Abstract
Microbial symbionts of honeybee colony are considered as promising tools to support the honeybee population welfare. The majority of existing honeybee microbiota studies is focused on genetic description of the honeybee-associated microbiome fingerprints. The lack of a deeper knowledge on the bacterial community colonizing the honeybee niche, which may be helpful in encouraging industrial applications of this microbiota, led us to undertake this study. The biodiversity of the cultivable fructophilic lactic acid bacteria (FLAB) isolated from adult honeybee intestine and beebread samples was studied. Phenotypic properties of probiotic interest, such as the adhesive potential using in vitro models and adhesion determinants, were also investigated. Antibiotic resistance profiles as reliable markers to evaluate the impact of long-term and current exposure of honeybees to antibiotics were phenotypically determined on the isolated lactic acid bacteria (LAB). The mannose-specific adhesion and high cell surface hydrophobicity found in the studied FLAB isolates sheds light on the effective adaptation of microbiota to specific ecologic niche. It is the first report of phenotypically detected antibiotic resistance profiles of honeybee endogenous bacteria and the first account of minimum inhibitory concentration (MIC) values for four antibiotics used in beekeeping practice.
Similar content being viewed by others
Introduction
The honeybee, Apis mellifera, together with other pollinator insects, provides critical services to ecosystem thanks to the pollination of flowering plants. The decline of honeybee colonies, the consequent decrease of their productivity, and the impaired pollination efficacy became a major concern for modern agriculture, which foresees world food shortages in the future if no remedy against all or even part of honeybee pathogens is found (Klein et al. 2007; Becher et al. 2013; Tirado et al. 2013). This pollinator crisis has stimulated scientific interest and new series of trials to understand the etiology of the diseases and the countermeasures against the honeybee pathologies, which are decimating their populations. A combination of multiple stress components is responsible for the increased mortality of honeybee colonies. The protection of honeybee populations requires a strategy targeting simultaneously several causes that might exert synergistically detrimental effects (Becher et al. 2013).
Research on honeybee gut symbiotic microbial composition, which is important for the protection of honeybees from different stressors (Wu et al. 2013), resulted in the elaboration of a microbial resource management (MRM) approach, analogous to what is successfully applied in different biological systems (Crotti et al. 2012). Bacteria, along with fungi residing in the gastrointestinal tract (GIT) of insects, represent a part of this particular and dynamically changing ecosystem. Recent findings showed that honeybee microbiome is different from what can be observed in other living organisms (Endo and Salminen 2013). This peculiar and unique set of intestinal honeybee bacteria is the result of a long-term co-evolution based on symbiotic interactions between the colonizers and their host (Evans and Armstrong 2006; Engel et al. 2012).
Functions of honeybee gut microbiota remain largely unexplored. Recent studies indicate the possible involvement of honeybee symbionts, particularly lactic acid bacteria (LAB), in the beebread production and its protective role from deterioration during storage. Antimicrobial activities expressed against causative agents of American foulbrood (AFB) and European foulbrood (EFB), Paenibacillus larvae and Melissococcus plutonius, respectively (Forsgren et al. 2010; Vásquez et al. 2012), demonstrate that MRM may be a promising tool for the control of bacterial diseases in the beekeeping. LAB were identified as active immunomodulators, inducing the generation of various antibacterial peptides in the host honeybees (Evans and Lopez 2004; Yoshiyama et al. 2013). Comparative analysis of the microbiome contents of the honeybee gut suggests that different microbial species display peculiar functional characteristics, such as the formation of biofilms and uptake of nutrients, which are expressed in response to interactions with the host (Engel et al. 2012).
