Abstract
Environmental exposure to toxicants/heavy metals during critical periods of development can influence changes in embryo and germline of the offspring; and later on affect the disease susceptibility in adults. Exposures to toxic metals or endocrine disruptors are particularly harmful during fetal development. Arsenic, a well-known toxic metalloid and reproductive toxicant is one the major concern because of its adverse and delayed health effects. Considering the complex and numerous adverse health effects of prenatal arsenic exposure, it is very difficult to identify the one single mechanism for arsenic-induced toxicity. This is further complicated due to biphasic response reported where arsenic has very different effects at low and high doses particularly during early life exposure scenario. In this review, we are focusing on prenatal arsenic exposure and its lifelong adverse effects, and their association with endocrine disruption and epigenetic changes. We provide evidence that developmental arsenic exposure alters the functional fetal epigenome in a tissue-specific manner by alterations in DNA methylation patterns, histone modifications and changes in micro RNA. Arsenic as an endocrine disruptor also affects the reproductive potential of the organism. These adverse effects of arsenic could manifest directly through classical hormone imprinting or through irreversible epigenetic modulation. Thus, understanding the association of epigenetic changes and endocrine disruption by prenatal arsenic exposure may help unravel the crucial mechanism for the development of disease later in life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental factors such as heavy metals, dietary macro and micro-nutrients, xenochemicals and endocrine disruptors are known to affect human health. It was established early in the last century that polluting chemical compounds such as pesticides and heavy metals had multifaceted effects on the health and wellbeing of animals and humans. The first comprehensive report on this phenomenon was from Silent Spring by Rachel Carson [21]. The book explored various ill effects of organic pesticides, herbicides and other persistent organic pollutants (POPs) in ecological niches, creating a huge public sensation. Nonetheless, the observations opened up a window for scientific community to explore the side effects of organic pesticides. Several evidences suggested serious side effects such as cancer of various organs, metabolic disorders and endocrine disruption through estrogen or gestagen mimicking compounds. Among this class of compounds, perhaps the most studied endocrine disrupting chemical is BPA, which acted as a xenoestrogen, and disrupted the various cellular signalling pathways associated with estrogen signalling in the responsive tissues. Previously only estrogen mimetic compounds were the focus of scientific research, but recent experimental evidences link a wide array of environmental pollutants including heavy metals (e.g. cadmium and lead) and metalloid (arsenic) contaminants with endocrine disruption. These compounds act upon the endocrine system either directly or indirectly in synergy with other EDCs. Toxic effects of heavy metals have been proven to be a major threat and there are several health risks associated with them [65, 124]. Among the heavy metals, arsenic is the twentieth most abundant element on earth and its inorganic forms are lethal to the living creatures at moderately high concentrations. Arsenic is also known as a protoplasmic poison [52] and teratogen [32, 41]. Inorganic Arsenic is classified as a group-1 human carcinogen by the International Agency for Research on Cancer (IARC) and its association have been found with several types of cancer [27, 31, 43]. Several non-carcinogenic effects, including cardiovascular disease [126], hypertension [1] and diabetes [138, 139] have also been observed in chronic exposure victims. Arsenic is also known to cross the blood-placenta barrier and hence is responsible for adverse birth and developmental outcomes after prenatal exposure [3, 33, 95, 113, 133].
At low doses, arsenic can disrupt the hormonal homeostasis by direct or indirect interactions and cause reproductive toxicity thus, it can also function as an endocrine disruptor [13, 14, 80]. The body of scientific literature regarding this phenomenon is constantly growing, but the existing research shows that arsenic acts on gonadal, adrenal and thyroid endocrine systems, which are vital for development and metabolic function. Most studies focus on the adult exposure aspect when observing endocrine disrupting properties of arsenic but an emerging body of scientific literature suggests that arsenic can also cause endocrine disruption during gestational exposure. Although, it has been well established that arsenic acts as a transplacental carcinogen [135] or co-carcinogen along with environmental carcinogenic agent i.e. UV-radiation [62], there are only a handful of reports on gestational or developmental endocrine disrupting properties of arsenic.
The environmental exposure during critical periods of development can influence development of the offspring and disease susceptibility later on in adults. This aspect has been integrated in developmental origins of health and disease (DOHaD) hypothesis [10, 47]. The classical example of the unfortunate Dutch famine suggested that maternal nutrient stress during gestation influenced the disease susceptibility in adulthood [94]. Embryonic environmental exposure, endocrine disruptors and nutrition influence the phenotype of F1 generation. Prenatal exposure to environmental insults (heavy metals, diet, xenochemicals, endorcrine disruptor) can modify the epigenetic regulation of the genome and hence can change the response of organism towards the adaptation in that particular environment. Thus, a single genotype to produce a broad range of adult phenotypes, as a consequence of the developmental plasticity occurs when environmental influences affect cellular pathways during gestation. The paradigm of adult-onset disease following prenatal exposure is rooted in the process of developmental plasticity. Developmental perturbations due to prenatal exposure of arsenic may lead to severe diseases [145]. Inorganic arsenic can pass the placental barrier and alter the different epigenetic marks in the foetus and further can increase the chances of early onset of disease [67]. Alteration in the maternal environment could adversely affect the functionality of epigenetic regulators which later on increase the disease susceptibility in adults [9]. Cellular memory modules play an important role in the reprogramming of genetic imprint and any amendment in this process leads to disruption of various signalling pathways and lead to disease conditions [36]. In the light of the above mentioned studies, present review is an attempt to summarize the role of prenatal arsenic exposure and its effect on alteration of epigenetic machinery or disruption of the endocrine system and its relation to adult onset diseases.
Adverse effects of prenatal exposure to arsenic
Both arsenic and its methylated metabolites can cross the blood-placenta barrier and thus prenatal arsenic exposure can lead to impaired fetal growth, fetal loss during pregnancy, or even increased post-birth infant mortality (reviewed in [102]). Arsenic is considered as reproductive toxicant in animals since early 90’s when low level arsenate exposure were found to cause various developmental anomalies in chick embryos [101]. The teratogenic effects of arsenic were explained by various groups in late 90’s [12, 50, 79]. These studies showed various developmental impairment, congenital malformation and adverse reproductive outcomes after prenatal exposure to arsenic.
Numerous epidemiological studies suggested that gestational arsenic exposure lead to significant increase in mortality and several adverse health effects [36, 37, 113, 105, 108] Various population cohort studies suggest prenatal arsenic associated diseases include disruption of immune system [38,39,40, 77]; heart disease [41, 118]; lung disease [42,120]; metabolic related disease [43] and nervous system related diseases [44,45,46,47,48,49, 97, 128] These adverse effects of prenatal arsenic exposure suggested that maternal/fetal environment is involved in the regulation of some transgenerational effects that are translated into disease phenotypes later in life of the offspring. For example, late-onset alterations and tumors in the livers of the F2 males arose after maternal arsenite exposure to pregnant mice [50]. Studies both in animal as well as population cohorts suggest that male infertility occurred after prenatal arsenic exposure [51,52,53,54]. Several reports suggest that the maternal exposure to endocrine disrupting chemicals during gestation also alter the growth and development of foetus [15, 55, 56, 83]. Thus, it would be interesting to delineate the associations of arsenic as an endocrine disruptor and epigenetic regulator after in utero exposure.
