Abstract
Polyethyleneimine (PEI) glutaraldehyde-refined calcium pectinate (CaP)-agar beads were presented as improved covalent immobilization matrices. The CaP-agar beads exhibited incremented mechanical stability which facilitated their handling. The beads’ concoction and activation processes were honed using the Box–Behnken design which recommended utilizing 5.4% agar, and a 2.95% PEI solution of pH 8.67. The honed CaP-agar beads established a more efficient ionic interaction with PEI which enabled the immobilization of more enzyme while utilizing less PEI than that required to activate the neat CaP beads. Furthermore, the activated CaP-agar beads granted superior operational stability to the immobilized enzyme, β-d-galactosidase (βgal), where it preserved 86.84 ± 0.37% of its precursive activity during its thirteenth reusability round. The CaP-agar immobilized βgal (iβgal) also showed incremented storage stability where it preserved 85.05 ± 3.32% of its precursive activity after 38 days of storage. The thermal stability of the iβgal was shown to be superior to that of the free enzyme as the iβgal exhibited incremented thermodynamic parameters, such as the t1/2 values, the D values, the thermal denaturation activation energy, the enthalpies, and the Gibb’s free energies. The βgal’s immobilization onto the activated CaP-agar beads also shifted the enzyme’s optimal pH from 4.6–5.1 to 3.3–4.9, whereas its optimal temperature was retained at 55 °C. The procured biocatalyst was exploited to efficiently hydrolyze the lactose in whey permeate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pectin is a cost-effective, safe, abundant, and renewable biomaterial that constitutes a propitious candidate for variable applications, such as tissue regeneration, drug delivery, cell entrapment, and enzymes’ entrapment (Contesini et al. 2012; Munarin et al. 2014; Sriamornsak et al. 2005; Wu and Yu 2007). It was also efficiently exploited as a matrix for enzymes’ covalent immobilization (Wahba 2016). Pectin is principally constituted from 1–4 linked α-d-galacturonic acid entities (Sriamornsak et al. 2005). Around 93.3% of these galacturonic acid entities exist as free carboxylic acids in the low-methoxy pectin utilized herein (according to manufacturer data). The presence of such a copious amount of the anionic galacturonic acid entities (> 50%) enables the cross-linking amongst low-methoxy pectin and Ca2+ cations which leads to the concoction of calcium pectinate (CaP) beads (Sriamornsak et al. 2005). The anionic galacturonic acid entities also enabled the activation of the CaP beads and their exploitation as covalent immobilization matrices. Initially, these anionic entities cross-linked the cationic polyethyleneimine’s (PEI) amino entities, and this led to the establishment of a PEI casing around the CaP beads. The PEI casing mechanically stabilized the CaP beads (Wahba 2016) and surmounted their native meager mechanical stability (Mollaei et al. 2010; Wu and Yu 2007). Moreover, the nucleophilic amino entities in such a PEI casing reacted with glutaraldehyde (GA). This reaction further incremented the mechanical stability of the CaP beads and also provided them with the functional residues necessary to covalently link enzymes. The procured GA-PEI processed CaP beads were efficacious carriers that granted incremented thermal and operational stabilities to their immobilized enzyme (Wahba 2016). Nevertheless, the preparation process of the activated CaP beads suffers from some hurdles that should be surmounted to be capable of up-scaling it. The meager mechanical stability of the neat CaP beads (Mollaei et al. 2010; Wahba 2016; Wu and Yu 2007) could cause the smashing and the loss of a substantial proportion of the beads, during their handling, prior to the mechanical-stabilizing PEI processing step. Moreover, the interaction amongst the neat CaP beads and the stabilizing PEI is limited. This interaction is an ionic interaction amongst the anionic entities of CaP and the cationic amino entities of PEI (Wahba 2016). However, cationic entities can displace the Ca2+ from within the CaP beads and disrupt their gelling egg-box construction (Sriamornsak et al. 2005). Thus, critical restrains were put on the PEI pH, and it was raised to the value of 10.55 so as to provide only minute amounts of cationic amino entities and preserve the integrity of the CaP beads (Barkovec and Koper 1997; Wahba 2016). This prohibited other possible PEI–CaP ionic interactions although these other interactions could have been superior to the interaction with the 10.55 pH PEI. The PEI chains were debated to establish flexible beds around the matrices, and these flexible beds could be penetrated by the enzymes (Torres et al. 2006) to be immobilized where these enzymes would be adsorbed to the charged entities (Pessela et al. 2003) of PEI. Thus, the enzymes would be adsorbed by these flexible PEI beds and would also be covalently linked via GA. It should be noted that if the ionic interaction amongst PEI and the carrier was altered, the 3-D orientation of the flexible PEI beds would definitely be altered. Consequently, this would alter the bead interaction with the target enzyme and could even alter the amount of the immobilized activity. On one occasion, the altered PEI orientation was debated to escalate the immobilized activity attained by the carrageenan-agar disks relative to that attained by the agar disks (Wahba and Hassan 2017).
To surmount the aforesaid hurdles, the CaP was blended with agar. Agar is known for its incremented mechanical strength (Makwana et al. 2020); thus, the concocted CaP-agar beads would exhibit sufficient mechanical stability to enable their handling prior to the PEI mechanical-stabilizing step. Moreover, agar gels upon cooling following a coil to helix transformation amongst its chains (Delattre et al. 2011). Hence, its gel construction would not be disrupted by the PEI’s cationic entities, and the integrity of the CaP-agar beads wouldn’t be disrupted if the PEI pH was lowered, and its cationic density was incremented. Accordingly, the CaP-agar beads would be capable of establishing a carrier–PEI ionic interaction different from that recommended for the CaP beads. The chemical construction of agar would also contribute to such a different ionic interaction as agar contains anionic entities (Delattre et al. 2011) capable of ionically cross-linking PEI. It should also be noted that agar is an approved food adjunct that is naturally procured, and it is also cost effective (Delattre et al. 2011; Fine-Chem Limited 2020).
Environmental issues are linked to dairy industries owing to their chief byproduct, whey, whose lactose content was reported, by various studies, to be chiefly responsible for its incremented biochemical oxygen demand (Geiger et al. 2016). Accordingly, lactose should be eliminated from whey earlier to its discarding to circumvent environmental pollution. β-d-Galactosidase (βgal) could eliminate lactose via hydrolyzing it. Furthermore, the βgal-mediated lactose hydrolysis would valorize whey, and thereby, encourage its exploitation rather than its discarding. The βgal-mediated lactose hydrolysis increments the whey’s sweetness; hence, it could be exploited as sweet syrup in food industries, such as soft drink and baking industries (Panesar et al. 2010).
