Abstract
Introduction
Tricuspid regurgitation (TR) affects approximately 1.6 million Americans and is associated with just a 63.9% 1-year survival rate in its moderate to severe forms due to its asymptomatic nature and late diagnosis and surgical referral. As a result, industrial fervor has begun to broach this topic, with several percutaneous treatment devices currently under development. As much remains unknown about the tricuspid apparatus, the mechanics of these procedures remain unquantified. In this study, a testing apparatus and technique for the evaluation of percutaneous tricuspid valve (TV) bicuspidization were developed for the evaluation of these parameters in twelve porcine hearts.
Methods
The passive relaxed myocardial state and the active contracted state were each induced in six porcine hearts and the bicuspidization experiment was run twice, the second time after induction of TR. TV annular area, cinching force, static leakage through the TV annulus, and annular ellipticity were quantified and compared among the groups.
Results
The use of phenol was effective to induce functional TR by increased annular area. Cinching force was not found to differ between any of the testing states, but the bicuspidization experiment was able to reduce the TR annular area to that of its healthy counterpart in addition to reducing static leakage through the TV annulus. Despite appropriately reducing the area, bicuspidization was found to induce a more circular TV annular shape.
Conclusion
Taken together, these results provide a first mechanical analysis of the TV bicuspidization mechanism and may serve as a point of reference for future clinical animal studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart valve disease, which refers to any abnormal condition involving one or more of the heart valves, affects about 2.5% of Americans.43 Academia and industry have long focused their efforts on the investigation and treatment of left heart valve diseases (aortic and mitral valves), which were thought to be the most prevalent and most catastrophic valvular conditions. Recent research, however, shows that diseases of the tricuspid valve (TV) are also extremely prevalent (affecting as many as 25% of people over the age of 6051), may occur secondary to mitral valve (MV) disease, but are not always properly diagnosed and/or treated.38,51
Tricuspid regurgitation (TR), or improper coaptation of the TV leaflets, affects roughly 1.6 million people in the U.S. (217,000 patients per year) and 4.7 million people in the European Union (331,000) and is associated with poor 1-year survival rates (just 63.9% for moderate to severe TR).6,40,57 The most common form of TR, in the absence of structural TV lesions of the tricuspid valve,19 is known as “functional” (FTR) and accounts for over 80% of such cases6,14,49 and may also occur secondary to MV disease in approximately 50% of MV regurgitation (MR) patients.55 TV diseases secondary to MV diseases were previously believed to clear up on their own once the primary MV disease was addressed. However, TR recurrence after concomitant MV surgery has been shown to reach levels as high as 15% and 1 month and 31% at 8 years, on average.9,11,28,49 In fact, close to 50% of patients have been found to develop significant TR after MV replacement.16 Additionally, due to its primarily asymptomatic nature in its mild to moderate forms, TR diagnosis typically occurs very late, once significant remodeling has occurred and the patient is approaching irreversible heart failure. These patients are deemed too high-risk for surgical implantation of a tricuspid annuloplasty ring (the current gold standard approach), which carries high rates of operative mortality (up to 25% and as high as 37% after reoperation) and TR recurrence (up to 60%)28,39,42; as a result, only 1% of patients undergo surgery,22,26,53 about 8,000 per year.54,55
In order to address this issue, several transcatheter devices are currently under development, leveraging the principles behind the surgical annuloplasty, edge-to-edge repair, and bicuspidization procedures. TR is an ideal scenario for the development of such devices due to the poor outcome of current therapies and the ability of the venous system to accommodate larger devices.6 Many of the devices seek to adapt approaches that have shown promise in the mitral space to the tricuspid environment. However, the large elliptical TV anatomy without anchoring calcification (which provides support for transcatheter aortic valve replacement devices), along with decreased flow and loading conditions in the right ventricle (RV) as compared to the left, provide unique challenges and increase the risk of adverse effects such as device dislodging, thrombosis, and endocarditis.6 The fundamental differences in these environments highlight the need for further analysis.
