Abstract
In mice, inorganic arsenic in the drinking water in the parts per million range via the dam during in utero life or with whole-life exposure is a multi-site carcinogen in the offspring. However, human arsenic exposure is typically in the parts per billion (ppb) range. Thus, we studied “whole-life” inorganic arsenic carcinogenesis in mice at levels more relevant to humans. Breeder male and female CD1 mice were exposed to 0, 50, 500 or 5,000 ppb arsenic (as sodium arsenite) in the drinking water for 3 weeks prior to breeding, during pregnancy and lactation, and after weaning (at week 3) groups of male and female offspring (initial n = 40) were exposed for up to 2 years. Tumors were assessed in these offspring. Arsenic exposure had no effect on pregnant dam weights or water consumption, litter size, offspring birthweight or weight at weaning compared to control. In male offspring mice, arsenic exposure increased (p < 0.05) bronchiolo-alveolar tumor (adenoma or carcinoma) incidence at 50-ppb group (51 %) and 500-ppb group (54 %), but not at 5,000-ppb group (28 %) compared to control (22 %). These arsenic-induced bronchiolo-alveolar tumors included increased (p < 0.05) carcinoma at 50-ppb group (27 %) compared to controls (8 %). An increase (p < 0.05) in lung adenoma (25 %) in the 50-ppb group compared to control (11 %) occurred in female offspring. Thus, in CD1 mice whole-life arsenic exposure induced lung tumors at human-relevant doses (i.e., 50 and 500 ppb).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inorganic arsenic is a multi-site human carcinogen with targets including the lung, skin and urinary bladder, and drinking water is a major route of exposure (IARC 2004, 2012). Estimates place tens of millions of people worldwide at risk for chronic exposure to potentially harmful levels of inorganic arsenic primarily through drinking water (Naujokas et al. 2013). In humans, in utero or early life inorganic arsenic exposure from contaminated drinking water has been associated with subsequent lung cancer in adulthood (Smith et al. 2006; Marshall et al. 2007) or other cancers (Smith et al. 2012; Liaw et al. 2008; Yuan et al. 2010) recognized as targets of inorganic arsenic in humans (IARC 2004, 2012). Similarly, in mice, the lung is a common target site after in utero exposure via inorganic arsenic in the maternal drinking water (Waalkes et al. 2003, 2004, 2006a, b; Tokar et al. 2012). Whole-life inorganic arsenic exposure in mice, which includes gestational exposure, produces a generally similar spectrum of tumors as in utero exposure, only at lower doses, and includes dose-related increases in lung cancers (Tokar et al. 2011). Thus, it appears inorganic arsenic exposure during development stimulates lung carcinogenesis that manifests in adulthood in both humans and rodents (Smith et al. 2006; Marshall et al. 2007; Waalkes et al. 2003, 2004, 2006a, b; Tokar et al. 2012), although most human populations are generally exposed during their whole life (IARC 2004, 2012).
Thus far, for in utero exposures to inorganic arsenic to result in adulthood cancer in mice, the exposure levels used in the maternal drinking water have been in the moderate parts per million (ppm) level (42.5 or 85 ppm; Waalkes et al. 2003, 2004, 2006a, b; Tokar et al. 2012), while human arsenic-associated cancers generally occur with exposures in the parts per billion (ppb) range (for examples see: IARC 2004, 2012). We developed a “whole-life” exposure model in mice which involves inorganic arsenic exposure via the drinking water to breeders 3 weeks prior to breeding, to the dams during pregnancy and lactation, and then to the offspring after weaning through adulthood to about 2 years of age (Tokar et al. 2011). With this whole-life exposure, lower inorganic arsenic doses result in tumor formation as in, for instance, lung cancers that occur to excess at 24 ppm but match the control rates at 6 ppm (Tokar et al. 2011). Rodent tumor endpoint assays can be insensitive, and their sensitivity can vary with a variety of parameters such as group size. Moreover, some have proposed that arsenic has complex dose–response characteristics with biological activity, like steroid receptor gene activation, that occurs at low doses but is lost at higher doses (Bodwell et al. 2004, 2006). This dose–response dichotomy seems to exist within oncogenesis as inorganic arsenic is a very effective cancer chemotherapeutic at pharmacologic doses, but can also enhance xenograft tumor growth and angiogenesis at lower doses (Soucy et al. 2003; Kamat et al. 2005; Liu et al. 2006). In addition, functional or genetic changes relevant to chronic lung disease and cancer have been reported after exposure in early life (including in utero exposure) to inorganic arsenic at doses as low as 100–500 ppb in rodents (Petrick et al. 2009; Lantz et al. 2009; Kozul et al. 2009; Ramsey et al. 2013a, b; Farzan et al. 2013).
