Abstract
Tricuspid valve repair durability often requires successful restoration and support with an annuloplasty ring. Throughout the cardiac cycle the annular plane changes shape and size. The stress distribution in these repair devices indirectly determines the success of the repair. Annuloplasty ring force distribution has previously been reported in the apical direction of the mitral valve. It was hypothesized that the force distribution in a stiff, flat annuloplasty ring in the tricuspid annulus plane throughout the cardiac cycle could be measured. A D-shaped rigid flat annuloplasty ring was designed and instrumented with strain gauges on the inner portion of the annulus. This system was capable of measuring in-plane mechanical strain. The calibration system was designed to convert the measured strain to force, with a scale ranging from −5 to 5 N. One animal was included in an acute porcine pilot study to test the transducer implanted in the tricuspid valve. The transducer and calibration system demonstrated that it is feasible to convert the measured mechanical strain in the annuloplasty ring to in-plane forces as a response to right ventricular and atrial pressure and muscle activity during the cardiac cycle. In the pilot experiment, a regional force of up to 0.4 N was observed at the tricuspid valve anteroseptal commissural area during systole. The feasibility of measuring in-plane calibrated mechanical force applied to the annuloplasty ring from the tricuspid valve will facilitate further in vivo work to illuminate any non-physiological loading of the right heart myocardium following valve repair with an annuloplasty ring. This information can be useful for optimization of tricuspid valve annuloplasty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tricuspid valve (TV) is positioned in the annular plane where it separates the right atrium from the right ventricle and ensures unidirectional flow during the cardiac cycle. The TV consists of three leaflets named after their location, the septal, anterior, and posterior leaflets. The leaflets are connected to the right ventricular papillary muscles by the chordea tendineae. Situated at the annular plane between the leaflets are the commissures; the anteroseptal, anteroposterior and posteroseptal commissures. Throughout the cardiac cycle the tricuspid annular plane changes shape and size.5,8 In TV disease, such as annular dilatation and regurgitation, valve repair with the use of either a flat or curved annuloplasty ring is often performed in order to stabilize the valve and ensure sufficient leaflet coaptation.2,3,11,12 This tricuspid annuloplasty can be performed with a subset of the rings traditionally used in mitral valve repair. Recently, annuloplasty rings have been developed to support the TV annulus in a 3D configuration that is more relevant to the tricuspid valve systolic configuration such as the Edwards Lifesciences MC3™ and Carpentier-Edwards Physio Tricuspid™ or the Medtronic Tri-Ad™ Adams Tricuspid Annuloplasty rings.1–4,6,12
Mitral valve annular force distribution has previously been reported in the apical direction9,10 as well as the in-plane direction7,14–16 but no studies have so far evaluated the tricuspid annular force distribution in annuloplasty rings. In order to obtain the force balance of the tricuspid valve both in and out of the annular plane for future modeling and device development purposes, it is important that this force is measured by restraining the annulus in the same flat configuration for the out of and in-plane measurements. This force can be referred to as the restraining force,9,10 and we have chosen to refer to it in the following as the force applied to the ring. The aim of this study was to develop a force transducer annuloplasty ring capable of measuring the in-plane force distribution of the tricuspid annulus.
Materials and Methods
Annuloplasty Ring Force Transducers
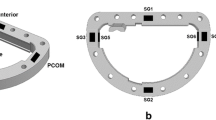
The annuloplasty ring was designed with 12 suture holes placed on top to simplify implantation and facilitate the strain gauge placement and force transduction (Fig. 1a). Rapid Prototyping technology (DAVINCI Development, Billund, Denmark and Danish Technology Institute, Aarhus, Denmark) was used to manufacture the annuloplasty ring (Fig. 1b). The source material for the rings was DuraForm (3D-systems, Valencia, Spain), which is commonly used in Selective Laser Sintering (SLS) rapid prototyping systems to produce rugged parts that withstand in vivo use in rapid prototyping and manufacturing applications. Duraform is approved for short-term in vivo experiments; hence the material does not change properties under these conditions. The glass transition temperature of Duraform is higher than the operating temperature of the experiment. From the Duraform datasheet it is also confirmed that we are expecting to operate well within the elastic range of the material, which will be confirmed by evaluating the linearity of the system during calibration.
