Abstract
The purpose of this research was to evaluate the ability of four strains of Saccharomyces cerevisiae endowed with β-glucosidase activity to improve the Trebbiano wine aroma profiles and quality. In particular, the strain effects on fermentation kinetics, sugar and acid concentrations, volatile aroma compound profiles, colour, and catechins parameters were evaluated. The four strains of S. cerevisiae were tested in comparison with a commercial strain. The results showed that the four wild strains had high fermentation rates associated with an efficient conversion of grape sugars to alcohol. In addition, each strain imparted specific features to the wine. In fact, significant differences in relation to the inoculated strain were observed mainly in residual sugar content, succinic and tartaric acid concentrations, and volatile aroma compound profiles as esters, higher alcohols, and terpenes. In particular, the products obtained with S. cerevisiae BV12 and BV14 were characterized by lowest residual sugar contents. Moreover, BV14 and AS15 produced the wines with the highest concentrations of succinic and tartaric acids. Also, the wine obtained with the strains AS11 and AS15 showed high concentrations of monoterpenes and particularly linalool and geraniol having a strong sensorial impact. Furthermore, the wine obtained with the strain AS11 showed a minor decrease in colour intensity, probably due to the highest content of total SO2. These results outlined the importance of the strain selection, tailor made based on the wine feature and the wine making process adopted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The new tendency of the market for white wines is presently directed to full-bodied and aromatic products. To fulfill this goal, technological and or biotechnological tools to enhance extract and flavor of white wines are requested to enological research (Antonelli et al. 2010). The influence of yeast strain on wine composition and quality is well-known. In fact, besides ethanol and CO2, the metabolism of yeasts yields a great number of by-products, for example, glycerol, acetic acid, succinic acid, and lactic acid. Moreover, the aromatic wine properties can be deeply affected by production of higher alcohols and other volatile substances including terpenic volatile compounds (Ugliano and Henschke 2009). The main role of yeast fermentation in the wine aroma formation is recognized particularly for products like Chardonnay, Semillon, Pinot Gris, most of the red varieties, and Trebbiano (Ugliano and Henschke 2009).
Trebbiano is an Italian white wine of widespread consumption in Italy and Europe (Spagna et al. 2000). This white wine is obtained from Trebbiano Romagnolo classified as a neutral variety not dependent upon monoterpenes for its flavor (Mateo and Jimenez 2000). It is well kwon that the grape contributes to wine aroma through free volatile terpenes (i.e. linalool, geraniol, nerol, citronellol, α-terpineol, linalool oxide, etc.) and several odourless glycoconjugate precursors (Maicas and Mateo 2005; Ribereau-Gayon et al. 2006; Vernocchi et al. 2011). These precursors can contribute to the wine aroma profiles after their hydrolysis by naturally occurring grape enzymes or enzymes produced by the microorganisms involved in the process (Sanchez-Torres et al. 1998; Manzanares et al. 2000). Although the greatest β-glucosidase activity is recognized as belonging to the non-Saccharomyces strains involved in wine making, β-glucosidase activity is reported also in some S. cerevisiae strains (Hernandez et al. 2003; Vernocchi et al. 2011). In fact, β-glucosidase activity of some S. cerevisiae strains has been detected during winemaking resulting less sensible to low pH, high sugar and ethanol contents indicating the potential of the starter culture to improve the sensorial profile of wine adding terpenic notes indicating the potential of the starter culture to improve the sensorial profile of wine (Hernandez et al. 2003; Tosi et al. 2009). Vernocchi et al. (2011) studied suitability of four S. cerevisiae strains endowed with in vitro β-glucosidase to improve the Sangiovese wine quality demonstrating that all the strains were able to impart a specific volatile profile increasing the concentration of volatile terpenes. However, the best performances were detected in two of the four.
