Abstract
The Fontan surgery for single ventricle heart defects is a typical example of a clinical intervention in which patient-specific computational modeling can improve patient outcome: with the functional heterogeneity of the presenting patients, which precludes generic solutions, and the clear influence of the surgically-created Fontan connection on hemodynamics, it is acknowledged that individualized computational optimization of the post-operative hemodynamics can be of clinical value. A large body of literature has thus emerged seeking to provide clinically relevant answers and innovative solutions, with an increasing emphasis on patient-specific approaches. In this review we discuss the benefits and challenges of patient-specific simulations for the Fontan surgery, reviewing state of the art solutions and avenues for future development. We first discuss the clinical impact of patient-specific simulations, notably how they have contributed to our understanding of the link between Fontan hemodynamics and patient outcome. This is followed by a survey of methodologies for capturing patient-specific hemodynamics, with an emphasis on the challenges of defining patient-specific boundary conditions and their extension for prediction of post-operative outcome. We conclude with insights into potential future directions, noting that one of the most pressing issues might be the validation of the predictive capabilities of the developed framework.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Simulation-based optimization is a concept that is progressively making its way from engineering and industrial applications to the biomedical area as a promising tool to plan and/or optimize clinical treatments. The idea is in principle quite simple: model the initial state of the system and clinical intervention, compute the resultant performance and iterate until an optimum is reached. In practice, each of these steps represents a challenge of its own, especially for biomedical applications where the system to be modeled (i.e., the human body or part of it) involves numerous feedback mechanisms, dynamic adaptation and remodeling, growth and aging, most of which are still incompletely understood. However, this is not to mean that computer-aided intervention planning is a doomed enterprise. Indeed, precisely because of the complexity of living systems, numerical models can be a strong asset by isolating the effect of different treatments and procedures, provided that (1) the optimization problem is properly set, and (2) the numerical model captures the mechanisms relevant for that problem.
These aspects are at the core of the current review. We will support our discussion with an example from the cardiovascular area, namely the Fontan surgery for congenital heart defects, which has attracted significant attention in terms of numerical modeling and virtual surgical planning and also offers valuable discussion points in terms of the interaction between local surgical alterations and global system adaptation. After a brief introduction to the Fontan procedure, we will provide a concrete example of patient-specific surgical planning followed by a discussion that focuses on what can be learnt from existing studies to reduce the parameter space or guide the optimization process, and strategies developed to impose patient-specific boundary conditions.
Single-Ventricle Heart Defects and Fontan Hemodynamics
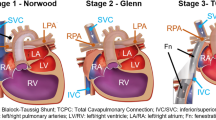
Single ventricle heart defects describe a set of congenital conditions that result in oxygenated and deoxygenated blood mixing (Fig. 1), cyanosis, reduced systemic perfusion pressure and poor prognosis if left untreated.17 The Fontan procedure seeks to restore normal oxygenation levels through a complete bypass of the right side of the heart via the connection of the venae cavae to the pulmonary arteries (PAs), see Fig. 1. In its current form, the procedure consists of a total cavopulmonary connection (TCPC) performed in two to three surgical stages. The first stage, performed in neonates, entails the reconstruction of the aortic root if hypoplastic and insertion of a systemic to pulmonary shunt, bridging patients to the actual TCPC. The superior vena cava (SVC) is connected to the PAs in the second stage followed by that of the inferior vena cava (IVC) in stage three. The third stage is typically completed between 2 and 5 years of age, the main variants being intra-atrial or extra-cardiac Fontan baffles or Y-grafts (Fig. 2).
Schematic representation of the normal, single ventricle and Fontan circulations. The single ventricle heart schematic illustrates a hypoplastic left heart syndrome with an atrial septal defect (1), a hypoplastic aorta (2), patent ductus arteriosus (3) and a hypoplastic left ventricle (4). Normal and single ventricle schematics from: https://en.wikipedia.org/wiki/Norwood_procedure#/media/File:Hypoplastic_left_heart_syndrome.svg, released to the public domain.
Most common surgical variations used to connect the IVC to the PAs. (a) Intra-atrial TCPC, wherein a prosthetic patch is placed within the atrium to form a channel with controlled diameter. (b) Extracardiac TCPC using a cylindrical graft to route the IVC around the heart up to the PAs. (c) Extracardiac Y-graft including a bifurcation in the conduit to try and optimize hepatic flow distribution. IVC inferior vena cava, SVC superior vena cava, RPA right pulmonary artery, LPA left pulmonary artery, PAs pulmonary arteries, TCPC total cavopulmonary connection.
The continuous improvements in surgical techniques and peri-operative care of the single ventricle patients have reduced post-operative mortality rates to about 1% and allowed patients with increasingly complex etiologies to benefit from the procedure.15 Yet, these improvements also came with the sobering observation that patients were prone to numerous long-term complications.17 While no doubt multi-factorial, a number of these have been associated with the hemodynamics of the surgically created bypass connection, driving an increasing number of fluid dynamic investigations with the hope of ultimately improving patient outcome.
Long-Term Complications and Their Relation to Fontan Hemodynamics
The Fontan circulation presents a drastic variation from the biventricular one, in that pulmonary and systemic circulations are placed in series without an intercalated pumping chamber. When compared to healthy subjects, these patients typically feature high central venous pressures, low trans-pulmonary gradients, limited preload due to low trans-pulmonary blood flow and low cardiac indices. Vascular resistance upstream of the atrium is one of the most important determinants of preload and cardiac output at rest and during exercise in these patients,9 calling for minimization of both pulmonary and TCPC resistances. Reducing these resistances should also lower the central venous pressure and therewith the risks of protein-losing enteropathy and liver dysfunction.32 Pulmonary arteriovenous malformations (PAVMs) have been associated with the deprivation of a hepatic factor, either due to liver dysfunction or to mal-distribution of the hepatic flow to one or both of the lungs as a result of suboptimal TCPC geometry.36 The reviews in Refs. 17, 32 are good starting points for further reading on Fontan complications and failure modes.
