Abstract
Annulus tension (AT) is defined as leaflet tension per unit length of the annulus circumference. AT was investigated to understand tricuspid valve (TV) annulus mechanics. Ten porcine TVs were mounted on a right ventricle rig with an annulus plate to simulate TV closure. The TVs were mounted on the annulus plate in a normal and dilated TV annulus sizes, and closed under transvalvular pressure of 40 mmHg with the annulus held peripherally by wires. Anterior papillary muscle (PM) and septal PM were displaced in the dilated annulus. Wire forces were measured, and ATs (N/m) were calculated. Clover repair was performed in the dilated TV state subsequently, and AT was calculated again. A one-way ANOVA and Tukey’s HSD test were used to test significances between the different TV states along each annulus segment with p < 0.05. Average ATs for the normal annulus, dilated annulus, and clover repair were 10.75 ± 1.87, 28.81 ± 10.51, and 26.93 ± 11.44 N/m, respectively. Septal annulus segments had the highest ATs when compared to the other segments. For the clover repair, there were no significant changes in AT values. ATs and leaflet forces increased roughly 3–4 times with annulus dilation. AT decelerates annulus dilation as previously shown in the mitral valve. Clover repair does not prevent further annulus dilation by AT change and should be accompanied by annuloplasty. AT improves annulus contraction during a cardiac cycle and should be considered when designing annuloplasty in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The annulus of the tricuspid valve (TV) is an intricate structure that is bounded by three main leaflets: septal, posterior, and anterior leaflets.11,16,21 The normal annulus has always been shown to be a “saddle-shape” form.17,28 Under systole, transvalvular pressure induces leaflet tension that is transferred to the annulus and chords on the TV.1,8 The chords pull the TV leaflets apically which prevent prolapsing and transfers the force to the papillary muscles.1,8 The leaflet tension (N) at the annulus is called annulus tension (AT). AT is defined as leaflet tension per unit length of the circumference of the annulus (N/m).1,8 AT is located in the annulus plane and pulls the annulus structure towards the center of the valve orifice during TV closure. AT may be displaced from the annulus plane when the TV is tethered by the displaced papillary muscles (PMs) or prolapsed, depending on the leaflet position.1,8,9 AT in the annulus plane contributes to annulus contraction and restricts annulus size, thereby preventing expansion of the myocardium around the annulus.1 Annulus geometry is determined by the interaction between the leaflets and the myocardium around the annulus, especially in ventricular development or remodeling.1 AT directly interacts with the myocardial force per unit length of the annulus circumference. This force per unit length is present around the annulus on the myocardium as an equal and opposite force.1,8,9 Imbalance of the AT and myocardial forces per unit length of the annulus due to right ventricular remodeling causes annulus dilation and subsequent tricuspid regurgitation, which is a common pathological disorder affecting the TV.8
When the right ventricle is enlarged, it is difficult for the leaflets of the TV to touch due to leaflet tethering and annulus dilation.25,26 The clover repair is used to suture central leaflet edges together to facilitate coaptation.4,5,12,14 Clover repair has been shown to abate tricuspid regurgitation with the addition of an annuloplasty ring.5 The MitraClip device on the mitral valve reduces mitral regurgitation, but still causes residual mitral regurgitation due to uncorrected or further annulus dilation.29,30 A similar device on the TV can be developed and is estimated to reduce tricuspid regurgitation. However, further annulus dilation abates efficacy of clover repair. It is unknown whether this clover repair without annuloplasty can prevent further annulus dilation. Therefore, AT was measured to predict whether this repair without annuloplasty can prevent further annulus dilation in terms of annulus mechanics. The objective of this study was to quantify AT in the TV annulus in order to understand TV annulus mechanics.