Presently, beside various pests and pathogens, honeybees are facing multiple new stressors, such as climate change, habitat loss, environmental pollutants, and contaminants from beekeeping practice (Potts et al. 2010; Smodis Skerl et al. 2010; Pilatic 2012). These new factors can also unbalance, to some extent, the endogenous microbial communities residing in bee GIT. Also, the antibiotic treatments can reduce the diversity of endogenous microbes, enriching the pool of antibiotic resistance genes, hence reinforcing the pathogens and impairing the community of microbes beneficial to the host bee (Tian et al. 2012). Despite the efficacy of antibiotics against AFB and EFB or nosemosis, their usage in beekeeping is limited in the EU by the established maximum residue level (MRL) in the bee products, resulting in a zero tolerance for the presence of traces of antimicrobial agents in honey (Reybroeck et al. 2012; Levy and Marshall 2013). Nevertheless, some antibiotics are still widely used outside Europe, e.g., oxytetracycline and tylosin in the USA (Murray et al. 2009) and in other parts of the world (Kaufmann and Kaenzig 2004; Ortelli et al. 2004; Vidal et al. 2009). In this regard, the application of probiotics may be a reliable problem-solving, soft, and biocompatible approach helping the honeybee colonies to preserve their unique microbiota, diminishing, thus, the necessity of drastic use of antibiotics. The candidate probiotic bacteria should possess highly specific properties to strengthen or maintain the health of the host, including: modulation of immune responses, inhibition of epithelial invasion, production of antimicrobial substances, and adhesive properties (Rolfe 2000; Mercenier et al. 2008; Duary et al. 2011; Salminen and van Loveren 2012).
Fructophilic lactic acid bacteria (FLAB) is a specific group of LAB which has been characterized and described only recently. They prefer fructose as growth substrate and inhabit only fructose-rich niches (Endo and Salminen 2013). Because honeybees are high-fructose-consuming insects, probiotic candidates were searched within FLAB. For the isolation of FLAB strains, foreguts separated from adult honeybee GIT and stored beebread samples were chosen. The foregut is an important organ which is used by bees for food production, collection, transport, and disposal of nectar in the hive. Furthermore, the honeybee nurses feed the larvae with foregut (crop) contents and this is the mechanism by which larval food is inoculated with LAB (Vásquez et al. 2012).
The geographic localization and biological diversity, including microbial diversity, are making the Caucasus range a key Euro-Asiatic ecologic niche. Its diversity of elevations, climates, and humidities presents to the dwelling insects huge adaptive challenges. Hence, the study of honeybee microbiota from Georgian bees may provide results that are also valid for the honeybee populations from other parts of the world.
The aim of the present study was to identify the cultivable FLAB isolated from the Georgian honeybees and to evaluate their diversity, according to the genotypic profiles, the adhesive properties, and the antibiotic resistance.
Materials and methods
Isolation of bacterial strains and growth conditions
Fifty-seven samples obtained from adult worker bee intestines and 25 samples of beebread were collected from 16 apiaries located mainly in the Caucasus Mountains and Kolkheti valley. For sampling, bee colonies without any visible signs of bacterial diseases and nosemosis were chosen. The bees were dissected, and their foregut was separated and homogenized in physiological solution. The beebread samples were homogenized in sterile physiological solution. For isolation of the honeybee FLAB, homogenized samples were spread on Lactic Bacteria Differential Agar medium (LBD, HiMedia, M1087) containing fructose as the unique source of carbohydrates. Plates were incubated in anaerobic conditions at 37 °C during a period of 48 h. Different bacterial colonies were collected and purified on de Man, Rogosa, and Sharpe (MRS) agar plates (Oxoid) supplemented with 0.1 % L-cysteine and 2.0 % fructose (Vásquez et al. 2012). Selected bacterial isolates were propagated in MRS broth supplemented with the same components and then maintained as frozen stocks at −80 °C in the presence of 150 ml/l glycerol as the cryoprotective agent.
DNA extraction and RAPD-PCR
Total bacterial DNA was extracted from overnight MRS cultures using a Chelex-based method described in the MicroSeq protocol of the manufacturer’s instruction (Life Technologies, Monza, Italy). The genomic DNA was used as a template in random amplified polymorphic DNA polymerase chain reaction (RAPD-PCR) fingerprinting using the M13 minisatellite core sequence (5′-GAGGGTGGCGGTTCT-3′) (Huey and Hall 1989). Amplification reactions were performed following the protocol described by Giraffa and Neviani 2000. The presence of PCR products was confirmed by 1 % agarose gel electrophoresis, and 1 Kb Plus DNA Ladder (Invitrogen, Milan, Italy) was used as a DNA molecular weight marker. The gels were stained with GelRed™ (Biotium, Hayward, CA, USA), according to the manufacturer’s instructions. Gel images from RAPD-PCR analysis were photographed after overnight electrophoresis (1.5 V cm−1) using the Kodak Electrophoresis Documentation and Analysis System 290 (EDAS 290, Celbio, Milan, Italy), equipped with the EDAS 290 imaging cabinet. The images were saved as TIFF files and processed with the pattern analysis software package BioNumerics™ (version 7.1; Applied Maths, Sint-Martens-Latem, Belgium).