One procedure that has seen success in early compassionate use cases is the percutaneous bicuspidization procedure. The surgical Kay bicuspidization procedure involves the suture plication of the posterior leaflet in order to reduce the tricuspid valve to one with two leaflets, and thus reduce annular area.13,24 While the surgical technique has lost popularity in favor of more physiologic repair techniques, its percutaneous counterpart, the Trialign device (Mitralign, Inc., Massachusetts, US) has shown promise in recent studies.17,29,32 With these promising results, however, comes a need to better understand the mechanics of device-tissue interaction.
Much remains unknown regarding the mechanics of not only TR repair, but also of the tricuspid apparatus (TV, annulus, and chordae tendineae) and RV; this, accompanied by the need and increased industrial push towards the development of effective percutaneous TR repair techniques, prompt a need for further mechanical understanding of the tricuspid apparatus, in order to better understand the implications and possible postoperative complications stemming from percutaneous intervention. As a result, in this study, we have developed a testing device and technique for evaluating the mechanics of TV bicuspidization in six porcine hearts, upon which we induced both “healthy” and “FTR” states. Since percutaneous procedures are performed on beating hearts, we also induced ventricular contraction in the porcine hearts to observe the differences between “passive” (referring to the relaxed configuration) and “active” (referring to the contracted configuration) states. It is hoped that these results offer mechanistic insights into TR repair as well as facilitate the development of computational models of TR.
Methods
Sample Procurement and Preparation
Twelve adult porcine hearts (age: 2–4 years) were obtained from the local slaughterhouse (Holifield Farms, Covington, GA). Immediately upon explantation from the animal, the hearts were submerged in a passive EDTA solution (n = 6) or in a 100 mM KCl (n = 6) solution for several hours to induce either the passive state (relaxed configuration)58 or active state (contracted configuration)20,23 of the right ventricle, respectively.
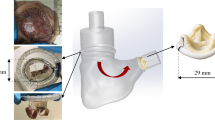
The atria were removed to expose the TV annulus, around which graphite markers were placed. Cinching lines were placed through the TV annulus on either side of the posterior TV leaflet as per current procedural indications (Fig. 1, 3 cm apart, 5 mm superior to leaflet attachment edge).4,29 Silicone pledgets were utilized to support the cinching lines on the inferior side of the TV annulus.
Bicuspidization Experiment
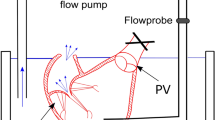
A quasi-static system was chosen as the TV experiences a highest force during peak systole or when it is fully closed.5 The prepped heart was affixed to a custom-built support structure through the left ventricle, so as not to disturb RV motion (Fig. 1b). The cinching lines were passed through a narrow tube acting as a catheter in order to simulate the percutaneous nature of the device and guide motion during the experiment—one suture line (closer to the septal annulus) was prevented from moving so that the other could be pulled towards it as shown in device guidelines. The free end of the moving cinching line was affixed to a load cell (TestResources SM-500-294, ± 1 g accuracy) mounted on a linear slider (± 1 mm resolution) in order to control and measure the cinching length as well as record the associated cinching force. A CCD camera was placed normal to the annular plane for cinching area tracking, and another camera was used to monitor the static leakage through the TV annulus by tracking the water level drop in the tube affixed to the pulmonary artery.
The RV was hydrostatically pressurized to 30 mmHg15,31 through the pulmonary artery to induce TV closure. Once secured to the load cell, the cinching lines were tensioned as in procedural specifications by pulling in 1 cm increments up to 4 cm. At each point, the cinching force was correlated with the images tracking static leakage and annular area and recorded through a custom LabVIEW code.
The FTR state was then induced by topical application of 95% Phenol on the posterior annulus in order to cause sufficient area dilation along the septal-lateral direction8,12 equivalent with induction of moderate FTR (> 25%), and the experiment was repeated in all 12 hearts (see Fig. 2).