Materials and Methods
TV Preparation and Right Ventricle Rig
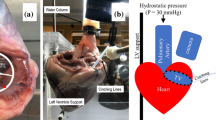
Pig hearts were obtained 4 h from a slaughterhouse hours after their death. Immediately, TVs with an annulus size of approximately 9.9 cm2 were obtained and extracted and placed in saline solution overnight in a refrigerator. Second morning, the TV was positioned on a right ventricle rig which consisted of an annulus plate, ventricular chamber, atrium reservoir, and PM holders as shown in Figs. 1a–1d. The TV was mounted on the right ventricle rig with the annulus plate. Stainless steel hooks were placed in the surrounding myocardium peripherally at a distance of 3 mm away from the annulus. Each stainless steel hook had a mass of approximately 0.15 g as shown in Fig. 2. The tricuspid annulus touched the annulus support ring in the annulus plate under transvalvular pressure. The annulus freely slipped because there was no restrictive connection between it and the support ring. Each hook was connected to a force transducer (LBB200/FSH00887, FUTEK Advanced Sensor Technology, Inc. Irvine, CA) via a wire. After the right ventricle rig was organized, the ventricular and atrial chambers were filled with saline (weight salt concentration of 0.9%) to close the TV. The TV was immersed in the top ventricular reservoir. A PVC pipe was attached to the bottom atrial reservoir to build transvalvular pressure of 40 mmHg.1,26 This pressure limit is set based on common pathological conditions in a dilated right heart.22,23,26
(a) Setup with right ventricle chamber consisting of the atrium chamber and reservoir, (PVC pipe from the atrium to ventricle). (b) Picture of the RV rig consisting of atrium chamber, TV, transducers, PM holder, and annulus plate. (c) Top cross-section view of the right ventricle rig. (d) Schematic of ventricle and atrium chamber along with right ventricle rig. The transvalvular pressure P1 − P2 = 40 mmHg.
Adjustments were made to support the in vitro setup of the TV annulus. Under transvalvular pressure, the TV closed and the annulus was positioned on the annulus plate.1,8,9 The wire lengths and anchor positions on the right ventricle rig were adjusted until all the wires were approximately perpendicular to the annulus. This reassured that the TV annulus coincided with the annulus support ring in the annulus plate. The PMs were adjusted apically and laterally via PM holders in rods as shown in Figs. 1b and 1d to simulate the normal and displaced PM positions that coincide with the normal and dilated right ventricle, respectively.24–27
TV Annulus Plate
The annulus plate had the supporting ring made of Plexiglas, matching a normal TV annulus shape and size with an area of approximately 9.9 cm2, annulus circumference of approximately 12 cm, and septal length of 3.4–4 cm.10,15,16,28 Figures 3a–3d shows two annulus plates used for this study. The normal annulus plate was saddle-shaped based on 3-dimensional echocardiographic studies of the normal TV as shown in Figs. 3a and 3b.10,11,13,15–17,28] The other plate was made for the dilated annulus with an area of approximately 16.8 cm2 and an annulus circumference of approximately 15 cm, as shown in Figs. 3c and 3d.16 28 This dilated annulus was planar to mimic the pathological dilated annulus state.7,11,28 The septal annulus segment on the normal and dilated plates was a straight line of approximately 4 cm.7,15,16,28 When the TV closed, the native annulus touched the supporting ring of the annulus plate.
(a) Front layout of the normal plate with the 4 cm septal annulus segment length. (b) Lateral septal view of the normal plate indicating the saddle shape and the height difference from the septoanterior commissure (dashed line). The height difference is around 3 mm. (c) Front layout of the dilated plate with the 4 cm septal annulus segment. (d) Lateral view of the dilated plate. All commissures are along the same plane in the dilated plate. (SA: septoanterior, SP: septoposterior, AP: anteroposterior).
A position in the annulus was normalized by dividing the distance starting from the septoanterior commissure by the whole annulus perimeter. The normalized annulus position starts and ends at the septoanterior commissure (0 and 100%). The entire annulus hook layout is shown in Fig. 4. The 1st, 5th, and 7th hooks were in the three commissures. For the septoanterior commissure, there were two adjacent hooks applied 2 mm apart on the septal and anterior annulus segments since the septoanterior region is sharply curved.6,21 The septoposterior and anteroposterior commissure positions were 32 and 61%, respectively, in the normal annulus, and 27.5 and 59%, respectively, in the dilated annulus. For the clover repair, the septoposterior and anteroposterior commissure positions were 28 and 60%, respectively.