In utero arsenic exposure has long-term adverse effects on human health (supported by both animal and human population associations study). Detrimental effects of developmental arsenic exposure have been found in lower organisms e.g. chick [57] and in killfish—Fundulus heteroclitus [58] to higher animal model and humans. Various studies in different model systems suggested that there is an association of alteration in the gene expression and adverse effects i.e. reduced growth, placental angiogenesis, early onset of puberty, and deficiencies in cognitive development, early onset of atherosclerosis and learning and memory deficit later in life (Table 1). Prenatal arsenic reduced growth of the embryo by increasing both insulin-like growth factors (IGF-1 and IGF-1R) levels in skeletal muscle in Killifish—F. heteroclitus [58]. Placental angiogenesis pathway is linked to arsenic induced reduced birth weight [60]. Correlation of maternal exposure to As during gestational period with early onset of puberty in offspring females has been shown by altered mRNA expression of signalling genes [84] (Table 1). In utero exposure to arsenite altered development of the mammary gland by increased expression of the ERα transcripts [96]. Altered GR signaling following gestation arsenic exposure is linked to induce glitch in cognitive development [18, 86]. Transplacental arsenic exposure alters transcriptome of liver and about 16% of the differential expressed genes have active SREBP1 (3 sterol regulatory element binding protein isoforms) which regulates lipid homeostasis in vertebrates [119]. These amendments in developmental programming of liver functions might be enough to induce pro-inflammatory response later in life and may contribute to early onset of atherosclerosis. Further, gestational arsenic exposure mimicked stress condition in liver which can later develop liver disease in mice [92].
Adult hippocampal neurogenesis and related gene expression during development had shown in arsenic exposed animals [84, 127, 143]. Arsenic targets the central nitrergic system, decreased the nitric oxide markers and altered brain structure [110]. Differential expression of behaviour and neurobiological markers with prolonged adverse effects has been shown in mice exposed to low level of arsenic [84]. Thus, differential gene expression and adverse health effects after prenatal exposure is associated (Table 1).
Arsenic and endocrine disruption
Due to its worldwide distribution and carcinogenic toxicity, arsenic is considered as a global health threat. A wide range of pathological symptoms has been defined for chronic arsenic exposure in human populations from the endemic areas. The symptoms of chronic exposure to arsenic in drinking water include the progression of various malignant and non-malignant skin lesions [66, 98]; peripheral neuropathy [40, 70] and cancers of liver, lung and urinary bladder [81, 121, 137, 138]. It has also been established that arsenic acts to attenuate the immune system following chronic exposure, rendering the individual susceptible towards pathogenic invasion [39, 90, 107]. Arsenic induced toxicity have been very well documented and reviewed in animal systems by various research groups across the globe, resulting in strict quality control measures by authorities, which limited the maximum tolerable level of inorganic arsenic in drinking water to 10 parts per billion (10 μg/L) in the developed countries. However, in India, the maximum tolerable limit of arsenic in drinking water is 50 parts per billion (50 μg/L). Although the advent of advance water purification systems and strict regulatory measures have curbed the exposure levels significantly, emerging evidence suggests that inorganic arsenic exposure in very low doses may induce harmful effects on mammalian endocrine systems. The reports published by various groups on the role of Arsenic as an endocrine disruptor after gestational exposure of arsenic are listed in Tables 2 and 3.
Arsenic toxicity on gonadal endocrine system
Gonadal endocrine system is an important player in regulating the reproductive behavior of living organisms through regulating gonadal hormones by Hypothalamus–pituitary–Gonadal (HPG) axis [57]. Hypothalamic–pituitary–ovarian (HPO) axis in females and hypothalamic–pituitary–testicular (HPTT) in males regulate gonadal gametogenesis [117]. Accumulation and inhibitory effect of arsenic in the gonadal glands has been reported in animal model [142]. Further, arsenic might interact with the hormone receptor, and induces inhibitory effects on gametogenesis in both sexes.
Despite all of these evidences, there are only a handful of studies that indicate gestational endocrine disruption of gonadal endocrine system by arsenic (Table 2). These changes are shown to be perceived during the exposure period but established later in life. The effects of gestational exposure to arsenic in both male and female gonadal endocrine system is diverse while some researchers demonstrate proliferative lesions of ovary, oviduct hyperplasia in offspring females and testicular lesions and interstitial cell tumors in male offspring [125], others have reported early onset of puberty in the female offspring as judged by vaginal opening time and estradiol levels [78]. Elevated gonadotrophin-releasing hormone (GnRH), FSH and testosterone in female offspring has been reported only once [16] (Table 2).
Effects of arsenic on estrogen and glucocorticoid signaling
Among other endocrine disrupting effects, arsenic exposure modulates the estrogen signaling pathway in a number of tissues (Table 3). Reproduction and development in males and females are regulated by endogenous estrogens [75]. The action of estrogens are activated by binding to nuclear receptors i.e. estrogen receptor alpha [54] and beta [72] as well as the membrane-bound receptor, GPR30 [20]. Kumar et al. explored the binding of arsenic trioxide to 17-β-estradiol in cancerous epithelial cell line of the breast (MCF-7) [73]. The binding titrated the natural activity of estrogen in these cells and promoted cell survival, proliferation and migration. In other words, this report demonstrated the estrogenic properties of arsenic trioxide which may explain numerous other disorders associated with low level exposure to arsenic. However, the studies in this area are often contradictory e.g. one report demonstrated the ability of arsenic and cadmium to bind the membrane bound estrogen receptor as well as G-protein coupled estrogen receptors in human lung adenocarcinoma cell line [61]. In their study, this group observed that exposure to environmentally relevant concentration of arsenic and cadmium resulted in increased proliferation of these cells like the exposure of these cells to physiologically relevant concentrations of 17-β-estradiol. In contrast, in the MCF-7 cells, arsenic at low concentrations (0.25–1 μM) inhibited cell proliferation [28]. The findings in these reports suggest that chronic exposure to low levels of arsenic can disrupt the estrogen signalling via interacting with estrogen receptor as well as the hormone itself. In the uterus of rat, disruption of circulating levels of gonadotropins and estradiol; degeneration of luminal epithelial, stromal and myometrial cells; and downregulation of the downstream components of the estrogen signaling pathway were found after exposure of high dose (4 ppm arsenic) of arsenic [22] Although, there are evidences of arsenic mediated disruption of estrogen signalling in vitro and in adult exposure models, there are only a handful of reports on the prenatal exposure to arsenic and its effects on estrogen signaling. In hepatocellular carcinoma of mice exposed to prenatal arsenic increased mRNA levels of ER-α and cyclin D1 has been observed (42.5 and 85 ppm of arsenic) [134]. Further, study by the same group targeting global alteration of gene expression profiles by gestational arsenic exposure suggested a case of “liver feminization” in in utero arsenic exposed male C3H mice, which showed upregulation of estrogen receptor mediated signaling in their liver and consequently, higher incidence of hepatocellular carcinoma [82]. Parodi et al., suggested early onset of mammary gland development, early vaginal opening (collectively, early onset of puberty) in rats, potentially increasing susceptibility of the exposed animals (5 μg As/kg bodyweight) towards breast cancer [96]. Waalkes et al. suggested that transplacental exposure to arsenic led to exacerbated postnatal urogenital carcinogenesis induced by diethylstilbestrol treatment [136] (Table 3).