In the current study, we concocted and activated CaP-agar beads to provide a more proficient immobilization carrier that would surmount the drawbacks of the activated CaP beads. This carrier was then exploited to immobilize βgal and hydrolyze the problematic whey permeate’s lactose.
Materials and methods
Materials
Aspergillus oryzae βgal, PEI (750 kDa), citrus peel pectin, and GA were attained from Sigma-Aldrich (Germany). Agar was supplied from S.D. Fine chemicals (Mumbai).
Methods
CaP-agar beads’ concoction and processing
Agar was dissolved via stirring in a boiling water bath. Afterwards, the heat was turned off, and pectin was added (3%, w/v final concentration). The stirring was carried on until all the pectin dissolved. The blend was then placed in the fridge for at least 18 h. To concoct the CaP-agar beads, the blend was liquefied via placing it in a ~ 90 °C water bath, and it was dripped onto 0.2 M CaCl2. The CaP-agar beads were rigorously washed with distilled water prior to their immersion in the respective PEI solutions (Table 1) for 2 h. The beads were then washed and immersed into 5% (v/v) GA solution for 1.5 h. Finally, the beads were rigorously washed and stored in distilled water in the fridge. It should be noted that the ratio amongst the beads and the processing solutions (PEI or GA) was not lower than 1:5. Neat CaP beads [− 1 level of agar concentration (Table 1)] were similarly processed; however, no heat was utilized during their preparation.
Enzyme’s loading
One gram of the processed CaP-agar beads was placed in a glass vial containing a βgal solution (~ 18.5U). The vial was rotated overnight on a roller stirrer. Finally, the beads were rigorously washed and their βgal activity was assessed.
βgal activity assessment
The free βgal or the βgal loaded CaP-agar beads (0.06 g) were dissolved or suspended in 0.5 ml pH 4.6 citrate–phosphate buffer, respectively. A 3.5 ml volume of a 200 mM lactose solution, which was dissolved in 0.1 M citrate–phosphate buffer of pH 4.6, was then added to the enzymes, and the reaction was left to proceed in 37 °C thermo-stated shaking water bath for 15 min. An aliquot of the reaction mixture was then withdrawn, and it was subjected to ~ 10 min heating process in a boiling water bath. Afterwards, it was cooled and its glucose content was assessed via commercial glucose kits. One unit of βgal (U) activity was recorded upon procuring 1 μmol glucose per minute under the aforesaid assay conditions. The immobilized βgal’s (iβgal’s) activity was presented herein as U/g beads.
Mechanical stability assessment
This was performed as per Wahba (2016). Initially, an accurately weighed 0.5 g of the beads sample (the CaP-agar or the GA-PEI-CaP-agar beads) was put in a 5 ml glass vial which contained 1 g of tiny glass beads (diameter 1.05 mm) and 1 ml distilled water. The vial was then vortexed for designated intervals to break-down and fragment the beads. Afterwards, the vial’s contents were sieved through a standard sieve (No. 16, aperture 1.18 mm) so as to wash away any diminutive beads’ fragments. Finally, the beads retained by the sieve were weighed and this weight was expressed as a percent from the precursive 0.5 g beads’ weight.
Shrinkage assessment
The CaP-agar beads were accurately weighed before their immersion in the optimal PEI solution, and this was denoted 100%. After being immersed in the PEI solution for 2 h, the beads were washed and weighed again. This weight was expressed relative to the 100% precursive weight.
Estimation of the Km and the Vmax
The values of the Michaelis–Menten constant (Km) and the uttermost reaction rate (Vmax) were procured from the straight line equations in the Hanes–Woolf plot. This plot was constructed following enzymatic assays that were accomplished as in “βgal activity assessment” section. However, the concentrations of the utilized lactose solutions were varied among a 50–200 mM range so that their final concentrations were 43.75–175 mM.
pH profile
The regular activity assay was accomplished (“βgal activity assessment” section); nevertheless, the pH of the utilized buffer was altered amongst a 2.7–7.5 range. The uttermost activity was denoted as the 100% and all the other activities were presented relative to it.
Temperature profile and assessment of activation energy
The regular activity assay was accomplished (“βgal activity assessment” section); nonetheless, the temperature was varied within a 30–70 °C range, and the uttermost activity was denoted as the 100%. Additionally, the assay duration was extended (up to 1 h) at the temperatures 30–55 °C to estimate the lactose hydrolysis rates at such temperatures. ln (lactose hydrolysis rate) was then plotted vs 1/temperature (in Kelvin scale), and the Arrhenius plot was constructed (Granjeiro et al. 2004). The Arrhenius plot’s slope amounts to −Ea/R where R is the universal gas constant (8.314 JK−1 mol−1) and Ea is the enzyme’s activation energy.
Thermal stability and assessment of thermodynamic parameters
The thermal stabilities of both the free and the activated CaP-agar iβgals were assessed via incubating the enzymes at varying temperatures (40–70 °C). At designated intervals the enzymes were withdrawn from these temperatures and were allowed to air cool for 30 s. Directly afterwards, the enzymes’ activities were assessed via the regular assay (“βgal activity assessment” section). These activities were presented as percents of the precursive enzyme activity that was estimated prior to the thermal incubation. The thermodynamic parameters were also estimated from the thermal stability profiles acquired at the temperatures 60–70 °C (Abdel-Wahab et al. 2018). Initially, plots of log (residual activity percents) vs time were created. The first order thermal-denaturation rate constants (kd) were then estimated from the slopes in these plots. Such kd values permitted the calculation of the t1/2 (enzymes’ half-lives) and the D values (decimal reduction times which correspond to the incubation periods that would elicit 90% loss of the enzyme’s precursive activity) at each given temperature as follows:
A plot of the ln kd values vs 1/temperature (in Kelvin scale) was also created. The slopes in this plot corresponded to –Ed/R where Ed is the activation energy for the βgal’s thermal denaturation. The variations in enthalpy (ΔH), Gibb’s free energy (ΔG) and entropy (ΔS) amongst the structures of the active enzymes and the thermally denatured ones were also acquired as follows:
T was the temperature in K, R was the universal gas constant (8.314 J mol−1 K−1), h was the Planck constant (11.04 × 10–36 J min), and kB was the Boltzman constant (1.38 × 10−23 JK−1).
Operational stability
In this experiment, the selfsame βgal loaded CaP-agar beads were repeatedly assayed (“βgal activity assessment” section) for 13 times. Six of these assays were performed on day 1, and the remaining seven assays were performed on day 2. In between the assays, the beads were washed thrice with the assay buffer.
Storage stability
An amount of the βgal loaded CaP-agar beads was dedicated for this experiment. Some of these beads were assayed, directly after being loaded with the enzyme, and their activity was designated as 100%. The remaining beads were stored in distilled water in the fridge. At designated intervals, a portion of the beads was assayed and the activity was calculated relative to the precursive 100% activity.