Tissue-Ring Friction
When the TV closed, the transvalvular pressure pushed the TV annulus onto the annulus plate. The friction exists between the native TV annulus and the supporting ring. AT in the loading and unloading processes were averaged to find the AT without involving friction.1 The loading process increased transvalvular pressure from 30 to 40 mmHg and unloading pressure decreased transvalvular pressure from 50 to 40 mmHg. The native TV annulus was allowed to slide slightly on the support ring during the loading and unloading process. The wire forces were recorded twice at the transvalvular pressure of 40 mmHg at the end of the processes and averaged to eliminate friction.1,9
Experimental Procedure and Clover Repair
Force transducers were connected to a LabView module (NI SC-2345 & NI SCC-SG24, National Instruments Corporation, Austin, TX) which was connected to a computer to measure wire forces on the annulus. Transducers were calibrated before the experiment. The wire forces were measured during loading and then unloading when the saline level in the reservoir was changed as shown in Fig. 1d.1,9 Subsequently, AT was obtained (N/m) using the equations as shown in Fig. 5. Normal PM position was set up in the commissural positions to maintain the leaflets coapting approximately on the annulus plane. After the test of the normal TV was completed, the dilated annulus plate was switched onto the right ventricle rig. The anterior PM and septal PM were displaced 15 mm apically and laterally to represent a dilated ventricle.24,25,27 Figure 6 explains the displacement of the PMs in the dilated ventricle.
After AT in the dilated case was measured, the clover repair technique was applied on the same porcine TV approximately 3 mm from the free edge of the leaflets.4,5,12,14 A 4.0 polypropylene suture was used. This procedure created the TV clover shape as shown in Fig. 7. AT was measured with the same procedure.
Leaflet Force and Average AT for Annulus Segments
The leaflet forces of the septal, posterior, and anterior annulus segments were calculated by resolving the wire forces in the x and y components, which were summed to obtain the resultant force for each segment. The resultant force values were averaged to obtain a final resultant leaflet force for each segment. The wire force in the commissure was assumed to be shared between two adjacent segments. Average AT for each segment was calculated by dividing the leaflet force by the average circumference of each annulus segment. The average and standard deviation of AT of each segment were calculated based on 10 valves.
Statistical Analysis
Statistical analyzes were performed using SPSS (v. 23, Chicago, IL). Since the AT data along each TV segment were not normally distributed initially, a natural log transformation was performed in order to stabilize the variances in the data which were analyzed using one-way ANOVA.25 Post hoc mean comparisons were made to test differences between the normal annulus, dilated annulus, and clover repair for each annulus segment using Tukey’s HSD test.18,25 Statistical significance was set at p < 0.05.
Results
Figure 8 shows the average AT distribution on the normal annulus, dilated annulus and clover repair, respectively. A “U-shape” pattern was observed for the AT distribution. The greatest AT value was on the septoanterior commissure. ATs in the other annulus segments did not adjust much. The average ATs in the normal annulus, dilated annulus, and clover repair were 10.75 ± 1.87, 28.81 ± 10.51, and 26.93 ± 11.44 N/m, respectively (mean ± standard deviation). The dilated annulus and clover repair showed a comparable AT. Figures 9a and 9b are a bar chart summary of the average AT values and average leaflet forces values for each segment and TV state. For each annulus segment, the AT in the dilated annulus was greater than the normal AT (all p < 0.005). When comparing the dilated annulus to the clover repair, there was no statistical difference between the AT values for each annulus segment. The TV state comparisons along each segment are summarized in Table 1.