Another endocrine pathway which is particularly vulnerable to arsenic toxicity, is the glucocorticoid signalling pathway (GR signaling—Table 3). Glucocorticoids are steroid hormones secreted from the adrenal gland in response to stress. Once in circulation, the glucocorticoids can exert a wide array of tissue specific effects. They are traditionally seen as anti-inflammatory molecules, but emerging evidence suggests more towards a dual mode of action of glucocorticoids [30]. An aberrant expression of glucocorticoid receptor or any disruption in GR signalling may get translated into a wide range of health effects including impaired reproduction [141], stress response [89] and adipogenesis and the induction of central obesity [76]. It was reported by several groups that arsenic modulates GR function both in vitro and in vivo. Moderate dose of arsenic has been shown to perturb the GR signalling in in vitro studies leading to a complex dose–response relationship. An interesting study illustrated that lower doses of arsenic (< 1 μM) cause stimulation of GR and tyrosine aminotransferase (TAT) genes, whereas higher concentrations (1–3 μM) cause repression across GRs under human, mouse and rat promoter control, suggesting a role of arsenic interference in the DNA binding domain of these receptors [13]. This study, conducted on the EDR3 cell line, also inferred that arsenic may also modulate the intracellular progesterone, mineralocorticoid and aldosterone receptor, as they all share a common/similar DNA binding domain. Thus, the exposure to arsenic in critical developmental periods may induce a hormonal imprinting, which persists throughout adult life. Several studies indicated the modulation of hormonal imprinting by arsenic, e.g. altered GR signaling following gestational arsenic exposure have been observed throughout adulthood [18] and in another study, arsenic exposure has been shown to induce glitches in cognitive development via transcriptional alteration in MAPK/ERK pathway [86]. Further, additional research work is required to explore estrogen receptor/signaling and its interaction to arsenic.
Developmental arsenic exposure and epigenetic modulations
Considering the complex and numerous adverse health effects of prenatal arsenic exposure, it is very difficult to identify the one single mechanism for the arsenic induced toxicity. Mechanisms pertaining to arsenic toxicity have been reviewed by many authors [60, 76, 115, 116, 123]. The mechanism of arsenic induced carcinogenesis has been the most intensely studied facet of arsenic toxicity, in which several molecular mechanisms are predicted to play a role. There are several recent reviews which suggested the epigenetic mechanisms for arsenic induced carcinogenesis after chronic or long term exposure [5, 23, 25, 59, 112, 140] but little is known about the gestational exposure. The interplay between genes and environment modulate disease susceptibility through epigenetic reprogramming at early development which could be possible cause of origins of adult onset disease. A heritable change (transgenerationally inheritable) in gene expressions without alteration in the DNA sequence is defined as epigenetics [6, 106]. Disease development is well characterized by disruption of the epigenome [34, 48]. There is a complex interplay between one’s genetic architecture and epigenetic marks “imprinted” by endogenous or exogenous factors which can be responsible for the disease susceptibility [64]. Regulation of the “epigenome” occurs by intertwined mechanisms of small-interfering RNAs, DNA methylation, and histone modifications [26, 38, 64, 88]. These mechanisms cooperatively decide when and where the gene expression is to be silenced or activated. Thus, instead of primary nucleotide sequence of a gene, the regulation of gene expression by epigenetics has greatly expanded our understanding the context of toxicities induced by the environmental factors i.e. arsenic associated toxicities.
Role of DNA methylation in arsenic associated early onset of disease
Altered expression of genes after prenatal arsenic exposure can be regulated by epigenetic mechanisms. Time and again, epigenetic dysfunction particularly differential DNA methylation and its role in arsenic induced toxicity and carcinogenesis has been reviewed [8, 109]. A review on the comparison of genes associated in prenatal arsenic induced toxicity in human population cohort studies, suggested a conserved biological response for arsenic induced toxicity [74]. However, till today, researchers are trying to decipher the sequence of events involved in the prenatal arsenic induced early onset of disease. Prenatal arsenic exposure in human and epigenetic alteration i.e. DNA methylation have been examined for arsenic endemic populations (Table 4). Developmental arsenic exposure affects almost all the organs and is associated with several diseases starting from reduced birth weight to early onset of cardiovascular disease, diabetes and cancer (Table 4). The complex interplay between DNA methylation and functional changes in gene expression in newborn cord blood leucocytes had shown in BEAR Pregnancy Cohort of Mexico [111]. The associations between early exposure of arsenic and DNA methylation suggest interference of arsenic with de novo DNA methylation in Matlab population in Bangladesh [17]. Low dose of Arsenic can also alter the DNA methylation globally [71]. Higher levels of arsenic in mother’s urine are correlated with the increase Global DNA hypomethylation in infants cord blood. [100]. In Taiwan cohort, altered DNA methylation at various CpGs associated with low density lipoproteins (LDL) have been identified (cg25189764, cg04986899, cg04903360, cg08198265 and cg10473311) that may help to determine pathological epigenetic mechanisms linked to LDL later in life [67]. In cord blood lymphocytes of gestationally arsenic-exposed newborns had promoter hypomethylation of inflammatory genes [69]. Further, DNA methylation has been altered with exposure dependent manner in cord blood [70]. Newborn proteome analysis after prenatal Arsenic exposure suggested the role of TNF signalling pathway for inter-individual differences [8]. Comparative analysis of Infectious Disease Genes (IDGs) and Exposure Responsive Genes (ERGs), identified genes including TNF and IFNγ (GR-associated genes) which is link to cause infectious disease after gestational As in humans [104]. Further, altered DNA methylation pattern ofplacental tissues suggested placental subpopulations changes is associated with As exposure in the New Hampshire birth cohort [53]. Cardenas et al., 2015 evidenced that in utero exposure to arsenic can alter DNA methylation of artery and placenta tissues [19]. In addition, DNA methylation at promoter region of tumor suppressor gene p16, were positively associated with higher level exposure of arsenic in both maternal and fetal leukocytes [68]. Hypermethylation in the promoter region of p53 increases in infants exposed in utero to arsenic [63]. DNA methylation of extracellular matrix remodeling genes was positively associated with arsenic levels, which in turn might be the cause of predisposing of lung diseases in children [51].