Data Analysis
All results are reported as mean ± standard deviation, and the analysis of variance test followed by a post-hoc t-test were employed to determine statistical significance. Non-parametric datasets were tested for significance using the Kruskal Wallis and post-hoc Mann-Whitney U Tests. Cinching force, cinching length, annular area, and static leakage were recorded. The cinching length is defined as the distance the slider moved to induce TAA reduction (1–4 cm); the force recorded by the load cell at each cinching point was defined as the cinching force. The annular area was derived through a custom MATLAB code by tracking the motion of the graphite markers, and static leakage (SL) was measured by recording the loss of water through the TV annulus. While SL is akin to a regurgitant volume, the lack of active beating in the hearts does not allow for direct comparison to clinical parameters.
Ellipticity of the TV annulus was also tracked and compared and relates the large and small radii of the geometry through the equation:
An e approaching 0 is indicative of a more circular geometry, with 0 being a perfect circle.
Regional stretch ratios and strains were computed by determining the displacement of the markers and computing the distance between each marker pair. The Green strain was then derived from the stretch ratios through the equation:
The regional measurements were then compiled according to anatomic location (i.e. Anterior, Septal, Posterior).
Results
The twelve porcine hearts used in this study were divided into four groups: Active Healthy (n = 6), Active TR (n = 6), Passive Healthy (n = 6), and Passive TR (n = 6), based on the muscular state of the ventricular tissue as well as the level of dilation of the TV annulus.
Induction of FTR
Appropriate induction of FTR was evaluated by TV annular area dilation due to phenol application, with successful induction of moderate TR achieved at approximately 25% area increase. An area increase of 22.5 ± 6.1 and 27.2 ± 3.0% was achieved for the passive and active hearts, respectively. Additionally, pre-experimental SL in the TR induced hearts was increased by a factor of 434 ± 74 and 444 ± 180% for the passive and active hearts, respectively, from an average of 3.36 ± 3.29 mL/s (passive, healthy) and 1.14 ± 1.50 mL/s (active, healthy) to 17.81 ± 5.72 mL/s (passive, TR) and 6.20 ± 4.24 mL/s (active, TR).
Bicuspidization Experiment
Figure 3 shows cinching force and normalized annular area for all four testing group at each cinching length. No statistically significant difference in cinching force was found at any cinching point between the testing groups. However, a significantly higher annular area was found in the TR groups as compared to their healthy counterparts at every cinching point, except for one, whose p value was 0.056, indicative of a trend. Additionally, for both the passive and active hearts, cinching the TR heart at 4 cm was able to reduce the annular area (see Figs. 4, 5, and 7a) to values not significantly different from those of the uncinched healthy heart (total annular area reduction of 19.0 and 21.1% for the passive and active TR hearts, respectively), indicative of the success of the experiment (p = 0.45 and 0.42 for the passive and active cases, respectively). The TR reduction was also corroborated by the 43.6 and 52.0% reduction of static leakage in the passive and active TR hearts, respectively (see Figs. 4 and 7b).
Morphology
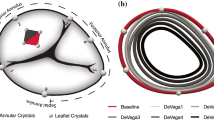
Figure 6 shows representative TV annular morphology for one heart throughout the entire experiment, as well as the superimposed annular geometries as used for ellipticity calculation.
Ellipticity was utilized to evaluate valvular shape throughout the cinching experiment (Fig. 7c). On average, the TV annulus trended towards a more circular shape with increased cinching (Fig. 7c), with ellipticity at the maximum cinching point being significantly lower than that of the starting shape for all groups except the active TR case (p = 0.04, 0.01, 0.01, and 0.10 for the passive healthy and TR and the active healthy and TR cases, respectively). Additionally, TR induction did not significantly alter the ellipticity of the TV annulus (p = 0.27 and 0.40 for the passive and active hearts, respectively).
Tables 1 and 2 summarize the average calculated regional stretch ratio and strain values for the porcine hearts in the anterior, septal, and posterior regions. On average, the anterior and posterior regions had higher strains than the septal (which experienced slight negative strains), with the highest strain in the anterior region. The septal region had significantly lower stretch values than the anterior and posterior regions.
Discussion
Termed “the forgotten valve,” the TV has long been neglected by research and industrial development due to the belief that diseases of the left heart were more prevalent. However, it has been shown that mild to severe TR can affect as many as 25% of people over age 60 and its prevalence is on par with that of aortic and mitral diseases.38,51 Unlike in the aortic valve space, the unique TV anatomy, the presence of immediate adjacent anatomic landmarks such as the atrioventricular node and the decreased blood pressure pose a challenge for the development of effective treatment strategies and require further investigation, particularly from a mechanical perspective.