Therefore, we designed the present study to expose mice to inorganic arsenic throughout gestation and then for the bulk of their adult life to a range of arsenic levels encompassing much lower doses such as those encountered commonly in the human environment. The original intent of this work was to provide rodent data potentially useful in more clearly defining levels of concern for inorganic arsenic. We used our “whole-life” protocol that exposes mice to inorganic arsenic via the drinking water through their entire in utero life stage and subsequently through adulthood until 2 years of age (Tokar et al. 2011) as this would duplicate how humans most likely would be exposed via the environment. The lower two dosage levels of drinking water inorganic arsenic used in the present study (50 and 500 ppb arsenic) are much more commonly seen in human drinking water (IARC 2004, 2012), and the lowest level (50 ppb) approaches the USEPA maximum contaminant level in drinking water for arsenic in the United States (10 ppb).
Materials and methods
Animals and treatment
This study started in June of 2009. The animal portion of the present study was designed and performed entirely within the National Cancer Institute facility at Fort Detrick in Frederick, MD. Animal care was provided in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources 1996). The NCI-Frederick animal facility and its animal program are accredited by the American Association for Accreditation of Laboratory Animal Care, and the animals were treated humanely and with regard for alleviation of suffering. The CD1 mice used for breeders and for initial dosing were obtained from Charles River South (Raleigh, NC). Mice were housed under conditions of controlled temperature, humidity and light cycle.
A basal diet (5L79; Ralston Purina, St. Louis, MO) and acidified water were provided ad libitum. The chow used was not archived but is made of the same components as Purina’s certified rodent diet which in its most recent testing was listed as below detection limits in total arsenic (<20 ppb; 2007; http://www.labdiet.com/cs/groups/lolweb/@labdiet/documents). In June 2013, we tested 5L79 diet sent to NIEHS from the Fort Detrick animal facility building where this bioassay was performed. We found arsenic to be below the level of detection by graphite furnace-atomic absorption spectrometry (GF-AAS; model AAnalyst 600 PerkinElmer with AS 800 auto sampler and End-capped THGA graphite tubes) after digestion of the feed in 50 % of a 2:1 ultrapure nitric acid (70 %; Aldrich, St. Louis, MO): perchloric acid (70 %; Aldrich) mixture and 50 % water. By a Method Detection Limit Study for our GF-AAS, we could consistently detect 5 ppb of inorganic arsenic standard. A mixture of palladium and magnesium was used as a matrix modifier to prevent premature volatilization before vaporization and therefore loss of signal (IARC 2004). Recovery was 99–101 % for spiked arsenic in digested diet samples (n = 8) indicating minimal matrix effects. Although the bioavailability of arsenicals from the diet can be highly variable depending on the arsenical form, our data suggest that any contributions from the feed were negligible compared to administered doses in the drinking water. Thus, the precise amount and form of arsenic that the diet added to the exposure in the present study is not known but is likely only a small fraction of the intentional exposure of inorganic arsenic from the drinking water. In addition, the dietary exposure would be equal between control and treated animals.
The water for this study was from the Fort Detrich water system. It is tested yearly for arsenic and is consistently reported to have non-detectable arsenic levels in compliance with USEPA and Maryland Department of the Environment testing regulations and limits and was so during the period of this study (<0.5 ppb by ICP-MS; personal communication, M. Schrader, NCI-Frederick). For arsenic treatment, sodium arsenite (NaAsO2; purity 99 %; Sigma Chemical Co., St. Louis, MO) was added to the drinking water. The levels added were 0 (control), 50, 500 and 5,000 ppb arsenic.
The CD1 mouse (Charles River) strain was used. Breeder males (10 per dose) and females (20 per dose) received arsenic in the drinking water for at least 3 weeks before successful breeding. Once pregnant, dams received arsenic during the entire course of pregnancy, and after birth, the mothers received arsenic in the drinking water through the period of lactation. At weaning (3 weeks of age), offspring in initial groups of 40 per dose continued to receive arsenic in the drinking water through adulthood to planned killing at 104 weeks (birth was considered time = 0). This treatment is termed “whole life” although it did not go to the natural end of life for the individual animal. Tumors were assessed only in the offspring. At birth, litters were culled to no more than eight pups, consisting of four males and four females if possible. At weaning, offspring were randomly selected to continue in the offspring treatment groups, but it was made sure that they represented animals from at least 10 litters treated with the same dosage. Within each treatment group, mice were allocated for further treatment into adulthood by random selection of 3–4 offspring mice/sex/litter from 10 to 12 litters of the 20 possible litters in each group.