Calculation of similarity of the PCR fingerprinting profiles was based on the Pearson product-moment correlation coefficient. A dendrogram was deduced from the matrix of similarities by the unweighted pair group method using arithmetic average (UPGMA) clustering algorithm (Vauterin and Vauterin 1992). The reproducibility of the RAPD-PCR fingerprinting patterns was evaluated by repeated running of DNA samples from duplicate amplifications of a control (Rossetti and Giraffa 2005).
Species identification by DNA sequencing
Representative isolates for each RAPD cluster were identified at the species level by sequencing a 500-bp fragment of the 16S rRNA. The 500-bp 16S rRNA fragment was amplified from the 5′ end of the gene using the forward primer 5′-GCYTAACACATGCAAGTCGA-3′ (46 Escherichia coli numbering) and reverse primer 5′-GTATTACCGCGGCTGCTGG-3′ (536 Escherichia coli numbering) (Carminati et al. 2014). For a few isolates, the whole 16S rRNA gene was sequenced by using the MicroSEQ Full Gene 16S rDNA PCR Kit (Life Technologies), according to the manufacturer’s protocol. The DNA sequencing was performed using an ABI PRISM 310 automated DNA sequencer (Life Technologies), as previously described (Suárez et al. 2009). The sequence data were processed, and the alignments were performed using the pattern analysis software package BioNumerics™ (version 7.1; Applied Maths). The obtained sequences were compared with closely related sequences from NCBI GenBank using the BLASTN program (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and aligned using the CLUSTALW software (http://www.ebi.ac.uk/Tools/msa/clustalw2/).
Study of adhesive properties
Yeast agglutination
Bacterial cells were washed and dissolved in 0.1 M of phosphate buffer saline (PBS, pH 7.2). Fifty microliters of a four-fold dilution of the initial bacterial suspensions in PBS was transferred to microtiter plates (Iwaki brand, SciTech Div. Asahi Techno Glass, Japan). To each well, 50 μl of buffer or buffer with methyl-a-D-mannopyranoside (final concentration 100 mM, Sigma Aldrich), as well as 100 μl of 1 % (v/w) of Saccharomyces cerevisiae cells, grown in yeast extract peptone dextrose (YPD) broth at 30 °C under ambient aeration and suspended in PBS, were added. The same suspension of S. cerevisiae alone was dispensed in the microtiter plates and used as a control for autoagglutination. Microtiter plates were shaken for 30 min at 28 °C. The induced visible yeast cell agglutination was assessed by visible light microscopy at 40-fold magnification. Agglutination with or without the addition of methyl-a-D-mannopyranoside was compared to evaluate the role of mannose in the adhesion mechanism (Zago et al. 2011). Tests were carried out in triplicate.
Agglutination with concanavalin A
Bacterial strains were propagated in MRS broth (supplemented with 0.1 % L-cysteine and 2.0 % fructose) at 37 °C for 18 h. The cells were harvested by centrifugation (2000 × g, 10 min, 20 °C) and washed three times with sterile 0.01 M PBS. Pellets were suspended in PBS to reach about 6 × 1010 cells/ml. Concanavalin A (ConA, Sigma Aldrich) was dissolved in PBS at a concentration of 0.1563 mg/ml. Twenty-five microliters of cell suspensions were dispensed in the microtiter plates (Iwaki brand, Sci Tech Div. Asahi Techno Glass, Japan) and mixed by pipetting up and down with an equal volume of ConA solution; sterile PBS was used as a negative control. The plates were left at room temperature for a period of 1 h. Agglutination of bacterial cells was observed under visible-light microscopy at 40-fold magnification and, after visual evaluation, was expressed as no agglutination (−), weak agglutination (+), and strong agglutination (++).