Need for Individualized Solutions
Based on all of the above, central venous pressures, TCPC resistance or energy dissipation (quantified in terms of power losses, PL, indexed PL, iPL, or power efficiency) and hepatic flow distribution (HFD) are the key factors that have been considered to quantify the performance of alternate surgical designs. Wall shear stresses (WSS), particle shear stress histories and residence times have also been characterized to assess the risk of hemolysis or thrombus formation. However, the anatomic heterogeneity associated with single-ventricle heart defects precludes a ‘‘one-size-fits-all’’ approach to Fontan surgery. In addition, different performance targets (for example minimizing PL vs. optimizing HFD) may lead to conflicting recommendations, making it challenging to identify the hemodynamically optimal surgical design for a patient, especially in complex cases. Simulation-based virtual surgical planning has thus emerged as an attractive option to optimize the TCPC design on a patient-specific basis.
Surgical Planning Example
The surgical planning paradigm for the Fontan surgery consists of five major steps: (1) clinical data acquisition and processing, (2) simulation of the pre-operative state when relevant, (3) virtual surgery, and (4) simulation of the post-operative state and performance prediction. These are illustrated in Figs. 3 and 4, based on data from6 for a patient with an interrupted IVC and a persistent left SVC (Fig. 3a). The patient had previously received an extra-cardiac connection of the hepatic veins to the PAs, which was taken down due to a clot in the hepatic baffle. After removal of the extra-cardiac connection, this patient was diagnosed with severe bilateral PAVMs and was recommended for a second TCPC to restore hepatic flow to the PAs. A total of fourteen options were investigated (Fig. 3b), looking into the impact of the Fontan baffle type (extra-cardiac vs. intra-atrial) and offset (Options 1 through 9), as well as more innovative solutions such as different combination of hepatic and AZ flows (Options 10–12) and options dividing the hepatic flow in two branches (Option 13–14). 3D reconstructions of the patient’s heart and great vessels (Fig. 3a) were included in the virtual surgery interface to ensure that the designed options met the anatomical constraints imposed by surrounding organs. Post-operative conditions were modeled using time-averaged pre-operative inlet flow rates and parametrically varying the right to left pulmonary flow ratio (respectively RPA and LPA) to capture possible post-operative evolutions of the outlet boundary conditions as a result of lung remodeling (Fig. 4). Deprivation of hepatic blood (low HFD) induces PAVMs which in effect reduce the resistance of the affected lung and thereby increase the share of the total blood flow going through it. Arrows on the HFD plots (Figs. 4a and 4b) depict possible evolutions of the RPA/LPA flow ratio based on the predicted HFD. Optimization objectives were to minimize PL and achieve close to 50/50 HFD for global flow distributions close to 50/50 as well. PL was not a strong discriminant among investigated options except for option 10, in which combining all lower venous returns into the relatively narrow AZ significantly increased PL. In terms of HFD, aiming for the mid-PA appeared as a robust strategy, with little sensitivity to the exact baffle design and anastomosis location. Recombining azygous and hepatic flow into the Fontan baffle could also be recommended as a mean to reduce HFD sensitivity to the global flow distribution and avoid detrimental flow stagnation in the Fontan baffle.
Virtual surgery example. (a) In vivo anatomy. Structures involved in the TCPC construct are shown in grey while the heart and aorta are shown in red. The patient featured a persistent LSVC and an interrupted IVC with azygous continuation. The latter implies that most of the inferior venous return flows through the azygous vein, while only the hepatic venous return reaches the inferior aspect of the atrium. (b) Virtual surgical options. Figure modified from Ref. 6. (L)SVC (left) superior vena cava, RPA right pulmonary artery, LPA left pulmonary artery, Mid-PA pulmonary artery segment lying in between the SVC and LSVC, HepV hepatic veins, AZ azygous vein.
Comparative performance maps for the thirteen surgical options shown in Fig. 3b. (a, b) Quality of the hepatic flow distribution as a function of the global flow distribution. Points falling in the grey area (i.e., HFD levels below 20% or GFD levels below 30% for one of the two lungs) are considered as unacceptable. The desired operating points are less clearly defined but should fall within the white area. Linear regressions are provided for groups of options with similar behavior. The blue arrows indicate expected GFD evolution for extreme HFD values. (c–e) Power loss performance as a function of the GFD to the RPA. With the exception of option 9, all options yielded similar power loss levels. Figure based on data from Ref. 6. HFD hepatic flow distribution, GFD global flow distribution, RPA right pulmonary artery, HFD to RPA share of the hepatic flow going to the RPA, GFD to RPA share of the total venous return going to the RPA.
Similar frameworks have been developed by different groups. Differences include methods used for clinical data reconstruction, the use of manual anatomical modification or automated shape optimization,44 numerical solvers, performance parameters, boundary conditions and approaches to possible post-operative adaptation. In this paper, we focus on the latter points. We will review prior patient-specific investigations of the TCPC hemodynamics in an effort to identify directions for the optimization process, and discuss strategies to include patient-specific boundary conditions and extend them to the post-operative state.
Patient-Specific Simulations of the Fontan, What have we Learned?
Optimizing the Energetic Efficiency of the TCPC as Means to Improve Patient Outcome
At the core of the Fontan surgical planning approach is the idea that optimizing TCPC hemodynamics will ultimately lead to improved patient outcome. Countering that hypothesis is the ongoing debate about extra-cardiac and intra-atrial connections. While extra-cardiacs had been expected to outperform intra-atrials due to their smoother Fontan pathway, patient-specific simulations did not report any significant differences in PL between the two modalities12,42 raising the question as to whether the energetics of TCPC connection could be optimized any further.
Yet, two additional facts should be considered: first, PL are closely related to flow rates and body surface area (BSA), which may be confounding factors. Second intra-atrial and extra-cardiac connections share quite a few design characteristics so that a finer geometrical description might be required. Based on the above, Dasi et al. 4 derived a normalized expression for the TCPC PL, which offers a single metric (iPL) representative of the resistance offered by a given design but independent of BSA and systemic venous return. Correlation of the geometrical characteristics and results of more than 100 patient-specific simulations38 demonstrated that minimum PA and Fontan pathway diameters were the strongest predictors for iPL, while other design parameters such as curvature, offset or flaring only had second order effects. These results emphasize that more than the connection type, vessel dimensions (and through them the TCPC energetics) might be better candidates to search for clinical correlates.