In animal model, in utero exposure to arsenic induces epigenetic change i.e. alteration in DNA methylation [85, 86, 92, 144]. Further, genes involved in neuronal plasticity showed hypomethylation in hippocampus and frontal cortex region of Wistar rats and suggested that memory deficit behaviour is associated with arsenic exposure [85, 86]. Thus, these results suggest that there are tissue-specific effects of As exposure on the fetal epigenome (Table 4). Moreover, the effects of iAs exposure vary across individuals and populations, may be as a result of genotype and/or hormonal factors.
Role of histone modifications and micro RNA in arsenic associated early onset of disease
Studies on mice suggested a link between DNA methylation and impaired effects on several tissues following prenatal arsenic exposure [87, 127]. Gestational arsenic exposure and their association with post translational histone modifications for adverse health effects have shown in Table 5. Embryonic arsenic exposure caused global hypo-acetylation at H3K9 and overexpression of Krüppel associated box (KRAB) transcription factors in altered genes in brain [29]. Sex specific alteration in H3K4me3 levels, however, there is no change in the level of H3K9me3 in the brain tissue [129]. Further, enrichment of H3K4me3 levels for genes associated with neuropathy and cancer in brain has been observed in C57BL/6 J after prenatal arsenic exposure [131]. Further, developmental arsenic exposure altered histone deacetyltransferase levels in brain and lead to aberrant cognitive capabilities in a sex dependent manner [132].
In cord blood of an arsenic endemic Mexican population, alterations in the miRNAs expression were found in response to prenatal arsenic exposure [103] (Table 5). Exposure of in utero arsenic changes the developmental trajectory of microRNAs in mouse liver and might cause to early onset of atherosclerosis due to persistent pro-inflammatory state in the liver [119]. Aberrant programming of neural stem cells (NSCs) function is associated with arsenic-induced deregulation of REST/NRSF and its target microRNAs which might induce glitches in neurogenesis [130]. Thus, iAs-associated miRNA deregulation may cause disease by alter the functional consequences for downstream gene expression.
Looking forward: link between endocrine disruption and epigenetics
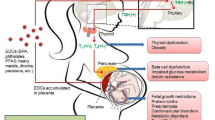
Arsenic can induce alteration in epigenome of the foetus which may result into various disease outcomes later in life. Arsenic associated changes in DNA methylation, histone modifications, miRNA has been studied well in both human populations cohort and in animal model as shown in Tables 4 and 5. Majority of human cohort studies are on cord blood cells or leukocytes populations, while majority of animal studies are on brain and liver tissue (Tables 4, 5). However, these changes are not in developmental stage specific manner. Development timings are crucial for the imprinting of epigenetic marks and associated with the hormonal imbalance in the maternal or foetal microenvironment during pregnancy. Adverse effects of prenatal arsenic exposure suggested that maternal/fetal environment is involved in the regulation of some transgenerational effects that are translated into disease phenotypes later in life of the offspring [93]. Arsenic as an endocrine disruptor may modulate the microenvironment of the foetus by alteration in various cell signalling pathways regulated by hormones (estrogens and glucocorticoids; Tables 2, 3). However, the downstream targets analysis and their regulatory mechanisms are still awaited. Apart from DNA methylation, histone modifications and miRNA (Tables 4, 5), cellular memory modules (PcG and TrxG proteins) could be the master regulators as these proteins are known to memorize the genomic imprints during development [44]. So far, we can suggest that low level arsenic exposure during development leads to change in the microenvironment of the developing foetus (hormonal imbalance), imprinting of the differential marks (hormonal imprinting/genetic imprinting), maintenance of the imprint marks by PcG/TrxG proteins and as a result that maternal the hormonal imbalance may maintain in the offspring, alteration in the cell signalling, alteration in gene/protein expression, their regulation by epigenome which leads to disease in the later life (Fig. 1). In addition, arsenic induced lifelong diseased state may persist because of the predisposition of the hormonal or genetic or epigenetic imprinting which can be identified by development stage specific experiments. Thus, the association of epigenetic changes and endocrine disruption by prenatal arsenic exposure may represent a critical mechanism for the development of disease later in life and need more attention in future.
References
Abhyankar LN, Jones MR, Guallar E, Navas-Acien A. Arsenic exposure and hypertension: a systematic review. Environ Health Perspect. 2012;120:494–500.
Ahir BK, Sanders AP, Rager JE, Fry RC. Systems biology and birth defects prevention: blockade of the glucocorticoid receptor prevents arsenic-induced birth defects. Environ Health Perspect. 2013;121:332–8.
Ahmad SA, Sayed MH, Barua S, Khan MH, Faruquee MH, Jalil A, et al. Arsenic in drinking water and pregnancy outcomes. Environ Health Perspect. 2001;109:629–31.
Allan AM, Hafez AK, Labrecque MT, Solomon ER, Shaikh MN, Zheng X, et al. Sex-dependent effects of developmental arsenic exposure on methylation capacity and methylation regulation of the glucocorticoid receptor system in the embryonic mouse brain. Toxicol Rep. 2015;2:1376–90.
Al-Eryani L, Jenkins SF, States VA, Pan J, Malone JC, Rai SN, et al. miRNA expression profiles of premalignant and malignant arsenic induced skin lesions. PLoS ONE. 2018;13(8):e0202579.
Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–9.
Bae-Jump VL, Zhou C, Boggess JF, Gehrig PA. Arsenic trioxide (As2O3) inhibits expression of estrogen receptor-alpha through regulation of the mitogen-activated protein kinase (MAPK) pathway in endometrial cancer cells. Reprod Sci. 2008;15:1011–7.
Bailey K, Fry RC. Long-term health consequences of prenatal arsenic exposure: links to the genome and the epigenome. Rev Environ Health. 2014;29:9–12.
Banik A, Kandilya D, Ramya S, Stünkel W, Chong YS, Dheen ST. Maternal factors that induce epigenetic changes contribute to neurological disorders in offspring. Genes. 2017;8(6):150.
Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: implications for research and public health. Environ Health. 2012;11:42.
Beaver LM, Truong L, Barton CL, Chase TT, Gonnerman GD, Wong CP, et al. Combinatorial effects of zinc deficiency and arsenic exposure on zebrafish (Danio rerio) development. PLoS ONE. 2017;12(8):e0183831.
Bencko V. Carcinogenic, teratogenic, and mutagenic effects of arsenic. Environ Health Perspect. 1977;19:179–82.
Bodwell JE, Gosse JA, Nomikos AP, Hamilton JW. Arsenic disruption of steroid receptor gene activation: complex dose-response effects are shared by several steroid receptors. Chem Res Toxicol. 2006;19:1619–29.