AT distribution for the TV annulus. The 0/100% perimeter represents the beginning of the AT curve. For the septoposterior (32/27.5/28%) and anteroposterior (61/59/60%) commissures, the perimeter percentage represents the normal annulus, dilated annulus, and clover repair points in the perimeter (normal/dilated/clover). (SA: septoanterior, SP: septoposterior, AP: anteroposterior).
Figure 10 shows the direction of the average resultant leaflet force from the annulus segments. According to Fig. 10, the average leaflet force pulled the annulus towards the TV orifice center in systole. This force ranged approximately from 0.32 to 0.51 N in the normal annulus, in which the septal and anterior forces were approximately the same. The posterior force was the lowest force average for all three states. The dilated annulus and clover repair showed a similar leaflet force average which was roughly 3–4 times that of each segment in the normal annulus. The average septal leaflet forces in the dilated annulus and clover repair were 1.94 ± 0.64 and 1.77 ± 0.70 N (mean ± standard deviation), respectively, making them the greatest among the three segments.
Leaflet forces of the TV annulus on the annulus plane when viewed from the right ventricle. The solid line/arrow and dashed line/arrow represent the normal and dilated states, respectively. The dotted arrow represents the clover repair force direction. (SA: septoanterior, SP: septoposterior, AP: anteroposterior).
Discussion
This is the first study on TV annulus mechanics with a focus on the leaflet force on the annulus to elucidate the interaction of the TV leaflets with the myocardium. The results highlight that overall AT was 10.75 N/m in the normal state and was highest at the septoanterior commissure. The septal annulus segment had the greatest AT in comparison to other annulus segments. AT in the dilated annulus was roughly 3–4 times that of the normal annulus and was comparable to that in the clover repair. The large AT increase from the normal to dilated annulus/clover repair decelerated the annulus dilation process.
Annulus Mechanics
We propose that the annulus tension plays an essential role in the overall biomechanical response of the annulus. We define that an annulus is a border line separating TV leaflets and the myocardium from which the leaflets originate. According to this definition, there is no tissue volume in the annulus. Further, we define myocardial tissue surrounding the annulus (with a width of 10–20 mm) as the annulus tissue which is adjacent to the annulus. We focus on annulus mechanics which is interaction between the annulus tissue and leaflets.
Annulus dynamics in the clinical study demonstrates a larger annulus in diastole and a smaller annulus in systole, which is called annulus contraction.6,7,17 Since AT is built when the leaflets undergo coaptation via transvalvular pressure, no leaflet tension exists when the TV is open during diastole. Myocardium is relaxed during diastole. Therefore, the annulus tissue is assumed to be a stress-free state in diastole. During systole, one could postulate that three forces are in development during systole: (1) leaflet stress (N/m2) at the annulus; (2) annulus tissue stress due to passive deformation (compression) of the annulus tissue; (3) annulus tissue contraction stress due to active contraction of the circumferential muscle fibers. Three resultant forces from the three stresses are leaflet tension towards the TV orifice center, the annulus tissue compressive force (TCF (N)) directed away from the TV orifice center, and the annulus tissue myocardial contraction force (MCF (N)) towards the TV orifice center. Figure 11 shows these forces in the annulus. Leaflet tension and MCF are the inward forces and TCF is the outward force. MCF and TCF are both in the annulus tissue; combined, they are called myocardial force. Stable annulus size indicated balanced annulus mechanics: TCF = Leaflet tension + MCF or leaflet tension = TCF − MCF. This equilibrium is a “tug-of-war” between the leaflet and the myocardium around the annulus. This force balance maintains a stable normal annulus geometry in the annulus plane during systole.8,9 We proposed a new mechanism of annulus dilation: annulus dilation is a consequence of the imbalance between the myocardial force and leaflet tension in the annulus plane.1,8 Imbalanced annulus mechanics can explain reduced MCF due to ventricular remodeling and/or reduced leaflet tension due to TV prolapse. TV prolapse is rare and is not investigated in the current study. TV annulus dilation is mainly accompanied by right ventricular dilation due to ischemic