Hydrolyzing the lactose in whey permeate
The pH of the whey permeate (WP) was adjusted to 4.9 via dilute HCl. Afterwards 6 ml of the WP were added to 0.58U of the CaP-agar iβgal, and they were incubated in a thermo-stated shaking water bath at 55 °C. At designated intervals, aliquots were withdrawn to assess their glucose contents. This experiment was allowed to proceed for 12 h, divided on two separate days. At the end of day one, the beads were separated from the WP and were washed with 0.1 M citrate–phosphate buffer (pH 4.6) prior to storing them in the fridge. On the other hand, the WP was stored in the freezer.
It should be noted that the BBD’s data were analyzed via the trial version of the Design Expert statistical software. As for any other data, Microsoft Excel was exploited to perform one-way ANOVA and assess the existence of significant differences amongst the experimental results. The assays conducted herein were performed in triplicates and their data was presented as mean ± SE.
Results and discussion
Box–Behnken design (BBD)
A quadratic model was utilized to fit the results of the BBD (Table 1), and the model’s equation was given as Eq. 6. The validity of the proposed model was evinced from its 0.0074 p value and its 0.9054 R2 (Table 2). As regards to the terms comprised within the model, the most significant term was the interaction among the agar concentration (A) and the PEI pH (C) where its p value amounted to 0.0058. The significance of the AC interaction could be elucidated from Fig. 1a, which predicted that incrementing the PEI pH from 8 (− 1 level) to 8.6 and finally to 10 (+ 1 level) would elicit big increments in the attainable iβgal’s activity from 5.55 to 6.65U/g, and finally to 7.60U/g, respectively, if no agar was added. On the other hand, if 5.4% agar was incorporated into the CaP beads, incrementing the PEI pH, in a similar manner, from 8 to 8.6 would elicit a slight increment in the attainable iβgal’s activity from 7.77 to 7.98U/g whereas further incrementing the PEI pH to 10 would reduce the attainable iβgal’s activity to 7.17U/g. The occurrence of such different alterations in the iβgal’s activity in response to the same alterations in the PEI pH could be attributed to the agar; its presence or absence. If no agar was added, the CaP beads would require lesser amounts of the cationic PEI’s amino groups to retain their integrity. The PEI’s cationic amino groups were previously debated to compete with and replace the Ca2+ in the CaP beads. Such replacements led to the concoction of collapsed beads whose surface groups were heavily distorted and incapable of efficiently binding to enzymes (Sriamornsak et al. 2005; Wahba 2016). In order for the hyper-branched PEI to provide only minute amounts of cationic amino groups, its pH should be incremented (Barkovec and Koper 1997). Hence, the CaP beads favored the incremented PEI pH and their highest iβgal’s activity would be attained at the highest utilized pH 10. On the other hand, when agar was blended into the CaP beads, it preserved the beads integrity. Such integrity wasn’t disrupted even when the CaP-agar beads were exposed to the loftiest amount of the PEI’s cationic amino-groups at the lowest pH 8 [~ 40% of the PEI’s amino groups would be protonated (Barkovec and Koper 1997)], and this was evident from the superior functionality of the 6%CaP-agar beads (run 15, Table 1) which attained a whole of 7.78U/g iβgal. Thus, it could be concluded that the agar eliminated the effect of the PEI pH on the beads’ integrity. This diminished the overall effect of the PEI pH to the extent that only slight variations (7.17–7.98U/g) were predicted to be attained in the activity of the βgal immobilized by the 5.4% CaP-agar beads upon altering the PEI pH over its entire tested range (Fig. 1a). Such slight induced variations caused the PEI pH to become the only insignificant factor in this study (p value = 0.0660) although it was the most significant factor when the neat CaP beads were investigated (Wahba 2016).
Contour plots exploring the effects of A: agar concentration; B: PEI concentration; C: PEI pH on the iβgal’s activity. The color gradient from blue to red reflected the increase in the attainable iβgal’s activity. The textboxes unveiled the predicted iβgal’s activities that would be attained at the specified factors’ settings
Both the agar concentration and the PEI concentration were significant as they attained 0.0138 and 0.0429 p values, respectively (Table 2). The interaction among the agar concentration (A) and the PEI concentration (B) was also significant (p value = 0.0385). Such a significant interaction could be elucidated from Fig. 1b which explored the effects of altering both the agar and the PEI concentrations while retaining the PEI pH constant at the central value of 9. Figure 1b predicted that incrementing the PEI concentration from 2% (− level) to 2.95% would elicit a 1.25-fold increment in the attainable iβgal’s activity if no agar was utilized, whereas only a 1.08-fold increment would be attained if 5.4% agar was incorporated into the CaP beads. Incrementing the concentration of the PEI would increment the amount of the functional groups that could react with GA, and this would eventually increment the quantity of iβgal. Moreover, the entanglement among the PEI’s chains would be incremented upon incrementing the concentration of this hyper-branched compound (Awad et al. 2020). Such entanglements could shield some of the PEI’s cationic charges. This would lessen the amount of the cationic charges that the neat CaP beads would be exposed to, and would help preserve the beads’ integrity and boost their functionality (Wahba 2016). Thus, it could be concluded that incrementing the PEI concentration would help increment the amount of iβgal by the CaP beads via the two aforementioned postulates. However, in case of the CaP-agar beads, the second postulate wouldn’t be applicable since the agar served to preserve the integrity of the beads against the presence of excessive cationic groups. Thus, incrementing the PEI concentration would boost the functionality of the CaP-agar beads via only one mechanism, and this would lead to the attainment of lower increments in the amount of iβgal as compared to those attained by the CaP beads.
Optimization of the CaP-agar beads’ processing
The statistical software recommended formulating 5.4% CaP-agar beads and processing such beads with a 2.95% PEI of pH 8.67 to attain an optimal amount of 7.98U/g iβgal. Upon implementing these recommendations 7.96 ± 0.18 U/g iβgal was recorded. This amount of iβgal represented a 43.07% activity recovery percent as each gram of the beads was loaded with 18.48 ± 0.05 U/g βgal. Such an activity recovery percent was loftier than the 38.09% activity recovered by the processed CaP beads (Wahba 2016). Moreover, it was much superior to the 27.71% activity recovered by the processed carrageenan-agar disks (Wahba and Hassan 2017).