To evaluate the role of different carbohydrates in the bacterial agglutination with ConA, tests were carried out with the addition of D-glucose, D-mannose, and D-galactose (Sigma) using sterile water as a negative control. The carbohydrate solutions in PBS buffer (1 % w/v) were mixed separately with equal volumes of ConA solution (0.3120 mg/ml concentration) and left at room temperature for 10 min. Next, they were mixed with equal volumes of bacterial cell suspensions and left at room temperature for a period of 1 h. The reaction was observed and assessed as described above (Kim et al. 2006). The experiments were carried out in triplicate.
Cell surface hydrophobicity
Cell surface hydrophobicity was determined according to the method of microbial adhesion to hydrocarbons (MATH) as described by Vinderola and Reinheimer (2003), using hexadecane as the solvent. The isolates were grown in MRS broth for 16–18 h at 37 °C. Bacterial cells were harvested by centrifugation (9500 rpm, 5 min, 5 °C) from overnight MRS cultures, washed twice, and suspended in 60 mM of potassium phosphate buffer (pH 6.5). The initial absorbance (A0) at 560 nm of the suspension was adjusted to 0.90–1.10 optical units. Three milliliters of cell suspension was dispensed in clean and dry round-bottom test tubes, followed by the addition of 600 μl of hexadecane. The tubes were vortexed for 2 min. The tubes were left for 1 h at 37 °C to allow phase separation. The lower aqueous phase was carefully removed with a sterile Pasteur pipette and absorbance (A1) at 560 nm was recorded. Cell surface hydrophobicity in terms of percent (H %) was calculated using the following formula: H % = (1 − A1/A0) × 100.
Determination of antibiotic resistance
Antibiotic susceptibility and the minimum inhibitory concentration (MIC) of different antibiotics were determined by the microdilution test, with some modifications. Growth medium was optimized by mixing Iso-Sensitest broth (70 % v/v, Oxoid) and MRS broth (30 % v/v, Merck) supplemented with L-cysteine (0.1 %) and fructose (2 %) pH 6.7. Inoculation of MIC microtiter test plates containing different antibiotics (test ranges: 2–1024 μg ml−1 for oxytetracycline and lincomycin; 0.0625–32 μg ml−1 for rifampicin and kanamycin) and preparation of inocula to reach a final inoculum density of 104 bacteria/ml were performed according to Rossetti et al. (2009). After anaerobic incubation of the inoculated plates at 37 °C for 48 h, the MIC of a given antibiotic was evaluated visually as the lowest concentration at which no growth was observed. All tested antibiotics were purchased from Sigma.
Results
Identification of bacterial strains
A total of 86 strains were studied. They were composed of 25 strains isolated from pollen samples and 61 strains from gut samples. Each strain corresponded to a distinct colony morphotype. RAPD-PCR fingerprinting allowed to genotype LAB isolates, which were grouped into 30 clusters (with an intracluster similarity coefficient >79 %, which corresponded to the reproducibility of the applied RAPD-PCR method). Fifteen clusters out of 30 were represented by single strains. Thirty strains representing each RAPD cluster were selected and identified by 500-bp 16S rRNA sequencing: Lactobacillus kunkeei 1 (KX180927), 6 (KX180928), 14p (KU158309), 18 (KX180929), 21 (KX180943), 22 (KX197199), 23a (KX180930), 23p (KX226337), 28 (KX180931), 38 (KX226339), 48b (KX180932), 55 (KX180933), 59 (KX180934), and 63b (KX226342); L. casei 45 (KX180935); Fructobacillus fructosus 32 (KX180939) and 49a (KU158312); F. tropaeoli 21p (KX180940) and 46 (KU158311); F. pseudoficulneus 57 (KU158313); Enterococcus durans 42s (KX180941); E. faecalis 31 (KX226338), 40 (KX226340), 43 (KX180942); Bifidobacterium. asteroides 26p (KU158310); and five unidentified strains belonging to Lactobacillus spp. 60 (KX180936), 61 (KX226341), 62s (KX180937), 63s (KX180938), and 64s (KX226343).