disease, for which reduced myocardial contraction is the probable cause.11,18 Reduced MCF causes the annulus to move away from the orifice center which increases AT or leaflet tension to reach new annulus equilibrium in the dilated annulus state. AT increased up to 3–4 times when the annulus is dilated by 70% as shown in our experiment. According to our annulus mechanics theory, if MCF is reduced to zero in an extreme condition which means no myocardial contraction, TCF would be 3–4 times the normal leaflet tension. Leaflet tension change can be used to indicate MCF change. MCF is hard to measure whereas leaflet tension is measurable and therefore provides an advantage to investigate MCF. Once annulus dilation is initiated, it does not stop. Leaflet tension is the only force that counteracts annulus dilation from ventricular remodeling. Increase in leaflet tension decelerates the annulus dilation process. Annuloplasty or annulus cinch suture are techniques that exert an extra inward force on the annulus to restrict annulus dilation. Their interaction with the annulus has been investigated on the mitral valve.3,19,20
(a) Top view of the three forces in the annulus tissue. (b) Leaflet tension direction and free body diagram force analysis on a small annulus tissue segment. The angle dθ can be approximated as a small angle. (c) Cross-section view of the myocardium-TV leaflet showing the forces in the free body diagram on the annulus plane. TCF: tissue compressive force, MCF: myocardial contraction force, r: radius from the annulus to its center.
Clover Repair on the TV
The clover repair is a surgical technique that sutures the TV at the mid-point of the free edges of the leaflets to form a “clover shape” for the TV.4,12 This technique forces leaflet coaptation and counteracts the effects of leaflet tethering.5,14 It was initially hypothesized that AT would increase and overcome the effects of annulus dilation with the addition of the clover repair. However, our results indicated that the slight decrease or no change in AT did not hold the annulus firmly and is not able to prevent further annulus dilation. The slight decrease in AT might be due to alteration of the TV leaflet geometry. Previous studies showed that the clover repair affected leaflet coaptation and stresses on the leaflets.14 However, it was not the case in our study. The forced leaflet coaptation from clover repair did not increase AT or leaflet tension which will restrict further annulus dilation. Therefore, clover repair will probably exacerbate regurgitation. Residual mitral regurgitation after MitraClip repair, which is similar to clover repair, suggests little or no effect on improving the annulus mechanics of the mitral valve.2,30 Our analysis on annulus mechanics of the clover repair highlights a long-term repair efficacy of a similar device on the TV. An annuloplasty ring in addition to the clover repair technique may be required.
Mitral Valve AT
Some of the results obtained in this study are similar to the prior work done on the mitral valve.1,8,9 When comparing the results of the mitral valve studies, increase in TV orifice area was directly correlated to increase in AT. Leaflet tension differed among TV leaflets. The underlying reason was that the leaflet force was proportional to the area of the leaflets covering the TV orifice. AT was also influenced by leaflet tethering or displacement of PMs shifting away from the orifice due to right ventricle remodeling.24–27 The PM displacements were in the apical and lateral direction in the right ventricle and were similar to those in the left ventricle with the exception of the posterior PM.1,8,9,24–27 Subsequently, the displaced PMs contributed to the increase in AT and leaflet forces as shown in the mitral valve.1,8 For the PM displacements, the mitral valve had three different PM positions: slack, normal, and taut. The slack PM was used to simulate mitral valve prolapse. For the TV, prolapse is rare from most of the recent clinical studies.11 Hence, the slack PM was not used in this study.
When the leaflets touch each other at the center of the orifice, a length is shared from all leaflets called the coaptation redundancy length. This length was reduced when the leaflets were tethered in a dilated annulus via the annulus or PM displacement.11,25,26 Leaflet redundancy is helpful in annulus dilation to prevent regurgitation to some extent.8 Like the mitral valve, the TV can overcome some of the effects of dilation with leaflet redundancy length. With increases in AT in dilation, leaflet forces are increased along the annulus plane like the mitral valve.