Lumped-parameter models of the whole circulation have been useful in assessing the above hypothesis. A multi-scale study of two 2nd stage anatomies21 showed that PL in the superior cavopulmonary connection represented up to 13% of the total PL in the pulmonary circulation and less than 2% of the total ventricular power. The authors argue that these values should have minimal impact on the overall cardiac performance. Yet, these numbers do not appear entirely negligible, all the more that they only represent the contribution of the superior cavopulmonary connection. The resistances of completed patient-specific TCPCs were shown to account for 15–20% of the PVR at rest.12,37 When included in an albeit simple lumped parameter model of the Fontan circulation, higher resistance TCPCs significantly limited the ventricular preload, leading to a blunted increase in cardiac output with heart rate suggesting a limited exercise capacity.37 But it was only very recently that clinical relationships were established demonstrating that TCPC iPL were negatively correlated to aerobic threshold for exercise19 and to resting ventricular end-diastolic and stroke volumes.13 Lack of correlation between TCPC PL and clinical outcome was attributed to the confounding effects of BSA and cardiac output, which are effectively removed in the normalized iPL expression.4 Similarly, power efficiency (defined as the ratio of output to input power) was noted to introduce a spurious dependence on central venous pressure (due to the division by the input power),39 again emphasizing the importance of appropriate metrics to reach clinically significant conclusions.
Take Home Message Although not the sole contributor, TCPC energetics do have an impact on single-ventricle function and “highly” dissipative connection designs can limit patients’ exercise capacity. Optimizing Fontan hemodynamics can thus contribute to improving patients’ quality of life, potentially allowing them to play and do sports like other children their age. The definition of what “too high” really means still remains an open question and warrants further exploration. Another important message is that hemodynamic metrics should be properly normalized in order to remove confounding effects. The ever increasing scientific understanding of the main parameters influencing these metrics should be incorporated into the clinical analysis to better define patient subgroups and underlying relationships.
Bigger is not Always Better
As mentioned above, minimum baffle and PA diameters have been identified as the two main determinants of iPL,12,38 setting the priority on the correction of local stenoses and avoidance of undersized baffles. However, as highlighted both in idealized14 and patient-specific43 configurations, bigger is not always better as excessive vessel dimensions favor the onset of flow stagnation regions and potential thrombosis. The challenge arises from the fact that the notion of “excessive” is not purely geometric but also depends on flow rates.7 In patients with an interrupted IVC, for example, most of the lower systemic venous return is redirected to the azygous vein and from there to the superior aspect of the TCPC, so that the Fontan pathway only carries the hepatic blood (i.e., only 10–20% of the cardiac output as opposed to 50–60% in other patients). When combined with regular-sized Fontan pathways, this results in a low energy stream that cannot counteract nor mix properly with the superior venous returns leading to recurrent cases of PAVMs (Fig. 5). Accordingly, surgical options suggested for these patients compromised an increase in PL in favor of increased energy and mixing.7
Flow interactions and distributions do not only depend on geometry but also on velocity and energy: comparison of two virtual surgery options in a patient with interrupted IVC. In this patient, the hepatic veins carried 10% of the cardiac output, while the azygous, SVC and left SVC respectively carried 31, 40 and 19% of the cardiac output. Results are shown for a global flow distribution of 50/50 RPA/LPA. (a) Because of the low hepatic flow rate, a simple Y graft resulted in deep penetration of the SVC flow into the right branch of the graft, flow stagnation and unilateral HFD to the LPA. (b) Recombining all lower systemic venous returns into the Fontan pathway may avoid such detrimental flow patterns. Figure modified from Ref. 6. (L)SVC (left) superior vena cava, HepV hepatic veins, AZ azygous vein, RPA right pulmonary artery, LPA left pulmonary artery, HFD to RPA hepatic flow distribution to the RPA.
Establishment of the optimal vessel size is further challenged by the fact that vessel dimensions are not only governed by the surgical intervention but also by post-operative growth and remodeling. On the pulmonary side, for example, Nakata indices (defined as the sum of the PA diameters normalized by body surface area) have generally been noted to decrease after each stage of the Fontan procedure. Originally perceived as the result of constrained growth due to the surgical procedure, recent evidence hint towards a natural remodeling response to the post-operative reduction in cardiac-output and absence of pulsatility, which may induce both Nakata index increase or decrease depending on the original vessel dimensions and post-operative hemodynamics.16 This observation has implications both for the need (or lack thereof) to enlarge small native PAs and for the evolution of the PA geometry and resistances in the post-operative period. Similar decrease in post-operative normalized diameters has been noted for the SVC and intra-atrial Fontan baffles34 but at different rates, suggesting that the TCPC geometry may not be assumed to simply scale up as the patient grows nor to scale with body surface area. While extra-cardiac baffles do not grow, intra-atrial ones do, but the pattern and extent of their growth is heterogeneous.34 Dimension and placement of the prosthetic intra-atrial patch, suture lines, compression by other structures, growth failure of a segment of the native right atrium, or thrombus formation in regions of flow stagnation may affect the growth of the intra-atrial flow area and even lead to baffle stenosis. Impact of the surgical reconstructions on the growth potential of surrounding organs is also one to consider. For example, oversized aortic root reconstructions in the first surgical stage (meant to allow unobstructed flow to the aorta) have been shown to constrain LPA development, leading to LPA stenosis with normalized LPA diameter just above 10 mm/m in some patients.5
Take Home Message Minimum baffle and PA diameters are the strongest determinant of PL. However, optimal vessel and baffle dimension should compromise between PL and risks of flow stagnation, thrombus formation, suboptimal HFD and potential compression of surrounding organs. Better understanding of growth and remodeling after the Fontan is critical for ensuring that the optimal TCPC design at the time of surgery will still perform well as the patient reaches adulthood. Development constraints imposed by vascular reconstructions, prosthetic material and suture lines are also ones to keep in mind.
The Key Drivers of Hepatic Flow Distribution
Pulmonary flow was found to be the dominant performance predictor for HFD in intra-atrial TCPCs, while its effect (although present) was dominated by the impact of IVC-SVC offset in extra-cardiac TCPCs.12 This difference is not unexpected given that, by construction, intra-atrial TCPCs typically feature no or little offset, so that the effect of that variable is only weakly present across intra-atrial patients. In fact, when grouping intra-atrial (n = 67) and extra-cardiac (n = 41) TCPCs together, Tang et al. 38 demonstrated that IVC-SVC offset and angle and pulmonary flow distribution were the independent predictors of HFD across TCPC templates. Given that the pulmonary flow distribution has primarily been correlated to the LPA/RPA area ratio,38 these studies imply that an optimal HFD depends upon the presence of equal LPA and RPA diameters to ensure even pulmonary blood flow, and minimal IVC-SVC offset and angle at the connection site to enhance mixing. Yet, although it may be beneficial for HFD, increased mixing has also been shown to be energetically detrimental. Y-grafts have thus been suggested as a means to evenly distribute the lower systemic return while minimizing energy dissipation. As demonstrated by the recent clinical and computational experiences,11,43 optimal flow distribution with that option requires additional control on the surgical side. Ideally, the branches should be placed sufficiently far away from the SVC with a rather symmetrical angle with the IVC trunk and be as parallel as possible with the PA axes. The optimal placement might also depend on the orientation of the SVC, constraints imposed by the surrounding organs (notably the aorta and the distance of the PA branches) and vessel flow rates, again calling for patient-specific optimization.
