Abstract
This study deals with lipase immobilization on micro- and mesoporous silica-based materials. The effects of the type of support (silica MCM-41, zeolite HZSM-5 (SAR 25), zeolite HZSM-5 (SAR 280), and the silica-aluminas Siral 10, Siral 20, and Siral 40) were investigated on the immobilization of lipase B from Candida antarctica (CALB) and lipase from Rhizomucor miehei (RML). The supports that allowed the highest immobilization efficiencies for the CALB were Siral 40 (91.4%), HZSM-5 (SAR 280) (90.6%), and MCM-41 (89.4%). Siral 20 allowed the highest immobilization efficiency for RML (97.6%), followed by HZSM-5 (SAR 25) (77.1%) and HZSM-5 (SAR 280) (62.7%). The effect of protein concentration on lipase immobilization was investigated, and the results adjusted well on the Langmuir isotherm model (R2 > 0.9). The maximum protein adsorption capacity of the support determined by the Langmuir model was equal to 10.64 and 20.97 mgprotein gsupport−1 for CALB and RML, respectively. The effects of pH (pH 7.0 and pH 11.0) and phosphate buffer solution concentration (5 and 100 mmol L−1) were also investigated on lipase immobilization. The immobilization efficiency for both lipases was similar for the different pH values. The use of 100 mmol L−1 phosphate buffer decreased the lipase immobilization efficiency. The biocatalysts (CALB-Siral 40 and RML-Siral 20) were tested in the ethyl oleate synthesis. The conversion of 61.7% was obtained at 60 °C in the reaction catalyzed by CALB-Siral 40. Both heterogeneous biocatalysts showed increased thermal stability compared with their free form. Finally, the reuse of the biocatalysts was studied. CALB-Siral 40 and RML-Siral 20 maintained about 30% of the initial conversion after 3 batches of ethyl oleate synthesis. Silica-aluminas (Siral 20 and 40) proved to be a support that allowed a high efficiency of immobilization of lipases and activity for esterification reaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lipases (triacylglycerol ester hydrolases E.C.3.1.1.3) are a main group of biocatalysts in the biotechnology field, belonging to the class of hydrolases (Kumar et al. 2023). They can be obtained from animal, plant, and microbial sources. Lipases from microbial sources are the most used enzymes industrially (Gog et al. 2012; Chandra et al. 2020). Aspergillus niger, Candida rugosa, Humicola lanuginosa, Mucor miehei, Rhizopus arrhizus, Rhizopus delemar, Rhizopus japonicus, Rhizopus niveus, and Rhizopus oryzae are the principal sources of commercial lipases (Chandra et al. 2020; Kumar et al. 2023). Candida antarctica lipase B (CALB) is the most common lipase used in biocatalytic processes (Chandra et al. 2020).
Lipases are versatile catalysts that can be used in various applications, not only on a laboratory level but also on an industrial scale, such as detergent production, food industries, oleochemical industries, bioremediation, cosmetics, biosensors, probiotics, biofuels, paper industry, leather industry, and ester synthesis (Rashmi and Gayathri 2014; Pedro et al. 2019; Dutra et al. 2022; Everton et al. 2022; Kumar et al. 2023).
This group of enzymes can catalyze hydrolysis and synthesis reactions like esterification, interesterification, alcoholysis and acidolysis (Reis et al. 2009). Lipases have been widely used as a biocatalyst in ester synthesis because they accept a wide range of substrates, have high activity, and are quite stable in non-aqueous media (Campisano et al. 2021). The biotechnological approach to ester synthesis using lipases has many advantages over the conventional chemical process (Reis et al. 2009; Brautaset and Ellingsen 2011; Sá et al. 2017; Fasim et al. 2021). Lipases can catalyze these reactions in mild conditions, producing a smaller quantity of by-products and effluents when compared to chemical catalysts since enzymes are highly selective catalysts (Ansorge-Schumacher and Thum 2013; Christopher et al. 2014). Although the downstream process is easier in industrial enzyme applications due to the high selectivity of enzymes, which generate fewer by-products, the recovery of enzymes in their free form at the end of industrial processes is difficult or even unfeasible (Krajewska 2004). Therefore, the immobilization of enzymes can be proposed as one of the most relevant alternatives to enzyme catalysis (Mateo et al. 2007; Cipolatti et al. 2014; Boudrant et al. 2020).