It could be noticed that the recommended agar concentration (5.4%) was close to its highest inspected level (+ 1 level, 6%). The higher agar concentrations provide more compact structures with smaller pores (Mitchell and Wimpenny 1997; Wahba and Hassan 2017). These compact structures would be favored in our case as they would confer enough physical stability to the CaP-agar beads so that these beads would not collapse or shrink upon being subjected to the PEI’s cationic entities which could break down the CaP structure after replacing its gelling Ca2+ ions (Wahba 2016). On the other hand, when agar gels were employed as an entrapment matrix for myrosinase, 3.5% was the optimal agar concentration (Rai et al. 2013). Higher agar concentrations could have been disfavored owing to their smaller pores (Mitchell and Wimpenny 1997) which would hinder the efficient diffusion of the substrates and products through the entrapment matrices.
The optimal PEI pH served to provide a competent ionic interaction amongst the PEI’s cationic entities and the anionic entities of agar and CaP without any restrains concerning the beads’ integrity as such integrity was preserved by the presence of agar. To verify that the beads’ integrity was not compromised by the pH 8.67 optimal PEI solution, a shrinkage study was accomplished where the CaP-agar beads were accurately weighed before and after the PEI processing. The PEI processed CaP-agar beads’ weight was 99.96 ± 2.15% of the weight of the CaP-agar beads, thereby verifying that the beads’ integrity was preserved. Conversely, the weight of the neat CaP beads dropped by 63.83% secondary to the pH 9.3 PEI processing step which disrupted the beads’ integrity and caused them to shrink excessively (Wahba 2016) (Table 3). As regards to the CaP-agar’s anionic traits, agar possesses minute amounts of anionic entities (0.12–0.65% of pyruvate (Freile-Pelegrín et al. 1996) and ~ 2% of sulfate (Delattre et al. 2011)), whereas ~ 93.3% (based on manufacturer’s data) of the galacturonic acid residues, which are the main constituents of pectin (Sriamornsak et al. 2005), are present as free carboxylic acids in the low methoxy pectin utilized herein. Moreover, the pectin’s pKa was reported to be within the 3.5–4.0 range (Martínez et al. 2013), and the agar’s anionic traits were prevalent at pHs as low as 3.5 (Toyama et al. 2011). Thus, the CaP-agar matrix would offer copious amounts of anionic entities throughout the entire tested pH range (8–10). These copious anionic entities would require a fairly big amount of cationic entities to establish a competent ionic interaction. This big amount of cationic PEI’s entities was provided when the PEI pH was lowered to 8.67 as at such pH ~ 32% of the PEI’s amino groups would be in their cationic state (Barkovec and Koper 1997). It is worth mentioning that a PEI pH of 8.5 was also recommended to process a somewhat analogous gel blend; the carrageenan-agar disks. The carrageenan-agar was also debated to provide a copious amount of anionic entities owing to κ-carrageenan (Wahba and Hassan 2017) which exhibits one sulfate group in each of its κ-carrabiose building units (Delattre et al. 2011). On the other hand, an escalated PEI pH of 10.55 was the optimal when processing the neat CaP beads so as to diminish the PEI’s cationic entities and preserve the CaP beads’ integrity (Wahba 2016).
The optimal PEI concentration that should be utilized to process the CaP-agar beads was 2.95%, and this was close to the 3% optimal PEI concentration that was utilized during the processing of agar disks (Wahba and Hassan 2015). Noteworthy, further incrementing the PEI concentration from this optimal 2.95% to 3.49%, and finally to 4% was predicted to dwindle the attainable iβgal’s activity from 7.94 to 7.76 U/g, and finally to 7.25U/g (Fig. 1b). These dwindles could be regarded to the occurrence of excessive entanglements among the chains of the high molecular weight (750 kDa), hyper-branched PEI upon incrementing its concentration beyond 2.95%. Such excessive entanglements would negatively affect the interaction of the beads with an enzyme as bulky as the Aspergillus oryzae βgal (110 kDa (Maksimainen et al. 2013), and this would eventually dwindle the attainable iβgal’s activity. It is worth mentioning that a higher PEI concentration of 3.49% was optimal for processing the neat CaP beads (Wahba 2016) (Table 3). This implied that the excessive entanglements among the PEI’s chains, which coated the neat CaP beads, were brought about by higher PEI concentrations than the case in hand. This could be regarded to the differences among the 3-D orientations of the PEI chains that coated the neat CaP beads and those coating the CaP-agar beads as each of the two aforementioned cases provided a different anionic–cationic environment. The CaP-agar beads were more anionic than the CaP beads owing to the presence of agar. Moreover, the PEI pH adopted during the processing of the neat CaP beads was 10.55 which greatly diminished the cationic traits of the PEI (Wahba 2016). It should be noted that the lower PEI concentration recommended for processing the CaP-agar beads made the preparation of such beads more economic than the preparation of the CaP processed beads despite the incorporation of agar. The preparation of 1 kg of the processed CaP-agar beads required an additional 54 g agar that was not utilized in case of the neat CaP beads. Nevertheless, it cut down the amount of the utilized 50% PEI stock solution, which is around threefold more expensive than the utilized agar (S.D. Fine-Chem. Limited 2020; Sigma-Aldrich 2020), by 54 g.
Elemental
The CaP-agar beads comprised 29.12% C, 4.20% H, 0.02% S, and no N. This was dissimilar to the elemental composition of the neat CaP beads which comprised a lesser amount of C (16.63%) and no S (Wahba 2016). Such increment in the C content of the CaP-agar beads and the emergence of S within their construction proved the coalescence of agar into the beads as agar is a polysaccharide with a sulfation percent of ~ 2% (Delattre et al. 2011). The PEI-CaP-agar beads comprised 34.97% C, 10.73% H, 0.0046% S, and 6.27% N. The arising of N in the elemental composition of the PEI-CaP-agar beads and the increments in their C and H contents as compared to the CaP-agar beads verified the coalescence of PEI (H(NHCH2CH2)nNH2) into the beads. Similarly, the arising of N in the composition of the PEI processed CaP beads was utilized to verify the coalescence of PEI into the beads. Nevertheless, the PEI-processed CaP beads exhibited a whole of 11.69% N (Wahba 2016) which was 1.86-fold higher than the N content of the PEI-CaP-agar beads (Table 3). Hence, it could be concluded that a lower amount of PEI coalesced into the structure of the CaP-agar beads. This could be regarded to the incremented cationic charge density of the employed pH 8.67 PEI solution (Barkovec and Koper 1997) which could have caused each PEI moiety to occupy several anionic locations on the CaP-agar beads, thereby blocking the way for other PEI moieties to interact with the CaP-agar beads. Noteworthy, the coalescence of PEI into the CaP-agar beads could also be visually verified where the beads acquired an opaque white color after their interaction with PEI (Fig. 2).