While achieving the absolute best HFD may appear technically challenging, it can be relativized by the following: first, obtaining a perfectly even HFD might not be necessary since PAVMs have mostly been reported in second stage patients (i.e., before connecting the IVC and hepatic flows to the PAs) or in patients with highly skewed HFD to one side,7 suggesting that there might be a range of acceptable HFDs. Second, HFD sensitivity to the placement of the IVC reduces with increasing IVC/SVC flow ratio,43 the latter being a normal evolution with growth and stabilizing round 6 or 7 years of age. The observed reduced sensitivity stems from both mass conservation and energy considerations. The increase in flow rate confers more energy to the IVC stream allowing it to more easily overcome the momentum barrier of the SVC.
This argument reaches its limit for patients with interrupted IVC in whom somatic growth does not translate into an increased flow rate through the Fontan pathway, but rather into increased superior venous returns. Such additional complications are the typical setting where virtual surgical planning can help, allowing for the exploration of innovative solutions such as Y-grafts or re-association of the venous returns via hepatic to azygous shunts.7 Issues with the relative energy of the superior and inferior streams have also emerged in the context of the Y-graft where, depending on the diameters retained for the two branches, the energy of an individual branch may drop below that of the SVC leading to detrimental flow competition and suboptimal HFD,43 again calling for individualized optimization.
Take Home Message Optimal hepatic flow distribution in intra-atrial and extra-cardiac TCPCs requires interaction of the IVC and SVC streams (i.e., minimal IVC-SVC offset and angle), conflicting with the requirements for minimal energy dissipation. Y-grafts might offer an interesting alternative, although optimum size and placement warrant further investigation. TCPC design for patients with low inferior venous return, such as those with interrupted IVC, requires special attention as reduced baffle flow rates increase HFD sensitivity to design variations. Surgical planning may be especially useful for patients with such additional complications, allowing for the exploration of innovative solutions.
Patient-Specific Boundary Conditions—A Critical Requirement to Capture In Vivo TCPC Dynamics
The previous sections clearly demonstrate the potential benefits of patient-specific simulations. Because blood flow dynamics within a given geometry are strongly dependent on boundary conditions, these constitute an important research front, establishing a crucial link between the simulations and clinical data.
Inflow Conditions
Arguing that the TCPC only featured low levels of pulsatility, a large core of the patient-specific studies have been based on the mean flow rates derived from PCMRI. This simplification has been shown to systematically underestimate PL and WSS12,18,29 compared to pulsatile simulations. Seeking to quantify this error, Khiabini et al. 18 compared results obtained under mean and pulsatile flow conditions for a cohort of 24 patients, using in vivo flow waveforms from cardiac gated PC MRI. Results are reported as a function of the pulsatility index of the patient-specific flow waveforms (defined as the sum of the amplitude of the flow rate variations in all TCPC vessels normalized by cardiac output). For patients with a pulsatility index below 30% (which accounted for most of their cohort), mean flow conditions yielded less than 10% error in PL, which the authors deemed reasonable. On the other hand, when pulsatility exceeded 30%, PL errors quickly rose up to 50% and HFD could no longer be accurately predicted.
This last point becomes all the more significant when considering not only cardiac pulsations (as in18) but also respiratory waves. Using real-time PC MRI to monitor the effects of free breathing, Körperich et al. 20 reported an increase in SVC and IVC flow rates of 10.5 and 22.5%, respectively, during inspiration and decrease of about 10% during expiration. These fluctuations are drastically amplified in the passive Fontan circulation, ranging between +22.2 and −12.8% for the SVC and between +69.8% and −78.5% for the IVC. From a modeling standpoint, an inherent difficulty stems from the fact that patient-specific inflow waveforms have typically been derived from cardiac-gated PC MRI, thereby blunting the respiration component. Marsden et al. 29 thus devised a generic respiration waveform for the IVC based on previous real-time MRI measurements and then combined it to the in vivo MRI measurements of the patient to produce pseudo-patient-specific inflow boundary conditions. As an alternative, Liu et al. 26 used echo Doppler to obtain real-time velocity recordings over multiple cardiac and respiratory cycles. Both approaches could be combined for improved patient-specificity and accuracy, or replaced by real-time PC MRI once finds clinical acceptance.
Outflow Boundary Conditions
In vivo PC MRI measurements in the PAs have been quite widely used to impose patient-specific outflow boundary conditions (using either mean values or pulsatile waveforms). One drawback, however, is that the associated pressure fields only describe pressure differences to a set reference point (typically the IVC inlet), whereas both pressure drops and absolute pressures factor into the clinical decision. On the numerical side, accurate pressure predictions are also essential for the accuracy of simulations with multiple pulmonary branches (i.e., that cannot be solely based on the PC MRI measurements in the main PA branches), and to capture vessel wall deformations and wave propagation.