Clinical data and pathological assessment
Individual offspring body weights were recorded once per week for the first 10 weeks after weaning and then once every month. Clinical signs were checked daily. Mice were killed with CO2 when moribund or at the end of the study. A complete necropsy was performed on all moribund animals, animals found dead or at the end of the study. Tissues taken and processed for histological analysis included liver, kidneys, lungs, adrenals, spleen, thymus, thyroid, pituitary, urinary bladder, gonads (testes, ovary), uterus, vagina, oviduct, mammary gland, skin, brain and all grossly abnormal tissues. The skin and paws were carefully inspected for any lesions. Tissues were fixed in 10 % neutral buffered formalin, embedded in paraffin, sectioned at 5 μm and stained with hematoxylin and eosin for histological analysis. The urinary bladder was inflated with fixative prior to being embedded. The pathologists were unaware of the treatment group during pathological assessments. Pathological peer review was conducted and provided consensus diagnoses. Significant increases were seen in lung tumors in several arsenic-treated groups. In order to expedite the reporting of these data, only primary lung lesions are reported in this work. There were tumors and non-neoplastic lesions in other tissues, but in no other site were tumors found to be related to arsenic exposure. Non-pulmonary lesions will be reported in a future publication.
Pathological evaluations and diagnoses of lung tumors and associated lung lesions were evaluated and diagnosed using standard diagnostic criteria as outlined in the International Harmonization of Nomenclature and Diagnostic Criteria in rats and mice (INHAND) document for respiratory tract lesions (Renne et al. 2009) in addition to criteria outlined in a descriptive document on the morphology of spontaneous and chemically induced pulmonary lesions in rats and mice (Dixon et al. 2008). Diagnostic criteria for lung bronchiolo-alveolar adenoma and bronchiolo-alveolar carcinoma included typical morphologic characteristics used for tumor diagnosis such as cell size and shape, degree of cellular atypia, cellular pleomorphism, invasive properties of tumor cells, tumor growth pattern and mitotic activity.
Data analysis
Data are given as lesion incidence (number of affected mice/total mice examined) or survival rate or as mean ± standard deviation or standard error of the mean (SEM), as appropriate. Data on males and females were analyzed separately. A probability level of p < 0.05 was considered to indicate a significant difference in all cases. The survival analyses excluded those animals that were found dead and too autolyzed to assess for lung tumors. In the analyses of tumor incidence, litter effects and survival were taken into account by use of a mixed effects logistic regression model that included survival time and a normally distributed random litter effect. Tumor incidence analyses p values were one-sided. Tumor incidence analyses is based on numbers of animals available for pathological examination, and loss of animals to observation was typically due to post-death autolysis that was considered too advanced for appropriate diagnosis. Lung tumors were considered part of a pathological continuum (adenoma and carcinoma) and were combined for analysis as “adenoma or carcinoma.” When a separate lung adenoma and carcinoma occurred in a single mouse, it is counted as a single case in this either/or category and noted as co-occurring in the “multiple tumors” category. There were also several instances of multiple primary lung adenoma in individual mice, and these are also discussed as cases of multiple tumors. For multiple comparisons of maternal body weight during pregnancy, maternal water consumption during pregnancy, average litter size at birth, newborn pup weight at birth and male or female weanling body weight (day 21), an ANOVA followed by a two-sided Dunnett’s test was used. These analyses of measurements prior to and at weaning used the litters as the basis of comparison and first averaged within litters then averaged these litter means for summary statistics. Survival analyses were performed by Cox regression and Tarone’s test according to sex and arsenic dose, and all p values for these tests are two-sided. Individual adult body weights were analyzed by repeated-measures ANOVA followed by a two-sided Dunnett’s test.
Results
Male and female breeder CD1 mice were exposed to 0, 50, 500 or 5,000 ppb arsenic in the drinking water at least 3 weeks prior to successful breeding. Arsenic exposure continued for the mothers during pregnancy and lactation and for the individual offspring in gender-based groups after weaning (at 3 weeks of age) until 2 years of age (see “Materials and methods”). Tumors were assessed in the offspring. This protocol was termed “whole-life” arsenic exposure although it did not go to the natural end of life of the individual mouse in all cases. All tumor incidence data comparisons are adjusted for survival and litter effects (see “Materials and methods”).
Survival and body weights
The arsenic exposure during early life did not impact maternal water intake or maternal body weight growth during pregnancy (Table 1). In utero fetal growth was unaffected by these levels of arsenic exposure as the number of newborns/litter and newborn body weight did not differ based on arsenic treatment. Trans-lactational arsenic exposure after gestational exposure via the dam to these levels of arsenic had no effect on neonatal growth as assessed by offspring body weight at weaning (Table 1).
