Abstract
Manure products fermented underground in cow horns and commonly used as field spray (preparation 500) in the biodynamic farming system, were characterized for molecular composition by solid-state nuclear magnetic resonance [13 C cross-polarization magic-angle-spinning NMR (13 C-CPMAS-NMR)] spectroscopy and offline tetramethylammonium hydroxide thermochemolysis gas chromatography-mass spectrometry. Both thermochemolysis and NMR spectroscopy revealed a complex molecular structure, with lignin aromatic derivatives, polysaccharides, and alkyl compounds as the predominant components. CPMAS-NMR spectra of biodynamic preparations showed a carbon distribution with an overall low hydrophobic character and significant contribution of lignocellulosic derivatives. The results of thermochemolysis confirmed the characteristic highlighted by NMR spectroscopy, revealing a molecular composition based on alkyl components of plant and microbial origin and the stable incorporation of lignin derivatives. The presence of biolabile components and of undecomposed lignin compounds in the preparation 500 should be accounted to its particularly slow maturation process, as compared to common composting procedures. Our results provide, for the first time, a scientific characterization of an essential product in biodynamic agriculture, and show that biodynamic products appear to be enriched of biolabile components and, therefore, potentially conducive to plant growth stimulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biodynamic (BD) agriculture is a unique organic farming system that, within soil organic matter (SOM) management practices and as an alternative to mineral fertilization and crop rotation, aims at improving the chemical, physical, and biological properties of cultivated soils upon application of these organic materials. The different managements used in BD farming system implies the application of six specific preparations (numbered 502–507) as compost additive, and two field spray preparations (500 and 501) (Koepf et al. 1976). Long-term field trials proved that such a BD system, as in the case of ordinary organic farming, allows for sustainable crop production and improves soil biological activity (Zaller and Köpke 2004; Birkhofer et al. 2008). In fact, the determination of soil quality indicators, such as aggregate stability, flux of phosphorus from soil matrix to soils solution, soil microbial biomass and diversity, ability of microbial communities to use organic matter, has shown larger responses in the BD systems than in conventional soil management techniques (Ryan and Ash 1999; Mader et al. 2002). Other studies suggested that, although BD farming provides lower crop yields than conventional systems, it generally improves soil quality, and ensures equal or greater net returns per hectare (Reganold 1955). It is noteworthy that the European Commission has included BD preparations in the list of products permitted in organic farming systems, since they are liable to maintain and increase soil fertility and biological activity (EC Regulation 834 2007).
Among the two common BD field spray applications (named 500 and 501), preparation 500 is sprayed on damp soil before sowing or immediately after plant emergence, while preparation 501 is sprayed several times on growing plants. These two BD preparations are believed to work synergistically, with preparation 500 mainly improving the overall soil fertility, and preparation 501 being active in enhancing the plant physiological response to the light radiation (Koepf et al. 1976). The BD preparation 500 consists of cow manure placed in a cow horn that is left to ferment while buried under the soil for six months throughout autumn and winter, whereas BD preparation 501 is made of powdered quartz packed in a cow horn that is also buried under the soil for six months, but over spring and summer. Besides these peculiar production methods, the effects of BD field spray preparations appear to rely on very low amounts for either soil or plant applications. The actual quantities for preparations 500 and 501 are based on the early recommendations by Steiner (1972), and are commonly applied as water solutions of 150 and 3 g ha-1, respectively (Koepf et al. 1976). Applications of field spray preparations have been found to correlate with lower N content in lentil, larger NO -3 content in soft white spring wheat, and greater NH +4 content in soil (Carpenter-Boggs et al. 2000a), compared to control treatments. In a two year-long trial, the field spray preparations favored carbon mineralization and reduced the differences in soil microbial fatty acid profiles (Carpenter-Boggs et al. 2000b).
Recent studies extensively investigated the effect of preparation 500 on soil chemical and biological fertility, as well as on crop yield (Birkhofer et al. 2008; Joergensen et al. 2010; Ngosong et al. 2010; Reeve et al. 2010). However, no attention has been so far devoted to evaluate the molecular composition of this BD material, whereas the composition of organic products used in conventional organic farming systems (e.g., manure, green and urban waste composts, pig slurry) has been instead thoroughly studied (Tang et al. 2006; Spaccini and Piccolo 2007; Albrecht et al. 2008). In fact, a detailed molecular characterization of organic materials applied to agricultural soils is a basic prerequisite to assess the mechanisms involved in the restoration and improvement of SOM quantity and quality (Gerzabek et al. 2006; Spaccini et al. 2009). Thus, the lack of analytical information on the compositions of BD preparations has not allowed yet for the standardization of production processes, optimization of methods for field application, and assessment of transformation in soil.
Both 13 C cross-polarization magic-angle-spinning nuclear magnetic resonance (13 C-CPMAS-NMR) spectroscopy and offline pyrolysis in the presence of tetramethylammonium hydroxide (TMAH) followed by gas chromatography-mass spectrometry (Pyr-TMAH-GC-MS) have been applied to identify the content and distribution of organic molecules in a wide range of solid organic matrices (Spaccini and Piccolo 2007; Šmejkalová et al. 2008). While CPMAS-NMR spectra offer a qualitative and semiquantitative evaluation of the different carbon types in a matrix, the offline pyrolysis enables the molecular characterization of complex organic materials, such as SOM and composted matter (Spaccini and Piccolo 2009). The latter technique involves the sample treatment with TMAH that favors the concomitant solvolysis and methylation of ester and ether bonds in the organic matter and thus enhances both thermal stability and chromatographic detection of the resulting methylated acidic, alcoholic, and phenolic groups. Moreover, the Pyr-TMAH-GC-MS technique in the offline mode allows the analysis of large quantities of solid material and, hence, a more effective qualitative and quantitative measurement of pyrolytic products.