The use of resistance29 and Windkessel-type28,31,43,45 boundary conditions has thus become an essential component of modeling TCPC hemodynamics, allowing simulations to achieve physiologic pressure levels. Simple resistance boundary conditions are adjusted to match both in vivo pulmonary flow distributions and catheterization pressure measurements.29 While more accurate, three-level Windkessel models require additional information on pressure pulse propagation that cannot be readily obtained from clinical measurements. Incorporating human pulmonary morphometry data to determine the impedance of the downstream vascular trees with patient-specific resistances determined from catheterization data has emerged as a successful strategy, providing physiologically sound boundary conditions and successfully capturing patient-specific pressure levels and flow characteristics.28,43 These types of boundary conditions also allow for the straightforward modeling of PVR reduction during exercise.29,45
Moving Walls
Finally, while most studies have assumed rigid walls, two recent studies have looked into the impact of wall motion in patient-specific TCPCs. Long et al. 27 conducted an FSI study for two patient-specific extra-cardiac TCPC, dividing the domain of interest into multiple regions to account for the different material properties and wall thicknesses of the veins, arteries and artificial Fontan pathway. Comparing FSI and rigid wall results, local differences were noted in WSS but not in global measures, such as time averaged pressures, energy efficiency, or hepatic flow distribution. Focusing on an intra-atrial example, Mirabella et al. 31 made use of MRI measurements to reproduce the in vivo wall motion. The authors provide a detailed procedure to address the mapping of consecutive geometrical configurations and extraction of the associated wall displacement with the smoothness required for CFD simulations. Neglecting wall motion for that patient resulted in 30% underestimation of the PL, 20% error in HFD and 60% overestimation of the particle residence times. All of these effects resulted from flow changes in the intra-atrial section of the connection, where motion induced by the atrium was significant. Taken together, these results suggest that neglecting wall motion may be acceptable for extra-cardiac but not for intra-atrial conduits. However, it should be emphasized that both studies were limited to only one or two patients. Quantification of the magnitude and effect of wall motion across a wider patient population might help the definition of procedure specific FSI models.
Choice of Boundary Conditions
From the above discussion, two main strategies emerge to capture the current TCPC hemodynamics of a patient: either using in vivo clinical measurements (inlet flow rates velocity profiles and/or pressures, outlet flow rates, wall motion), or modeling the underlying mechanisms through additional constitutive relationships (lumped-parameter models, FSI). If the corresponding measurements are available, the former approach should allow for an accurate match to the in vivo state. The latter option allows for boundary condition estimations when clinical measurements are unavailable and as such carries more potential for prospective applications. Parameter fitting to the limited patient data and validation of the added lumped-parameter or FSI models remains an issue.
Patient-Specific Simulations for Prospective Surgical Planning
Even more challenging than capturing the current hemodynamic state of a given patient is the question of the prospective prediction of post-operative outcome, especially for the Fontan surgery which results in a complete alteration of the cardiovascular circuit. In this section we cover the different sources of variability between pre- and post-operative states and strategies that have been devised to cope with them.
Changes in Outflow Boundary Conditions
What really set emphasis on outflow variations is the referral of patients with PAVMs. For these patients, the key objective of the surgery and, thereby, of the virtual surgery, is to increase hepatic venous return to the diseased lung to reduce the PAVMs. In practice PAVMs create low resistance arteriovenous shunts, thereby significantly lowering the effective resistance of the affected lung. Their disappearance should thus increase the resistance of the formerly diseased lung and change the outflow distribution. As a result, pre-operative outlet conditions will no longer hold. The ideal solution would thus be the development of an adaptive model of the pulmonary vasculature and associated resistance, but this has been pushed back by lack of clinical or biological data to fit a mathematical model. Although subjective, the only reported alternative has been to parametrically vary the boundary conditions and observe the corresponding performance variations, seeking to estimate both the robustness of different designs and possible temporal evolutions.6
Changes in Inflow Boundary Conditions
Changes in inflow boundary conditions are equally important and can occur in one or several of the following categories: (1) cardiac output, (2) flow distribution among the different systemic venous returns, and (3) pulsatile content. Clinical studies have demonstrated a reduction in cardiac output going from the second to the third stage, due to an increase in afterload and volume unloading of the ventricle.8 Using iPL rather than PL could help address that issue by removing the dependence on cardiac output. On the other hand, changes in the relative inflow distributions have been associated with significant differences in all performance metrics, including PL or iPL and HFD.7,10 For patients with bilateral SVCs, for example, the optimal configuration is inherently dependent on the left to right flow balance and significant shifts in this ratio might transform an apparently optimal approach into a sub-optimal one. A better understanding of these variations is of paramount importance for the reliability of the predicted performances.
Multiscale Modeling
Lumped parameter models are being pursued as a means to address the above adaptation in inlet/outlet conditions. Sample applications include modeling the effect of different medication and management protocols,24 cardiovascular adaptation after surgery,41 or prediction of exercise performance.22,23 A few models also take into account the intra-abdominal and intra-thoracic pressures to capture the effects of respiration.41 In line with these developments, multi-scale models that couple such lumped parameter models of the whole circulation with detailed 3D simulations of the TCPC are now being developed. Multi-scale approaches offer distinct advantages for surgical planning: (1) they allow for the direct quantification of cardiac output or central venous pressure associated with a given TCPC design rather than using surrogate metrics such as PL; (2) they can, in principle, capture dynamic feedback mechanisms, such as changes in cardiovascular performance or PVR after the Fontan surgery, or under exercise conditions. Using such multi-scale framework, Baretta et al. 1 observed a decrease in cardiac output after virtual completion of the TCPC due to the sudden increase in both afterload and preload of the single ventricle, consistent with clinical observations.
However, the theoretical advantages listed above should be nuanced by the fact that parameter fitting to a patient-specific state remains a challenge because of limited access to in vivo information. Liang et al. 25 reported a thorough sensitivity analysis to reduce the parameter space to only the most sensitive ones and a detailed parameter fitting procedure. Kung et al. 22 made use of literature data on cardiovascular characteristics and cohort analyses of Fontan cardiovascular response to establish functional relationships between different model parameters, allowing them to adjust their model solely based on patient size and reference heart rate/oxygen consumption. While all of these strategies are valid, it should be kept in mind that their representation is only as good as the underlying parameter relationships. As acknowledged by the authors, the model described in22 can only represent typical Fontan patients and cannot capture a failing Fontan morphology. Accordingly, while both lumped-parameter and multi-scale studies have successfully captured general trends reported in literature, a detailed evaluation of their predictive capabilities for a given patient has not been reported yet.
Clinical Validation and Uncertainty Quantification
While potential benefits of patient-specific simulations are clear, it should not be taken for granted that they represent the patient’s hemodynamics accurately. Accuracy of the in vivo measurements and subsequent post-processing (to obtain the in vivo anatomies and flows for example), of the numerical solvers and boundary conditions all factor into the accuracy of the final results. Reasonable agreement between simulated velocity fields and in vivo PC MRI12 or between HFD and lung perfusion data43 are encouraging with respect to our ability to capture the current in vivo state of the patient. But validation efforts are still very much needed, especially to assess the accuracy and robustness of the post-operative predictions. Although limited to 6 patients, the study of Haggerty et al. 10 is the most comprehensive attempt to assess the accuracy of the entire surgical planning framework. The authors compared (1) the suggested and actual in vivo post-op geometries and (2) the performance rankings obtained under pre- and post-operative flow conditions. Geometrical mismatch and discrepancies in flow distributions were identified as the most sensitive parameters. Unfortunately, differences between the virtually designed procedure and its actual surgical implementation are currently unavoidable. More pragmatic approaches have thus been pursued to try and account for variations during the optimization procedure.