As regards to the GA-PEI-CaP-agar beads, they comprised 43.10% C, 3.81% H, 0.057% S, and 4.36% N. The GA-PEI-CaP-agar beads’ incremented carbon content, as compared to the PEI-CaP-agar beads, verified the coalescence of GA (C5H8O2) into the beads. Moreover, the beads acquired a brown color after interacting with GA (Fig. 2). This color was regarded to the Schiff base (–C=N–) which is amongst the possible GA-amine reactions’ products (Wahba 2017). Noteworthy, the increments in the beads’ carbon contents were formerly employed to verify the coalescence of GA into the PEI processed CaP beads (Wahba 2016) and into the chitosan beads (Wahba 2017). It is worth mentioning that both the PEI-CaP-agar beads and the PEI processed CaP beads exhibited analogous increments in their C contents secondary to their reaction with GA (1.23- and 1.26-fold (Wahba 2016), respectively). This implied that analogous amounts of GA were bound to both kinds of beads although the PEI-CaP-agar beads comprised much lesser PEI, and accordingly, much lesser amine entities. In a similar situation, both the PEI processed agar disks and carrageenan-agar disks were shown to bind to analogous amounts of GA although the PEI-processed carrageenan-agar disks exhibited a much lower PEI content. The authors debated that the 3-D orientation of PEI, in case of the PEI processed carrageenan-agar disks, provided the disks with a more proficient flexible polymer bed of PEI that allowed escalated amounts of GA to bind to these disks (Wahba and Hassan 2017). This debate could also be applied to the case in hand as the anionic–cationic interactions established among the CaP-agar and the pH 8.67 PEI would be different from those established among the neat CaP beads and the pH 10.55 PEI (Wahba 2016), and accordingly, the 3-D orientation of the bound PEI would differ in both cases. The elemental composition of the βgal loaded GA-PEI-CaP-agar beads was 40.64% C, 4.96% H, 0.04% S, and 4.46% N. Similarly, increments in the N and H contents of the activated CaP beads’ together with the dwindling in the beads’ C content were observed secondary to the βgal’s immobilization onto such beads (Wahba 2016).
Mechanical stability assessment
The CaP-agar beads preserved 82.70 ± 2.14% of their precursive weight following a 20 s vortexing procedure. Conversely, the neat CaP beads were totally fragmented secondary to an analogous 20 s vortexing procedure (Wahba 2016). The incremented mechanical stability of the CaP-agar beads could be regarded to the presence of agar which is known to possess escalated mechanical stability (Makwana et al. 2020). Such an escalated mechanical stability would facilitate the handling of the CaP-agar beads and would allow for the up-scaling of the beads’ concoction and activation processes.
As regards to the GA-PEI-CaP-agar beads, they preserved 99.51 ± 0.49% and 81.97 ± 1.54% of their precursive weight following 20 s and 3 min 20 s vortexing procedures, respectively. By comparison, the GA-PEI-CaP beads preserved 96.57 ± 0.89% and 94.69 ± 1.42% of their precursive weight, respectively, following analogous vortexing procedures (Wahba 2016) (Table 3). Thus, it could be concluded that the processed CaP-agar beads exhibited a somewhat inferior mechanical stability relative to the processed CaP beads. Nevertheless, the mechanical stability of the processed CaP-agar beads was still incremented enough to allow for the efficient utilization of the biocatalyst. This mechanical stability was even superior to that of the Na2CO3-GA processed chitosan beads, which were prepared to overcome the meager mechanical stability of chitosan, where these processed CS beads preserved 82.42 ± 0.53% of their precursive weight after an analogous vortexing procedure that lasted only for 1 min (Wahba 2017). The variations amongst the mechanical stabilities of the processed CaP-agar beads and the processed CaP beads could be regarded to the differences in the amounts of their bound PEI. The processed CaP-agar beads were shown in “Elemental” section. to exhibit much lesser amount of bound PEI as compared to the processed CaP beads. This bound PEI was formerly shown to be the major stabilizing agent that incremented the mechanical stability of the GA-PEI-CaP beads after establishing a tough shell around the CaP beads (Wahba 2016).
Estimation of the Km and the Vmax
The Hanes–Woolf plot (Fig. 3A) disclosed that the immobilization onto the processed CaP-agar diminished the βgal’s Vmax from 29.72 to 8.28 μmol/min mg enzyme. Conversely, the βgal’s Km was incremented from 76.37 to 104.10 mM secondary to its immobilization. The incremented iβgal’s Km implied that its affinity for lactose was inferior to that of the free βgal. This inferior affinity could have been caused by the immobilized enzyme’s lower capability to build the enzyme–substrate complex (Fatarella et al. 2014). The immobilization rigidifies the enzymes’ construction secondary to the multipoint covalent linkages established amongst such enzymes and their carriers (Rodrigues et al. 2013). This rigidified construction could have compromised the iβgal’s ability to build the enzyme–substrate complex. It is worth mentioning that similar alterations in the βgal’s Vmax and Km were recorded following its covalent immobilization onto cellulose-acetate membranes (Güleç et al. 2010).
pH profile
The free βgal’s optimal activity was recorded at a pH range of 4.6–5.1 (Fig. 3B). This was consistent with the formerly reported 4.5–5 optimal pH range (Klein et al. 2016). The βgal’s immobilization onto the processed CaP-agar beads widened its optimal pH range and shifted it towards acidity where it became 3.3–4.9. Likewise, the βgal’s optimal pH was widened and shifted towards acidity (from 5.3 to 3.2–5.4) following its immobilization onto the activated CaP beads (Wahba 2016). Acidic shifts in the optimal A. oryzae βgal’s pH were also formerly reported following its immobilization onto chitosan beads. These acidic shifts were regarded to the poly-cationic traits of chitosan (Klein et al. 2016). The poly-cationic traits of PEI at the tested pH range (2.6–7.5) could have also triggered such acidic shifts.
Temperature profile and assessment of activation energy
The uttermost activity of the free βgal was observed at 55 °C (Fig. 4A). Similarly, Güleç et al. (2010) reported 55 °C as the optimal temperature for the A. oryzae βgal. The optimal βgal’s temperature wasn’t altered following its immobilization onto the activated CaP-agar beads. Nevertheless, the iβgal demonstrated superior thermal tolerance where it maintained 27.80 ± 1.90% of its optimal activity at 70 °C. On the other hand, only 17.18 ± 0.31% of the free enzyme’s optimal activity was maintained at 70 °C. This implied that the iβgal was more thermally stable than its free counterpart, and this will be verified in the following section. It is worth mentioning that the A. oryzae βgal’s covalent immobilization onto concanavalin-A-layered calcium alginate-cellulose beads also didn’t alter its optimal temperature but caused the iβgal to demonstrate superior thermal tolerance (Ansari and Husain 2011).