The objective of this work was thus to obtain, for the first time, information on the molecular composition of three different BD preparations 500, by applying a combination of NMR spectroscopy and offline pyrolysis (Pyr/TMAH-GC-MS).
Material and methods
Production of the BD preparation 500
Different commercial samples of BD preparation 500 from three leading Italian producers were studied. Sample A was produced by Società agricola biodinamica (Labico, Roma), sample B by La Farnia (Rolo, Reggio Emilia), and sample C by Biodynamic Agriculture Section (Bolzano). Briefly, the routine production comprises the following procedure: in early autumn, hollow cow horns are filled with cow manure from organic farming and buried underneath a biodynamically managed soil. The organic material is left to decompose during winter and cow horns are recovered in the following spring after almost 150–180 days of maturation. The compost collected from cow horns is a moist, dark, odorless, humic-like material.
Solid state 13 C-NMR spectroscopy
Solid state NMR spectroscopy (13 C-CPMAS-NMR) was conducted on a Bruker AV-300, equipped with a 4 mm wide-bore MAS probe. NMR spectra were obtained by applying the following conditions: 13,000 Hz of rotor spin rate, 1 s of recycle time, 1 ms of contact time, 20 ms of acquisition time, and 4000 scans. Samples were packed in 4 mm zirconia rotors with Kel-F caps. The pulse sequence was applied with a 1 H Ramp pulse to account for nonhomogeneity of the Hartmann–Hahn condition at high spin rotor rates. For the interpretation of the 13 C-CPMAS-NMR spectra, the overall chemical shift range was divided in resonance regions that identify different carbon structures: alkyl-C (0–45 ppm), methoxyl-C and N-C (45-60 ppm), O-alkyl-C (60–110 ppm), aromatic-C (110–145 ppm), phenolic-C (145–160 ppm), carboxyl- and carbonyl-C (160–200 ppm). Integration of these regions allows to develop useful indexes to compare different materials: 1. hydrophobic index (HB) as the ratio of hydrophobic over hydrophilic carbons [(0–45) + (45–60) + (110–160)/[(45–60) + (60–110) + (160–200)]; 2. lignin ratio (LR) as the ratio of Methoxyl-C ÷ C–N over phenolic carbon: (45–60)/(145–160); 3. alkyl ratio (AR) as the ratio of alkyl over O-alkyl carbon: (0–45)/(60–110).
Offline pirolysis TMAH-GC-MS
About 100 mg of the BD preparation 500 were placed in a quartz boat and moistened with 1 ml of TMAH (25 % in methanol) solution. After drying the mixture under a gentle stream of nitrogen, the quartz boat was introduced into a Pyrex tubular reactor (50 cm × 3.5 cm i.d.) and heated at 400 °C for 30 min in a round furnace (Barnstead Thermolyne 21100). The released gaseous products were continuously transferred by a helium flow (20 ml min-1) into a series of two chloroform (50 ml) traps kept in ice/salt baths. The chloroform solutions were combined and concentrated by rotoevaporation. The residue was redissolved in 1 ml of chloroform and transferred in a glass vial for GC-MS analysis. The GC-MS analyses were conducted with a Perkin-Elmer Autosystem XL by using a RTX-5MS WCOT capillary column, (Restek, 30 m × 0.25 mm; film thickness, 0.25 μm) that was coupled, through a heated transfer line (250 °C), to a PE Turbomass-Gold quadrupole mass spectrometer. The chromatographic separation was achieved with the following temperature program: 60 °C (1 min isothermal), rate 7 °C min-1 to 320 °C (10 min isothermal). Helium was used as carrier gas at 1.90 mil min-1, the injector temperature was at 250 °C, the split-injection mode had a 30 ml min-1 of split flow. Mass spectra were obtained in EI mode (70 eV), scanning in the range 45–650 m/z, with a cycle time of 1 s. Compound identification was based on comparison of mass spectra with the NIST library database, published spectra, and real standards.
For quantitative analysis, external calibration curves were built by mixing methyl-esters and/or methyl-ethers of the following standards: heptadecane, octadecanoic acid, cinnamic acid, octadecanol, 16-hydroxy hexadecanoic acid, docosandioic acid, and beta-sitosterol. Increasing amount of standards mixture were placed in the quartz boat and moistened with 0.5 ml of TMAH (25 % in methanol) solution. The same thermochemolysis conditions as for BD preparation 500 were applied to the standards. The standards percentage recovery ranged from 82 % to 91 %.
Result and discussion
Spectroscopic characterisation
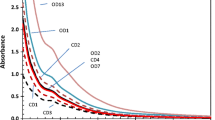
The 13 C-CPMAS-NMR spectra of the three BD preparations 500 are reported in Fig. 1, while the relative distribution of carbon types is listed in Table 1. The carbon distribution of NMR spectra reveals that the preparations 500 contained mainly lignocellulosic materials and lower but significant amounts of hydrophobic alkyl molecules.