If AT magnitude would have been compared between the mitral valve and TV, AT decrease mainly would have been caused by low transvalvular pressure. ATs on the mitral valve in the normal PM position were 54 and 36 N/m for the anterior and posterior segments in the normal state with a trans-mitral pressure of 122 mmHg.8 TV AT was 10 N/m at 40 mmHg in the normal annulus, which is lower than one-third of the AT on the mitral valve. Hence, the AT magnitude was close to that of the TV, allowing for change in valve orifice area. These results are consistent when considering the differences in PM and annulus shape settings between the mitral valve and TV. Note that the TV annulus area was larger than that of the mitral valve. The value of the annulus tension is based on higher than normal transvalvular pressure which is lower than 40 mmHg. Actual AT of the normal heart can also be interpolated by transvalvular pressure because AT is directly proportional to transvalvular pressure according to prior study.8
Limitations
There are some limitations in this study. The annulus size and geometry could be different and might cause some minor differences in AT. The annuli of the pig TVs were triangular, whereas the typical human TV annulus is elliptical, according to most clinical studies.16,28 There are chordal forces in the out-of-annulus plane which can potentially influence the AT in the annulus plane. This effect should be minor because they are in different planes.
Other AT differences can come from the experimental procedure. With the displacement of the PMs to represent dilation, displacement of the posterior PM has also been shown to be correlated with annulus dilation.25,27 If the posterior PM was displaced, there might be minor changes to the ATs (septal, posterior, and anterior) for the dilated annulus. The clover repair in the current study might be different from that in many surgical procedures based on the surgeon’s professional opinion. This difference affects AT on the TV. For this study, a static experiment was performed which measured the peak AT in an entire cardiac cycle. For a dynamic heart, there will be differences in AT values.1,8,13
Conclusion
This study is a comprehensive analysis of the mechanics of the TV annulus using the definition of AT. The average AT is 10.75 ± 1.87, 28.81 ± 10.51, and 26.93 ± 11.44 N/m at transvalvular pressure of 40 mmHg for the normal annulus, dilated annulus, and subsequent clover repair, respectively. AT increases approximately 3–4 times with annulus dilation and decelerates annulus dilation as the counteracting force like that in the mitral valve. Overall, the clover repair does not affect AT, and therefore does not prevent further annulus dilation. This study of leaflet force and TV AT elucidates annulus mechanics, which can improve the annuloplasty technique and potentially mitigate tissue tear-off.
References
Bhattacharya, S., and Z. He. Role of annulus tension in annular dilatation. J. Heart Valve Dis. 18(5):481–487, 2009.
Bhattacharya, S., and Z. He. Annulus tension of the prolapsed mitral valve corrected by edge-to-edge repair. J. Biomech. 45(3):562–568, 2012. doi:10.1016/j.jbiomech.2011.11.005.
Bhattacharya, S., T. Pham, Z. He, and W. Sun. Tension to passively cinch the mitral annulus through coronary sinus access: an ex vivo study in ovine model. J. Biomech. 47(6):1382–1388, 2014. doi:10.1016/j.jbiomech.2014.01.044.
Castedo, E., A. Canas, R. A. Cabo, R. Burgos, and J. Ugarte. Edge-to-edge tricuspid repair for redeveloped valve incompetence after DeVega’s annuloplasty. Ann. Thorac. Surg. 75(2):605–606, 2003.
De Bonis, M., E. Lapenna, G. La Canna, A. Grimaldi, F. Maisano, L. Torracca, et al. A novel technique for correction of severe tricuspid valve regurgitation due to complex lesions. Eur. J. Cardio Thorac. Surg. 25(5):760–765, 2004. doi:10.1016/j.ejcts.2004.01.051.
Fawzy, H., K. Fukamachi, C. D. Mazer, A. Harrington, D. Latter, D. Bonneau, et al. Complete mapping of the tricuspid valve apparatus using three-dimensional sonomicrometry. J. Thorac. Cardiovasc. Surg. 141(4):1037–1043, 2011. doi:10.1016/j.jtcvs.2010.05.039.