A Influence of temperature on the relative activities of the free and the CaP-agar iβgals (data recorded while adopting the regular 15 min assays); B Arrhenius plot of ln (lactose hydrolysis rate) vs 1/temperature (T) which was exploited to calculate Ea (enzyme’s activation energy) of both βgal samples
The βgals’ activation energies (Ea) were calculated after estimating their lactose hydrolysis rates at a temperature range of 30–55 °C (Fig. 5) Such a temperature range was selected as it didn’t trigger any thermal deactivation during the regular 15 min incubation (Fig. 4A). However, upon extending the incubation of the free βgal at 55 °C, thermal deactivation was observed. The glucose released, at 55 °C, by the free βgal was first loftier than those released at all the other temperatures (Fig. 5A), and it should have continued as so if the free βgal did not suffer from thermal deactivation. Nevertheless, after 30 min, the glucose released at 55 °C was nearly analogous to that released at 50 °C. After 60 min, the glucose released at 55 °C was nearly analogous to that released at 45 °C. Accordingly, the overall free βgal’s 55 °C rate (5.71E−05 ML−1 sec−1) dropped below both its 50 °C (6.84E−05 ML−1 sec−1) and 45 °C (6.39E−05 ML−1 sec−1) rates, and it was not incorporated into the Arrhenius plot. The slopes of the Arrhenius plots of the two tested βgals were then utilized to calculate their Ea (Fig. 4B). The Ea of the activated CaP-agar iβgal was 14.36 kJ mol−1 whereas that of its free analogue was 18.53 kJ mol−1. The reduction of the βgal’s Ea following its immobilization implied that the iβgal would utilize a lesser quantity of energy to construct its activated complex during lactose hydrolysis; thus, it would offer a superior hydrolytic capacity (Da Silva et al. 2018). Dwindles in the enzymes’ Ea were formerly reported following their immobilization (Abdel-Wahab et al. 2018).
Thermal stability and assessment of thermodynamic parameters
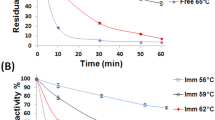
The activated CaP-agar iβgal exhibited incremented thermal stability relative to its free counterpart. The iβgal conserved 48.60 ± 3.18% and 27.78 ± 0.88% of its precursory activity after 1 h incubation at 63 and 65 °C, respectively (Fig. 6B). Conversely, the free βgal conserved only 15.29 ± 0.36% and 2.01 ± 0.56% of its precursory activity, respectively, after analogous incubations (Fig. 6A). To verify the incremented thermal stability of the activated CaP-agar iβgal, the thermodynamic parameters were estimated along a temperature range of 60 to 70 °C (Table 4). It should be noted that the thermodynamic parameters were not estimated for temperatures 40 and 50 °C as no statistically significant alterations (p value > 0.0500) were recorded in the activities of either of the tested βgals (Fig. 6). Initially, the kd values for both enzyme preparations were estimated from the slopes in Fig. 7, and it was revealed that, at all the given temperatures, the iβgal offered lesser kd values than its free counterpart (Table 4) which verified its incremented thermal stability (Abdel-Wahab et al. 2018). The iβgal also exhibited incremented t1/2 and D values relative to its free counterpart. Thus, at any of the given temperatures, longer durations would be needed to elicit 50% and 90% dwindles in the iβgal’s activity, respectively, and this would be in favor of its industrial exploitation. Noteworthy, a 0.0211 min−1 kd and a 62.85 min t1/2 were reported for the A. oryzae βgal which was covalently immobilized via Duolite®A568 at 60 °C (Soares et al. 2017). The kd and t1/2 attained by the activated CaP-agar iβgal at the selfsame temperature were superior as they amounted to 0.0013 min−1 and 518.24 min, respectively.
The activation energies of the thermal denaturation (Ed) of the two tested βgals were calculated from Fig. 8 slopes. The free βgal exhibited an Ed of 278.00 kJ mol−1 whereas an Ed of 282.15 kJ mol−1 was recorded for the activated CaP-agar iβgal. The incremented Ed of the iβgal further verified its superior thermal stability as Ed is the energy necessary to elicit enzyme denaturation (Ferreira et al. 2018). Likewise, immobilization incremented the Ed values and the thermal stabilities of pectinase and lipase (Abdel-Wahab et al. 2018; Ferreira et al. 2018). It is worth mentioning that the Ed of the activated CaP-agar iβgal was also superior to the 278.15 kJ mol−1 Ed formerly reported for the A. oryzae βgal which was covalently immobilized via Duolite®A568 (Guidini et al. 2011).
Being straightforwardly connected to the Ed (Eq. 3), the enthalpy of denaturation (ΔH) indicates the total quantity of energy needed to elicit enzyme denaturation (Da Silva et al. 2018) at any given temperature. Table 4 disclosed that the ΔH values of the two tested βgals dwindled gradually upon incrementing the temperature which implied that lower energy amounts were necessary to denature such enzymes at incremented incubation temperatures. Similarly, the ΔH values of the free protease (Da Silva et al. 2018) and of the free and immobilized pectinases (Abdel-Wahab et al. 2018) dwindled gradually upon incrementing their incubation temperatures. Comparing the ΔH values of the free and the activated CaP-agar iβgals revealed that the iβgal provided loftier ΔH values at all the given temperatures. The iβgal was more thermo-stable and required loftier energy amounts to get denatured. Immobilization was formerly debated to increment the thermo-stability of the A. oryzae βgal via rigidifying (Wahba 2017) its monomeric structure (Maksimainen et al. 2013). This rigidification was secondary to the multipoint covalent linkages created amongst the immobilization carrier and the loaded enzyme (Rodrigues et al. 2013). The enzyme’s structural rigidification could also be coupled with conformational variations (Abdel-Wahab et al. 2018) which could promote the establishment of new non-covalent linkages amongst the enzyme moieties. Thermal denaturation, being principally accomplished via the breaking of non-covalent linkages (Da Silva et al. 2018), would then need loftier energy amounts to break these new non-covalent linkages and denature the enzyme. Thus, the iβgal offered incremented ΔH values relative to its free counterpart.
The activation entropy (ΔS), or the randomness and disorder, is another parameter that was reported to escalate during thermal denaturation (Da Silva et al. 2018; Ferreira et al. 2018). The activated CaP-agar iβgal offered loftier ΔS values relative to its free counterpart (Table 4), which reflected the escalated disorder of the CaP-agar iβgal’s denatured states. Likewise, the immobilized lipase offered loftier ΔS values relative to its free counterpart (Ferreira et al. 2018). The Gibb’s free energy of denaturation (ΔG) is a pivotal thermodynamic parameter as it combines the enthalpic and the entropic contributions. Accordingly, it is a more dependable parameter when determining an enzyme’s thermal stability. Incremented ΔG values are coupled to non-spontaneous reactions, and they imply incremented thermal denaturation resistance and incremented thermal stability (Da Silva et al. 2018). The activated CaP-agar iβgal provided loftier ΔG values than the free βgal at all the given temperatures. This further verified the incremented thermal stability of the CaP-agar iβgal. Noteworthy, the immobilized pectinase and lipase also provided loftier ΔG values than their free counterparts (Abdel- Wahab et al. 2018; Ferreira et al. 2018).