The broad peak in the alkyl-C region (0–45 ppm) revealed the inclusion of methylenic chains deriving from various lipid compounds, plant waxes, and biopolyester (Deshmukh et al. 2005), which accounted for about the 20 % of total spectral area in all BD preparations. A larger amount of carbon was found in the O-alkyl-C (60–110 ppm) interval (Table 1) that implies a molecular composition dominated by carbohydrates (Fig. 1). In particular, the different resonances in the 60–110 ppm region, accounting for 40 % to 46 % of total spectral area (Table 1), are currently assigned to monomeric units in oligosaccharidic and polysaccharidic chains of plant woody tissues (Johnson et al. 2005). The intense signal around 73 ppm is due to the overlapping resonances of C2, C3, and C5/C4 carbons in the pyranoside structure of cellulose and hemicellulose, whereas the signal at 104 ppm is assigned to the anomeric C1 carbon of cellulose. The signals at 62–64 ppm and the shoulders at 83–87 ppm represent the C6 and C4 carbons of monomeric units, respectively, being the larger and smaller chemical shift resonance in both intervals attributed to crystalline and amorphous forms of cellulose, respectively (Atalla and VanderHart 1999). In addition to the signals usually assigned to cellulose, the spectra of preparations 500 display two resonance shoulders around 21 and 100 ppm (Fig. 1) assigned to the methyl group in acetyl substituents and the di-O-alkyl-C of hemicellulose chains, respectively, contained in cell walls of the vascular tissues like xylan, glucomannans, etc. (Wikberg and Maunu 2004; Vane et al. 2005).
A significant content of lignin derivatives in the BD preparations 500 is suggested by the signals centered at 56 ppm, in the O-alkyl C region (Fig. 1). This resonance is currently associated with methoxy substituents on the aromatic ring of guaiacyl and siringyl units of lignin structures (Preston et al. 2009), although C–N bonds in aminoacids could also contribute to this resonance. Moreover, the various O-alkyl regions may also include signals related to ether and epoxy groups of plant biopolyesters (Deshmukh et al. 2005), whose 56, 62–67, and 74 ppm resonances in complex matrices may be indeed masked by lignin and polysaccharides.
Signals in the 110–160 ppm interval revealed the presence of aromatic compounds in the BD preparations 500. The large resonance around 129 ppm (Fig. 1) may be related to either unsubstituted and/or alkyl-substituted carbons in aromatic rings of both lignin and cynnamic and p-coumaric units of suberin biopolymers. Partially degraded lignin structures and olefinic carbons may also be included in this spectral region (Gilardi et al. 1995). The resonance for methoxyl groups at 56 ppm should be matched by signals in the phenolic C region (145–160 ppm) that are related to O-substituted carbons in aromatic rings. Both observations suggest the presence of lignin derivatives in the three BD preparations 500 (Table 1). In fact, resonances around 148 and 154 ppm (Fig. 1) are usually assigned to C3 and C5 ring carbons coupled to methoxyl substituents and to O-substituted C4 carbon in lignin monomers (Preston et al. 2009). However, since the 56 ppm signal may be also due to resonance of C–N containing compounds, a better index for the extent of lignin content in humified material may be given by the LR (Table 1), where the area under the 56 ppm is divided by that under the phenolic C region (145–160 ppm; Spaccini and Piccolo 2011). Finally, the prominent signal at 174 ppm indicates a large content of carboxyl groups in aliphatic acids of plant and microbial origin, as well as the presence of amide groups in peptidic moieties.
Although no NMR spectra on biodynamic products are presently available in literature, the spectra shown here for the preparations 500 appear similar to those reported for different recycled biomasses currently used in organic farming systems such as green compost (Spaccini and Piccolo 2007), municipal solid waste (González-Vila et al. 1999), green manure (Albrecht et al. 2008), and cattle manure (Tang et al. 2006). However, with respect to the data reported in the literature for common mature composts, a lower humification degree is suggested by the results of NMR analyses for the three manure preparations.
The NMR spectra of fresh cattle manure are usually characterized by a predominance of carbohydrates and polysaccharides contents, which represent about the 60 % of the C distribution, with lower but significative amount of lignin components and minor contribution of alkyl carbons (Inbar et al. 1989; Gómez et al. 2007). The biological stabilization through composting processes result in a large decomposition of labile material, such as carbohydrates, and the accumulation of aromatic and aliphatic recalcitrant compounds. The NMR spectra of composted manures are therefore characterized by both a progressive increase of alkyl-C/O-alkyl-C ratio and an improvement of the overall hydrophobic characters of final organic products (Tang et al. 2006; Gómez et al. 2007, 2011).
Conversely, the AR calculated from NMR spectra of preparation 500 indicates an uneven distribution between alkyl and O-alkyl carbons, with a prevalence of polar aliphatic functional groups (Table 1), thereby revealing a low recalcitrant character of organic material (Baldock et al. 1997). Moreover the relatively lower intensity (Fig. 1) found for the C4 carbons (84–87 ppm) of the β1 → 4 glycosidic bonds of cellulose units, and the detection, in the O-alkyl region, of signals related to hemicellulose components, suggest the presence of biolabile carbohydrate derivatives in the preparation 500. The lower humification level of the biodynamic products, is also indicated by the hydrophobic index (HB), whose values resembles those obtained for green compost and vermicompost in the earlier stage of maturation (Spaccini and Piccolo 2007; Aguiar 2011), while higher HB values are currently found in mature composts typically used for SOM management (Spaccini and Piccolo 2011). Furthermore, the low value of LRs (Table 1) obtained from the NMR data suggest that a considerable amount of lignin was still present in the three preparations 500 at final maturation (Spaccini and Piccolo 2011).