Sankaran et al. 35 developed a stochastic collocation method for error analysis, so that variables may be reported with a confidence interval accounting for the different variations in input parameters, such as uncertainties in the location of the pressure measurements used for the determination of the pulmonary vascular resistances. Along the same lines, Restrepo et al. 33 assessed the sensitivity of the different performance metrics (HFD and PL) to prescribed geometrical deviations and from there derived an additional “robustness” parameter for the optimization. Similar approaches could be used to probe the sensitivity to all input parameters, notably inflow conditions. A critical step for such error assessment to be meaningful is to first understand the possible deviation ranges. While measurement uncertainties can be estimated and propagated,35 deviations due to post-operative adaptation are more challenging. Further refinement in lumped parameter modeling (even if still generic) and analyses of clinical patient databases might provide a first answer.
Future Directions
Two main challenges emerge from the above presented studies: the need to capture cardiovascular adaptation as a result of growth and remodeling or changes in operating conditions (exercise, pregnancy), and the need for validation. Models of pulmonary vascular adaptation and remodeling as a function of pressure and WSS40 might be very relevant to the Fontan area. As emphasized by Dasi et al.,3 mechanical constraints imposed by the surrounding organs should also be accounted for. Lower limb exercise has typically been modeled by imposing a generic 2 to 3-fold increase in IVC flow rate and constant reduction in pulmonary resistance.45 In practice, changes in inlet flow rates result from changes in heart rate and contractility and should be modulated by the vascular and TCPC resistances. Enhanced respiratory effects, flow pulsatility and wall motion may also be expected. Exercise hemodynamics need to be more accurately modeled and further investigated to understand the possible causes of exercise intolerance in Fontan patients and their relation to TCPC hemodynamics. Multi-scale modeling coupling 3D FSI to lumped-parameter models of the whole circulation carry high potential in that regard. Of specific interest is the closed looped lumped parameter framework for Fontan exercise physiology developed by Kung et al.,22 which notably accounts for modulations in heart rate and contractility, changes in vascular resistances and respiratory effects.
Going hand in hand with these modeling strategies is the burning issue of boundary condition validation. This endeavor has in part been limited by (1) the amount of available patient data, (2) the retrospective aspect of most of the studies and heterogeneity of the clinical data acquired across centers and patients, and (3) the very limited number of patient-cases considered in most numerical studies. Validation of patient-specific multi-scale models and post-operative predictions will thus require coordinated clinical and computational efforts for the definition of data acquisition protocols and their application in longitudinal clinical studies, and prospective computational validation efforts on relatively large patient cohorts.
Other directions for developments may also arise from the application side. Recent applications have started addressing other unusually complicated anatomical configurations.30 Extension of the virtual surgical paradigm to earlier stages of the procedure is also gaining interest. Corsini et al. 2 presented the first predictive study for the 2nd stage, using a coupled multiscale model. Last but not least, these environments also have a huge potential as hypothesis test bed, as illustrated by the case of the Y-graft that was first explored numerically and is now being deployed in patients.
Summary and Conclusions
Methods for patient-specific simulations have made major strides forward in the past decade, notably through the refinement of boundary conditions. The emergence of multi-scale solutions including closed-loop representations of the entire cardiovascular system carry a lot of potential for the prospective assessment of post-operative performance and optimization of the Fontan connection on a patient-specific basis. However, much work remains to be done to validate these models and ensure that the results can be confidently used in a clinical setting. This will require both a prospective validation efforts on the computational side and detailed longitudinal studies on the clinical side. Despite these limitations, current publications demonstrate that the existing frameworks already benefit specific patient populations, especially in the context of unusual anatomic configurations. Refining our clinical understanding and simulation/optimization methodologies may allow a larger number of patients to benefit from such frameworks.
References
Baretta, A., C. Corsini, W. Yang, I. E. Vignon-Clementel, A. L. Marsden, J. A. Feinstein, T. Y. Hsia, G. Dubini, F. Migliavacca, and G. Pennati. Virtual surgeries in patients with congenital heart disease: a multi-scale modelling test case. Philos Trans A Math Phys Eng Sci 369(1954):4316–4330, 2011.
Corsini, C., C. Baker, E. Kung, S. Schievano, G. Arbia, A. Baretta, G. Biglino, F. Migliavacca, G. Dubini, G. Pennati, A. Marsden, I. Vignon-Clementel, A. Taylor, T. Y. Hsia, and A. Dorfman. An integrated approach to patient-specific predictive modeling for single ventricle heart palliation. Comput Methods Biomech Biomed Engin 17(14):1572–1589, 2014.
Dasi, L. P., R. KrishnankuttyRema, H. D. Kitajima, K. Pekkan, K. S. Sundareswaran, M. Fogel, S. Sharma, K. Whitehead, K. Kanter, and A. P. Yoganathan. Fontan hemodynamics: importance of pulmonary artery diameter. J. Thorac. Cardiovasc. Surg. 137(3):560–564, 2009.
Dasi, L. P., K. Pekkan, H. D. Katajima, and A. P. Yoganathan. Functional analysis of Fontan energy dissipation. J. Biomech. 41(10):2246–2252, 2008.
Dasi, L. P., K. S. Sundareswaran, C. Sherwin, D. de Zelicourt, K. Kanter, M. A. Fogel, and A. P. Yoganathan. Larger aortic reconstruction corresponds to diminished left pulmonary artery size in patients with single-ventricle physiology. J. Thorac. Cardiovasc. Surg. 139(3):557–561, 2010.
de Zélicourt, D. A. Pulsatile Fontan hemodynamics and patient-specific surgical planning: a numerical investigation. Atlanta: Georgia Institute of Technology, 2010. https://smartech.gatech.edu/handle/1853/39549.
de Zélicourt, D. A., C. M. Haggerty, K. S. Sundareswaran, B. S. Whited, J. R. Rossignac, K. R. Kanter, J. W. Gaynor, T. L. Spray, F. Sotiropoulos, M. A. Fogel, and A. P. Yoganathan. Individualized computer-based surgical planning to address pulmonary arteriovenous malformations in patients with a single ventricle with an interrupted inferior vena cava and azygous continuation. J. Thorac. Cardiovasc. Surg. 141(5):1170–1177, 2011.