Operational stability
The activated CaP-agar iβgal exhibited superior operational stability compared to the neat CaP iβgal. The CaP-agar iβgal preserved 98.44 ± 0.31% and 86.84 ± 0.37% of its precursive activity during its second and thirteenth reusability rounds, respectively (Fig. 9A). On the other hand, only 89% and 79.34% of the precursive activity of the neat CaP iβgal were preserved during its second and fourteenth reusability rounds, respectively (Wahba 2016) (Table 3). Such a superior operational stability would further favor the exploitation of the activated CaP-agar beads presented herein. It is worth mentioning that the distortions that could have occurred in the active sites of immobilized enzymes following such enzymes’ recurrent reactions with their substrates could account for the activity diminutions observed during re-using immobilized enzymes (Kishore and Kayastha 2012).
Storage stability
The activated CaP-agar iβgal preserved 85.05 ± 3.32% of its precursive activity after 38 days of storage (Fig. 9B). This result was superior to ~ 71% activity preserved by the A. oryzae βgal, which was covalently immobilized to poly (glycidylmethacrylate-methylmethacrylate) magnetic beads, after 35 days of storage (Bayramoglu et al. 2007). The incremented storage stability of the CaP-agar iβgal could be referred to the rigidification of its structure secondary to the multipoint covalent linkages created amongst it and its immobilization carrier (Rodrigues et al. 2013). The iβgal’s rigidified structure would be less prone to unfolding and denaturation.
Hydrolyzing the lactose in whey permeate
This experiment was executed at 55 °C as this was the optimal temperature for the activated CaP-agar iβgal. Such an incremented temperature would guard against the propagation of pathogenic microbes as they propagate at ~ 37 °C (Dutra Rosolen et al. 2015), and this would be favorable specially that lactose hydrolysis processes proceed for extended periods. Figure 10 disclosed that the quantity of procured glucose incremented progressively with time until 64.32 mM glucose was procured after 12 h. Noteworthy, the quantities of glucose procured after 10, 11, and 12 h were statistically insignificant from one another (p value = 0.559). It should also be noted that the 64.32 mM glucose procured herein was superior to the uttermost quantity of glucose procured (~ 45 mM) by a thermo-stable A. niger βgal while hydrolyzing the WP’s lactose at 55 °C although the incubation period was extended to 60 h (Hatzinikolaou et al. 2005).
Conclusions
An improved CaP-based immobilization carrier was concocted after the merger of agar. The CaP-agar beads’ offered the sufficient mechanical stability that would permit their efficient handling without concerns regarding their integrity. The CaP-agar beads also established an ionic interaction with PEI which was different and also superior to that provided by the neat CaP beads as it permitted the immobilization of more enzyme while utilizing lesser PEI. This made the preparation of the CaP-agar carriers more economic than the preparation of the neat CaP carriers. The CaP-agar carriers granted their immobilized enzyme incremented thermal stability as was verified from its incremented thermodynamic parameters. The CaP-agar carriers also granted their immobilized enzyme incremented storage and operational stabilities.
References
Abdel-Wahab WA, Karam EA, Hassan ME, Kansoh AL, Esawy MA, Awad GEA (2018) Optimization of pectinase immobilization on grafted alginate-agar gel beads by 24 full factorial CCD and thermodynamic profiling for evaluating of operational covalent immobilization. Int J Biol Macromol 113(2018):159–170
Ansari SA, Husain Q (2011) Immobilization of Aspergillus oryzae β-galactosidase on concanavalin A-layered calcium alginate-cellulose beads and its application in lactose hydrolysis in continuous spiral bed reactors. Pol J Chem Technol 13(4):15–20
Awad GEA, Ghanem AF, Abdel-Wahab WA, Wahba MI (2020) Functionalized κ-carrageenan/hyperbranched poly(amidoamine) for protease immobilization: thermodynamics and stability studies. Int J Biol Macromol 148(2020):1140–1155
Barkovec M, Koper GJM (1997) Proton binding characteristics of branched polyelectrolytes. Macromolecules 30(7):2151–2158
Bayramoglu G, Tunali Y, Arica MY (2007) Immobilization of β-galactosidase onto magnetic poly(GMA–MMA) beads for hydrolysis of lactose in bed reactor. Catal Commun 8(7):1094–1101
Contesini FJ, Ibarguren C, Grosso CRF, Carvalho PDO, Sato HH (2012) Immobilization of glucosyltransferase from Erwinia sp. using two different techniques. J Biotechnol 158(3):137–143
Da Silva OS, de Oliveira RL, Silva JDC, Converti A, Porto TS (2018) Thermodynamic investigation of an alkaline protease from Aspergillus tamarii URM4634: a comparative approach between crude extract and purified enzyme. Int J Biol Macromol 109:1039–1044
Delattre C, Fenoradosoa TA, Michaud P (2011) Galactans: an overview of their most important sourcing and applications as natural polysaccharides. Braz Arch Biol Technol 54(6):1075–1092
Dutra Rosolen M, Gennari A, Volpato G, de Souza CFV (2015) Lactose hydrolysis in milk and dairy whey using microbial β-galactosidases. Enzyme Res 2015:806240
Fatarella E, Spinelli D, Ruzzante M, Pogni R (2014) Nylon 6 film and nanofiber carriers: preparation and laccase immobilization performance. J Mol Catal B: Enzym 102:41–47
Ferreira MM, Santiago FLB, da Silva NAG, Luiz JHH, Fernandéz-Lafuente R, Mendes AA, Hirata DB (2018) Different strategies to immobilize lipase from Geotrichum candidum: Kinetic and thermodynamic studies. Process Biochem 67:55–63
Freile-Pelegrín Y, Robledo D, Armisén R, García-Reina G (1996) Seasonal changes in agar characteristics of two populations of Pterocladia capillacea in Gran Canaria. Spain J Appl Phycol 8(3):239–246