These findings suggest that the organic preparation obtained by biodynamic procedures maintained a lower overall hydrophobicity than those usually found for composted organic materials, with a larger content of both carbohydrates and polysaccharides and of slight decomposed lignin compounds. A similar amount of labile and recalcitrant components was found, after 4 months of maturation, by Tang et al. (2006) for aerobic processed compost, whose initial composition was based, however, on a mixture of cattle manure and rice straw residues. On the other hand, the C distribution shown in the NMR spectra of preparations 500 after 180 days of stabilization is comparable with that found in pure cattle manure composts after either 40 or 70 days of maturating processes (Inbar et al. 1989; Gómez et al. 2007, 2011), thereby revealing the slower decomposition activity of biodynamic procedures. These differences imply that the biodynamic products may result in less recalcitrant organic matter than in mature compost, thereby resulting in a higher content of bioavailable labile molecules and of lignin-derived aromatic compounds, which may confer a potentially larger plant growth promoting effect (Canellas et al. 2010; Zancani et al. 2011).
Offline Pyr-TMAH-GC-MS
The total ion chromatograms (TICs) obtained by Pyr-TMAH-GC-MS for the BD preparations 500 are shown in Fig. 2. The compounds identified in the TIC are listed in Table 2, while the total yield of main components, their dimensional range, and dominant homologs are shown in Table 3. The thermochemolysis of the three preparations 500 released more than 100 different molecules, which were mainly identified as methyl ethers and esters of natural compounds (Table 2). The most abundant compounds were lignin derivatives and fatty acids, followed by hydrophobic aliphatic molecules derived from plant waxes and biopolyesters, whereas minor components were alicyclic lipidic components of microbial bioproducts (Table 3).
In contrast to the results of CPMAS-NMR spectra, a relative lower amount of carbohydrates derivatives were found among the pyrolysis products of preparations 500 (Table 2). The low thermochemolysis yield of polysaccharide and oligosaccharide derivatives has also been reported in case of plant woody tissues, SOM, and composted materials (Chefetz et al. 2000; Spaccini and Piccolo 2007). This finding has been related to the lower efficiency of offline pyrolysis techniques to detect carbohydrate units of polysaccharides in complex matrices (Chefetz et al. 2000). The thermal behavior and pyrolitic rearrangement of polyhydroxy compounds combined with the lower temperature of TAHM analysis, in fact, are believed to prevent the complete release of polysaccharides (Schwarzinger et al. 2002). A dedicated design of pyrolysis conditions, such as TMAH content, temperature, and pyrolysis time, specific for different substrates, was found to be important to improve the role of TMAH for the detection of carbohydrates in complex geochemical matrices (Schwarzinger et al. 2002).
In accordance with the NMR results, a large range of lignin compounds were detected in the preparations 500 by thermochemolysis (Table 3). The lignin products (Table 2) are identified by current symbols used for lignin basic structures: P for p-hydroxyphenyl, G for guaiacyl (3-methoxy, 4-hydroxyphenyl), and S for syringyl (3,5-dimethoxy, 4-hydroxyphenyl) (Vane et al. 2001; Spaccini and Piccolo 2007). The distribution of various methylated p-hydroxyphenyl, guaiacyl, and syringyl derivatives released from the three samples 500 confirms their origin from lignin of higher plants. The softwood of gymnosperms is composed almost exclusively of guaiacyl subunits, whereas both syringyl and guaiacyl units constitute the hardwood of perennial angiosperm, and all various components including the p-hydroxyphenyl units are building blocks of grass lignin (Goñi and Hedges 1992). The wide range of lignin components released from the preparations 500 (Table 2) indicates the presence of both microbial processed compounds as well as those of fresh decaying plant residues (Vane et al. 2001, 2005). The degraded materials were represented by the oxidized products of di- and tri-methoxy phenylpropane molecules, which included the aldehydic (G4, S4), ketonic (G5, S5), and the benzoic acid (G6, S6) units. Conversely, the identification, among lignin monomers, of the cis- and trans-isomers of 1-(3,4-dimethoxyphenyl)-1(3)-methoxy-propene (G10, G13) and 1-(3,4,5-trimethoxyphenyl)-1(3)-methoxy-propene (S10, S13) are currently associated with the presence of slightly decomposed material (Vane et al. 2005). Moreover, the detection of the enantiomers of 1-(3,4-dimethoxyphenyl)-1,2,3-trimethoxypropane (G14 and G15) and 1-(3,4,5-trimethoxyphenyl)-1,2,3-trimethoxypropane (S14 and S15) indicates the persistence of integral undecomposed lignified plant tissues. While the aldehydic (G4, S4) and acidic forms (G6, S6) of guaiacyl and syringyl structures result from progressive lignin oxidation, the corresponding homologs with a methoxylated side chain (G14/15, S14/15) are indicative of unaltered lignin components, which retain the propyl ether intermolecular linkages. Therefore, the ratio of peak areas of acidic structures over that of the corresponding aldehydes [Ad/Al = (G6/G4, S6/S4)] and over the sum of peak areas for the threo/erythro isomers [Γ G = G6/(G14 + G15); Γ S = S6/(S14 + S15)] are considered useful indicators of the biooxidative transformation of lignin polymers (Vane et al. 2001). The low values of both Ad/Al and Γ ratios of the three preparations 500 (Table 3), as compared to analogous data reported in the literature for both fresh and decomposed woody tissues (Vane et al. 2001, 2005), mature green compost and SOM (Spaccini and Piccolo 2007; Spaccini et al. 2009), suggest an limited structural decomposition and the stable incorporation of slightly decomposed lignin molecules. The higher retained lignin monomers were represented by the 3-(4,5-dimethoxyphenyl)-2-propenoic (G18) and 3-(3,4,5-trimethoxyphenyl)-2-propenoic (S18) acid forms, which could originate from either the oxidation of guaiacyl and siringyl units as well as from the partial decomposition of aromatic domains of suberin biopolymers in plant tissues.