In this perspective, the main goal of this work was to evaluate the suitability of the same four strains of S. cerevisiae to improve the varietal aroma of Trebbiano obtained from the variety Trebbiano Romagnolo. This grape variety is classified as a neutral variety not dependent upon monoterpenes for its flavor (Mateo and Jimenez 2000). In particular, the effects of the strains on fermentation kinetics, wine sugar and acid concentrations, GC-MS/SPME profiles, and catechin content index were evaluated. The four wild strains of S. cerevisiae were tested in comparison with a commercial S. cerevisiae strain.
Material and methods
Strains
The Saccharomyces cerevisiae strains AS11, AS15, BV12, and BV14, belonging to the collection of Department of Food Science, University of Teramo (Italy), were used. They were previously characterized for their in vitro β-D-glucosidase activity according to Vernocchi et al. (2011). All strains were grown on YPD medium composed of 1 % (w/v) yeast extract, 2 % peptone, and 2 % glucose (all chemicals provided by Oxoid, Milan, Italy). In addition, a commercial strain of S. cerevisiae (“Fermol complet killer”, AEB Group, Brescia, Italy) was used as a control.
Micro-vinification
Grape must of Trebbiano variety of 2008 vintage was used to test the effect of different strains of S.cerevisiae on wine characteristics. The fermentations were carried out in 130-mL Erlenmeyer flasks filled with 100 mL of 0.2 μm filtered Trebbiano must inoculated with the different yeast strains at levels of about 6 Log CFU mL−1. The inoculations were performed using 48-h pre-cultures in the same must. The temperature was kept at 22 °C during alcoholic fermentation. The weight lost, as parameters to follow the fermentation process, was used. After the completion of alcoholic fermentation, the different wine samples were clarified with bentonite (1 g L−1) by stirring them very slightly for about 30 min at room temperature. The wine was then separated by filtration. Three samples for each condition tested were prepared.
Microbiological analysis
Viable cell counts were evaluated at sampling time by plate counting using YPD agar (Oxoid) incubated at 28 °C for 48 h. Three repetitions for each sample were considered.
Chemical-physical analysis
Sugars (glucose and fructose) and organic acids (tartaric, malic, succinic, citric, lactic, and acetic acids) were detected by HPLC using a Jasco HPLC system with a pump (PU980) (Jasco Inc, Tokyo, Japan) equipped with a variable wavelength detector (UV970) or a refractive index detector (RI830) for the determination of organic acids and sugars, respectively (Vernocchi et al. 2011). Furthermore, SAX cartridges were used in order to eliminate organic acids from grape juices and wines, according to Castellari et al. (2000). Peak identification and quantification were based on retention times (Rt) and spiking technique, and relied on the external standard method. Three repetitions for each condition were analysed.
Determination of ethanol, percent decrease of sugars, volatile acidity, and pH were performed on the wines according to the official EU methods (EC 2000). SO2 was determined using iodine by colorimetric titration according to Lowinsohn and Bertotti (2001).
Evaluation of colour intensity and total catechins
To evaluate colour intensity, three repetitions for each sample were used according to the spectrophotometric methods such as absorbance of a wine directly measured at 420, 520, and 620 nm as proposed by Glories (1984).
The concentration of total catechins was determined using the p-(dimethylamino) cinamaldehyde (p-DMACA) method. Briefly, 1 mL of an appropriately diluted sample was added to a 10 mL volumetric flask followed by the addition of three drops of glycerol and 5 mL p-DMACA reagent. The total volume was made up to 10 mL with methanol and after 7 min, the absorbance was read at 640 nm against a methanol blank. The DMACA reagent was prepared immediately before use, and contained 1 % (w/v) DMACA in a cold mixture of methanol and HCl (4:1) as proposed by Ivanova et al. (2010).