Enzyme immobilization is required for their use as industrial biocatalysts, as immobilization allows enzyme reuse (lowering the cost of the biocatalyst) and simplifies bioreactors’ overall design and performance control (Rodrigues et al. 2013). In addition, an immobilized enzyme is usually more stable than its free form and can withstand a wider range of reaction conditions, showing higher resistance to inhibitors and chemical reagents (Cipolatti et al. 2014; Manoel et al. 2016; Pinto et al. 2020; Rodrigues et al. 2013). There are several ways to immobilize an enzyme. The adsorption of enzymes to solid supports is a technique commonly used for its relative simplicity, with no need for support activation, low cost, and also for the possibility of reusing the support through protein desorption (Fernandez-Lafuente et al. 1998; Mateo et al. 2007; Sheldon and van Pelt 2013). Protein adsorption on solid surfaces is a spontaneous process resulting from several contributions, such as hydrophobic dehydration, structural changes, electrostatic interactions, van der Waals interactions, and specific bonds (Reis et al. 2009). The enzyme is spontaneously immobilized on a preferential and energetic favorable orientation without significant change in the tridimensional structure of the enzyme and preventing its denaturation (Zhou and Hartmann 2013).
It is known that the material used as support directly influences the performance of the biocatalyst (Cunha et al. 2014; Cipolatti et al. 2015; Pinto et al. 2020), and its choice is fundamental for the success of the reaction. Several supports are used to immobilize enzymes: polymers, silica, zeolites, activated carbon, hydrotalcite, gold, mica, metal–organic frameworks (MOFs), and magnetic nanoparticles (Thangaraj and Solomon 2019). The choice of support for enzyme immobilization takes into account some characteristics, such as particle size, surface area, pore diameter, thermal stability, chemical durability, hydrophobic/hydrophilic character, the presence of chemical moieties, good sorption properties, mechanical strength, microbial resistance, non-toxicity, loading capacity, and cost (Serralha et al. 1998; Cipolatti et al. 2014; Thangaraj and Solomon 2019). To make industrial products, silica-based carriers provide excellent matrices for immobilizing enzymes because they have great mechanical strength, thermal stability, and resistance to organic solvents. Various mesoporous silica materials, such as MCM-41 and SBA-15, are extensively used as a carrier for enzyme immobilization (Thangaraj and Solomon 2019). Many inorganics materials, such as zeolites and highly ordered mesoporous solids doped with aluminas (silica-aluminas), have large specific areas and high mechanical and thermal stability. These are important features to optimize immobilization procedures and obtain solids with high enzyme loading (Wang et al. 2012; Gustafsson et al. 2012; Yu et al. 2013a). Silica-doped aluminas have more Brønsted and Lewis acid sites on their surface than aluminas (Hensen et al. 2010). Thus, the electrostatic interactions between the support acid sites and enzyme amino groups could be enhanced.
Moreover, the increase of silica content on silica-aluminas results in increased hydrophobicity (Chen 1976; Nakamoto and Takahashi 1982; Tsutsumi et al. 1994), making this material promising support for immobilization, especially for lipases that hydrophobic surfaces can hyperactivate. Concerning the zeolites, their variable, and wide-range framework topology, the presence of exchangeable cations that neutralize the framework charge and reactive silanol groups on the surface are important aspects of enzyme immobilization. They also provide several routes for optimizing enzyme–support interaction and biocatalyst performance. In addition, zeolites may act as a water source essential for enzymatic activity when applied to organic phase catalysis (Mitchell and Pérez-Ramírez 2011).