Fukuda, S., G. Saracino, Y. Matsumura, M. Daimon, H. Tran, N. L. Greenberg, et al. Three-dimensional geometry of the tricuspid annulus in healthy subjects and in patients with functional tricuspid regurgitation: a real-time, 3-dimensional echocardiographic study. Circulation 114(1 Suppl):I492–I498, 2006. doi:10.1161/CIRCULATIONAHA.105.000257.
He, Z., and S. Bhattacharya. Papillary muscle and annulus size effect on anterior and posterior annulus tension of the mitral valve: an insight into annulus dilatation. J. Biomech. 41(11):2524–2532, 2008. doi:10.1016/j.jbiomech.2008.05.006.
He, Z., and S. Bhattacharya. Mitral valve annulus tension and the mechanism of annular dilation: an in vitro study. J. Heart Valve Dis. 19(6):701–707, 2010.
Hiro, M. E., J. Jouan, M. R. Pagel, E. Lansac, K. H. Lim, H. S. Lim, et al. Sonometric study of the normal tricuspid valve annulus in sheep. J. Heart Valve Dis. 13(3):452–460, 2004.
Hung, J. The pathogenesis of functional tricuspid regurgitation. Semin. Thorac. Cardiovasc. Surg. 22(1):76–78, 2010. doi:10.1053/j.semtcvs.2010.05.004.
Kotoulas, C., R. P. Jones, W. Turkie, and R. Hasan. Edge-to-edge repair of tricuspid valve in a corrected transposition of the great vessels. Hellenic J. Cardiol. 49(6):434–436, 2008.
Kragsnaes, E. S., J. L. Honge, J. B. Askov, J. M. Hasenkam, H. Nygaard, S. L. Nielsen, et al. In-plane tricuspid valve force measurements: development of a strain gauge instrumented annuloplasty ring. Cardiovasc. Eng. Technol. 4(2):131–138, 2013. doi:10.1007/s13239-013-0135-9.
Lapenna, E., M. De Bonis, A. Verzini, G. La Canna, D. Ferrara, M. C. Calabrese, et al. The clover technique for the treatment of complex tricuspid valve insufficiency: midterm clinical and echocardiographic results in 66 patients. Eur. J. Cardio Thorac. Surg. 37(6):1297–1303, 2010. doi:10.1016/j.ejcts.2009.12.020.
Mahmood, F., H. Kim, B. Chaudary, R. Bergman, R. Matyal, J. Gerstle, et al. Tricuspid annular geometry: a three-dimensional transesophageal echocardiographic study. J. Cardiothorac. Vasc. Anesth. 27(4):639–646, 2013. doi:10.1053/j.jvca.2012.12.014.
Ring, L., B. S. Rana, A. Kydd, J. Boyd, K. Parker, and R. A. Rusk. Dynamics of the tricuspid valve annulus in normal and dilated right hearts: a three-dimensional transoesophageal echocardiography study. Eur. Heart J. Cardiovasc. Imaging 13(9):756–762, 2012. doi:10.1093/ehjci/jes040.
Rogers, J. H., and S. F. Bolling. The tricuspid valve: current perspective and evolving management of tricuspidregurgitation. Circulation 119(20):2718–2725, 2009. doi:10.1161/CIRCULATIONAHA.108.842773.
Siefert, A. W., J. H. Jimenez, K. J. Koomalsingh, F. Aguel, D. S. West, T. Shuto, et al. Contractile mitral annular forces are reduced with ischemic mitral regurgitation. J. Thorac. Cardiovasc. Surg. 146(2):422–428, 2013. doi:10.1016/j.jtcvs.2012.10.006.
Siefert, A. W., J. H. Jimenez, D. S. West, K. J. Koomalsingh, R. C. Gorman, J. H. Gorman, 3rd, et al. In-vivo transducer to measure dynamic mitral annular forces. J. Biomech. 45(8):1514–1516, 2012. doi:10.1016/j.jbiomech.2012.03.009.