The 86 strains studied belonged to the genera Lactobacillus (72 strains), Fructobacillus (eight strains), and Enterococcus (five strains). Only one strain was identified as B. asteroides. Among the 72 Lactobacillus strains, L. kunkeei was found to be the most frequently isolated species (66 strains, which were grouped into 16 different RAPD clusters), followed by L. casei (one strain), while five strains remained unidentified. A complete 16S rRNA gene sequencing of two out of the five unidentified strains allowed to identify one of them as L. kunkeei, while the sequence of the other strains perfectly matched with Lactobacillus sp. M4, a strain isolated from honeybee gut by a Korean group (GenBank accession number KF543103.1) (unpublished data). The Fructobacillus strains belonged to the species F. fructosus (three strains), F. tropaeoli (three strains), and F. pseudoficulneus (two strains). The Enterococcus strains belonged to the species E. faecalis (four strains) and E. durans (one strain).
Twenty-four strains representing all identified species and covering the most distinguishable RAPD clusters were selected for further characterization.
Adhesive properties
Yeast agglutination
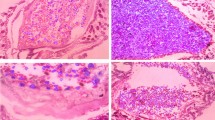
Among the 24 strains tested, only one strain of F. fructosus (strain 49a) was able to agglutinate S. cerevisiae cells (Fig. 1 left). This ability was lost after the addition of methyl-D-mannopyranoside (Fig. 1 right).
Agglutination with concanavalin A
All 24 strains were unable to self-agglutinate, while 17 strains (ten L. kunkeei, two F. fructosus, three F. tropaeoli, and two Enterococcus) showed agglutination properties in the presence of ConA (Fig. 2 left; strain L. kunkeei 21p shown as an example; Table 1). Strains of L. casei, Lactobacillus spp., F. pseudoficulneus, and B. asteroides did not agglutinate with ConA. The agglutination was always inhibited in the presence of D-mannose (Fig. 2 right), while it always took place in the presence of D-glucose and D-galactose (Table 1).
Hydrophobicity
Tests on the microbial adhesion to hydrocarbons (MATH test) demonstrated genus-dependent differences in cell surface hydrophobicity among the tested strains, with the highest values for L. kunkeei, F. tropaeoli, and F. pseudoficulneus (about 90 %), followed by F. fructosus and Lactobacillus spp. (about 80 %). Lactobacillus casei and E. faecalis had intermediate values (about 50 %), while E. durans showed the lowest hydrophobicity (6 %).
Antibiotic resistance
Due to the lack of approved standards applicable for antibiotic susceptibilities in honeybee gut bacteria, the isolated FLAB were tested with the most frequently exploited antibiotics used in Georgia for beekeeping manipulations (Table 2). Overall, the strains of L. kunkeei showed the highest resistance to oxytetracycline and kanamycin, with MICs of about 256–512 and 128–256 μg/ml, respectively. The strains of Fructobacillus species were less resistant, with MIC values between 64 and 128 μg/ml for both oxytetracycline and kanamycin. Lactobacillus casei, Lactobacillus spp., B. asteroides, E. durans, and E. faecalis appeared to be the most sensitive (Table 2). Conversely, all the strains tested resulted, with few exceptions, highly sensitive to rifampicin and lincomycin.
Discussion
The 86 cultivable fructose-fermenting LAB isolated from Georgian honeybee and beebread samples were found to belong to four bacterial genera, with Lactobacillus as the most prevalent. Among lactobacilli, the species L. kunkeei was the most frequently isolated, as also demonstrated in other similar studies (Olofsson and Vásquez 2008; Vásquez and Olofsson 2009; Neveling et al. 2012). The genera Fructobacillus and Enterococcus were less represented, and only one strain of Bifidobacterium was identified.
The abundance and ratio of Fructobacillus and bifidobacteria were in agreement with the data of Vojvodic et al. (2013) on bacteria isolated from honeybee larval instars. Fructobacillus prefers fructose as growth substrate and inhabits only fructose-rich niches, such as the honeybee ecosystem (Endo et al. 2009; Endo and Salminen 2013). Even Enterococcus strains were usually associated with the intestinal tract of the honeybee (Carina Audisio et al. 2011). In regards to bifidobacteria, B. asteroides is the only species frequently isolated from the hindgut of social insects (Bottacini et al. 2012).