(a) Design of the annuloplasty ring used to measure the force applied to the ring in the tricuspidal valve annular plane. POST: Posterior segment, ANT: Anterior segment, SEP: Septal segment. Dark squares on inside of ring mark the strain gauge locations (two out of six shown here). (b) The annuloplasty ring produced with rapid prototyping technology and mounted with strain gauges, ready for implantation. (c) Atrial view identifying the positions of the strain gauge [SG1-6], [P] = Posterior leaflet, [A] = Anterior leaflet, [S] = Septal leaflet
Six quarter-bridge strain gauges were mounted (Vishay Measurement Group UK Ltd., Basingstoke, UK), placed corresponding to the central part of the septal, posterior, and anterior leaflets respectively and at each of the commissural positions (Fig. 1c). All were mounted around the inside of the ring and placed such that the calibrated radial forces were measured, with less distance between the strain gauges in the posterior and anterior area, where the largest deformation of the TV annulus has been observed.5,8 The wires connecting the strain gauges were covered and sealed with silicone (Fig. 1b). The lead wire was fed through a stabilizing support frame extrusion (Figs. 1a, 1b) which was placed at the anteroseptal side with an angle to facilitate exteriorizing of the lead wire through the right atriotomy. A three-lead-wire strain gauge wiring scheme was utilized in order to minimize the effects of leadwire desensitization of the gauge arm and temperature changes between the calibration and measurement process.
By calibrating the rings it was possible to convert the measured strain to forces and determine the direction of these forces relative to the calibration set-up. Hence, a custom made calibration stand was designed and used to convert the regionally measured unit (strain) to force applied to the ring from the TV annulus (Fig. 2). The ring was calibrated in the annular plane with a scale ranging from −5 N to 5 N with 1 N increments based on experience from force measurements in the mitral valve.7,9,15 The tricuspid annulus structural integrity along the annular perimeter is complex and the fixation arrangement of annuloplasty rings in the annulus could not be precisely replicated in this calibration setup. Hence, all data reported on force applied to the ring from the experiments in this study were adjusted according to this calibration. Force applied to the annuloplasty ring acting in the direction away from the center of the ring were defined as negative (Figs. 3a, 3c) Thus, calibration forces acting in the opposite direction, towards the center of the ring were defined as positive (Figs. 3b, 3d).
(a), (b) Location of strain gauges [SG1] and [SG4], marked with solid black squares. The direction of the forces used to calibrate [SG1] and [SG4] in tension (defined as negative force direction, −FA) and compression (defined as positive force direction, +FB) are marked. (c), (d) Location of strain gauges [SG2], [SG3], [SG5] and [SG6], marked with solid black squares. The direction of the forces used to calibrate [SG2], [SG3], [SG5] and [SG6] in tension (defined as negative force direction, −FC) and compression (defined as positive force direction, +FD) are marked. See text for further explanation
The calibration system consisted of a calibration force arm to each hole in the annuloplasty ring (Fig. 2). It was decided to use the average signal from the strain resulting from forces from two opposite positioned calibration arms to calibrate the strain gauges. Accordingly, SG1 and SG4 were calibrated by using known forces in the positive (+FB) or negative (−FA) configuration of the ring (Figs. 3a, 3b). Negative force or pull [−FA] would apply strain to the area onto which the gauges SG1 and SG4 were mounted (Fig. 3a). Likewise, positive force or push [+FB] would apply compression (negative strain) onto the area in which the gauges SG1 and SG4 were mounted (Fig. 3b).