Offspring body weights during adulthood are shown in Fig. 1 (numerical data are available in Supplemental Table 1 and 2). In the adult male offspring, exposure to arsenic had some transient effects (weeks 50–74) on adult offspring body weights but only in the groups exposed to the highest two doses (500 and 5,000 ppb). Body weights were significantly (p < 0.05) reduced in male offspring exposed to 500 or 5,000 ppb as assessed on weeks 50, 54, 58, 62, 66 and in the 5,000-ppb group additionally on weeks 70 and 74 but returned to control levels for the remainder of the study (Supplemental Table 1). Only in one case did these differences exceed 10 % of the control mean, specifically on week 62 when the difference between control (average 65.2 g) and 5,000-ppb-treated male mice (average 58.4 g) was 10.4 %. In male offspring treated with the lowest arsenic dose (50 ppb), there were no significant differences in body weight compared to control at any time in the study. In female offspring, reduced post-weaning body weights occurred only at 78 and 82 weeks for the group receiving 50 ppb compared to control (Supplemental Table 2). Increased body weight compared to control occurred at 62 weeks of treatment for the group receiving 5,000 ppb. The lack of consistent body weight differences in arsenic-treated females makes these changes appear unrelated to treatment.
Body weights during adulthood in male (top) and female (bottom) CD1 mice exposed to whole-life inorganic arsenic. See “Materials and methods” for details of exposure. Standard deviations are omitted for clarity. Because of concurrent unrelated demands on trained necropsy personnel, terminal killing was staggered over week 104 and 105, and the week 104 body weights would not be a true time-based mean and are not included. The occasional differences that occurred in treated animals compared to control rarely exceed 10 % and are detailed in the “Results” text. For precise numerical values, see Supplemental Tables 1 and 2
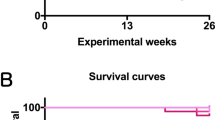
Survival of progeny mice is shown in Fig. 2 and Table 2. There were no significant differences in survival from control in the males treated with any of the levels of arsenic. Control male average survival in the present study (595 days or 85 weeks) is essentially the same as the survival seen in control males in our prior whole-life study (85 weeks; Tokar et al. 2011). In females, the 5,000 and 500 ppb doses had no impact on survival, but there was a significant reduction in survival for the mice treated with the lowest dose of arsenic (50 ppb) compared to females controls. Most of these deaths in the female 50-ppb group occurred relatively late in the study (>70 weeks). The reasons for the loss of females at the lowest dose are unclear.
Kaplan–Meier survival curves for male (top) and female (bottom) CD1 mice exposed to whole-life inorganic arsenic. Survival was reduced in females exposed to 50 ppb. See Table 2 for details and statistical analyses
Lung tumors and other lung lesions
Table 3 shows the effect of whole-life arsenic exposure on incidence of lung tumors in male mice. In males, whole-life arsenic exposure induced significant increases (p < 0.05) in bronchiolo-alveolar tumor (adenoma or carcinoma) incidence at 500 ppb (54 %) and 50 ppb (51 %) but not at 5,000 ppb (28 %), compared to control incidence (22 %). Arsenic-induced bronchiolo-alveolar tumors included significantly increased (p < 0.05) carcinoma at 50 ppb (27 %) compared to controls (8 %). At the 500-ppb level, adenoma incidence (38 %) was significantly increased (p < 0.05) over the control rate (14 %).
In male mice, the first lung tumor, a bronchiolo-alveolar carcinoma, was observed at 46 weeks in a mouse treated with 5,000 ppb arsenic and was the cause of early termination. All male control mice survived to this point in the study.
The control rates in male CD1 mice of lung adenoma (14 %), carcinoma (8 %) and adenoma or carcinoma (22 %) in the present study were comparable with control lung tumor rates from our most recent tumor endpoint study concerning inorganic arsenic using CD1 male mice from the same source and conducted at the same facility (adenoma, 14 %; carcinoma, 6 %; adenoma or carcinoma, 20 %; Tokar et al. 2012).
In arsenic-treated males, multiple lung tumors (multiple primaries in a single mouse) did not show significant differences but occurred to a numerically greater extent in the 50 ppb (14 %) compared to control (3 %) or the other arsenic treatment groups (5,000 ppb, 3 %; 500 ppb, 5 %; Table 3). Also in the 50-ppb group, one case of multiple lung tumors was a case of multiple adenomas along with a carcinoma. The 50-ppb group also showed numerically more cases of alveolar hyperplasia (11 %) than control (3 %) or the other arsenic treatment groups (5,000 ppb 3 %; 500 ppb, 5 %).