The qualitative distribution of lignin components released by thermochemolysis from preparations 500 closely resembles that obtained for lignocellulose fractions of plant tissues, green compost, and vermicompost, which were characterized, however, with respect to BD products by an advanced oxidation state of lignin monomers (Vane et al. 2001; Spaccini and Piccolo 2007). No data are currently available in literature on the application of thermochemolysis for the molecular characterization of aerobic composted cattle manure. Analytical flash (online) pyrolysis was applied to evaluate the transformation of buffalo manure during aerobic composting and vermicomposting processes. After 90 days of biological stabilization, both compost and vermicomposts showed an improvement of humification stage with a large decrease of polysaccharides and strong biological transformation of lignin components (Ngo et al. 2011). Compared to aerobic processed composts, the large amount of lignin monomers found in preparation 500 and the persistence of nondecomposed derivatives may, hence, be ascribed to the oxygen-limited conditions which promote the slow maturation process of biodynamic procedures.
In addition to lignin components, thermochemolysis of preparations 500 released a large range of alkyl compounds (Table 2). Methyl esters of linear fatty acids (FAME), with chain length ranging from C10 to C30 were the most abundant alkyl compounds (Table 3). About 50–60 % of total FAME was accounted by both saturated and unsaturated hexadecanoic and octadecanoic acids, which are ubiquitous components in living and decayed organisms. The marked predominance of even over odd carbon atoms in FAME suggests a contribution of higher plant lipids to these BD preparations (Amblès et al. 1994). The prevalent plant origin of FAME in thermochemolysis products is also indicated by a relatively large amount (26–29 %) of longer-chain FAME (Table 3). These compounds originate from early degradation of long-chain aliphatic esters, which constitute, together with n-alkanes and sterols, the external protective wax layer of aerial plant tissues (Naafs et al. 2004).
The contribution of microbial input is revealed by the identification of structural components of microbial cells represented by phospholipid fatty acids (PLFA) and by the 2-hydroxy aliphatic acids (Tables 2 and 3), which are important microbial biomarkers of natural organic matter found in soils, sediments, and recycled biomasses (Puglisi et al. 2005). The most abundant PLFA were identified as 13 and 12 methyl-tetradecanoic acids (iso and anteiso pentadecanoic acids), 14 and 15 methyl hexadecanoic acids (iso and anteiso heptadecanoic acids), and the cyclopropane-(2-hexyl)-octanoic acid. It is noteworthy that such metabolites of microbial activity were found (Table 3) in the biodynamic products in larger amount (1075 μg g-1) than usually found in mature green compost (Spaccini and Piccolo 2007). This finding is in line with recent results obtained in green house experiments which revealed a significantly higher microbial activity in biodynamic treated samples as compared to that with traditional compost (Reeve et al. 2010). However, although the soil managements with biodynamic systems was correlated with an improved stimulation of microbial biomass with respect to conventional fertilizers and manure composts (Zaller and Köpke 2004), no unequivocal response on selected biochemical parameters could be drawn in long-term field experiments by comparison between traditional organic farming and BD preparation (Carpenter-Boggs et al. 2000a; Ngosong et al. 2010).
Other pyrolitic products were the methylated form of a wide range of even carbon-numbered ω-hydroxy alkanoic acids and alkandioic acids (Tables 2 and 3). Both classes showed the same distribution of long-chain compounds ranging from C16 to C24 homologs, with the monounsaturated C18:1 acid as the main product (Table 2). Alkane dioic acids included also short-chain (C6–C10) homologs, which are likely to be derived from microbial oxidation of long-chain unsaturated fatty acids or midchain oxygen-bearing hydroxy acids. In fact, some representative compounds of midchain hydroxy acids were found among thermochemolysis products of the preparations 500 (Tables 2 and 3). Variable amounts of C16 and C18 components, mainly the 9,16–10,16-dihydroxy-hexadecanoic and 9,10,18 trihydroxy-octadecanoic isomers, are currently detected in pyrolysis products of plant residues, SOM, and compost (Riederer et al. 1993; Spaccini and Piccolo 2007). The intrinsic biolability of such compounds with polar oxygen functionalities usually results in a preferential microbial degradation of these molecules (Riederer et al. 1993; Opsahl and Benner 1995). Therefore, the particular condition used for the stabilization of preparation 500 may have allowed a stable incorporation of the biolabile polyhydroxy fatty acids.