GC–SPME analysis and mass spectrometry
In this study a polyacrylate coated fiber (85 μm) and a manual SPME holder (Supelco Inc., Bellefonte, PA, USA) were used after a preconditioning according to the manufacturer’s instruction manual. Before each head space sampling, the fiber was exposed to the GC inlet for 5 min for thermal desorption at 250 °C in a blank sample. Five-milliliter wine samples were placed in 10-mL glass vials, to which 1 g NaCl and 10 μL of 4-methyl-2-pentanol were added (final concentration 4 mg L−1) as internal standard. The samples were then equilibrated for 10 min at 45 °C. The SPME fiber was exposed to each sample for 40 min. Both the equilibration and absorption phases were carried out under stirring condition. The fiber was then inserted into the injection port of the GC for a 5 min sample desorption. The instrumental conditions were performend according to Vernocchi et al. (2011).
Molecule identification was based on comparison of their retention times with those of pure compounds (Sigma–Aldrich, Milan, Italy) analyzed in the same conditions. The identification was further confirmed by comparing mass spectra of compounds with those contained in the available database (NIST version 2005) and those of pure standards. The concentration of volatile compounds, without pure references, was obtained by using the same calibration graphs as one of the compounds with the most similar chemical structure (Li et al. 2008). Quantitative data of the identified compounds were obtained by interpolation of the relative areas versus the internal standard area, in calibration curves built with pure reference compounds.
Statistical analysis of data
The data reported are the means of three repetitions. The data were analyzed by Student’s t test independent for groups using the statistical package Statistica for Windows 8.0 (Statsoft Inc. Tulsa, OK, USA). Evaluations were based on significance level P ≤ 0.05.
Results
In order to evaluate the effects of yeast strain on the physico-chemical wine characteristics, Trebbiano musts were inoculated with about 6 Log CFU mL−1 of the four wild yeasts and with the commercial strain. During fermentation, the inoculated samples were maintained at 22 °C and periodically analyzed to assess yeast growth and fermentation kinetics.
At the end of fermentation, ethanol, sugars, organic acids, volatile molecule profiles, colour parameters, and catechin concentrations were measured.
Course of fermentation and yeast cell load evolution
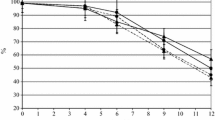
The fermentation kinetics were evaluated measuring the percent decrease of sugars, and, as shown by Fig. 1, significant differences (P > 0.05) were not observed for the considered strains. Also, the total yeast cell loads, recorded during the incubation at 22 °C (Fig. 2), supported the fermentation behaviors of the different samples. After 24 h of incubation at 22 °C, all samples inoculated with S.cerevisiae strains gave rise to cell loads higher than 7 Log CFU mL−1 without significant differences (P > 0.05). The cell loads of all the considered strains began to decrease after 36 h of incubation reaching levels ranging between 4 and 5 Log CFU mL−1. Significant differences (P < 0.05) in the death kinetics were detected for BV12 (4 Log CFU mL−1) and BV14 (5 Log CFU mL−1).
Wine analytical profile
Table 1 reports the concentration of glucose, fructose, acetic, succinic, citric, malic, and tartaric acids of must before alcoholic fermentation and wines obtained with the different strains. In addition, it includes the ethanol concentration and the wine pH at the end of fermentation in relation to the used strain.
The concentrations of glucose and fructose detected at the end of fermentation showed that the residual sugar content did not exceed 2.8 g L−1, independently of the employed strain. However, the strain B14, followed by the strains BV12, gave rise to the wine with the lowest glucose and fructose concentrations (P < 0.05) with respect to the other strains. No significant differences in residual sugar concentrations were detected among the strains AS11, AS14, and the commercial one (P > 0.05). As expected, fructose was always the main residual sugar and different ratios of glucose and fructose were observed in relation to the starter employed. No significant differences (P > 0.05) in ethanol, citric, and malic acid concentrations were observed. On the contrary, significant differences (P < 0.05) were observed for succinic and tartaric concentrations. In fact, the strains BV14 and AS15 produced the wines with the highest concentrations of the two acids. AS 15 produced also the wine with the highest content of total SO2 (34 mg L−1), while no difference in total SO2 content were observed in the other wines (26 ± 2 mg L−1). No significant differences (P > 0.05) in pH values were observed in relation to the strain used.