Fogel, M. A., P. M. Weinberg, A. J. Chin, K. E. Fellows, and E. A. Hoffman. Late ventricular geometry and performance changes of functional single ventricle throughout staged Fontan reconstruction assessed by magnetic resonance imaging. J. Am. Coll. Cardiol. 28(1):212–221, 1996.
Gewillig, M., S. C. Brown, B. Eyskens, R. Heying, J. Ganame, W. Budts, A. La Gerche, and M. Gorenflo. The Fontan circulation: who controls cardiac output? Interact. Cardiovasc. Thorac. Surg. 10(3):428–433, 2010.
Haggerty, C. M., D. A. de Zelicourt, M. Restrepo, J. Rossignac, T. L. Spray, K. R. Kanter, M. A. Fogel, and A. P. Yoganathan. Comparing pre- and post-operative Fontan hemodynamic simulations: implications for the reliability of surgical planning. Ann. Biomed. Eng. 40(12):2639–2651, 2012.
Haggerty, C. M., K. R. Kanter, M. Restrepo, D. A. de Zélicourt, W. J. Parks, J. Rossignac, M. A. Fogel, and A. P. Yoganathan. Simulating hemodynamics of the Fontan y-graft based on patient-specific in vivo connections. J. Thorac. Cardiovasc. Surg. 145(3):663–670, 2013.
Haggerty, C. M., M. Restrepo, E. Tang, D. A. de Zelicourt, K. S. Sundareswaran, L. Mirabella, J. Bethel, K. K. Whitehead, M. A. Fogel, and A. P. Yoganathan. Fontan hemodynamics from 100 patient-specific cardiac magnetic resonance studies: a computational fluid dynamics analysis. J. Thorac. Cardiovasc. Surg. 148(4):1481–1489, 2014.
Haggerty, C. M., K. K. Whitehead, J. Bethel, M. A. Fogel, and A. P. Yoganathan. Relationship of single ventricle filling and preload to total cavopulmonary connection hemodynamics. Ann. Thorac. Surg. 99(3):911–917, 2015.
Itatani, K., K. Miyaji, T. Tomoyasu, Y. Nakahata, K. Ohara, S. Takamoto, and M. Ishii. Optimal conduit size of the extracardiac Fontan operation based on energy loss and flow stagnation. Ann. Thorac. Surg. 88(2):565–572, 2009; (discussion 72–3).
Iyengar, A. J., D. S. Winlaw, J. C. Galati, D. S. Celermajer, G. R. Wheaton, T. L. Gentles, L. E. Grigg, R. G. Weintraub, A. Bullock, R. N. Justo, and Y. d’Udekem. Trends in Fontan surgery and risk factors for early adverse outcomes after Fontan surgery: the Australia and New Zealand Fontan registry experience. J. Thorac. Cardiovasc. Surg. 148(2):566–575, 2014.
Kansy, A., G. Brzezinska-Rajszys, M. Zubrzycka, M. Mirkowicz-Malek, P. Maruszewski, M. Manowska, and B. Maruszewski. Pulmonary artery growth in univentricular physiology patients. Kardiol. Pol. 71(6):581–587, 2013.
Khairy, P., N. Poirier, and L. A. Mercier. Univentricular heart. Circulation 115(6):800–812, 2007.
Khiabani, R. H., M. Restrepo, E. Tang, D. De Zélicourt, F. Sotiropoulos, M. Fogel, and A. P. Yoganathan. Effect of flow pulsatility on modeling the hemodynamics in the total cavopulmonary connection. J. Biomech. 45(14):2376–2381, 2012.
Khiabani, R. H., K. K. Whitehead, D. Han, M. Restrepo, E. Tang, J. Bethel, S. M. Paridon, M. A. Fogel, and A. P. Yoganathan. Exercise capacity in single-ventricle patients after Fontan correlates with haemodynamic energy loss in TCPC. Heart 101(2):139–143, 2015.
Korperich, H., P. Barth, J. Gieseke, K. Muller, W. Burchert, H. Esdorn, D. Kececioglu, P. Beerbaum, and K. T. Laser. Impact of respiration on stroke volumes in paediatric controls and in patients after Fontan procedure assessed by mr real-time phase-velocity mapping. Eur. Heart J. Cardiovasc. Imaging 16(2):198–209, 2015.
Kung, E., A. Baretta, C. Baker, G. Arbia, G. Biglino, C. Corsini, S. Schievano, I. E. Vignon-Clementel, G. Dubini, G. Pennati, A. Taylor, A. Dorfman, A. M. Hlavacek, A. L. Marsden, T. Y. Hsia, and F. Migliavacca. Predictive modeling of the virtual hemi-Fontan operation for second stage single ventricle palliation: two patient-specific cases. J. Biomech. 46(2):423–429, 2013.
Kung, E., G. Pennati, F. Migliavacca, T. Y. Hsia, R. Figliola, A. Marsden, and A. Giardini. A simulation protocol for exercise physiology in Fontan patients using a closed loop lumped-parameter model. J. Biomech. Eng. 136(8):081007, 2014.
Kung, E., J. C. Perry, C. Davis, F. Migliavacca, G. Pennati, A. Giardini, T. Y. Hsia, and A. Marsden. Computational modeling of pathophysiologic responses to exercise in Fontan patients. Ann Biomed Eng 43(6):1310–1320, 2014.
Liang, F., H. Senzaki, C. Kurishima, K. Sughimoto, R. Inuzuka, and H. Liu. Hemodynamic performance of the Fontan circulation compared with a normal biventricular circulation: a computational model study. Am. J. Physiol. Heart Circ. Physiol. 307(7):H1056–H1072, 2014.
Liang, F., K. Sughimoto, K. Matsuo, H. Liu, and S. Takagi. Patient-specific assessment of cardiovascular function by combination of clinical data and computational model with applications to patients undergoing Fontan operation. Int. J. Numer. Method Biomed. Eng. 30(10):1000–1018, 2014.
Liu, J., Y. Qian, Q. Sun, and M. Umezu. Use of computational fluid dynamics to estimate hemodynamic effects of respiration on hypoplastic left heart syndrome surgery: total cavopulmonary connection treatments. Sci. World J. 2013:131597, 2013.