By identifying the key mechanical and morphological factors involved in TR reduction, the results from this study may shed light on key mechanical and morphological parameters associated with this procedure, and provide preliminary results in an animal model, which will serve as baseline results for future human experiments and can inform insights from clinical animal testing.
Experimental Investigations of Tricuspid Annular Mechanics
Several studies have measured the forces applied on the tricuspid annulus under various conditions, specifically aimed at understanding the tricuspid annuloplasty procedure,27,33,44 but none to date have investigated the bicuspidization procedure, one of the primary mechanisms for development of percutaneous TR repair techniques. The high risk of recurrence and operative mortality for the annuloplasty procedure and the low rate of surgical intervention make such an investigation necessary.
Currently, little information is available regarding the TV annulus as well as device-tissue interaction, particularly in the context of percutaneous application. The Yoganathan group has investigated the variation of pullout forces of mitral and tricuspid annuloplasty sutures in different species (ovine, porcine, human) around the annulus and with ring shape and flexibility, but those methods are focused on the surgical therapy.34,44,45 Additionally, the Redaelli group has studied the transcatheter edge-to-edge repair technique from a flow and morphological perspective 21,60 using a passive beating heart model.30 While the passive beating heart model can provide a more accurate hemodynamic assessment of TV mechanics, such a setup requires significant intervention in RV mechanics through supports and would affect TV annular motion. For a mechanical investigation of the bicuspidization procedure, an uninhibited annulus and collection of mechanical force data are imperative and were not studied in these publications. Several studies have either collected mechanical data of the RV components of various animal tissues through common mechanical testing techniques3,25,46,47 or morphological and hemodynamic parameters through more sophisticated ex vivo models, but few have collected mechanical data in conjunction with morphological data for the tricuspid annulus,1,2,5 and none in the context of the TV bicuspidization procedure.
The studies of Bhattacharya et al employed a similar procedure to the one presented in this study to evaluate annular area reduction in ovine and porcine MV and TV annuli.1,2,5 These studies found mean cinching forces of 0.40 and 0.38 N in the respective ovine and porcine hearts,1,2 while our study found mean cinching forces to be around 1.2–1.5 N. The differences can be explained by key variations in the testing protocol of Bhattacharya et al as compared to the one presented in this study: (1) support was provided through both ventricles, while in our experiments the heart was supported through the left ventricle to avoid interfering with the mechanical environment of the RV and confounding the results, (2) annular dilation was significantly lower than in our studies (8.82 and 11.2% area increase vs. 22 and 27% in our studies), (3) the cinching mechanism of the Bhattacharya et al TV studies placed a running suture around the free wall of the RV with an exit between the mitral and aortic valves, whereas our study aims to mimic current bicuspidization approaches by placing two sutures anchored around the posterior leaflet, (4) the maximum cinching length in the Bhattacharya et al studies is approximately 2.4 cm, significantly lower than our maximum cinching length of 4 cm—at the 2 cm cinching length, in our study, the average cinching force was around 0.7 N. Additionally, our study also induced the active and passive tissue states, something not attempted in previous studies of either the mitral or tricuspid valves.1,2,5
Induction of FTR
The tricuspid annulus is known for being widely adaptable—specifically being able to withstand large changes in dimensions (as compared to other valves) without immediately undergoing significant changes in flow parameters. As a result, more and more studies have investigated the potential applicability of geometric parameters such as annular area and diameter as early indicators for surgical intervention.56,59 Current guidelines recommend tricuspid valve repair when there is significant annular dilatation of the tricuspid valve, approximately 25% (> 40 mm).7,10,59 It has also been shown that severe TR is possible at 40% dilation of annular area,52 while 25% may be more indicative of moderate TR.