Together with small traces of linear chain hydrocarbons, a significant amount of alkene/alkane peak doublets was detected in pyrograms of preparations 500 (Fig. 2, Table 3). The occurrence of such alkene/alkane chromatographic pattern is common when natural organic matter is analyzed by online flash or Curie-point pyrolysis techniques. High temperature and the absence of alkylating agents favor the loss of polar functional groups followed by a radical rearrangement and consequent appearance of alkene–alkane doublets (Gobé et al. 2000). Conversely, lower temperature and addition of the TMAH-methylating agent during offline thermochemolysis limit the rearrangement reactions and thereby avoid the occurrence of secondary products in the TIC (McKinney et al. 1996). Therefore, the observed series of alkene/alkane pairs shown by thermochemolysis of preparations 500 may be associated with products of highly hydrophobic organic components, similar to nonhydrolyzable aliphatic biomolecules such as the cutan and suberan found in both living and decaying plant tissues (Augris et al. 1998).
A short range of long-chain aliphatic alcohols (C24–C30) were also released during thermochemolysis of the three preparations 500 (Tables 2 and 3). The heavier linear alcohols, represented by the C26, C28, and C30 components, are typical constituents of plant waxes and have been used as biomarkers to trace organic inputs in long-term field experiments of organic farming systems based on manure addition (Bull et al. 2000).
A few components of tricyclic diterpenes and tetracyclic and pentacyclic triterpenes were also identified among the thermochemolysis products of preparations 500 (Tables 2 and 3). The diterpenic acids, especially those with abietane and pimarane skeletons, are the most representative components of natural diterpenoids, which are commonly found in resins of various higher plants (Pastorova et al. 1997). The abietic acid was the main original precursor found among diterpenoid molecules, although diagenetic products, such as dehydroabietane and dehydroabietic acid, were the most abundant derivatives (Table 3). Tetracyclic triterpenes were represented by methyl ethers of methyl/ethyl cholesten-3-ol derivatives, while the acidic forms of ursane, lupeane, and oleanane structures were the main pentacyclic triterpenes. Although sterol and triterpenol compounds are among the most abundant lipid components in plant tissues (Bull et al. 2000), it has been shown that they may undergo decomposition, once they are exposed to aerobic degradation processes, and their content rapidly decreases passing from plant litter to SOM (Naafs et al. 2004). Notwithstanding the overall biochemical lability of sterol and triterpenol derivatives when exposed to microbial activity, their persistence in the thermochemolysis products indicate the occurrence of incorporation and preservation of biolabile lipid compounds during the maturation process of biodynamic manure preparations.
Conclusion
The detailed molecular characterization of three different BD 500 preparations obtained by solid state NMR spectra and offline TMAH thermochemolysis reveals a complex molecular composition, whereby a wide range of different molecular structures are identified. Lignin derivatives, plant polysaccharides, and linear and cyclic lipid components of plant and microbial origin were recognized as the main components of the biodynamic preparation 500 obtained from cow dung. In particular, the identification of various lignin and lipid biomarkers by thermochemolysis may become useful to trace the origin of the biodynamic products, estimate their transformation in soil, and relate their structure to bioactivity on plants.
Both NMR spectroscopy and thermochemolysis indicate that lignocellulosic residues are incorporated to a large extent in biodynamic preparation 500, with a significant amount of labile slightly decomposed molecules. Concomitantly, the selective preservation of recalcitrant alkyl molecules into preparation 500 was also accompanied by a preservation of less stable alkyl molecules and microbial biomarkers, so that the biochemical recalcitrance of such biodynamic product may be less than that usually shown by mature compost having undergone full aerobic fermentation. These molecular properties suggest that preparation 500 may be more biolabile in soil than common compost and, due to a large content of aromatic lignin derivatives, become potentially more bioactive toward plant growth.
Despite the scepticism on the rationale of biodynamic agricultural preparations, this system increasingly finds practical application, mainly as a small-scale side technique to soil organic farming, with a recognized capacity to maintain crop yields and improve soil health. Our results on the molecular composition of three different preparations 500 not only scientifically provide the basis for a molecular distinction between these and other composted materials but also suggest that such differences may be the reason for the observed bioactivity of such biodynamic product.