For the strain gauges placed in the posterior and anterior areas (Figs. 3c, 3d); SG2, 3, 5 and 6 were combined into pairs of two: SG2 and SG3 in one pair and SG5 and SG6 in one pair, calibrated by using [−FC] (Fig. 3c) and [+FD] (Fig. 3d). The concept of calibrating SG2, 3, 5 and 6 was the same as calibrating SG1 and SG4. The calibration output is illustrated in Fig. 4. Careful design and testing of the ring ensured that a force of one Newton acting orthogonally out of the plane of the ring produced less than 10% of the strain that an equivalent force affecting the ring in the annulus plane would produce.
Calibration of the strain gauges [SG1] and [SG4] as well as the strain gauge sets [SG2 + SG3] and [SG5 + SG6]. Calibration was performed by applying forces as illustrated in Fig. 3 from −5 N to +5 N in increments of 1 N. The linear relationship of the strain as a function of force is illustrated
The noise level of the force transducer was measured and evaluated during a pilot animal experiment, as this was the environment in which it was most susceptible to surrounding electrical noise. In this case the noise floor was less than 0.1 N using a 50 Hz 3rd order Butterworth low-pass filter. Noise reduction was optimized by proper grounding between the animal and the data acquisition equipment, and thorough sealing of the SGs on the annuloplasty ring.
Data Acquisition
The strain gauges were connected to a Wheatstone Bridge completion circuit and measurements were acquired with data acquisition hardware (compact DAQ model 9172 and NI-9237; National Instruments, Austin, TX, USA). The right atrial and ventricular pressures signals were obtained with Microtip pressure catheters (SPC-350MR; Millar Instruments, Houston, TX, USA) and amplified with a two channel pressure control unit (PCU-2000; Millar Instruments). Analog signals from the pressure transducer and amplification system were acquired with data acquisition hardware (NI-9215; National Instruments). Strain and hemodynamic data were recorded with virtual instrumentation software (LabVIEW version 2010; National Instruments). The ECG was monitored with the CardioMed data acquisition system (Model 4008; CardioMed A/S, Oslo, Norway) and recorded with the data acquisition hardware and software.
For intracardiac measurements, a flat frequency response curve up to 30 Hz is recommended.13 The determinants of a mechanical system to enter global resonance frequency are structural design and the nature of a cyclic impact. The frequency response of a mechanical system may be virtually tested by numerical modal analysis in the design phase. However, the structure of the physical design of this ring is equivalent to the out-of-plane force measurement rings that have been used by our group previously,9 and the material used is identical. It is important to realize that these rings are affected by forces in all directions, and hence, the frequency content of the cyclic impact necessary to bring the transducer systems to resonance will be equivalent. It was shown that the resonance frequency of the ring when the strain measurements were performed on the top surface of the ring was orders of magnitude larger than the frequency content experienced in the cardiovascular system. Hence we can use the results from the previous frequency response test from a dedicated setup, in which the response curves were flat within ±3 dB up to approximately 150 Hz, which determines the upper limiting frequency.9 In addition, the phase frequency response curve identified that the delay from applied to measured force was acceptable, such that the load rate dependency of the calibration of the device was minimized.
Data Analysis
All data were post processed, analyzed offline, and reported as a 10-heart cycle ensemble average, with 1 heart cycle defined as the time from QRS peak to peak. All parameters are reported at mid systole and mid diastole, which was defined as time center points between right ventricular dp/dt max and dp/dt min.
Implantation and Measurements In vivo
The force transducer was evaluated in terms of its applicability and interference with the measured quantity in one animal pilot study using a mixed Yorkshire and Danish Landrace pig with a body weight of 80 kg in an acute setting. The animal was bred under standard laboratory conditions, and the experiment complied with the guidelines from the Danish Inspectorate of Animal Experimentation, which has also approved the study. Details of the animal model have been described previously.9,10 Initially, a sternotomy was performed and canulation was performed in a bi-caval fashion. During cardiopulmonary bypass and cardiac arrest the tricuspid valve was entered through the right atrium and the annuloplasty force ring was secured to the tricuspid annulus through the mounting holes using 11 interrupted 2–0 TI-CRON sutures. For pressure measurements, the Millar catheters were placed in the right atrium and ventricle.