The studies of Bhattacharya et al.,1,2,5 which also employed the phenol technique for induction of FMR and FTR, achieved only 8.82 and 11.2% increases in annular area for ovine and porcine hearts, which do not satisfy the significant annular dilatation requirement of FTR induction and may be instead related to milder forms of FTR. Due to the active contraction of the RV in vivo, it is impossible to compare experimental leakage through the TV to clinical values of regurgitant volume. However, these studies resulted in 81.73 and 275% increases in SL for ovine and porcine tissue, respectively, a significant increase in TV backflow, which was reduced by 56% after cinching in the passive FTR state, comparable to the values we have also presented.
Morphological Considerations
It is known that the atrioventricular valves have a complex three-dimensional geometry that changes with disease state. Studies from the Rausch lab as well as others35,36,–37,47,48 have investigated the 3D geometries of healthy and diseased animal TV valves. In this study, we also discussed the ellipticity of the TV annulus pre- and post- induction of TR as well as pre- and post- bicuspidization, finding that the TV annulus trends towards a more circular shape as it is cinched in this mechanism. The planar projections of the TV are measurements that, in the clinical setting, are obtained more quickly than the complex 3D anatomy—thus, having information regarding the ellipticity of the en face view of the TV annulus may provide an extra marker for disease classification and possible procedural endpoints.
While the Rausch group has primarily studied the tricuspid annulus in an ovine model, we found comparable ellipticity values, ranging between 0.6 and 0.8, annular strain values in healthy sheep (− 0.10 to +0.10)47 as well as sheep with induced pulmonary hypertension (− 0.15 to + 0.15).48 As in our study, the highest positive strains were observed in the anterior annulus region and negative strains were observed in the septal region. This correlates well with previously published mechanical data,34,46 which has shown increased stiffness in the tricuspid apparatus components (leaflets, chordae tendineae, annulus) located in the septal region.
We also found that, in the passive TR case, strains decreased uniformly, with negative strains increasing in all regions, especially in the septal region—a similar conclusion was reached by the Rausch group when analyzing the ovine tricuspid annulus post-annuloplasty. Taken together, these results provide additional mechanistic insight into the tendency for implanted devices to suffer from device dehiscence in the septal region and may help to guide future studies as well as the development of mitigation techniques.
Implications for the Bicuspidization Procedure
The first percutaneous bicuspidization procedure using the Trialign device was performed in an 89 year-old female in 2014, resulting in a 57% reduction in annular area, TR and right atrial pressure reduction, and an increase in LV stroke volume.14,50 Since then, the procedure has increased in use, with a completed FDA early feasibility study (SCOUT) and a multicenter CE Mark trial underway (SCOUT-II).41 The 30-day results of the SCOUT trial18 show a 10% decrease in TV annular area, a 28% reduction in regurgitant volume, and no significant change in ellipticity of the regurgitant orifice. While the final endpoints of our study showed increased reduction in TV annular area and static leakage compared to human results, the reported in-patient results are similar to our study’s TR results at the 2 cm cinching length, pointing to the applicability of the presented method as an efficient animal model for studying the percutaneous bicuspidization procedure. Additionally, at the 2 cm cinching length, no significant change in annular ellipticity was found in all groups except the active TR group. The SCOUT trial also reported single-pledget annular detachments without reintervention in 20% of treated patients,18 an adverse effect that should be investigated from a mechanical perspective in future studies.
Limitations
While the results from this study are promising, they are only the first step in assessing the mechanics of TR and its repair techniques, and this study carries several limitations. First, the sample size of six samples is relatively small, and a larger one would increase the statistical power of the study. Second, the atria were removed for visualization of the tricuspid annulus, which does affect the annular support structure and may have influenced the results. Additionally, the topical application of phenol to the RV free wall has been shown to change the tissue integrity, specifically in the realm of cell viability,16 and may have affected the results as well. Future studies comparing naturally-occurring chronic TR with dilated RA and/or RV in human hearts to healthy hearts or controlled induction of TR in a live animal model may shed more light on this matter. However, as typical RV remodeling that leads to TR also occurs on the free wall, the use of phenol was deemed an appropriate substitute for the purposes of this study. Lastly, while the planar projection of the TV annular shape was analyzed, the 3D geometry was not, and should be incorporated in future iterations of this study.