The highest levels (0.013 g L−1) of acetic acid recovered in this study were detected in the samples obtained with the commercial starter. On the contrary, BV12 (0.002 g L−1) and AS 11 (0.003 g L−1) strains gave rise to wine having the lower acetic acid levels (P < 0.05). The strains AS15 and BV14 produced wines with acetic acid concentration of 0.004 and 0.005 g L−1, respectively.
Colour intensity and catechin content
Table 2 shows the colour intensity and the catechin contents of the wines in relation to the used yeast strain. The data clearly indicated the stability of catechins, independently of strain (P > 0.05) and the suitability of the strains AS15 to improve the wine colour. In fact, the catechin contents remained quite stable in the wines obtained with the strains BV12 and BV14, while it weakly increased in those obtained with the remaining strains (P < 0.05). The increase observed can be attributed to the low sensitiveness of the method used and to the release of catechins from dispersed solids due to the incomplete clarification of wine.
GC–MS–SPME analysis and mass spectrometry
The gas-chromatographic method used allowed the identification of 30 molecules belonging to different chemical classes such as aldehydes, lactones, higher alcohols, esters, short chain fatty acids, and terpenes. The concentrations of the main molecules indicated the marked effects of strains on volatile molecule profiles of wines (Table 3). More specifically, the wine obtained with the inoculum of the commercial strain had highest concentrations of acetic acid, acethaldeyde, and esters (mainly ethyl acetate) (P < 0.05). In contrast, the wines obtained with BV12 and BV14 had high concentrations of higher alcohols associated to low levels of ethyl acetate. These strains gave rise also to wines with interesting concentrations of molecules such α-terpineol (P < 0.05) having a great sensorial impact. Moreover, AS11 and AS15 produce the highest levels of isoamylic and phenyl ethyl alcohol (P < 0.05) and interesting concentrations of volatile terpenes. In particular, the strain AS15 produced the wine with the highest content (P < 0.05) of linalool (1.62 mg L−1) and geraniol (0.38 mg L−1).
Discussion
The results of the present work showed that the yeast strain used influenced and drove wine features.
All the wild strains showed high fermentation and growth rates. These were absolutely comparable with those of the commercial strain generally employed for Trebbiano wine production. Moreover, they were able to convert grape sugars to alcohol efficiently, fitting the primary selection criteria of yeast strain selection (Henschke 1997; Ubeda Iranzo et al. 1998). In our experimental conditions, the inoculation with S. cerevisiae BV12 and BV14 resulted in wine characterized by lowest residual sugar contents. However, the other strains also left low concentrations of unfermented sugar, but with different ratios of fructose and glucose. The preference of S. cerevisiae for glucose and the wide variability in fructose utilization among S. cerecisiae strains are well documented (Berthels et al. 2004). However, the highest concentration of fructose was detected in the wine obtained with the commercial S. cerevisiae strain. The stains BV14 and AS15 produced the wines with the highest concentrations of succinic and tartaric acids. On the other hand, the strain dependence of succinic acid production is widely recognized. Moreover, the ability of S. cerevisiae strain to influence the tartaric stability throughout the release on mannoproteins during fermentation has been recognized (Rosi et al. 2000). The entity of the mannoprotein release, although influenced by physico-chemical conditions, is strain dependent (Rosi et al. 2000; Escot et al. 2001). In addition, each strain imparted specific features in volatile molecule profiles to the wine obtained.
In our experimental conditions, the inoculation with S. cerevisiae BV12 and BV14 resulted in wines characterized by lowest volatile acidity and the highest level of α-terpineol and higher alcohols without detrimental effects on colour intensity and catechin content. On the other hand, these strains proved to be suitable to improve the quality of Sangiovese wine (Vernocchi et al. 2011). Also, the wines obtained with the strains AS11 and AS15 showed high concentrations of monoterpenes and particularly of linalool and geraniol having a strong sensorial impact. Moreover, the wine obtained with the strain AS11 showed a minor decrease in color intensity, probably due to the high content of total SO2.
