doi.org/10.1016/j.jbiomech.2016.11.025.
Wei, Z., K. K. Whitehead, R. H. Khiabani, M. Tree, E. Tang, S. M. Paridon, et al. Respiratory effects on fontan circulation during rest and exercise using real-time cardiac magnetic resonance imaging. Ann. Thorac. Surg. 101(5):1818–1825, 2016. https://doi.org/10.1016/j.athoracsur.2015.11.011.
Wei, Z. L. A., and Z. Q. C. Zheng. Fluid-structure interaction simulation on energy harvesting from vortical flows by a passive heaving foil. J. Fluid Eng. Trans. ASME. 2018. https://doi.org/10.1115/1.4037661.
Whitehead, K. K., K. Pekkan, H. D. Kitajima, S. M. Paridon, A. P. Yoganathan, and M. A. Fogel. Nonlinear power loss during exercise in single-ventricle patients after the Fontan: insights from computational fluid dynamics. Circulation 116(11 Suppl):I165–I171, 2007. https://doi.org/10.1161/CIRCULATIONAHA.106.680827.
Xu, H. J., D. Baroli, F. Di Massimo, A. Quaini, and A. Veneziani. Backflow stabilization by deconvolution-based large eddy simulation modeling. J Comput Phys. 2020. https://doi.org/10.1016/j.jcp.2019.109103.
Mittal, R., J. H. Seo, V. Vedula, Y. J. Choi, H. Liu, H. H. W. Huang, et al. Computational modeling of cardiac hemodynamics: current status and future outlook. J Comput Phys. 305:1065–1082, 2016. https://doi.org/10.1016/j.jcp.2015.11.022.
Yang, W., F. P. Chan, V. M. Reddy, A. L. Marsden, and J. A. Feinstein. Flow simulations and validation for the first cohort of patients undergoing the Y-graft Fontan procedure. J. Thorac. Cardiovasc. Surg. 149(1):247–255, 2015. https://doi.org/10.1016/j.jtcvs.2014.08.069.
Yang, W., I. E. Vignon-Clementel, G. Troianowski, V. M. Reddy, J. A. Feinstein, and A. L. Marsden. Hepatic blood flow distribution and performance in conventional and novel Y-graft Fontan geometries: a case series computational fluid dynamics study. J. Thorac. Cardiovasc. Surg. 143(5):1086–1097, 2012. https://doi.org/10.1016/j.jtcvs.2011.06.042.
Yuan, C., L. M. Mitsumori, M. S. Ferguson, N. L. Polissar, D. Echelard, G. Ortiz, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation 104(17):2051–2056, 2001. https://doi.org/10.1161/hc4201.097839.
Zélicourt, Dd. Ge. L., C. Wang, F. Sotiropoulos, A. Gilmanov, A. Yoganathan. Flow simulations in arbitrarily complex cardiovascular anatomies—An unstructured Cartesian grid approach. Comput. Fluids. 2009;38(9):1749-62. https://doi.org/10.1016/j.compfluid.2009.03.005.
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Zhenglun Wei and Mark Fogel report no conflict of interest.
Additional information
Associate Editor John Oshinski oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wei, Z.A., Fogel, M.A. Engineering Perspective on Cardiovascular Simulations of Fontan Hemodynamics: Where Do We Stand with a Look Towards Clinical Application. Cardiovasc Eng Tech 12, 618–630 (2021). https://doi.org/10.1007/s13239-021-00541-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-021-00541-y