Experimental Protocol
The annuloplasty ring force transducer was zeroed after implantation while on cardiopulmonary bypass prior to performing the pilot experiment. Evaluating the performance of the system, annuloplasty force distribution measurements were performed after weaning from cardiopulmonary bypass at a right ventricular pressure of 28 mmHg. Tricuspid valve competence was confirmed with color Doppler ultrasound. Normal atrial and ventricular pressures were verified with online pressure measurements (see above).
Results
Using the transducer demonstrated that it is feasible to convert measured mechanical strain in the plane of annuloplasty rings to forces (relative to the calibration setup) with a high degree of linearity (See Fig. 4). The uncalibrated strain recorded in each annulus segment from individual strain gauges is shown in Fig. 5. Data for SG2 displaying the measured strain converted to force [N] relative to the calibration in relation to the right ventricular and right atrial pressures during the cardiac cycle can be seen in Fig. 6. It was here, at the anteroseptal commissural area, that the largest regional calibrated force applied to the ring of 0.4 N was observed at during systole.
Example of one heart cycle of right atrial and ventricular pressure measurements synchronized with direct strain data from the individual six strain gauges of the ring. [RVP] = right ventricular pressure, [RAP] = right atrial pressure. See Fig. 1c for placement of individual strain gauges on the annuloplasty ring. Data is an ensemble average of ten heart cycles
Discussion
The tricuspid annulus has been reported to be a non-homogeneous structure that changes in shape and size during the cardiac cycle.5,8 Implantation of a rigid annuloplasty ring on one hand obviously limits the active movement of the annulus but on the other hand allows us to study the distribution of the force applied to the annuloplasty ring present in the beating heart. The present study identifies a method to measure the in-plane biomechanical stresses on tricuspid annuloplasty rings by positioning the force transducers on the inner curvature of the annuloplasty ring. This allows continuous measurement of the strain and calibrated force distribution at six well defined regions of the tricuspid annulus. Furthermore, it was possible to place suture holes on top of the ring to facilitate proper and accurate implantation of the ring. No valve incompetence was observed, demonstrating that ring implantation did not adversely affect the tricuspid valve function. Future experiments with this equipment currently ongoing will enlighten the link between the tricuspid annulus and a potential non-homogeneous force distribution during the cardiac cycle in the tricuspid annuloplasty rings.
The fact that the observed largest deformation in the normal tricuspid annulus seen in the in the posterior and anterior areas5,8 does not necessarily match the location largest measured calibrated force in the anteroseptal commissural is an important concept of “images can be deceiving”. This was also experienced in the mitral valve9,10: It is essential to understand that a structure such as the annuloplasty ring acts as a fixed body within a dynamic annulus, and the creation of strain in this body is governed by both direct forces as well as secondary forces acting in other locations creating a moment at the point in which the body is suspended. And this suspension can be thought of as being primarily located around the fibrous bands surrounding the interface between the aortic and the atrioventricular valves. These observations are important to verify in future experiments with this device.
Naturally, a device that has the capability to simultaneously measure both in- and out-of-plane loads/stresses would be the optimal solution. However, the amount of surface area on the ring required for this type of setup for strain gauge mounting and wiring with the current suture hole locations would render the ring inappropriately large for animal implantation. Additionally, the wiring for more strain gauges would create a thicker and stiffer cable leading off the transducer. Also, heat development in the ring needs to be considered extra carefully if both in and out of plane loads/stresses were attempted measured simultaneously. One option would be to use the Rosette strain gauge configuration, which typically consists of three gauge grids mounted close to each other with known angles in-between, and the principal direction of the stain can be found. However, calibrating these types of setups would render ambiguous results in this fixation situation.