The ability to agglutinate yeast cells, the co-aggregation of bacterial cells in the presence of ConA, and the cell wall hydrophobicity overall showed a high adhesion propensity of the species L. kunkeei, as well as of the genus Fructobacillus. In particular, F. fructosus displayed yeast cell agglutination ability in a mannose-specific manner, and L. kunkeei, F. fructosus, and F. tropaeoli showed a mannose-specific co-aggregation ability with ConA as well as the highest cell wall hydrophobicity. Highly hydrophobic cell wall surfaces serve as an efficient tool for LAB to anchor and survive in the host gut.
The abundance of mannose-specific adhesive strains in honeybee GIT could be one possible explanation for the rare manifestation of bacterial diseases in adult bees, which are frequently found in honeybee larvae. The absence of intestinal bacterial diseases in adult bees may be attributed to inefficient competition of exogenous bacterial pathogens for the colonization of GIT surfaces with honeybee endogenous bacteria. It was proposed that the immune status of honeybees might be modulated by the presence of the endogenous symbionts in their GIT (Vásquez et al. 2012). The resistance against pathogens is different in larvae because of the differences between their immune profiles with those of adult individuals. The agglutination ability and a mannose-specific adhesion could create the possibility of selection of specific bacterial strains shielding honeybees against their unicellular microsporidian parasites, such as Nosema apis and Nosema ceranae. This could also allow to hypothesize that GIT microbiota might play a role in the protection of honeybees against their intracellular fungal pathogens. The efficiency of conjoint probiotic therapy against the mucosal opportunistic fungal pathogen Candida albicans was recently reported by Köhler et al. (2012).
The absence or scarcity of consensus for antibiotic resistance criteria with respect to honeybee GIT bacterial species (EFSA Panel on Additives and Products or Substances used in Animal Feed 2012) and the lack of microbiological cut-off values make it difficult to assess and compare with specific standards the results obtained. The MIC values of the 24 endogenous honeybee FLAB showed an overall resistance of these strains to high concentrations of oxytetracycline and kanamycin, and a sensitivity to low concentrations of rifampicin and lincomycin. The detection of oxytetracycline resistance phenotype in this study is coherent with the reported detection of genes encoding such resistance in the honeybee gut microbiota (Tian et al. 2012). Similarly to what is observed in human and animal-associated resistant bacteria, oxytetracycline resistance may have been acquired by horizontal gene transfer between and within commensal microflora because of frequent and extended use of this antibiotic in beekeeping practice (Murray et al. 2009; Tian et al. 2012). Co-resistance against kanamycin found in this study may be the effect of prolonged single-agent therapy with oxytetracycline and a co-mobilization of genetic determinants for both antibiotics (Tian et al. 2012).
High sensitivity of almost all the strains (except for the E. faecalis strain) to low concentrations of rifampicin and lincomycin could reveal a detrimental role of these antibiotics for honeybee gut microbiota, increasing the risks for depletion of resident FLAB strains and consequent exposure to fungal colonization. It should be pointed out that the use of rifampicin-based preparations as honeybee therapeutic agents was introduced only recently (Gurgulova et al. 2003). However, as already demonstrated for oxytetracycline, the use of rifampicin-based treatments in beehives could lead to the emergence of new resistance genes in honeybee gut microbiota.
The results of this study can improve the understanding of many fundamental aspects of bacteria–insect interactions in honeybee GIT. More studies on the biochemical and microbiological properties of honeybee microbiota, especially the FLAB community, are still needed for a possible use of these microorganisms as probiotics, immune boosters, replacements, or supplements to antibiotic treatments.