Bronchiolo-alveolar adenomas when compared to normal lung alveolar regions consisted of well-circumscribed areas of cuboidal tumor cells that tended to fill adjacent alveolar spaces. The benign tumor cells were relatively uniform, had little to no pleomorphism or atypia and had very low mitotic activity. The lung adenomas often compressed the surrounding tissue; however, there was no invasion, with defined borders that demarcated the tumor cells from the surrounding tissue. In contrast, the lung bronchiolo-alveolar carcinomas consisted of irregular to poorly circumscribed areas of tumor cells that occupied large regions of lung lobes and caused architectural distortion of the surrounding tissue. The malignant tumor cells were pleomorphic, ranging from low cuboidal to columnar with increased mitotic activity, and often formed papillary structures. The lung carcinoma cells were infiltrative and had ill-defined borders. Histopathological images of the various arsenic-induced bronchiolo-alveolar tumors compared to a control lung in males are shown in Fig. 3a–e. The representative images contain both low and high magnifications of a control lung (Fig. 3a, b), and an adenoma (Fig. 3c, d) and a carcinoma (Fig. 3e, f) from male mice exposed to 50 ppb arsenic. Criteria used for tumor diagnosis are provided in the Methods. The large lung carcinomas often nearly filled an entire lung lobe (Fig. 3e).
Histopathological depictions of the various arsenic-induced lung bronchiolo-alveolar tumors from male mice exposed to 50 ppb as compared to a control male mouse. a Control lung without space occupying bronchiolo-alveolar tumors. b Higher magnification of area outlined in 3A showing normal alveolar acinar structures. c Multiple bronchiolo-alveolar adenomas consisting of circumscribed areas of tumor cells that compress, but do not invade the surrounding tissue. d Higher magnification of tumor cells outlined in 3C; note uniform population of cuboidal tumor cells with minimal pleomorphism and no atypia or mitosis. e Large bronchiolo-alveolar carcinoma nearly occupying the entire lung lobe. f Higher magnification of tumor cells outlined in 3E, note pleomorphic cells, ranging from low cuboidal to columnar with increased mitotic activity, and formation of papillary structures. Note this tumor histopathology is representative of typical adenomas and carcinomas seen in arsenic-treated male mice
Pulmonary non-neoplastic lesions occurred in male mice but with no association with arsenic treatment. This included one case of bronchus epithelial hyperplasia each in the 5,000 and 50-ppb groups, one case of bronchiole epithelial hyperplasia in the 500-ppb group, one case of bronchus epithelial hyaline accumulation in the 50-ppb group, one case of lymphoid hyperplasia in controls, one in the 500-ppb group and three in the 5,000-ppb groups, and one case of osseous metaplasia in the 50-ppb group. Lung fibrosis (2 cases in control, 5 in the 50-ppb group, 3 in the 500-ppb group and 5 in the 5,000-ppb group), alveolar histiocytic infiltration (10 cases in control, 13 in the 50-ppb group, 14 in the 500-ppb group and 13 in the 5,000-ppb group) and hemorrhage (one case in control, 2 cases in the 50-ppb group, 2 in the 500-ppb group and one in the 5,000-ppb group) occurred as well but were not related to treatment.
In females, a significant (p < 0.05) increase (Table 4) in lung adenoma occurred at 50-ppb group (25 %) compared to control (11 %). Carcinoma and adenoma or carcinoma showed no treatment-related differences from control with arsenic exposure in female offspring. One female mouse in the 5,000-ppb treatment group had a case of multiple adenoma. Alveolar hyperplasia was unremarkable in females (Table 4).
The control rates of lung adenoma (11 %), carcinoma (5 %) and adenoma or carcinoma (16 %) in female CD1 mice in the present study were comparable with control lung tumor rates from our most recent tumor endpoint study concerning inorganic arsenic using CD1 female mice from the same source and conducted at the same facility (adenoma, 14 %; carcinoma, 6 %; adenoma or carcinoma, 20 %; Tokar et al. 2011).
Pulmonary non-neoplastic lesions occurred in female mice with no association with arsenic treatment. This included one case of chronic pleura inflammation in the 500-ppb group; one case of intima vascular proliferation in the 5,000-ppb group; one case of hemorrhage each in the 50-ppb, 500-ppb and 5,000-ppb groups; one case of vascular inflammation in control group; and one case of mesothelial hyperplasia in the 500-ppb group. Lung fibrosis (3 cases in control, 2 in the 50-ppb group, 3 in the 500-ppb group and 5 in the 5,000-ppb group), alveolar histiocytic infiltration (7 cases in control, 7 in the 50-ppb group, 8 in the 500-ppb group and 13 in the 5,000-ppb group), and lymphoid hyperplasia (7 cases in control, 6 in the 50-ppb group, 7 in the 500-ppb group and 6 in the 5,000-ppb group) occurred but were unrelated to treatment.