The colour instability and browning is a serious problem during white wine storage, shortening its commercial life. A comprehensive research proved that the choice of a yeast strain is an important factor to maintain the stability of wine colour since these microorganisms have different capacity to retain or adsorb phenolic compounds depending on the outer cell structure composition (Morata et al. 2003). Moreover, yeasts are reported to contribute to wine colour stabilization, as result of participating in the formation of vitisins during fermentation or liberating mannoproteins that have the capacity to bind to anthocyanins and tannins, protecting them from precipitation (Bautista-Ortin et al. 2007). Yeast can affect the colour of white wine, also competing with phenols for oxygen dissolved in the wine (Salomon et al. 2002). The strains AS11 showed great potentiality to improve Trebbiano wine colour. On the contrary, this strain, analogously to AS15, decreased colour intensity of Sangiovese wine (Vernocchi et al. 2011) probably due to its ability to adsorb anthocyanins or to an anthocyanin-β-glucosidase. Both mechanisms to decrease color parameters of red wine are documented in S. cerevisiae strains (Rodrıguez et al. 2004; Caridi 2007; Todaro et al. 2008; Tosi et al. 2009). These results outlined the importance of a strain selection tailor made on the wine feature and the wine making process adopted.
In conclusion, the data obtained showed that some S. cerevisiae wild strains endowed with high in vitro β-glucosidase activity can increase the volatile terpene concentrations and the colour stability of Trebbiano wine without detrimental effects on fermentation rates and conversion of grape sugars to alcohol.
References
Antonelli A, Arfelli G, Masino F, Sartini E (2010) Comparison of traditional and reductive winemaking: influence on some fixed components and sensorial characteristics. Eur Food Res Technol 231:85–91
Bautista-Ortin AB, Romero-Cascales I, Fernández-Fernández JI, López-Roca JM, Gómez-Plaza E (2007) Influence of the yeast strain on Monastrell wine colour. Innov Food Sci Emerg Technol 8:322–328
Berthels NJ, Cordero Otero RR, Bauer FF, Thevelein JM, Pretorius IS (2004) Discrepancy in glucose and fructose utilization during fermentation by Saccharomyces cerevisiae wine yeast strains. FEMS Yeast Res 4:683–689
Caridi A (2007) New perspectives in safety and quality enhancement of wine through selection of yeasts based on the parietal adsorption activity. Int J Food Microbiol 120:167–172
Castellari M, Versari A, Spinabelli U, Galassi S, Amati A (2000) An improved HPLC method for the analysis of organic acids, carbohydrates and alcohols in grape musts and wines. J Liq Chromatogr Related Technol 23:2047–2056
EC (2000) Community reference methods for the analysis of spirits drinks. Official Journal of the European Community (Reg EC No 2870/00. 19 Dec, Brussels).