Calibration is in general only valid when the analysis of results is based on the conditions of the calibration. When comparing the attachment in the calibration set-up with the valve insertion it is clear that the two supporting structures are not mechanically equal. Hence, it is essential to keep in mind that the direction and the force values are relative to the calibration. Reporting on forces from measurements with this ring will hence be ambiguous without referring back to the calibration setup. If the data from this device would be used in for example a computerized model of the right heart with annuloplasty devices implanted, it may be advantageous to use the actual strain measurements on the ring in a system where the ring including strain gauges, glue, wiring, and sealing can be included in the model. In addition, since the design of the annuloplasty ring includes a support mechanism for the lead wire, the thickness of the ring is not continuous. However, calibration will eliminate any error from this. Also, the transducer may experience a slight difference in temperature between calibration and in vivo ring implantation. This may influence the results, but is minimized by using a three-lead-wire setup as well as focusing the analysis on the difference between systole and diastole.
The calibration data displayed in Fig. 4 confirms that we are operating within the elastic range of the Duraform material used to create the ring, ensuring that we have linearity of the system. The frequency response test that is referenced in this study is performed with forces influencing the system from only one direction, which is a limitation. It would be optimal if a frequency response test could be designed to apply forces with varying frequency content in different points and directions of the ring—separated by 45° angles. However, this method of extrapolating frequency response tests has been used in the past by our own and other groups.10,16
We do not consider the drag of blood on the ring to be affecting the measurements considerably, since the highest forces are measured in systole when the valve is closed. However, we do focus on the difference between force values in systole and diastole, so the force that drag of blood incurring during diastole may have an impact on the results. This remains to be verified in future experiments with the device.
The annuloplasty ring used to measure strain and calibrated forces was designed as a flat rigid ring. Therefore, care must be taken to interpret and compare the results obtained with existing tricuspid annuloplasty devices, since most tricuspid annuloplasty rings such as the Edwards Lifesciences MC3™ and Carpentier-Edwards Physio Tricuspid™ or the Medtronic Tri-Ad™ Adams Tricuspid Annuloplasty rings are semi-flexible and non-planar. However, restraining the annulus in the flat configuration is necessary to study the distribution of forces applied to the ring present in the beating heart if both the in plane and out of plane strains are recorded.
Ring implantation is predominately performed in cases with a dilated annulus, whereas this study only investigated the in-plane force distribution in the healthy myocardium. Because of this we cannot leave out the possibility that a different force distribution pattern would be present in a dilated annulus.
Right atrial and ventricular filling and dimensions are dependent on both pre- and afterload conditions. Although fluid balance is planned to be adjusted throughout the experiments and both right atrial and ventricular pressures to be standardized by continuous measurements, differences between pre- and post bypass cannot be ruled out.
The annuloplasty ring force transducer was designed to fit the average size of an 80 kg pig. Differences in annular dimensions between different animals in an in vivo study could affect the measurements obtained. To eliminate this problem, rings with different sizes should be tested in different animal models.
To further investigate the tricuspid annular force distribution, a full scale animal study is needed to quantify the impact of tricuspid ring implantation, and this is currently ongoing at our institution. Data from these studies will provide a better understanding of the annular dynamics as well as aid in the design of new and improved tricuspid annuloplasty rings for clinical use.
References
Calafiore, A. M. A single-size band, 50 mm long, for tricuspid annuloplasty. Eur. J. Cardio-thorac. Surg. 34(3):677–679, 2008.
Carpentier, A. Surgical management of acquired tricuspid valve disease. J. Thorac. Cardiovasc. Surg. 67(1):53–65, 1974.
Chikwe, J. Surgical strategies for functional tricuspid regurgitation. Semin. Thorac. Cardiovasc. Surg. 22(1):90–96, 2010.
Cosgrove D. M., III, J. M. Arcidi, L. Rodriguez, W. J. Stewart, K. Powell, and J. D. Thomas. Initial experience with the Cosgrove-Edwards Annuloplasty System. Ann. Thorac. Surg. 60(3):499–503, 1995.
Fawzy, H., K. Fukamachi, C. D. Mazer, A. Harrington, D. Latter, D. Bonneau, et al. Complete mapping of the tricuspid valve apparatus using three-dimensional sonomicrometry. J. Thorac. Cardiovasc. Surg. 141(4):1037–1043, 2011.