Conclusions
This study investigated the mechanics of the percutaneous TV bicuspidization in six porcine hearts, comparing the passive and active states as well as the healthy and induced TR states in the same heart sets. A novel device and technique were employed to obtain mechanical parameters evaluating TV cinching, and the planar projection of the TV annulus shape was also analyzed. It was found that the use of topical phenol application was effective in inducing moderate TR by annular area dilatation, and that the developed apparatus and technique were able to reduce the TV annular area to pre-dilation levels, although the force required did not statistically differ between the passive and active tissue states. Additionally, it was found that bicuspidization leads to a more circular annular profile. Taken together, these results present a first look at ex vivo animal models of TR bicuspidization—they may serve as a point of reference for clinical animal studies and facilitate the development of a computational model of TV bicuspidization.
References
Adkins, A., et al. Force required to cinch the tricuspid annulus: an ex-vivo study. J. Heart Valve Dis. 24(5):644, 2015.
Aleman, J., et al. Effects of Cinching Force on the Tricuspid Annulus: A Species Comparison. J. Cardiovasc. Dis. Diagn. 5:4, 2017.
Amini Khoiy, K., et al. Surface strains of porcine tricuspid valve septal leaflets measured in ex vivo beating hearts. J. Biomech. Eng. 138(11):111006, 2016.
Besler, C., C. U. Meduri, and P. Lurz. Transcatheter treatment of functional tricuspid regurgitation using the trialign device. Interv. Cardiol. Rev. 13(1):8, 2018.
Bhattacharya, S., et al. Tension to passively cinch the mitral annulus through coronary sinus access: An ex vivo study in ovine model. J. Biomech. 47(6):1382–1388, 2014.
Cilingiroglu, M., and K. Marmagkiolis. Percutanous management of tricuspid regurgitation: The “Achilles tendon” of transcatheter valve interventions. Catheterization Cardiovasc. Interv. 88(2):294–295, 2016.
Colombo, T., et al. Tricuspid regurgitation secondary to mitral valve disease: tricuspid annulus function as guide to tricuspid valve repair. Cardiovasc. Surg. 9(4):369–377, 2001.
Correia, P., G. Coutinho, and M. Antunes. Tricuspid valve: a valve not to be forgotten. E-J. Cardol. Pract. 11:20, 2013.
Di Mauro, M., et al. Mitral valve surgery for functional mitral regurgitation: prognostic role of tricuspid regurgitation☆. Eur. J. Cardio-Thorac. Surg. 35(4):635–640, 2009.
Dreyfus, G. D., et al. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann. Thorac. Surg. 79(1):127–132, 2005.
Fender, E. A., R. A. Nishimura, and D. R. Holmes. Percutaneous therapies for tricuspid regurgitation. Expert review of medical devices 14(1):37–48, 2017.
Fukuda, S., et al. Three-dimensional geometry of the tricuspid annulus in healthy subjects and in patients with functional tricuspid regurgitation: a real-time, 3-dimensional echocardiographic study. Circulation 114(1_supplement):I-492-I–498, 2006.
Ghanta, R. K., et al. Suture bicuspidization of the tricuspid valve versus ring annuloplasty for repair of functional tricuspid regurgitation: Midterm results of 237 consecutive patients. J Thorac. Cardiovasc. Surg. 133(1):117–126, 2007.
Giannini, F. and Latib, A. (2016) The Next Frontier of Percutaneous Tricuspid Valve Repair.
Granegger, M., et al. A passive beating heart setup for interventional cardiology training. Curr. Dir. Biomed. Eng. 2(1):735–739, 2016.
Green, G. R., et al. Mitral annular dilatation and papillary muscle dislocation without mitral regurgitation in sheep. Circulation 100(2):II-95-II-102, 1999.
Hahn, R. T., et al. Early feasibility study of a transcatheter tricuspid valve annuloplasty: SCOUT trial 30-day results. J. Am. Coll. Cardiol. 69(14):1795–1806, 2017.
Hahn, R. T., et al. Early feasibility study of a transcatheter tricuspid valve annuloplasty: SCOUT Trial 30-day results. J. Am. Coll. Cardiol. 69(14):1795–1806, 2017.