Siefert, A. W., E. L. Pierce, M. Lee, M. O. Jensen, C. Aoki, S. Takebayashi, et al. Suture forces in undersized mitral annuloplasty: novel device and measurements. Ann. Thorac. Surg. 98(1):305–309, 2014. doi:10.1016/j.athoracsur.2014.02.036.
Silver, M. D., J. H. Lam, N. Ranganathan, and E. D. Wigle. Morphology of the human tricuspid valve. Circulation 43(3):333–348, 1971.
Simonneau, G., N. Galie, L. J. Rubin, D. Langleben, W. Seeger, G. Domenighetti, et al. Clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 43(12 Suppl S):5S–12S, 2004. doi:10.1016/j.jacc.2004.02.037.
Simonneau, G., M. A. Gatzoulis, I. Adatia, D. Celermajer, C. Denton, A. Ghofrani, et al. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 62(25 Suppl):D34–D41, 2013. doi:10.1016/j.jacc.2013.10.029.
Spinner, E. M., D. Buice, C. H. Yap, and A. P. Yoganathan. The effects of a three-dimensional, saddle-shaped annulus on anterior and posterior leaflet stretch and regurgitation of the tricuspid valve. Ann. Biomed. Eng. 40(5):996–1005, 2012. doi:10.1007/s10439-011-0471-6.
Spinner, E. M., S. Lerakis, J. Higginson, M. Pernetz, S. Howell, E. Veledar, et al. Correlates of tricuspid regurgitation as determined by 3D echocardiography: pulmonary arterial pressure, ventricle geometry, annular dilatation, and papillary muscle displacement. Circ. Cardiovasc. Imaging. 5(1):43–50, 2012. doi:10.1161/CIRCIMAGING.111.965707.
Spinner, E. M., P. Shannon, D. Buice, J. H. Jimenez, E. Veledar, P. J. Del Nido, et al. In vitro characterization of the mechanisms responsible for functional tricuspid regurgitation. Circulation 124(8):920–929, 2011. doi:10.1161/CIRCULATIONAHA.110.003897.
Spinner, E. M., K. Sundareswaran, L. P. Dasi, V. H. Thourani, J. Oshinski, and A. P. Yoganathan. Altered right ventricular papillary muscle position and orientation in patients with a dilated left ventricle. J. Thorac. Cardiovasc. Surg. 141(3):744–749, 2011. doi:10.1016/j.jtcvs.2010.05.034.
Ton-Nu, T. T., R. A. Levine, M. D. Handschumacher, D. J. Dorer, C. Yosefy, D. Fan, et al. Geometric determinants of functional tricuspid regurgitation: insights from 3-dimensional echocardiography. Circulation 114(2):143–149, 2006. doi:10.1161/CIRCULATIONAHA.106.611889.
Vakil, K., H. Roukoz, M. Sarraf, B. Krishnan, M. Reisman, W. C. Levy, et al. Safety and efficacy of the MitraClip(R) system for severe mitral regurgitation: a systematic review. Catheter. Cardiovasc. Interv. 84(1):129–136, 2014. doi:10.1002/ccd.25347.
Wan, B., M. Rahnavardi, D. H. Tian, K. Phan, S. Munkholm-Larsen, P. G. Bannon, et al. A meta-analysis of MitraClip system versus surgery for treatment of severe mitral regurgitation. Ann. Cardiothorac. Surg. 2(6):683–692, 2013. doi:10.3978/j.issn.2225-319X.2013.11.02.
Acknowledgements
The pig hearts were donated by the Klemke Sausage Haus in Slaton, Texas, USA. The authors would like to thank the ARCS Foundation for their generous support. The authors would also like to thank Philip Henry for providing assistance with construction of the normal and dilated annulus plates.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ajit P. Yoganathan oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Basu, A., He, Z. Annulus Tension on the Tricuspid Valve: An In-Vitro Study. Cardiovasc Eng Tech 7, 270–279 (2016). https://doi.org/10.1007/s13239-016-0267-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-016-0267-9