Bodwell JE, Kingsley LA, Hamilton JW. Arsenic at very low concentrations alters glucocorticoid receptor (GR)-mediated gene activation but not GR-mediated gene repression: complex dose-response effects are closely correlated with levels of activated GR and require a functional GR DNA binding domain. Chem Res Toxicol. 2004;17:1064–76.
Bommarito PA, Martin E, Fry RC. Effects of prenatal exposure to endocrine disruptors and toxic metals on the fetal epigenome. Epigenomics. 2017;9:333–50.
Bourguignon NS, Bonaventura MM, Rodriguez D, Bizzozzero M, Ventura C, Nunez M, et al. Evaluation of sodium arsenite exposure on reproductive competence in pregnant and postlactational dams and their offspring. Reprod Toxicol. 2017;69:1–12.
Broberg K, Ahmed S, Engstrom K, Hossain MB, Jurkovic Mlakar S, Bottai M, et al. Arsenic exposure in early pregnancy alters genome-wide DNA methylation in cord blood, particularly in boys. J Dev Orig Health Dis. 2014;5:288–98.
Caldwell KE, Labrecque MT, Solomon BR, Ali A, Allan AM. Prenatal arsenic exposure alters the programming of the glucocorticoid signaling system during embryonic development. Neurotoxicol Teratol. 2015;47:66–79.
Cardenas A, Houseman EA, Baccarelli AA, Quamruzzaman Q, Rahman M, Mostofa G, et al. In utero arsenic exposure and epigenome-wide associations in placenta, umbilical artery, and human umbilical vein endothelial cells. Epigenetics. 2015;10:1054–63.
Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45:607–17.
Carson R. Silent spring. Boston: Houghton-Mifflin; 1962.
Chatterjee A, Chatterji U. Arsenic abrogates the estrogen-signaling pathway in the rat uterus. Reprod Biol Endocrinol. 2010;8:80.
Chatterjee D, Bandyopadhyay A, Sarma N, Basu S, Roychowdhury T, Roy SS, Giri AK. Role of microRNAs in senescence and its contribution to peripheral neuropathy in the arsenic exposed population of West Bengal, India. Environ Pollut. 2018;233:596–603.
Chen GC, Guan LS, Hu WL, Wang ZY. Functional repression of estrogen receptor a by arsenic trioxide in human breast cancer cells. Anticancer Res. 2002;22:633–8.
Chen QY, DesMarais T, Costa M. Metals and mechanisms of carcinogenesis. Annu Rev Pharmacol Toxicol. 2019;59:537–54.
Cheung P, Lau P. Epigenetic regulation by histone methylation and histone variants. Mol Endocrinol. 2005;19:563–73.
Chiou HY, Hsueh YM, Liaw KF, Horng SF, Chiang MH, Pu YS, et al. Incidence of internal cancers and ingested inorganic arsenic: a seven-year follow-up study in Taiwan. Cancer Res. 1995;55:1296–300.
Chow SK, Chan JY, Fung KP. Suppression of cell proliferation and regulation of estrogen receptor alpha signaling pathway by arsenic trioxide on human breast cancer MCF-7 cells. J Endocrinol. 2004;182:325–37.
Cronican AA, Fitz NF, Carter A, Saleem M, Shiva S, Barchowsky A, et al. Genome-wide alteration of histone H3K9 acetylation pattern in mouse offspring prenatally exposed to arsenic. PLoS ONE. 2013;8:e53478.
Cruz-Topete D, Cidlowski JA. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. NeuroImmunoModulation. 2015;22:20–32.
Dakeishi M, Murata K, Grandjean P. Long-term consequences of arsenic poisoning during infancy due to contaminated milk powder. Environ Health. 2006;5:31.
DeSesso JM. Teratogen update: inorganic arsenic. Teratology. 2001;64:170–3.
Ditzel EJ, Nguyen T, Parker P, Camenisch TD. Effects of arsenite exposure during fetal development on energy metabolism and susceptibility to diet-induced fatty liver disease in male mice. Environ Health Perspect. 2016;124:201–9.
Dolinoy DC, Jirtle RL. Environmental epigenomics in human health and disease. Environ Mol Mutagen. 2008;49:4–8.
Dong X, Shulzhenko N, Lemaitre J, Greer RL, Peremyslova K, Quamruzzaman Q, et al. Arsenic exposure and intestinal microbiota in children from Sirajdikhan, Bangladesh. PLoS ONE. 2017;12(12):e0188487.
Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–63.
Engel RR, Hopenhayn-Rich C, Receveur O, Smith AH. Vascular effects of chronic arsenic exposure: a review. Epidemiol Rev. 1994;16:184–209.
Esteller M. Aberrant DNA methylation as a cancer-inducing mechanism. Annu Rev Pharmacol Toxicol. 2005;45:629–56.
Farzan SF, Korrick S, Li Z, Enelow R, Gandolfi AJ, Madan J, et al. In utero arsenic exposure and infant infection in a United States cohort: a prospective study. Environ Res. 2013;126:24–30.
Feldman RG, Niles CA, Kelly-Hayes M, Sax DS, Dixon WJ, Thompson DJ, et al. Peripheral neuropathy in arsenic smelter workers. Neurology. 1979;29:939–44.
Ferm VH. Arsenic as a teratogenic agent. Environ Health Perspect. 1977;19:215–7.
Ferreira RT, Silva AR, Pimentel C, Batista-Nascimento L, Rodrigues-Pousada C, Menezes RA. Arsenic stress elicits cytosolic Ca(2 +) bursts and Crz1 activation in Saccharomyces cerevisiae. Microbiology. 2012;158:2293–302.
Flora S. Handbook of arsenic toxicology. Cambridge: Academic Press; 2015.
Geisler SJ, Paro R. Trithorax and Polycomb group-dependent regulation: a tale of opposing activities. Development. 2015;142:2876–87.
Fry RC, Navasumrit P, Valiathan C, Svensson JP, Hogan BJ, et al. Activation of inflammation/NF-jB signaling in infants born to arsenic-exposed mothers. PLoS Genet. 2007;3(11):e207.
Gliga AR, Engstrom K, Kippler M, Skroder H, Ahmed S, Vahter M, et al. Prenatal arsenic exposure is associated with increased plasma IGFBP3 concentrations in 9-year-old children partly via changes in DNA methylation. Arch Toxicol. 2018;92:2487–500.
Gluckman PD, Hanson MA, Mitchell MD. Developmental origins of health and disease: reducing the burden of chronic disease in the next generation. Genome Med. 2010;2:14.
Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD, Hanson MA. Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatr Res. 2007;61:5R–10R.
Goggin SL, Labrecque MT, Allan AM. Perinatal exposure to 50 ppb sodium arsenate induces hypothalamic-pituitary-adrenal axis dysregulation in male C57BL/6 mice. Neurotoxicology. 2012;33:1338–45.