References
Becher MA, Osborne JL, Thorbek P, Kennedy PJ, Grimm V (2013) Review: towards a systems approach for understanding honeybee decline: a stocktaking and synthesis of existing models. J Appl Ecol 50:868–880
Bottacini F, Milani C, Turroni F, Sánchez B, Foroni E, Duranti S et al (2012) Bifidobacterium asteroides PRL2011 genome analysis reveals clues for colonization of the insect gut. PLoS One 7:e44229. doi:10.1371/journal.pone.0044229
Carina Audisio M, Torres MJ, Sabaté DC, Ibarguren C, Apella MC (2011) Properties of different lactic acid bacteria isolated from Apis mellifera L. bee-gut. Microbiol Res 166:1–13
Carminati D, Tidona F, Fornasari ME, Rossetti L, Meucci A, Giraffa G (2014) Biotyping of cultivable lactic acid bacteria isolated from donkey milk. Lett Appl Microbiol 59:299–305
Crotti E, Balloi A, Hamdi C, Sansonno L, Marzorati M, Gonella E et al (2012) Microbial symbionts: a resource for the management of insect-related problems. Microb Biotechnol 5:307–317
Duary RK, Rajput YS, Batish VK, Grover S (2011) Assessing the adhesion of putative indigenous probiotic lactobacilli to human colonic epithelial cells. Indian J Med Res 134:664–671
EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP) (2012) Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J 10:2740. doi:10.2903/j.efsa.2012.2740
Endo A, Salminen S (2013) Honeybees and beehives are rich sources for fructophilic lactic acid bacteria. Syst Appl Microbiol 36:444–448
Endo A, Futagawa-Endo Y, Dicks LM (2009) Isolation and characterization of fructophilic lactic acid bacteria from fructose-rich niches. Syst Appl Microbiol 32:593–600
Engel P, Martinson VG, Moran NA (2012) Functional diversity within the simple gut microbiota of the honey bee. Proc Natl Acad Sci U S A 109:11002–11007
Evans JD, Armstrong TN (2006) Antagonistic interactions between honey bee bacterial symbionts and implications for disease. BMC Ecol 6:4. doi:10.1186/1472-6785-6-4
Evans JD, Lopez DL (2004) Bacterial probiotics induce an immune response in the honey bee (Hymenoptera: Apidae). J Econ Entomol 97:752–756
Forsgren E, Olofsson TC, Vásquez A, Fries I (2010) Novel lactic acid bacteria inhibiting Paenibacillus larvae in honey bee larvae. Apidologie 41:99–108
Giraffa G, Neviani E (2000) Molecular identification and characterization of food-associated lactobacilli. Ital J Food Sci 12:403–423
Gurgulova K, Panchev I, Stanchev P (2003) A study on antibacterial activity of Riphampizyn against bee disease microorganisms. Uludag Bee J 11:40–41
Huey B, Hall J (1989) Hypervariable DNA fingerprinting in Escherichia coli: minisatellite probe from bacteriophage M13. J Bacteriol 171:2528–2532
Kaufmann A, Kaenzig A (2004) Contamination of honey by the herbicide asulam and its antibacterial active metabolite sulfanilamide. Food Addit Contam 21:564–571
Kim SY, Ogawa Y, Adachi Y (2006) Canine intestinal lactic acid bacteria agglutinated with concanavalin A. J Vet Med Sci 68:1351–1354
Klein AM, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C et al (2007) Importance of pollinators in changing landscapes for world crops. Proc Biol Sci 274:303–313
Köhler GA, Assefa S, Reid G (2012) Probiotic interference of Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 with the opportunistic fungal pathogen Candida albicans. Infect Dis Obstet Gynecol 2012:636474. doi:10.1155/2012/636474
Levy SB, Marshall BM (2013) Honeybees and tetracycline resistance. MBio 4:e00045-13. doi:10.1128/mBio.00045-13
Mercenier A, Lenoir-Wijnkoop I, Sanders ME (2008) Physiological and functional properties of probiotics. Bull Int Dairy Fed 429:2–6
Murray KD, Aronstein KA, Eischen F (2009) Promiscuous DNA and terramycin resistance in American foulbrood bacteria. Am Bee J 149:577–581
Neveling DP, Endo A, Dicks LM (2012) Fructophilic Lactobacillus kunkeei and Lactobacillus brevis isolated from fresh flowers, bees and bee-hives. Curr Microbiol 65:507–515
Olofsson TC, Vásquez A (2008) Detection and identification of a novel lactic acid bacterial flora within the honey stomach of the honeybee Apis mellifera. Curr Microbiol 57:356–363
Ortelli D, Edder P, Corvi C (2004) Analysis of chloramphenicol residues in honey by liquid chromatography–tandem mass spectrometry. Chromatographia 59:61–64