Geiger B, Nguyen HM, Wenig S, Nguyen HA, Lorenz C, Kittl R, Mathiesen G, Eijsink VGH, Haltrich D, Nguyen TH (2016) From by-product to valuable components: Efficient enzymatic conversion of lactose in whey using β-galactosidase from Streptococcus thermophilus. Biochem Eng J 116:45–53
Guidini CZ, Fischer J, de Resende MM, Cardoso VL, Ribeiro EJ (2011) β-Galactosidase of Aspergillus oryzae immobilized in an ion exchange resin combining the ionic-binding and crosslinking methods: Kinetics and stability during the hydrolysis of lactose. J Mol Catal B: Enzym 71(3–4):139–145
Granjeiro PA, Cavagis ADM, Leite LDC, Ferreira CV, Granjeiro JM, Aoyama H (2004) The thermal stability of a castor bean seed acid phosphatase. Mol Cell Biochem 266(1–2):11–15
Güleç HA, Gürda S, Albayrak N, Mutlu M (2010) Immobilization of Aspergillus oryzae β-galactosidase on low pressure plasma-modified cellulose acetate membrane using polyethyleneimine for production of galactooligosaccharide. Biotechnol Bioprocess Eng 15:1006–1015
Hatzinikolaou DG, Katsifas E, Mamma D, Karagouni AD, Christakopoulos P, Kekos D (2005) Modeling of the simultaneous hydrolysis–ultrafiltration of whey permeate by a thermostable β-galactosidase from Aspergillus niger. Biochem Eng J 24(2):161–172
Kishore D, Kayastha AM (2012) Optimisation of immobilization conditions for chick pea β-galactosidase (CpGAL) to alkylamine glass using response surface methodology and its applications in lactose hydrolysis. Food Chem 134(3):1650–1657
Klein MP, Hackenhaar CR, Lorenzoni ASG, Rodrigues RC, Costa TMH, Ninow JL, Hertz PF (2016) Chitosan crosslinked with genipin as support matrix for application in food process: support characterization and β-d-galactosidase immobilization. Carbohydr Polym 137:184–190
Maksimainen MM, Lampio A, Mertanen M, Turunen O, Rouvinen J (2013) The crystal structure of acidic β-galactosidase from Aspergillus oryzae. Int J Biol Macromol 60:109–115
Makwana D, Castaňo J, Somani RS, Bajaj HC (2020) Characterization of Agar-CMC/Ag-MMT nanocomposite and evaluation of antibacterial and mechanical properties for packaging applications. Arab J Chem 13(1):3092–3099
Martínez YN, Cavello I, Hours R, Cavalitto S, Castro GR (2013) Immobilized keratinase and enrofloxacin loaded on pectin PVA cryogel patches for antimicrobial treatment. Bioresour Technol 145:280–284
Mollaei M, Abdollahpour S, Atashgahi S, Abbasi H, Masoomi F, Rad I, Lotfi AS, Zahiri HS, Vali H, Noghabi KA (2010) Enhanced phenol degradation by Pseudomonas sp SA01: gaining insight into the novel single and hybrid immobilizations. J Hazard Mater 175(1–3):284–292
Mitchell AJ, Wimpenny JWT (1997) The ffects of agar concentration on the growth and morphology of submerged colonies of motile and non-motile bacteria. J Appl Microbiol 83(1):76–84
Munarin F, Petrini P, Barcellona G, Roversi T, Piazza L, Visai L, Tanzi MC (2014) Reactive hydroxyapatite fillers for pectin biocomposites. Mater Sci Eng C 45:154–161
Panesar PS, Kumari S, Panesar R (2010) Potential applications of immobilized β-galactosidase in food processing industries. Enzyme Res 2010:473137
Pessela BCC, Fernández-Lafuente R, Fuentes M, Vián A, García JL, Carrascosa AV, Mateo C, Guisánb JM (2003) Reversible immobilization of a thermophilic β-galactosidase via ionic adsorption on PEI-coated Sepabeads. Enzyme Microb Technol 32(3–4):369–374
Rai AK, Prakash O, Singh J, Singh PM (2013) Immobilization of cauliflower myrosinase on agar agar matrix and its application with various effectors. Adv Biochem 1(3):51–56
Rodrigues RC, Ortiz C, Berenguer-Murcia A, Torres R, Fernández-Lafuente R (2013) Modifying enzyme activity and selectivity by immobilization. Chem Soc Rev 42(15):6290–6307
S. D. Fine-Chem Limited (2020) Price list 2019–2020. https://www.sdfine.com/pricelistpdf.pdf. Accessed 22 July 2020
Sigma-Aldrich (2020) Poly(ethyleneimine) solution. https://www.sigmaaldrich.com/catalog/product/sial/p3143?lang=en®ion=EG. Accessed 22 July 2020
Soares LN, Falleiros S, Cabral BV, Fischer J, Guidini CZ, Cardoso VL, De Resende MM, Ribeiro EJ (2017) Improvement of recovered activity and stability of the Aspergillus oryzae β-galactosidase immobilized on Duolite®A568 by combination of immobilization methods. Chem Ind Chem Eng Q 23:495–506
Sriamornsak P, Thirawong N, Puttipipatkhachorn S (2005) Emulsion gel beads of calcium pectinate capable of floating on the gastric fluid: effect of some additives hardening agent or coating on release behavior of metronidazole. Eur J Pharm Sci 24(4):363–373
Torres R, Pessela BCC, Fuentes M, Mateo C, Munilla R, Fernández- Lafuente R, Guisán J (2006) Supports coated with PEI as a new tool in chromatography. Enzyme Microb Technol 39(4):711–716
Toyama Y, Sahara R, Lino Y, Kubota K (2011) pH dependence of rheological properties of gelatin gel mixed with agar or agarose. Trans Mater Res Soc Jpn 36(3):383–386
Wahba MI (2016) Treated calcium pectinate beads for the covalent immobilization of β-d-galactosidase. Int J Biol Macromol 91:877–886
Wahba MI (2017) Porous chitosan beads of superior mechanical properties for the covalent immobilization of enzymes. Int J Biol Macromol 105(1):894–904
Wahba MI, Hassan ME (2015) Novel grafted agar disks for the covalent immobilization of β-d-galactosidase. Biopolymers 103(12):675–684
Wahba MI, Hassan ME (2017) Agar-carrageenan hydrogel blend as a carrier for the covalent immobilization of β-d-galactosidase. Macromol Res 25(9):913–923
Wu J, Yu HQ (2007) Biosorption of 2,4-dichlorophenol by immobilized white-rot fungus Phanerochaete chrysosporium from aqueous solutions. Bioresour Technol 98:253–259
Acknowledgements
This work was funded by the National Research Centre, Egypt.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wahba, M.I. Calcium pectinate-agar beads as improved carriers for β-d-galactosidase and their thermodynamics investigation. 3 Biotech 10, 356 (2020). https://doi.org/10.1007/s13205-020-02341-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-02341-y