Golub MS, Macintosh MS, Baumrind N. Developmental and reproductive toxicity of inorganic arsenic: animal studies and human concerns. J Toxicol Environ Health B Crit Rev. 1998;1:199–241.
Gonzalez-Cortes T, Recio-Vega R, Lantz RC, Chau BT. DNA methylation of extracellular matrix remodeling genes in children exposed to arsenic. Toxicol Appl Pharmacol. 2017;329:140–7.
Gordon JJ, Quastel JH. Effects of organic arsenicals on enzyme systems. Biochem J. 1948;42:337–50.
Green BB, Karagas MR, Punshon T, Jackson BP, Robbins DJ, Houseman EA, et al. Epigenome-wide assessment of DNA methylation in the placenta and arsenic exposure in the new hampshire birth cohort study (USA). Environ Health Perspect. 2016;124:1253–60.
Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, et al. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–9.
Gundert-Remy U, Damm G, Foth H, Freyberger A, Gebel T, Golka K, et al. High exposure to inorganic arsenic by food: the need for risk reduction. Arch Toxicol. 2015;89:2219–27.
Hill DS, Wlodarczyk BJ, Mitchell LE, Finnell RH. Arsenate-induced maternal glucose intolerance and neural tube defects in a mouse model. Toxicol Appl Pharmacol. 2009;239:29–36.
Hoffmann F, Kloas W. An environmentally relevant endocrine-disrupting antiandrogen, vinclozolin, affects calling behavior of male Xenopus laevis. Horm Behav. 2010;58:653–9.
Hopenhayn-Rich C, Browning SR, Hertz-Picciotto I, Ferreccio C, Peralta C, Gibb H. Chronic arsenic exposure and risk of infant mortality in two areas of Chile. Environ Health Perspect. 2000;108:667–73.
Howe CG, Gamble MV. Influence of arsenic on global levels of histone posttranslational modifications: a review of the literature and challenges in the field. Curr Environ Health Rep. 2016;3:225–37.
Huang MC, Douillet C, Dover EN, Styblo M. Prenatal arsenic exposure and dietary folate and methylcobalamin supplementation alter the metabolic phenotype of C57BL/6 J mice in a sex-specific manner. Arch Toxicol. 2018;92:1925–37.
Huff MO, Todd SL, Smith AL, Elpers JT, Smith AP, Murphy RD, et al. Arsenite and cadmium activate MAPK/ERK via membrane estrogen receptors and G-protein coupled estrogen receptor signaling in human lung adenocarcinoma cells. Toxicol Sci. 2016;152:62–71.
Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: a historical perspective. Toxicol Sci. 2011;123:305–32.
Intarasunanont P, Navasumrit P, Waraprasit S, Chaisatra K, Suk WA, Mahidol C, et al. Effects of arsenic exposure on DNA methylation in cord blood samples from newborn babies and in a human lymphoblast cell line. Environ Health. 2012;11:31.
Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–54.
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7:60–72.
Karagas MR, Gossai A, Pierce B, Ahsan H. Drinking water arsenic contamination, skin lesions, and malignancies: a systematic review of the global evidence. Curr Environ Health Rep. 2015;2:52–68.
Kaushal A, Zhang H, Karmaus WJJ, Everson TM, Marsit CJ, Karagas MR, et al. Genome-wide DNA methylation at birth in relation to in utero arsenic exposure and the associated health in later life. Environ Health. 2017;16:50.
Kile ML, Baccarelli A, Hoffman E, Tarantini L, Quamruzzaman Q, Rahman M, et al. Prenatal arsenic exposure and DNA methylation in maternal and umbilical cord blood leukocytes. Environ Health Perspect. 2012;120:1061–6.
Kile ML, Houseman EA, Baccarelli AA, Quamruzzaman Q, Rahman M, Mostofa G, et al. Effect of prenatal arsenic exposure on DNA methylation and leukocyte subpopulations in cord blood. Epigenetics. 2014;9:774–82.
Kim S, Takeuchi A, Kawasumi Y, Endo Y, Lee H, Kim Y. A Guillain-Barre syndrome-like neuropathy associated with arsenic exposure. J Occup Health. 2012;54:344–7.
Koestler DC, Avissar-Whiting M, Houseman EA, Karagas MR, Marsit CJ. Differential DNA methylation in umbilical cord blood of infants exposed to low levels of arsenic in utero. Environ Health Perspect. 2013;121:971–7.
Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–30.
Kumar S, Mukherjee TK, Guptasarma P. Arsenic and 17-beta-estradiol bind to each other and neutralize each other’s signaling effects. Biochem Biophys Res Commun. 2016;477:575–80.
Laine JE, Fry RC. A systems toxicology-based approach reveals biological pathways dysregulated by prenatal arsenic exposure. Ann Glob Health. 2016;82:189–96.
Law Smith MJ, Deady DK, Moore FR, Jones BC, Cornwell RE, Stirrat M, et al. Maternal tendencies in women are associated with estrogen levels and facial femininity. Horm Behav. 2012;61:12–6.
Lee MJ, Pramyothin P, Karastergiou K, Fried SK. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim Biophys Acta. 2014;1842:473–81.
Li H, Engstrom K, Vahter M, Broberg K. Arsenic exposure through drinking water is associated with longer telomeres in peripheral blood. Chem Res Toxicol. 2012;25:2333–9.
Li X, Sun Z, Manthari RK, Li M, Guo Q, Wang J. Effect of gestational exposure to arsenic on puberty in offspring female mice. Chemosphere. 2018;202:119–26.
Li Y, Zhu H, Pan Y. Teratogenic effects of arsenic on rats. Zhonghua Yu Fang Yi Xue Za Zhi. 1998;32:37–9.
Liu H, Lu S, Zhang B, Xia W, Liu W, Peng Y, et al. Maternal arsenic exposure and birth outcomes: a birth cohort study in Wuhan, China. Environ Pollut. 2018;236:817–23.
Liu J, Waalkes MP. Liver is a target of arsenic carcinogenesis. Toxicol Sci. 2008;105:24–32.
Liu J, Xie Y, Ducharme DM, Shen J, Diwan BA, Merrick BA, et al. Global gene expression associated with hepatocarcinogenesis in adult male mice induced by in utero arsenic exposure. Environ Health Perspect. 2006;114:404–11.
Mallozzi M, Bordi G, Garo C, Caserta D. The effect of maternal exposure to endocrine disrupting chemicals on fetal and neonatal development: a review on the major concerns. Birth Defects Res C Embryo Today. 2016;108:224–42.
Martinez EJ, Kolb BL, Bell A, Savage DD, Allan AM. Moderate perinatal arsenic exposure alters neuroendocrine markers associated with depression and increases depressive-like behaviors in adult mouse offspring. Neurotoxicology. 2008;29:647–55.
Martinez L, Jimenez V, Garcia-Sepulveda C, Ceballos F, Delgado JM, Nino-Moreno P, et al. Impact of early developmental arsenic exposure on promotor CpG-island methylation of genes involved in neuronal plasticity. Neurochem Int. 2011;58:574–81.