Discussion
The whole-life exposure mouse model for inorganic arsenic in the drinking water generally duplicates more typical human environmental arsenic exposure (IARC 2004, 2012). In prior work, use of this whole-life drinking water inorganic arsenic exposure model in CD1 mice used drinking water levels ranging from 6 to 24 ppm (Tokar et al. 2011), doses which are, generally speaking, at or well above the upper range reported for human inorganic arsenic exposure from the drinking water (IARC 2004, 2012). The lung tumor response in mice seen after whole-life exposure to 24 ppm (adenoma or carcinoma in females and carcinoma in males) was not seen at the lowest dose (6 ppm; Tokar et al. 2011). Consistent with those results, in the present study, the highest arsenic dose used (5,000 ppb or 5 ppm) was not linked to lung tumors. However, in males in the present study, the lower two doses of arsenic (500 and 50 ppb) induced bronchiolo-alveolar tumors as reflected by >230 % increases in lung adenoma or carcinoma incidence. At the lowest dose (50 ppb), this increased lung tumors included a significant increase in lung carcinoma and numerically the most multiple primary lung tumors and alveolar hyperplasia. These results were not expected, particularly the unusual dose–response. The lowest whole-life dose (50 ppb) used is only five times the USEPA maximum contaminant level in drinking water for arsenic in the US (10 ppb), and a response at this level is cause for serious concern. Nonetheless, these results should be interpreted with great care. For one, the reason for the absence of a typical dose–response for lung tumor formation is unknown and requires thoughtful scrutiny and confirmation in further study.
As a toxicant, arsenic has such a multitude of diverse biological actions, and evidence indicates inorganic arsenic can show atypical dose–response effects. For instance, complex dose–response effects occur in vitro for inorganic arsenic and glucocorticoid receptor-mediated transcription such that low arsenic concentrations (<0.7 µM) stimulate transcription but slightly higher non-toxic concentrations inhibit transcription (Bodwell et al. 2004). Complex arsenic dose–response curves are shared by the progesterone and mineralocorticoid receptors (Bodwell et al. 2006). Similarly, a clear dichotomy exists in that inorganic arsenic was widely used as a cancer chemotherapeutic (Bentley and Chasteen 2002; Chen et al. 2011) long before it was ever considered an environmental carcinogen. Arsenic has been reintroduced as a cancer chemotherapeutic and is considered a “magic bullet” against certain leukemias (Chen et al. 2011) and may be useful against other cancers (Kritharis et al. 2013). However, it is clear that inorganic arsenic can both kill tumors and stimulate tumor growth depending on the dose (Soucy et al. 2003; Kamat et al. 2005; Liu et al. 2006). In human xenograft tumor model systems, oral or injected inorganic arsenic in mice clearly can kill tumor cells and block tumor growth (e.g., Liu et al. 2006), but at lower doses, it will actually stimulate tumor growth and metastasis (Soucy et al. 2003; Kamat et al. 2005; Liu et al. 2006), and often the lower the dose of arsenic, the more effective it is in this stimulation (Soucy et al. 2003; Liu et al. 2006). Indeed, some find xenograft tumor growth and metastasis is stimulated by oral doses of inorganic arsenic as low as 10 ppb (Kamat et al. 2005), and others caution that low-dose metronomic arsenic chemotherapy should be used with “extreme caution” because of carcinogenic potential (Liu et al. 2006). The current whole-life exposure study showed no increase in lung tumors at the highest dose (5,000 ppb), and one might speculate that this level acted as a “pharmacological” dose for lung cancer. The net effects of a complex, dual action carcinogen/cancer chemotherapeutic agent likely result from an intricate interplay of various factors such as target site dosimetry and length and timing of exposure. Beyond this, the mechanism of arsenic-induced lung cancer is not defined, and it is not known whether the mechanism for lung tumor induction in the moderate ppm range seen in prior whole-life work (Tokar et al. 2011) is the same as in the ppb range in the present study. Regardless, it may be that at certain doses chronic arsenic exposure initiates cancer and then acts as a chemotherapeutic for the very cancers that were initiated. In essence, arsenic could target the same cells it had earlier transformed, with the tumor outcome dependent on the dose and window of exposure. Further study is clearly required to help define the complexities of the dose–response in lung tumor formation seen in the present study.
Lung tumors in mice are often a late life event and have a propensity to be observed in older mice (e.g., Wang et al. 2012). Survival therefore may impact their formation. However, there were no significant differences in survival in the arsenic-treated males compared to control males in the present study. In females, the group exposed to 50 ppb arsenic showed reduced survival for unknown reasons but, nonetheless, showed an increase in lung adenoma formation compared to control. In addition, survival was adjusted for in all statistical comparisons of tumor incidence. Thus, the increased lung tumors associated with arsenic exposure cannot be explained by differences in survival in the present study.