Hung, J. The pathogenesis of functional tricuspid regurgitation. Semin. Thorac. Cardiovasc. Surg. 22(1):76–78, 2010.
Huntington, A., et al. Anisotropy of the Passive and Active Rat Vagina Under Biaxial Loading. Ann. Biomed. Eng. 47(1):272–281, 2019.
Jaworek, M., et al. Long-arm Clip for Transcatheter Edge-to-Edge Treatment of Mitral and Tricuspid Regurgitation–Ex-Vivo Beating Heart Study. Struct. Heart 3(3):211–219, 2019.
Jeong, D. S., et al. Fate of functional tricuspid regurgitation in aortic stenosis after aortic valve replacement. J. Thorac. Cardiovasc. Surg. 148(4):1328–1333, 2014.
Kato, T., et al. Inhibitory effects and active constituents of Alisma rhizomes on vascular contraction induced by high concentration of KCl. Bull. Chem. Soc. Japan 67(5):1394–1398, 1994.
Kay, J. H., G. Maselli-Campagna, and H. K. Tsuji. Surgical treatment of tricuspid insufficiency. Ann. Surg. 162(1):53, 1965.
Khoiy, K. A., and R. Amini. On the biaxial mechanical response of porcine tricuspid valve leaflets. J. Biomech. Eng. 138(10):104504, 2016.
Kim, Y.-J., et al. Determinants of surgical outcome in patients with isolated tricuspid regurgitation. Circulation 120(17):1672–1678, 2009.
Kragsnaes, E. S., et al. In-plane tricuspid valve force measurements: development of a strain gauge instrumented annuloplasty ring. Cardiovas. Eng. Technol. 4(2):131–138, 2013.
Kuwaki, K., et al. Tricuspid valve surgery for functional tricuspid valve regurgitation associated with left-sided valvular disease. Eur. J. Cardio-Thorac. Surg. 20(3):577–582, 2001.
Latib, A., et al. Percutaneous bicuspidization of the tricuspid valve. JACC 10(4):488–489, 2017.
Leopaldi, A., et al. A novel passive left heart platform for device testing and research. Med. Eng. Phys. 37(4):361–366, 2015.
Li, R.-K., et al. Autologous porcine heart cell transplantation improved heart function after a myocardial infarction. J. Thorac. Cardiovasc. Surg. 119(1):62–68, 2000.
Lurz, P., et al. Early experience of the trialign system for catheter-based treatment of severe tricuspid regurgitation. Eur. Heart J. 37(47):3543–3543, 2016.
Madukauwa-David, I. D., et al. Suture dehiscence and collagen content in the human mitral and tricuspid annuli. Biomech. Model. Mechanobiol. 8(2):291–299, 2018.
Madukauwa-David, I. D., et al. Suture dehiscence and collagen content in the human mitral and tricuspid annuli. Biomech. Model. Mechanobiol. 18(2):291–299, 2019.
Malinowski, M., et al. Tricuspid annular geometry and strain after suture annuloplasty in acute ovine right Heart failure. Ann. Thorac. Surg. 106(6):1804–1811, 2018.
Malinowski, M., et al. Impact of tricuspid annular size reduction on right ventricular function, geometry and strain. Eur. J. Cardio-Thorac. Surg. 2019. https://doi.org/10.1093/ejcts/ezy484.
Malinowski, M., et al. Sonomicrometry-derived 3-dimensional geometry of the human tricuspid annulus. J. Thorac. Cardiovasc. Surg. 157(4):1452–1461, 2019.
Marciniak, A., K. Glover, and R. Sharma. Cohort profile: prevalence of valvular heart disease in community patients with suspected heart failure in UK. BMJ open 7(1):e012240, 2017.
McCarthy, P. M., et al. Tricuspid valve repair: durability and risk factors for failure. The Journal of Thoracic and Cardiovascular Surgery 127(3):674–685, 2004.
Nath, J., E. Foster, and P. A. Heidenreich. Impact of tricuspid regurgitation on long-term survival. J. Am. Coll. Cardiol. 43(3):405–409, 2004.