Therefore, the immobilization process depends on the enzyme molecule and the physicochemical characteristics of the support (specific area, particle size, pore structure, and nature of functional groups present on the surface) (Jesionowski et al. 2014; Boudrant et al. 2020). High loadings of enzymes and, consequently, higher activities are favored by the supports with higher and accessible specific areas (Mitchell and Pérez-Ramírez 2011). The low interaction between enzyme and support in the immobilized enzyme might contribute to enzyme desorption (Brady and Jordaan 2009).
Immobilized lipases should be highlighted in producing several products, such as different esters. Esters are organic compounds widely used to prepare other commercial products, from flavors to renewable biofuels (Stergiou et al. 2013; Antonopoulou et al. 2016; Ćorović et al. 2020). The production of esters by extraction from natural products or fermentation needs to be increased to meet current demand. Thus, these compounds can be obtained from the esterification reaction between fatty acid and alcohol catalyzed by chemical catalysts or enzymes. Ethyl oleate is an ester produced by the esterification of ethanol and oleic acid and is commercially relevant in many ways. For example, it is the main biodiesel component (Wenchao et al. 2020), a vehicle for intramuscular drug delivery (Date and Nagarsenker 2008), and an important solvent for pharmaceutical processes that involve steroids.
Lipase B from Candida antarctica (CALB), lipase from Rhizomucor miehei (RML) (commercially named Palatase 20000L), and their respective commercial immobilized forms, Novozym 435 and Lipozyme RM IM, are the most employed lipases in ethyl oleate synthesis (Tan et al. 2010; Bajaj et al. 2010; Gog et al. 2012; Aarthy et al. 2014; Amini et al. 2017). This work aimed to study the immobilization process of CALB and RML by adsorption using six different supports (zeolites and silica-aluminas). In addition, the influence of some immobilization parameters, such as pH, temperature, and initial protein concentration, was investigated. The production of ethyl oleate by esterification in a free-solvent system using the biocatalysts produced was also performed.
Materials and methods
Chemicals
Oleic acid (P.A) and ethanol (99.8%) were purchased from Vetec Química Fina Ltda. (Rio de Janeiro, Brazil). Novozymes Latin America Ltda (Araucária, Brazil) kindly provided the lipases commercial liquid preparations: RML (Palatase 20000L, free lipase from Rhizomucor miehei) and CALB (free lipase B from Candida antarctica).
The mesoporous silica MCM-41 was purchased from Tianjin Co (China). The HZSM-5 zeolite with chemical SAR (SiO2:Al2O3 molar ratio) equal to 25 was supplied by CENPES/Petrobras, and that with chemical SAR of 280 (HZSM-5 SAR = 280) was purchased from Zeolyst International. The silica-aluminas, Siral 10, 20, and 40, with Al2O3:SiO2 molar ratios of 90:10, 80:20, and 60:40, respectively, were kindly provided by SASOL (Germany).
N2 physisorption
The specific surface area (BET isotherm), the microporous volume (t-plot), and mesoporous volumes and pore size distribution (BJH method) of the upports for enzyme immobilization were measured by N2 physisorption at – 196 °C using an ASAP 2020 Micromeritics automatic analyzer. The samples were outgassed at 300 °C for 12 h before measuring the isotherm.
Immobilization of CALB and RML on zeolites and silica-aluminas
The support (1 g) was mixed with 10 mL of lipase solution (10%v/v of CALB or RML diluted in sodium phosphate buffer solution at 5 mmol L−1 pH 7) under mild stirring, using a roller stirrer at 25 ± 1 °C for 2 h. The supernatant samples (300 µL) were periodically withdrawn and analyzed for protein concentration and enzyme activity determination. Then, the immobilized derivatives (support-bound immobilized enzymes) were washed with 25 mL sodium phosphate buffer, filtered using a Bucher funnel, and stored in a desiccator at 8 °C. The immobilized derivatives refer to the enzyme derivatives that are chemically or physically bound to a support (or carrier). The studied supports were HZSM-5 zeolites with SAR of 25 and 280, MCM-41, and silica-aluminas (Siral 10, Siral 20, and Siral 40).