Pilatic H (2012) Pesticides and honey bees. State of the Science. Report from the Pesticide Action Network North America
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353. doi:10.1016/j.tree.2010.01.007
Reybroeck W, Daeseleire E, De Brabander HF, Herman L (2012) Antimicrobials in beekeeping. Vet Microbiol 158:1–11
Rolfe RD (2000) The role of probiotic cultures in the control of gastrointestinal health. J Nutr 130:396S–402S
Rossetti L, Giraffa G (2005) Rapid identification of dairy lactic acid bacteria by M13-generated, RAPD-PCR fingerprint databases. J Microbiol Methods 63:135–144
Rossetti L, Carminati D, Zago M, Giraffa G (2009) A qualified presumption of safety approach for the safety assessment of Grana Padano whey starters. Int J Food Microbiol 130:70–73
Salminen S, van Loveren H (2012) Probiotics and prebiotics: health claim substantiation. Microb Ecol Health Dis 23:18568
Smodis Skerl MI, Kmecl V, Gregorc A (2010) Exposure to pesticides at sublethal level and their distribution within a honey bee (Apis mellifera) colony. Bull Environ Contam Toxicol 85:125–128
Suárez V, Zago M, Giraffa G, Reinheimer J, Quiberoni A (2009) Evidence for the presence of restriction/modification systems in Lactobacillus delbrueckii. J Dairy Res 76:433–440
Tian B, Fadhil NH, Powell JE, Kwong WK, Moran NA (2012) Long-term exposure to antibiotics has caused accumulation of resistance determinants in the gut microbiota of honeybees. MBio 3:e00377-12. doi:10.1128/mBio.00377-12
Tirado R, Simon G, Johnston P (2013) Bees in decline. A review of factors that put pollinators and agriculture in Europe at risk. Greenpeace Research Laboratories Technical Report (Review)
Vásquez A, Olofsson TC (2009) The lactic acid bacteria involved in the production of bee pollen and bee bread. J Apicult Res 48:189–195
Vásquez A, Forsgren E, Fries I, Paxton RJ, Flaberg E, Szekely L et al (2012) Symbionts as major modulators of insect health: lactic acid bacteria and honeybees. PLoS One 7:e33188. doi:10.1371/journal.pone.0033188
Vauterin L, Vauterin P (1992) Computer-aided objective comparison of electrophoresis patterns for grouping and identification of microorganisms. Eur Microbiol 1:37–41
Vidal JLM, Aguilera-Luiz MDM, Romero-González R, Frenich AG (2009) Multiclass analysis of antibiotic residues in honey by ultraperformance liquid chromatography-tandem mass spectrometry. J Agric Food Chem 57:1760–1767
Vinderola CG, Reinheimer JA (2003) Lactic acid starter and probiotic bacteria: a comparative “in vitro” study of probiotic characteristics and biological barrier resistance. Food Res Int 36:895–904
Vojvodic S, Rehan SM, Anderson KE (2013) Microbial gut diversity of Africanized and European honey bee larval instars. PLoS One 8:e72106. doi:10.1371/journal.pone.0072106
Wu M, Sugimura Y, Taylor D, Yoshiyama M (2013) Honeybee gastrointestinal bacteria for novel and sustainable disease control strategies. J Dev Sust Agric 8:85–90
Yoshiyama M, Wu M, Sugimura Y, Takaya N, Kimoto-Nira H, Suzuki C (2013) Inhibition of Paenibacillus larvae by lactic acid bacteria isolated from fermented materials. J Invertebr Pathol 112:62–67
Zago M, Fornasari ME, Carminati D, Burns P, Suàrez V, Vinderola G et al (2011) Characterization and probiotic potential of Lactobacillus plantarum strains isolated from cheeses. Food Microbiol 28:1033–1040
Acknowledgments
The author I.J. would like to express his gratitude to the Shota Rustaveli National Science Foundation (SRNSF) for partial support allowing his PhD training in Italy. The author also expresses his gratitude to the Service of Science and Technology of the French Embassy in Tbilisi for the fellowship allowing his PhD training in France.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 71 kb)
Rights and permissions
About this article
Cite this article
Janashia, I., Carminati, D., Rossetti, L. et al. Characterization of fructophilic lactic microbiota of Apis mellifera from the Caucasus Mountains. Ann Microbiol 66, 1387–1395 (2016). https://doi.org/10.1007/s13213-016-1226-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-016-1226-2