Nijenhuis, V., et al. The last frontier: transcatheter devices for percutaneous or minimally invasive treatment of chronic heart failure. Netherlands Heart J. 25(10):536–544, 2017.
Nishimura, R. A., et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129(23):e521–643, 2014.
Nkomo, V. T., et al. Burden of valvular heart diseases: a population-based study. Lancet 368(9540):1005–1011, 2006.
Paul, D. M., et al. Suture dehiscence in the tricuspid annulus: an ex vivo analysis of tissue strength and composition. Ann. Thorac. Surg. 104(3):820–826, 2017.
Pierce, E. L., et al. How local annular force and collagen density govern mitral annuloplasty ring dehiscence risk. Ann. Thorac. Surg. 102(2):518–526, 2016.
Pokutta-Paskaleva, A., et al. Comparative mechanical, morphological, and microstructural characterization of porcine mitral and tricuspid leaflets and chordae tendineae. Acta Biomater. 85:241–252, 2019.
Rausch, M. K., et al. Engineering analysis of tricuspid annular dynamics in the beating ovine heart. Ann. Biomed. Eng. 46(3):443–451, 2018.
Rausch, M. K., et al. The effect of acute pulmonary hypertension on tricuspid annular height, strain, and curvature in sheep. Cardiovasc. Eng. Technol. 9(3):365–376, 2018.
Rodés-Cabau, J., et al. Transcatheter therapies for treating tricuspid regurgitation. Journal of the American College of Cardiology 67(15):1829–1845, 2016.
Schofer, J., et al. First-in-human transcatheter tricuspid valve repair in a patient with severely regurgitant tricuspid valve. J. Am. Coll. Cardiol. 65(12):1190–1195, 2015.
Singh, J. P., et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am. J. Cardiol. 83(6):897–902, 1999.
Spinner, E. M., et al. In vitro characterization of the mechanisms responsible for functional tricuspid regurgitation. Circulation 124(8):920–929, 2011.
Stuge, O., and J. Liddicoat. Emerging opportunities for cardiac surgeons within structural heart disease. J. Thorac. Cardiovasc. Surg. 132(6):1258–1261, 2006.
Stuge, O. and J. Liddicoat, Emerging opportunities for cardiac surgeons within structural heart disease. 2006, Mosby.
Taramasso, M., et al. Percutaneous tricuspid valve therapies: the new frontier. European heart journal 38(9):639–647, 2017.
Ton-Nu, T.-T., et al. CLINICAL PERSPECTIVE. Circulation 114(2):143–149, 2006.
Tricuspid Regurgitation Global Strategic Market Assessmen, D. Consulting, Editor. 2016.
Tritthart, H., et al. Effects of Ca-free and EDTA-containing Tyrode solution on transmembrane electrical activity and contraction in guinea pig papillary muscle. Pflügers Archiv 338(4):361–376, 1973.
Van de Veire, N. R., et al. Tricuspid annuloplasty prevents right ventricular dilatation and progression of tricuspid regurgitation in patients with tricuspid annular dilatation undergoing mitral valve repair. J. Thorac. Cardiovasc. Surg. 141(6):1431–1439, 2011.
Vismara, R., et al. Transcatheter edge-to-edge treatment of functional tricuspid regurgitation in an ex vivo pulsatile heart model. J. Am. Coll. Cardiol. 68(10):1024–1033, 2016.
Acknowledgments
Fatiesa Sulejmani is also supported by the Georgia Institute of Technology-Emory University-Peking University Global Biomedical Engineering Research and Education Fellowship, as well as the Friends of Bobby Jones Fund from Emory University’s Laney Graduate School.
Conflict of interest
Dr. Wei Sun is a co-founder and serves as the Chief Scientific Advisor of Dura Biotech. He has received compensation and owns equity in the company. Fatiesa Sulejmani and Joshua Pataky declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Changfu Wu oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sulejmani, F., Pataky, J. & Sun, W. Mechanical and Structural Evaluation of Tricuspid Bicuspidization in a Porcine Model. Cardiovasc Eng Tech 11, 522–531 (2020). https://doi.org/10.1007/s13239-020-00480-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-020-00480-0