The initial protein concentration of CALB and RML (C0) and the protein concentration of the supernatants, after immobilization time (Cf), were determined through the Bradford method (Bradford 1976). The immobilization efficiency (Ef) was defined as described in Eq. 1.
Effect of buffer concentration and pH on the immobilization process
The influence of two phosphate buffer concentrations (5 and 100 mmol L−1) and two different pH (7 and 11) was tested on lipase immobilization. Both buffer concentrations were studied at pH 7, and the adequate buffer concentration (5 mmol L−1) for immobilization was evaluated at pH 11. One gram of Siral 40 was mixed with 10 mL of the lipase solution previously diluted (1:10) in sodium phosphate buffer. The immobilization process occurred at room temperature (25 ± 1 °C) for 2 h under stirring.
Effect of protein concentration on immobilization process and Langmuir adsorption isotherm
The effect of protein concentration on immobilization efficiency was studied using the commercial lipase solution (CALB or RML) diluted in sodium phosphate buffer (5 mmol L−1) at pH 7.0. The effect of CALB concentration on Siral 40 immobilization was studied using CALB concentrations of 0.8, 1.3, 2.2, 3.3, 4.2, and 4.9 mg mL−1. Solutions of RML at 0.9, 1.5, 1.8, 2.4, and 2.7 mg mL−1 were employed to study the effect of RML concentration on Siral 20 immobilization. The experimental data were adjusted to the Langmuir isotherm model. The Langmuir adsorption isotherm is described as shown in Eq. 2, where Qe is the equilibrium concentration of adsorbed protein at the support (mg g−1), K is the equilibrium constant of adsorption (mL mg−1), Qm is the maximum adsorbed mass of protein in the support (mg g−1), and Ce is the equilibrium concentration of free protein in solution (mg mL−1).
The Langmuir isotherm model for adsorption states that the adsorption would occur on active sites at the support surface area. It also states that the surface would be homogeneous, and thus the adsorption energy of each adsorbate would be identical. Due to the limited quantity of active sites per g of support, there would be a limit of adsorbate that could be adsorbed in a specific support mass, that limit being called Qm.
The Levenberg–Marquardt method was used to estimate the parameter values which best describe the data. The non-linear fitting was made using Origin 8.1 software. The convergence criterion was the chi-square minimization with a tolerance of 10–9 and the maximum number of interactions equal to 50.
Determination of lipase esterification activity
Esterification reactions were carried out using oleic acid and ethanol (oleic acid: ethanol molar ratio of 1) (Pedro et al. 2019). The activities of the lipase-free and its immobilized preparations were determined at 30 °C. One esterification unit (U) was defined as the enzyme amount that consumes 1 μmol of oleic acid per minute per gram or milliliter of enzymatic preparation under the standard assay condition.
Ethyl oleate synthesis
The enzymatic synthesis of ethyl oleate was carried out in a closed batch reactor magnetically stirred and coupled to a condenser to avoid alcohol loss. The temperature was kept constant by the circulation of ethylene glycol in the reactor jacket. The reaction mixture consisted of 15 mmol of ethanol and 15 mmol of oleic acid. The progress of the reaction was verified by taking samples (50 µL) in duplicate that were analyzed by acid–base volumetric analysis for unreacted oleic acid. The samples were dissolved in an acetone/ethanol mixture (35 mL). The residual oleic acid was determined by titration with sodium hydroxide 0.02 mol L−1 using a Mettler Toledo T50 Titrator (Pedro et al. 2019). The blank tests were performed by adding the support to the reaction mixture (without enzyme), and no consumption of oleic acid was observed under the experimental conditions.