In the present work, whole-life arsenic exposure in the ppb range increased lung adenoma or carcinoma in male CD1 mice but appeared less effective in females, although females did show an increase in lung adenoma at 50 ppb. Various aspects of arsenic toxicity can have significant gender differences. For example, men appear more sensitive to arsenic-induced skin effects than women, and there can be gender-related differences in inorganic arsenic methylation (Vahter et al. 2007). Further, gender differences can occur in experimental carcinogenesis studies with inorganic arsenic, although females are not always less sensitive (Tokar et al. 2010; Vahter et al. 2007). In humans, males and females often are equally susceptible to lung cancers after inorganic arsenic exposure (IARC 2004, 2012), although some data indicate higher sensitivity to lung cancer in women after arsenic exposure (Aballay et al. 2012). In the lung, in utero exposure via maternal drinking water to 10 or 100 ppb results in impaired pulmonary mechanics in the offspring during infancy in mice to a greater extent in male offspring than female (Ramsey et al. 2013a). Thus, although we have no ready explanation for the observed gender relationship in lung tumors in this study, gender differences for inorganic arsenic are not uncommon but can be complex.
In summary, in male CD1 mice, lung tumors were associated with whole-life inorganic arsenic exposure in the ppb range, a level of exposure of concern to humans (IARC 2004, 2012). This response was lost at highest dose in the low ppm range for reasons that are not completely apparent. Further work should be performed in assessing the carcinogenic potential of inorganic arsenic in mice at human relevant doses. Such a study could include aspects such as more doses in the human relevant range, larger group sizes for even greater statistical power, and more extensive pathological analyses. We are hopeful that the present work will stimulate others to investigate the carcinogenic potential, and eventually the carcinogenic mechanisms, of human relevant inorganic arsenic doses in mice.
References
Aballay LR, Díaz Mdel P, Francisca FM et al (2012) Cancer incidence and pattern of arsenic concentration in drinking water wells in Córdoba, Argentina. Int J Environ Health Res 22:220–231
Bentley R, Chasteen TG (2002) Arsenic curiosa and humanity. Chem Educ 7:51–60
Bodwell JE, Kingsley LA, Hamilton JW (2004) Arsenic at very low concentrations alters glucocorticoid receptor (GR)-mediated gene activation but not GR-mediated gene repression: complex dose-response effects are closely correlated with levels of activated GR and require a functional GR DNA binding domain. Chem Res Toxicol 17:1064–1076
Bodwell JE, Gosse JA, Nomikos AP et al (2006) Arsenic disruption of steroid receptor gene activation: complex dose-response effects are shared by several steroid receptors. Chem Res Toxicol 19:1619–1629
Chen SJ, Zhou GB, Zhang XW et al (2011) From an old remedy to a magic bullet: molecular mechanisms underlying the therapeutic effects of arsenic in fighting leukemia. Blood 117:6425–6437
Dixon D, Herbert RA, Kissling GE et al (2008) Summary of chemically induced pulmonary lesions in the National Toxicology Program (NTP) toxicology and carcinogenesis studies. Toxicol Pathol 36:428–439
Farzan SF, Karagas MR, Chen Y (2013) In utero and early life arsenic exposure in relation to long-term health and disease. Toxicol Appl Pharmacol 272:384–390
Institute of Laboratory Animal Resources (1996) Guide for the care and use of laboratory animals, 7th edn. National Academy Press, Washington
International Agency for Research on Cancer (IARC) (2004) Arsenic in drinking water. In: Some drinking water disinfectants and contaminants, including arsenic; International agency for research on cancer monographs on the evaluation of carcinogenic risk to humans, vol 84. IARC Press, Lyon, pp 269–477
International Agency for Research on Cancer (IARC) (2012) Monographs on the evaluation of carcinogenic risks to humans. A review of human carcinogens: arsenic, metals, fibres, and dusts, vol 100C. IARC Press, Lyon, pp 41–93
Kamat CD, Green DE, Curilla S et al (2005) Role of HIF signaling on tumorigenesis in response to chronic low-dose arsenic administration. Toxicol Sci 86:248–257
Kozul CD, Hampton TH, Davey JC et al (2009) Chronic exposure to arsenic in the drinking water alters the expression of immune response genes in mouse lung. Environ Health Perspect 117:1108–1115
Kritharis A, Bradley TP, Budman DR (2013) The evolving use of arsenic in pharmacotherapy of malignant disease. Ann Hematol 92:719–730