Filsoufi, F., S. P. Salzberg, M. Coutu, and D. H. Adams. A three-dimensional ring annuloplasty for the treatment of tricuspid regurgitation. Ann. Thorac. Surg. 81(6):2273–2277, 2006.
Hasenkam, J. M., H. Nygaard, P. K. Paulsen, W. Y. Kim, and O. K. Hansen. What force can the myocardium generate on a prosthetic mitral valve ring? An animal experimental study. J. Heart Valve Dis. 3(3):324–329, 1994.
Hiro, M. E., J. Jouan, M. R. Pagel, E. Lansac, K. H. Lim, H. S. Lim, et al. Sonometric study of the normal tricuspid valve annulus in sheep. J. Heart Valve Dis. 13(3):452–460, 2004.
Jensen, M. O., H. Jensen, S. L. Nielsen, M. Smerup, P. Johansen, A. P. Yoganathan, et al. What forces act on a flat rigid mitral annuloplasty ring? J. Heart Valve Dis. 17(3):267–275, 2008.
Jensen, M. O., H. Jensen, M. Smerup, R. A. Levine, A. P. Yoganathan, H. Nygaard, et al. Saddle-shaped mitral valve annuloplasty rings experience lower forces compared with flat rings. Circulation 118(14 Suppl):S250–S255, 2008.
McCarthy, P. M., S. K. Bhudia, J. Rajeswaran, K. J. Hoercher, B. W. Lytle, D. M. Cosgrove, et al. Tricuspid valve repair: durability and risk factors for failure. J. Thorac. Cardiovasc. Surg. 127(3):674–685, 2004.
Milla, F., J. G. CasCllo, R. Varghese, J. Chikwe, A. C. Anyanwu, and D. H. Adams. Rationale and initial experience with the Tri-Ad Adams tricuspid annuloplasty ring. J. Thorac. Cardiovasc. Surg. 143(4 suppl):S71–S73, 2012.
Nichols, W. W., and M. F. O’Rourke. Mcdonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles, 5th edn., ed. by H. Arnold. 2007.
Shandas, R., M. Mitchell, C. Conrad, O. Knudson, J. Sorrell, S. Mahalingam, et al. A general method for estimating deformation and forces imposed in vivo on bioprosthetic heart valves with flexible annuli: in vitro and animal validation studies. J. Heart Valve Dis. 10(4):495–504, 2001.
Siefert, A. W., J. H. Jimenez, K. J. Koomalsingh, D. S. West, F. Aguel, T. Shuto, et al. Dynamic assessment of mitral annular force profile in an ovine model. Ann. Thorac. Surg. 94(1):59–65, 2012.
Siefert, A. W., J. H. Jimenez, D. S. West, K. J. Koomalsingh, R. C. Gorman, J. H. Gorman, III, et al. In-vivo transducer to measure dynamic mitral annular forces. J. Biomech. 45(8):1514–1516, 2012.
Acknowledgments
The authors wish to thank the staff at the Institute for Clinical Medicine and the Faculty of Health Sciences, Aarhus University for excellent surgical facilities and expertise. In addition, the following have supported the development of in vivo measurement transducers and calibration equipment: A. P. Møller Foundation for the Advancement of Medical Science, Snedkermester Sophus Jacobsen og hustru Astrid Jacobsens Fond, Hørslev Fonden, Aase og Ejnar Danielsens Fond, The Danish Medical Association Research Fund, Helga og Peter Kornings Fund. Dr. Morten Jensen’s participation in this research project was partially funded by the Central Denmark Region Health Science Research Fund.
Conflict of interest
This work was partially supported by Edwards Lifesciences Corporation. Sten L. Nielsen and J. Michael Hasenkam are co-owners of Enovacor ApS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ajit P. Yoganathan oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Kragsnaes, E.S., Honge, J.L., Askov, J.B. et al. In-plane Tricuspid Valve Force Measurements: Development of a Strain Gauge Instrumented Annuloplasty Ring. Cardiovasc Eng Tech 4, 131–138 (2013). https://doi.org/10.1007/s13239-013-0135-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-013-0135-9