Escot S, Feuillat M, Dulau L, Charpentier C (2001) Release of polysaccharides by yeasts and the influence of released polysaccharides on color stability and wine astringency. Aust J Grape Wine R 7:153–159
Glories Y (1984) La coleur des vins rouges: 11 partie. Les equilibres des anthocyanes et des tanins. Connaissance de la Vigne et du Vin 18:195–217
Henschke PA (1997) Yeast sugar metabolism: biochemistry, genetics, biotechnology and applications. In: Zimmermann FK, Entian KD (eds) Wine yeast. Technomic, Lancaster, pp 527–560
Hernandez LF, Espinosa JC, Fernandez-Gonzalez M, Briones A (2003) ß-Glucosidase activity in a Saccharomyces cerevisiae wine strain. Int J Food Microbiol 80:171–176
Ivanova V, Stefova M, Chinnici F (2010) Determination of the polyphenol contents in macedonian grapes and wines by standardize spectrophotometric methods. J Serbian Chem Soc 75:45–59
Li H, Tao YS, Wang H, Li Z (2008) Impact odorants of Chardonnay dry white wine from Changli County (China). Eur Food Res Technol 227:287–292
Lowinsohn D, Bertotti M (2001) Determination of sulphite in wine by coulometric titration. Food Addit Contam 18:773–777
Maicas S, Mateo JJ (2005) Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: a review. Appl Microb Biotechnol 67:322–335
Manzanares P, Orejas M, Ibanez E, Valles S, Ramon D (2000) Purification and characterization of an L-rhamnosidase from Aspergillus nidulans. Lett Appl Microbiol 31:198–202
Mateo JJ, Jimenez M (2000) Monoterpenes in grape juice and wines: review. J Chromatog A 881:557–567
Morata A, Gómez-Cordovés MC, Suberviola L, Bartolomé B, Colomo B, Suárez JA (2003) Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J Agr Food Chem 51:4084–4088
Ribereau-Gayon P, Glories Y, Maujean A, Dubourdieu D (2006) The chemistry of wine stabilization and treatments. In: handbook of enology, vol 2, 2nd eds. Wiley, Chichester, pp 205–227
Rodrıguez ME, Loopes CA, Van Broock M, Vallees S, Ramon D, Caballero AC (2004) Screening and typing of patagonian wine yeasts for glycosidase activities. J Appl Microbiol 96:89–95
Rosi I, Gheri A, Domizio P, Fia G (2000) Production de macromolecules pariétales de Saccharomyces cerevisiae au cours de la fermentation et leur influence sur la fermentation malolactique. Revue des Oenologues 94:18–20
Salomon JM, Fornairon-Bonnefond C, Mazauric JP (2002) Interactions between wine lees and polyphenols: influence on oxygen consumption capacity during simulation of wine aging. J Food Sci 67:1604–1609
Sanchez-Torres P, Gonzalez-Candelas L, Ramon D (1998) Heterologous expression of a Candida molischiana anthocyanin-bglucosidase in a wine yeast strain. J Agr Food Chem 46:354–360
Spagna G, Barbagallo RN, Pifferi PG (2000) Fining treatments of white wines by means of polymeric adjuvants for their stabilization against browning. J Agric Food Chem 48:4619–4627
Todaro A, Palmeri R, Barbagallo RN, Pifferi PG, Spagna G (2008) Increase of trans-resveratrol in typical Sicilian wine using β-glucosidase from various sources. Food Chem 107:1570–1575
Tosi E, Azzolini M, Guzzo F, Zapparoli G (2009) Evidence of different fermentation behaviours of two indigenous strains of Saccharomyces cerevisiae and Saccharomyces uvarum isolated from Amarone wine. J Appl Microbiol 107:210–221
Ubeda Iranzo JF, Briones Perez AI, Izquierdo Canas PM (1998) Study of the oenological characteristics and enzymatic activities of wine yeasts. Food Microbiol 15:399–406
Ugliano M, Henschke PA (2009) Yeast and wine flavour. In: Moreno-Arribas MV, Polo C (eds) Wine chemistry and biochemistry. Springer, Chapter 8D, New York, pp 313–392
Vernocchi P, Ndagijmana M, Serrazanetti DI, Chaves Lopez C, Fabiani A, Gardini F, Guerzoni ME, Lanciotti R (2011) Use of Saccharomyces cerevisiae strains endowed with β-glucosidase activity for the production of Sangiovese wine. World J Microb Biot 27:1423–1433
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vernocchi, P., Patrignani, F., Ndagijimana, M. et al. Trebbiano wine produced by using Saccharomyces cerevisiae strains endowed with β-glucosidase activity. Ann Microbiol 65, 1565–1571 (2015). https://doi.org/10.1007/s13213-014-0995-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13213-014-0995-8