References
Aguiar NO (2011) Características Químicas E Bioatividade De Ácidos Húmicos Isolados De Vermicompostos Em Diferentes Estádios De Maturação (Chemical characterization and bioactivity of humic acids isolated from different vermicompost at maturation stages). Master degree thesis. Universidade Estadual Do Norte Fluminense (UENF) Darcy Ribeiro-Campos Dos Goytacazes, Rio de Janeiro, Brasil
Albrecht R, Ziarelli F, Alarcón-Gutiérrez E, Le Petit J, Terrom G, Perissol C (2008) 13 C solid-state NMR assessment of decomposition pattern during co-composting of sewage sludge and green wastes. Eur J Soil Sci 59:445–452
Amblès A, Jambu P, Parlanti E, Joffre J, Riffe C (1994) Incorporation of natural monoacids from plant residues into a hydromorphic forest podzol. Eur J Soil Sci 45:175–182
Atalla RH, VanderHart DL (1999) The role of solid state 13 C NMR spectroscopy in studies of the nature of native celluloses. Solid State Nucl Mag 15:1–19
Augris N, Balesdent J, Mariotti A, Derenne S, Largeau C (1998) Structure and origin of insoluble and non hydrolysable, aliphatic organic matter in forest soil. Org Geochem 28:119–124
Baldock JA, Oades JM, Nelson PN, Skene TM, Golchin A, Clarke P (1997) Assessing the extent of decomposition of natural organic materials using solid-state C-13 NMR spectroscopy. Aust J Soil Res 35:1061–1083
Birkhofer K, Bezemer TM, Bloem J, Bonkowski M, Søren C, Dubois D et al (2008) Long-term organic farming fosters below and aboveground biota: Implications for soil quality, biological control and productivity. Soil Biol Biochem 40:2297–2308
Bull ID, Nott CJ, van Bergen PF, Bull ID, Poulton PR, Evershed RP (2000) Organic geochemical studies of soils from the Rothamsted classical experiments V. The fate of lipids in different long-term experiments. Org Geochem 31:389–408
Canellas LP, Piccolo A, Dobbss LB, Spaccini R, Olivares FL, Zandonadi DB, Façanha AR (2010) Chemical composition and bioactivity properties of size-fractions separated from a vermicompost humic acid. Chemosphere 78:457–466
Carpenter-Boggs L, Reganold JP, Kennedy AC (2000a) Biodynamic preparations: Short-term effects on crops, soils, and weed populations. Am J Altern Agric 15:96–104
Carpenter-Boggs L, Kennedy AC, Reganold JP (2000b) Organic and biodynamic management: effects on soil biology. Soil Sci Soc Am J 64:1651–1659
Chefetz B, Chen Y, Clapp CE, Hatcher PG (2000) Characterization of organic matter in soils by thermochemolysis using tetramethylammonium hydroxide (TMAH). Soil Sci Soc Am J 64:583–589
Deshmukh AP, Simpson AJ, Hadad CM, Hatcher PG (2005) Insights into the structure of cutin and cutan from Agave americana leaf cuticle using HRMAS NMR spectroscopy. Org Geochem 36:1072–1085
EC Regulation 834 (2007) Council regulation on organic production and labelling of organic products and repealing regulation, 28 June 2007
Gerzabek MH, Antil RS, Kögel-Knabner I, Knicker H, Kirchmann H, Haberhauer G (2006) How are soil use and management reflected by soil organic matter characteristics: a spectroscopic approach. Eur J Soil Sci 57:485–494
Gilardi G, Abis L, Cass AEG (1995) Carbon-13 CP/MAS solid state NMR and FT-IR spectroscopy of wood cell wall biodegradation. Enzyme Microb Tech 17:268–275
Gobé V, Lemée L, Amblès A (2000) Structure elucidation of soil macromolecular lipids by preparative pyrolysis and thermochemolysis. Org Geochem 31:409–419
Gómez X, Diaz MC, Cooper M, Blanco D, Morán A, Snape CE (2007) Study of biological stabilization processes of cattle and poultry manure by thermogravimetric analysis and 13 C NMR. Chemosphere 68:1889–1897
Gómez X, Blanco D, Lobato A, Calleja A, Martínez-Núñez F, Villacorta JM (2011) Digestion of cattle manure under mesophilic and thermophilic conditions: characterization of organic matter applying thermal analysis and 1 H NMR. Biodegradation 22:623–635
González-Vila FJ, Almendros G, Madrid F (1999) Molecular alteration of organic fractions from urban waste in the course of composting and their further transformation in amended soil. Sci Total Environ 236:215–229
Goñi MA, Hedges JI (1992) Lignin dimers: structures, distribution, and potential geochemical applications. Geochim Cosmochim Acta 49:2097–2107
Inbar Y, Chen Y, Hadar Y (1989) Solid-state carbon-13 nuclear magnetic resonance and infrared spectroscopy of composted organic matter. Soil Sci Soc Am J 53:1695–1701
Joergensen RG, Mäder P, Fließbach A (2010) Long-term effects of organic farming on fungal and bacterial residues in relation to microbial energy metabolism. Biol Fert Soils 46:303–307
Johnson CE, Smernik RJ, Siccama TG, Kiemle DK, Xu Z, Vogt DJ (2005) Using 13 C nuclear magnetic resonance spectroscopy for the study of northern hardwood tissues. Can J Forest Res 35:1821–1831
Koepf HH, Pettersson BB, Schaumann W (1976) Biodynamic agriculture. The Anthroposophic Press, Spring Valley, NY
Mader P, Fliessbach A, Dubois D, Gunst L, Fried P, Niggli U (2002) Soil fertility and biodiversity in organic farming. Science 296:1694–1697
McKinney DE, Bortiatynski JM, Carson DM, Clifford DJ, De Leeuw JW, Hatcher PG (1996) Tetramethylammonium hydroxide (TMAH) thermochemolysis of the aliphatic biopolymer cutan: insights into the chemical structure. Org Geochem 24:641–650