Martinez-Finley EJ, Goggin SL, Labrecque MT, Allan AM. Reduced expression of MAPK/ERK genes in perinatal arsenic-exposed offspring induced by glucocorticoid receptor deficits. Neurotoxicol Teratol. 2011;33:530–7.
Martinez-Pacheco M, Hidalgo-Miranda A, Romero-Cordoba S, Valverde M, Rojas E. MRNA and miRNA expression patterns associated to pathways linked to metal mixture health effects. Gene. 2014;533:508–14.
Morris JS, Schmid M, Newman S, Scheuer PJ, Sherlock S. Arsenic and noncirrhotic portal hypertension. Gastroenterology. 1974;66:86–94.
Myers B, McKlveen JM, Herman JP. Glucocorticoid actions on synapses, circuits, and behavior: implications for the energetics of stress. Front Neuroendocrinol. 2014;35:180–96.
Nadeau KC, Li Z, Farzan S, Koestler D, Robbins D, Fei DL, et al. In utero arsenic exposure and fetal immune repertoire in a US pregnancy cohort. Clin Immunol. 2014;155:188–97.
Nakareangrit W, Thiantanawat A, Visitnonthachai D, Watcharasit P, Satayavivad J. Sodium arsenite inhibited genomic estrogen signaling but induced pERalpha (Ser118) via MAPK pathway in breast cancer cells. Environ Toxicol. 2016;31:1133–46.
Ngalame NN, Micciche AF, Feil ME, States JC. Delayed temporal increase of hepatic Hsp70 in ApoE knockout mice after prenatal arsenic exposure. Toxicol Sci. 2013;131:225–33.
Nohara K, Okamura K, Suzuki T, Murai H, Ito T, Shinjo K, et al. Augmenting effects of gestational arsenite exposure of C3H mice on the hepatic tumors of the F(2) male offspring via the F(1) male offspring. J Appl Toxicol. 2016;36:105–12.
Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol. 2005;20:345–52.
Pant HH, Rao MV. Evaluation of in vitro anti-genotoxic potential of melatonin against arsenic and fluoride in human blood cultures. Ecotoxicol Environ Saf. 2010;73:1333–7.
Parodi DA, Greenfield M, Evans C, Chichura A, Alpaugh A, Williams J, et al. Alteration of mammary gland development and gene expression by in utero exposure to arsenic. Reprod Toxicol. 2015;54:66–75.
Parvez F, Wasserman GA, Factor-Litvak P, Liu X, Slavkovich V, Siddique AB, et al. Arsenic exposure and motor function among children in Bangladesh. Environ Health Perspect. 2011;119:1665–70.
Paul S, Majumdar S, Giri AK. Genetic susceptibility to arsenic-induced skin lesions and health effects: a review. Genes Environ. 2015;37:23.
Phookphan P, Navasumrit P, Waraprasit S, Promvijit J, Chaisatra K, Ngaotepprutaram T, et al. Hypomethylation of inflammatory genes (COX2, EGR1, and SOCS3) and increased urinary 8-nitroguanine in arsenic-exposed newborns and children. Toxicol Appl Pharmacol. 2017;316:36–47.
Pilsner JR, Hall MN, Liu X, Ilievski V, Slavkovich V, Levy D, et al. Influence of prenatal arsenic exposure and newborn sex on global methylation of cord blood DNA. PLoS ONE. 2012;7:e37147.
Puzanova L, Doskocil M. Mechanism of the development of malformations in chick embryos after administration of arsenic (author’s transl). Sb Lek. 1976;78:57–63.
Quansah R, Armah FA, Essumang DK, Luginaah I, Clarke E, Marfoh K, et al. Association of arsenic with adverse pregnancy outcomes/infant mortality: a systematic review and meta-analysis. Environ Health Perspect. 2015;123:412–21.
Rager JE, Bailey KA, Smeester L, Miller SK, Parker JS, Laine JE, et al. Prenatal arsenic exposure and the epigenome: altered microRNAs associated with innate and adaptive immune signaling in newborn cord blood. Environ Mol Mutagen. 2014;55:196–208.
Rager JE, Yosim A, Fry RC. Prenatal exposure to arsenic and cadmium impacts infectious disease-related genes within the glucocorticoid receptor signal transduction pathway. Int J Mol Sci. 2014;15:22374–91.
Rahman A, Vahter M, Ekstrom EC, Persson LA. Arsenic exposure in pregnancy increases the risk of lower respiratory tract infection and diarrhea during infancy in Bangladesh. Environ Health Perspect. 2011;119:719–24.
Rakyan VK, Chong S, Champ ME, Cuthbert PC, Morgan HD, Luu KV, et al. Transgenerational inheritance of epigenetic states at the murine Axin(Fu) allele occurs after maternal and paternal transmission. Proc Natl Acad Sci USA. 2003;100:2538–43.
Ramsey KA, Foong RE, Sly PD, Larcombe AN, Zosky GR. Early life arsenic exposure and acute and long-term responses to influenza A infection in mice. Environ Health Perspect. 2013;121:1187–93.
Raqib R, Ahmed S, Sultana R, Wagatsuma Y, Mondal D, Hoque AM, et al. Effects of in utero arsenic exposure on child immunity and morbidity in rural Bangladesh. Toxicol Lett. 2009;185:197–202.
Reichard JF, Puga A. Effects of arsenic exposure on DNA methylation and epigenetic gene regulation. Epigenomics. 2010;2:87–104.
Rios R, Zarazua S, Santoyo ME, Sepulveda-Saavedra J, Romero-Diaz V, Jimenez V, et al. Decreased nitric oxide markers and morphological changes in the brain of arsenic-exposed rats. Toxicology. 2009;261:68–75.
Rojas D, Rager JE, Smeester L, Bailey KA, Drobna Z, Rubio-Andrade M, et al. Prenatal arsenic exposure and the epigenome: identifying sites of 5-methylcytosine alterations that predict functional changes in gene expression in newborn cord blood and subsequent birth outcomes. Toxicol Sci. 2015;143:97–106.
Sage AP, Minatel BC, Ng KW, Stewart GL, Dummer TJB, Lam WL, Martinez VD. Oncogenomic disruptions in arsenic-induced carcinogenesis. Oncotarget. 2017;8:25736–55.
Saha KK, Engstrom A, Hamadani JD, Tofail F, Rasmussen KM, Vahter M. Pre- and postnatal arsenic exposure and body size to 2 years of age: a cohort study in rural Bangladesh. Environ Health Perspect. 2012;120:1208–14.
Shen J, Liu J, Xie Y, Diwan BA, Waalkes MP. Fetal onset of aberrant gene expression relevant to pulmonary carcinogenesis in lung adenocarcinoma development induced by in utero arsenic exposure. Toxicol Sci. 2007;95:313–20.