Long, C. C., M. C. Hsu, Y. Bazilevs, J. A. Feinstein, and A. L. Marsden. Fluid-structure interaction simulations of the Fontan procedure using variable wall properties. Int. J. Numer. Method Biomed. Eng. 28(5):513–527, 2012.
Marsden, A. L., A. J. Bernstein, V. M. Reddy, S. C. Shadden, R. L. Spilker, F. P. Chan, C. A. Taylor, and J. A. Feinstein. Evaluation of a novel y-shaped extracardiac Fontan baffle using computational fluid dynamics. J. Thorac. Cardiovasc. Surg. 137(2):394–403, 2009.
Marsden, A. L., I. E. Vignon-Clementel, F. P. Chan, J. A. Feinstein, and C. A. Taylor. Effects of exercise and respiration on hemodynamic efficiency in CFD simulations of the total cavopulmonary connection. Ann. Biomed. Eng. 35(2):250–263, 2007.
Menon, P. G., M. Yoshida, and K. Pekkan. Presurgical evaluation of Fontan connection options for patients with apicocaval juxtaposition using computational fluid dynamics. Artif. Organs 37(1):E1–E8, 2013.
Mirabella, L., C. M. Haggerty, T. Passerini, M. Piccinelli, A. J. Powell, P. J. Del Nido, A. Veneziani, and A. P. Yoganathan. Treatment planning for a TCPC test case: a numerical investigation under rigid and moving wall assumptions. Int. J. Numer. Method Biomed. Eng. 29(2):197–216, 2013.
Mori, M., A. J. Aguirre, R. W. Elder, A. Kashkouli, A. B. Farris, R. M. Ford, and W. M. Book. Beyond a broken heart: circulatory dysfunction in the failing Fontan. Pediatr. Cardiol. 35(4):569–579, 2014.
Restrepo, M., M. Luffel, J. Sebring, K. Kanter, P. Del Nido, A. Veneziani, J. Rossignac, and A. Yoganathan. Surgical planning of the total cavopulmonary connection: robustness analysis. Ann. Biomed. Eng. 43(6):1321–1334, 2014.
Restrepo, M., L. Mirabella, E. Tang, C. M. Haggerty, R. H. Khiabani, F. Fynn-Thompson, A. M. Valente, D. B. McElhinney, M. A. Fogel, and A. P. Yoganathan. Fontan pathway growth: a quantitative evaluation of lateral tunnel and extracardiac cavopulmonary connections using serial cardiac magnetic resonance. Ann. Thorac. Surg. 97(3):916–922, 2014.
Sankaran, S., and A. L. Marsden. A stochastic collocation method for uncertainty quantification and propagation in cardiovascular simulations. J. Biomech. Eng. 133(3):031001, 2011.
Srivastava, D., T. Preminger, J. E. Lock, V. Mandell, J. F. Keane, J. E. Mayer, Jr, H. Kozakewich, and P. J. Spevak. Hepatic venous blood and the development of pulmonary arteriovenous malformations in congenital heart disease. Circulation 92(5):1217–1222, 1995.
Sundareswaran, K. S., K. Pekkan, L. P. Dasi, K. Whitehead, S. Sharma, K. R. Kanter, M. A. Fogel, and A. P. Yoganathan. The total cavopulmonary connection resistance: a significant impact on single ventricle hemodynamics at rest and exercise. Am. J. Physiol. Heart Circ. Physiol. 295(6):H2427–H2435, 2008.
Tang, E., M. Restrepo, C. M. Haggerty, L. Mirabella, J. Bethel, K. K. Whitehead, M. A. Fogel, and A. P. Yoganathan. Geometric characterization of patient-specific total cavopulmonary connections and its relationship to hemodynamics. JACC Cardiovasc. Imaging 7(3):215–224, 2014.
Troianowski, G., C. A. Taylor, J. A. Feinstein, and I. E. Vignon-Clementel. Three-dimensional simulations in glenn patients: clinically based boundary conditions, hemodynamic results and sensitivity to input data. J. Biomech. Eng. 133(11):111006, 2011.
Valentin, A., L. Cardamone, S. Baek, and J. D. Humphrey. Complementary vasoactivity and matrix remodelling in arterial adaptations to altered flow and pressure. J. R. Soc. Interface 6(32):293–306, 2009.
Watrous, R. L., and A. J. Chin. Model-based comparison of the normal and Fontan circulatory systems: Part i: Development of a general purpose, interactive cardiovascular model. World J. Pediatr. Congenit. Heart Surg. 5(3):372–384, 2014.
Whitehead, K. K., K. Pekkan, H. D. Kitajima, S. M. Paridon, A. P. Yoganathan, and M. A. Fogel. Nonlinear power loss during exercise in single-ventricle patients after the Fontan: insights from computational fluid dynamics. Circulation 116(11 Suppl):I165–I171, 2007.
Yang, W., F. P. Chan, V. M. Reddy, A. L. Marsden, and J. A. Feinstein. Flow simulations and validation for the first cohort of patients undergoing the y-graft Fontan procedure. J. Thorac. Cardiovasc. Surg. 149(1):247–255, 2015.
Yang, W., J. A. Feinstein, and A. L. Marsden. Constrained optimization of an idealized y-shaped baffle for the Fontan surgery at rest and exercise. Comput. Methods Appl. Mech. Eng. 199(33–36):2135–2149, 2010.
Yang, W., I. E. Vignon-Clementel, G. Troianowski, V. M. Reddy, J. A. Feinstein, and A. L. Marsden. Hepatic blood flow distribution and performance in conventional and novel y-graft Fontan geometries: a case series computational fluid dynamics study. J. Thorac. Cardiovasc. Surg. 143(5):1086–1097, 2012.
Acknowledgments
The authors gratefully acknowledge the financial support provided by the Swiss National Science Foundation through the NCCR Kindey.CH and a Marie Heim-Vögtlin Fellowship (PMPDP2_151255).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Karol Miller oversaw the review of this article.
Rights and permissions
About this article
Cite this article
de Zélicourt, D.A., Kurtcuoglu, V. Patient-Specific Surgical Planning, Where Do We Stand? The Example of the Fontan Procedure. Ann Biomed Eng 44, 174–186 (2016). https://doi.org/10.1007/s10439-015-1381-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1381-9