Naafs DFW, van Bergen PF, de Jong MA, Oonincx A, de Leeuw JW (2004) Total lipid extracts from characteristic soil horizons in a podzol profile. Eur J Soil Sci 55:657–669
Ngo P-T, Rumpel C, Dignac M-F, Billou D, Duc T-T, Jouquet P (2011) Transformation of buffalo manure by composting or vermicomposting to rehabilitate degraded tropical soils. Ecol Eng 37:269–276
Ngosong C, Jarosch M, Raupp J, Neumann E, Ruess L (2010) The impact of farming practice on soil microorganisms and arbuscular mycorrhizal fungi: Crop type versus long-term mineral and organic fertilization. Appl Soil Ecol 46:134–142
Opsahl S, Benner R (1995) Early diagenesis of vascular plant tissues: lignin and cutin decomposition and biogeochemical implications. Geochim Cosmochim Acta 59:4889–4904
Pastorova I, van der Berg KJ, Boon JJ, Verhoeven JW (1997) Analysis of oxidised diterpenoid acids using thermally assisted methylation with TMAH. J Anal Appl Pyrol 43:51–57
Preston CM, Nault JR, Trofymov JA (2009) Chemical changes during 6 years of decomposition of 11 litters in some Canadian forest sites. Part 2. 13 C abundance, solid-state 13 C NMR spectroscopy and the meaning of “Lignin”. Ecosystems 12:1078–1102
Puglisi E, Nicelli M, Capri E, Trevisan M, Del Re AAM (2005) A soil alteration index based on phospholipid fatty acids. Chemosphere 61:1548–1557
Reeve JR, Carpenter-Boggs L, Reganold JP, York AL, Brinton WF (2010) Influence of biodynamic preparations on compost development and resultant compost extracts on wheat seedling growth. Bioresource Technol 101:5658–5666
Reganold JP (1955) Soil quality and profitability of biodynamic and conventional farming systems: a review. Am J Altern Agric 10(1):36–45
Riederer M, Matzke K, Ziegler F, Kögel-Knabner I (1993) Occurrence, distribution and fate of the lipid plant biopolymers cutin and suberin in temperate forest soils. Org Geochem 20:1063–1076
Ryan M, Ash J (1999) Effects of phosphorus and nitrogen on growth of pasture plants and VAM fungi in SE Australian soils with contrasting fertiliser histories (conventional and biodynamic. Agric Ecosyst Environ 73:51–62
Schwarzinger C, Tanczos I, Schmidt H (2002) Levoglucosan, cellobiose and their acetates as model compounds for the thermally assisted hydrolysis and methylation of cellulose and cellulose acetate. J Anal Appl Pyrol 62:179–196
Šmejkalová D, Spaccini R, Piccolo A (2008) Multivariate analysis of CPMAS 13 C-NMR spectra of soils and humic matter as a tool to evaluate organic carbon quality in natural systems. Eur J Soil Sci 59:496–504
Spaccini R, Piccolo A (2007) Molecular characterisation of compost at increasing stages of maturity: II. Thermochemolysis-GC-MS and 13 C-CPMAS-NMR spectroscopy. J Agric Food Chem 55:2303–2311
Spaccini R, Piccolo A (2009) Molecular characteristics of humic acids extracted from compost at increasing maturity stages. Soil Biol Biochem 41:1164–1172
Spaccini R, Piccolo A (2011) Carbon sequestration in soils by hydrophobic protection and in situ catalyzed photo-polymerization of soil organic matter (SOM): chemical and physical–chemical aspects of SOM in field plots. In: Piccolo A (ed) Carbon sequestration in agricultural soils — a multidisciplinary approach to innovative methods. Springer-Verlag, Berlin, Heidelberg, pp 61–106 (in press)
Spaccini R, Sannino D, Piccolo A, Fagnano M (2009) Molecular changes in organic matter of a compost-amended soil. Eur J Soil Sci 60:287–296
Steiner R (1972) Agriculture. A course of eight lectures. Biodynamic Agricultural Association, London
Tang JC, Maie N, Tada Y, Katayama A (2006) Characterization of the maturing process of cattle manure compost. Process Biochem 41:380–389
Vane CH, Martin SC, Snape CE, Abbott GD (2001) Degradation of lignin in wheat straw during growth of the oyster mushroom (Pleurotus ostreatus) using off-line thermochemolysis with tetramethylammonium hydroxyde and solid state 13 C NMR. J Agric Food Chem 49:2709–2716
Vane CH, Drage TC, Snape CL, Stephenson MH, Foster C (2005) Decay of cultivated apricot wood (Prunus armeniaca) by the ascomycete Hypocrea sulphurea, using solid state 13 C NMR and off-line TMAH thermochemolysis with GC-MS. Int Biodeter Biodegr 55:175–185
Wikberg H, Maunu SL (2004) Characterization of thermally modified hard- and softwoods by 13 C CPMAS NMR. Carbohydr Polym 58:461–466
Zaller JG, Köpke U (2004) Effects of traditional and biodynamic farmyard manure amendment on yields, soil chemical, biochemical and biological properties in a long-term field experiment. Biol Fertil Soils 40:222–229
Zancani M, Bertolini A, Petrussa E, Krajňàková J, Piccolo A, Spaccini R, Vianello A (2011) Fulvic acid affects proliferation and maturation phases in Abies cephalonica embryogenic cells. J Plant Physiol 168:1226–1233
Acknowledgments
We thank Carlo Noro (Società Agricola Biodinamica), Gianni Catellani (La Farnia), and the section of Italian Association for Biodynamic Agriculture for providing us samples of the BD preparations 500.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Spaccini, R., Mazzei, P., Squartini, A. et al. Molecular properties of a fermented manure preparation used as field spray in biodynamic agriculture. Environ Sci Pollut Res 19, 4214–4225 (2012). https://doi.org/10.1007/s11356-012-1022-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1022-x