Shi H, Shi X, Liu KJ. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem. 2004;255:67–78.
Singh AP, Goel RK, Kaur T. Mechanisms pertaining to arsenic toxicity. Toxicol Int. 2011;18:87–93.
Stafford DE. Altered hypothalamic-pituitary-ovarian axis function in young female athletes: implications and recommendations for management. Treat Endocrinol. 2005;4:147–54.
States JC, Srivastava S, Chen Y, Barchowsky A. Arsenic and cardiovascular disease. Toxicol Sci. 2009;107:312–23.
States JC, Singh AV, Knudsen TB, Rouchka EC, Ngalame NO, Arteel GE, et al. Prenatal arsenic exposure alters gene expression in the adult liver to a proinflammatory state contributing to accelerated atherosclerosis. PLoS ONE. 2012;7:e38713.
Steinmaus C, Ferreccio C, Acevedo J, Balmes JR, Liaw J, Troncoso P, et al. High risks of lung disease associated with early-life and moderate lifetime arsenic exposure in northern Chile. Toxicol Appl Pharmacol. 2016;313:10–5.
Steinmaus C, Ferreccio C, Yuan Y, Acevedo J, Gonzalez F, Perez L, et al. Elevated lung cancer in younger adults and low concentrations of arsenic in water. Am J Epidemiol. 2014;180:1082–7.
Szymkowicz DB, Sims KC, Castro NM, Bridges WC, Bain LJ. Embryonic-only arsenic exposure in killifish (Fundulus heteroclitus) reduces growth and alters muscle IGF levels one year later. Aquat Toxicol. 2017;186:1–10.
Tapio S, Grosche B. Arsenic in the aetiology of cancer. Mutat Res. 2006;612:215–46.
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Exs. 2012;101:133–64.
Tokar EJ, Diwan BA, Thomas DJ, Waalkes MP. Tumors and proliferative lesions in adult offspring after maternal exposure to methylarsonous acid during gestation in CD1 mice. Arch Toxicol. 2012;86:975–82.
Tsuji JS, Perez V, Garry MR, Alexander DD. Association of low-level arsenic exposure in drinking water with cardiovascular disease: a systematic review and risk assessment. Toxicology. 2014;323:78–94.
Tyler CR, Allan AM. Adult hippocampal neurogenesis and mRNA expression are altered by perinatal arsenic exposure in mice and restored by brief exposure to enrichment. PLoS ONE. 2013;8:e73720.
Tyler CR, Allan AM. The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr Environ Health Rep. 2014;1:132–47.
Tyler CR, Hafez AK, Solomon ER, Allan AM. Developmental exposure to 50 parts-per-billion arsenic influences histone modifications and associated epigenetic machinery in a region- and sex-specific manner in the adult mouse brain. Toxicol Appl Pharmacol. 2015;288:40–51.
Tyler CR, Labrecque MT, Solomon ER, Guo X, Allan AM. Prenatal arsenic exposure alters REST/NRSF and microRNA regulators of embryonic neural stem cell fate in a sex-dependent manner. Neurotoxicol Teratol. 2017;59:1–15.
Tyler CR, Weber JA, Labrecque M, Hessinger JM, Edwards JS, Allan AM. ChIP-Seq analysis of the adult male mouse brain after developmental exposure to arsenic. Data Brief. 2015;5:248–54.
Tyler CRS, Smoake JJW, Solomon ER, Villicana E, Caldwell KK, Allan AM. Sex-dependent effects of the histone deacetylase inhibitor, sodium valproate, on reversal learning after developmental arsenic exposure. Front Genet. 2018;9:200.
Vahter M. Effects of arsenic on maternal and fetal health. Annu Rev Nutr. 2009;29:381–99.
Waalkes MP, Liu J, Chen H, Xie Y, Achanzar WE, Zhou YS, et al. Estrogen signaling in livers of male mice with hepatocellular carcinoma induced by exposure to arsenic in utero. J Natl Cancer Inst. 2004;96:466–74.
Waalkes MP, Liu J, Diwan BA. Transplacental arsenic carcinogenesis in mice. Toxicol Appl Pharmacol. 2007;222:271–80.
Waalkes MP, Liu J, Ward JM, Powell DA, Diwan BA. Urogenital carcinogenesis in female CD1 mice induced by in utero arsenic exposure is exacerbated by postnatal diethylstilbestrol treatment. Cancer Res. 2006;66:1337–45.
Waalkes MP, Qu W, Tokar EJ, Kissling GE, Dixon D. Lung tumors in mice induced by “whole-life” inorganic arsenic exposure at human-relevant doses. Arch Toxicol. 2014;88:1619–29.
Wang W, Cheng S, Zhang D. Association of inorganic arsenic exposure with liver cancer mortality: a meta-analysis. Environ Res. 2014;135:120–5.
Wang W, Xie Z, Lin Y, Zhang D. Association of inorganic arsenic exposure with type 2 diabetes mellitus: a meta-analysis. J Epidemiol Community Health. 2014;68:176–84.
Wang Z, Yang C. Metal carcinogen exposure induces cancer stem cell-like property through epigenetic reprograming: a novel mechanism of metal carcinogenesis. Semin Cancer Biol. 2019. https://doi.org/10.1016/j.semcancer.2019.01.002.
Whirledge S, Cidlowski JA. Glucocorticoids and reproduction: traffic control on the road to reproduction. Trends Endocrinol Metab. 2017;28:399–415.
Willhite CC, Ferm VH. Prenatal and developmental toxicology of arsenicals. Adv Exp Med Biol. 1984;177:205–28.
Xi S, Jin Y, Lv X, Sun G. Distribution and speciation of arsenic by transplacental and early life exposure to inorganic arsenic in offspring rats. Biol Trace Elem Res. 2010;134:84–97.
Xie Y, Liu J, Benbrahim-Tallaa L, Ward JM, Logsdon D, Diwan BA, et al. Aberrant DNA methylation and gene expression in livers of newborn mice transplacentally exposed to a hepatocarcinogenic dose of inorganic arsenic. Toxicology. 2007;236:7–15.
Young JL, Cai L, States JC. Impact of prenatal arsenic exposure on chronic adult diseases. Syst Biol Reprod Med. 2018;64(6):469–83.
Acknowledgements
We acknowledge Prof. Alok Dhawan, Director CSIR-IITR for overall support. This work was supported by Department of Biotechnology (DBT) Grant, Project No. GAP-302. S.G was supported by CSIR junior and senior research fellowship. A.C was supported by DBT Inspire junior research fellowship. V.S was supported by SERB Project GAP-344 fellowship.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gangopadhyay, S., Sharma, V., Chauhan, A. et al. Potential facet for prenatal arsenic exposure paradigm: linking endocrine disruption and epigenetics. Nucleus 62, 127–142 (2019). https://doi.org/10.1007/s13237-019-00274-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-019-00274-3