Lantz RC, Chau B, Sarihan P et al (2009) In utero and postnatal exposure to arsenic alters pulmonary structure and function. Toxicol Appl Pharmacol 235:105–113
Liaw J, Marshall G, Yuan Y et al (2008) Increased childhood liver cancer mortality and arsenic in drinking water in northern Chile. Cancer Epidemiol Biomarkers Prev 17:1982–1987
Liu B, Pan S, Dong X et al (2006) Opposing effects of arsenic trioxide on hepatocellular carcinomas in mice. Cancer Sci 97:675–681
Marshall G, Ferreccio C, Yuan Y et al (2007) Fifty-year study of lung and bladder cancer mortality in Chile related to arsenic in drinking water. J Natl Cancer Inst 99:920–928
Naujokas MF, Anderson B, Ahsan H et al (2013) The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121:295–302
Petrick JS, Blanchere FM, Selmin O et al (2009) Inorganic arsenic as a developmental toxicant: in utero exposure and alterations in the developing rat lungs. Mol Nutr Food Res 53:583–591
Ramsey KA, Larcombe AN, Sly PD (2013a) In utero exposure to low dose arsenic via drinking water impairs early life lung mechanics in mice. BMC Pharmacol Toxicol 14:13
Ramsey KA, Bosco A, McKenna KL et al (2013b) In utero exposure to arsenic alters lung development and genes related to immune and mucociliary function in mice. Environ Health Perspect 121:244–250
Renne R, Brix A, Harkema J et al (2009) Proliferative and nonproliferative lesions of the rat and mouse respiratory tract. Toxicol Pathol 37(7 Suppl):5S–73S
Smith AH, Marshall G, Yuan Y et al (2006) Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Health Perspect 114:1293–1296
Smith AH, Marshall G, Liaw J et al (2012) Mortality in young adults following in utero and childhood exposure to arsenic in drinking water. Environ Health Perspect 120:1527–1531
Soucy NV, Ihnat MA, Kamat CD et al (2003) Arsenic stimulates angiogenesis and tumorigenesis in vivo. Toxicol Sci 76:271–279
Tokar EJ, Benbrahim-Tallaa L, Ward JM et al (2010) Cancer in experimental animals exposed to arsenic and arsenic compounds. Critical Rev Toxicol 40:912–927
Tokar EJ, Diwan BA, Ward JM et al (2011) Carcinogenic effects of “whole-life” exposure to inorganic arsenic in CD1 mice. Toxicol Sci 119:73–83
Tokar EJ, Diwan BA, Waalkes MP (2012) Renal, hepatic, pulmonary and adrenal tumors induced by prenatal inorganic arsenic followed by dimethylarsinic acid in adulthood in CD1 mice. Toxicol Lett 209:179–185
Vahter M, Akesson A, Lidén C et al (2007) Gender differences in the disposition and toxicity of metals. Environ Res 104:85–95
Waalkes MP, Ward JM, Liu J et al (2003) Transplacental carcinogenicity of inorganic arsenic in the drinking water: induction of hepatic, ovarian, pulmonary and adrenal tumors in mice. Toxicol Appl Pharmacol 186:7–17
Waalkes MP, Ward JM, Diwan BA (2004) Induction of tumors of the liver, lung, ovary and adrenal in adult mice after brief maternal gestational exposure to inorganic arsenic: promotional effects of postnatal phorbol ester exposure on hepatic and pulmonary, but not dermal cancers. Carcinogenesis 25:133–141
Waalkes MP, Liu J, Ward JM, Powell DA et al (2006a) Urogenital carcinogenesis in female CD1 mice induced by in utero arsenic exposure is exacerbated by postnatal diethylstilbestrol treatment. Cancer Res 66:1337–1345
Waalkes MP, Liu J, Ward JM et al (2006b) Enhanced urinary bladder and liver carcinogenesis in male CD1 mice exposed to transplacental inorganic arsenic and postnatal diethylstilbestrol or tamoxifen. Toxicol Appl Pharmacol 215:295–305
Wang Y, Rouggly L, You M et al (2012) Animal models of lung cancer characterization and use for chemoprevention research. Prog Mol Biol Transl Sci 105:211–226
Yuan Y, Marshall G, Ferreccio C et al (2010) Kidney cancer mortality: fifty-year latency patterns related to arsenic exposure. Epidemiol 21:103–108
Acknowledgments
The authors wish to thank Dr. Bhalchandra Diwan, NCI-Frederick for aid with experimental design, Drs. Thayer, Sills and Bucher, DNTP, for critical evaluation of this manuscript, Dan Logsdon and Mark Schrader NCI-Frederick for expert technical assistance in animal husbandry, for provision of critical information on diet and water, and provision of diet samples, and Matthew Bell, DNTP, for assistance with graphics. This research was supported in part by the DNTP, NIEHS and in part by the National Cancer Institute, Center for Cancer Research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Waalkes, M.P., Qu, W., Tokar, E.J. et al. Lung tumors in mice induced by “whole-life” inorganic arsenic exposure at human-relevant doses. Arch Toxicol 88, 1619–1629 (2014). https://doi.org/10.1007/s00204-014-1305-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-014-1305-8