Effect of temperature on ethyl oleate synthesis
The reactions were carried out at five temperatures (30, 40, 50, 60, and 75 °C) (Pedro et al. 2019) using 10% v/v of the free-liquid enzyme (0.6 mgprotein of CALB/mL or 0.4 mgprotein of RML/mL) or 20 w/v % of the immobilized derivative (1.2 mgprotein of CALB on Siral 40/mL or 0.8 mgprotein RML on Siral 20/mL). The decay of the activity was followed over time.
Effect of enzyme concentration on the ethyl oleate synthesis
Esterification reactions were carried out at 30 °C using five free enzyme (CALB or RML) concentrations (5, 10, 20, 30, and 40% v/v) and three concentrations (3, 10, and 20 w/v %) of the immobilized enzymes (CALB-Siral 40 or RML-Siral 20).
Reuse of immobilized enzyme on ethyl oleate synthesis
The reactions were carried out at 30 °C using 20 w/v % of immobilized lipase derivatives (CALB-Siral 40 or RML-Siral 20). After 30 min of reaction, the immobilized enzyme was separated from the reaction media, washed with 5 mL of ethanol, and filtered under a vacuum (Aguieiras et al. 2016). Then, the recovered enzyme was reused in further esterification cycles. The experiments were carried out in duplicate.
Fourier transform infrared attenuated total reflection (FTIR-ATR) spectroscopy
FTIR was used to analyze post-reaction immobilized derivatives, esterification reaction media, esterification reactants, and commercial enzymatic solutions. FTIR-ATR spectra were recorded on a Perkin Elmer spectrometer (Frontier model). The ATR accessory contained an expanded diamond window, and each spectrum was corrected after subtracting background signals from the window. All spectra were recorded at room temperature. A special dry system was constructed to prevent interference of atmospheric moisture in the spectra.
Results and discussion
Textural and acid properties of the supports for the immobilization process
Porous silicates are promising carriers for enzyme immobilization because they have a large surface area, narrow pore size distribution, well-defined pore geometry, thermal and mechanical stability, functional groups for enzyme linking, water insolubility, and non-toxicity (Costantini and Califano 2021). In this work, some microporous (HZSM-5 zeolites with SAR 25 and 280) and mesoporous (Si-MCM 41 and Siral 10, 20, 40) silica-based solids were used for CALB and RML immobilization. The supports’ textural characteristics were determined and shown in Table 1.
According to the results of Table 1, all supports have specific areas greater than 300 m2 g−1, favoring lipase adsorption. The HZSM-5 zeolites (SAR 25 and SAR 280) are microporous solids, and the other solids (MCM-41, Siral 10, Siral 20, Siral 40) are mesoporous.
Porous solids have been used for lipase immobilization due to their larger surface area compared to non-porous ones. The presence of porous increases the internal surface area for enzyme adsorption (Mokhtar et al. 2020). The disadvantage of porous support is related to mass transfer limitations inside the support structure. Diffusional restrictions can occur because of substrate and product transport inside the biocatalyst particle (internal diffusional restriction). However, the location of enzyme molecules inside the pores also protects against possible adverse conditions in the reaction medium (Mateo et al. 2007).
The diameter of the support pores must be large enough to accommodate the enzyme and allow access to the substrate (Kalantari et al. 2017). The ideal pore diameter is reported to be 50 to 70 nm (5–7 Å), allowing the immobilization of a higher protein load (Thangaraj and Solomon 2019). The pore diameters on microporous carriers are too small for enzyme diffusion, even though the supports have large surface areas (Mokhtar et al. 2020).
The HZSM-5 zeolite pore diameter (Dp) is around 5.5 Å. The HZSM-5 zeolites have a pore structure formed by two interconnected microporous systems with an aperture of 5.5 × 5.1 Å and 5.6 × 5.3 Å (Baerlocher and McCusker 2007). The lipases CALB and RML have an average spherical diameter of 39.2 Å and 41 Å, respectively (Dumitriu et al. 2003; Macario et al. 2005). Therefore, enzyme immobilization should occur mostly on the HZSM-5 particle surface, considering the enzyme diameter.
The other supports (MCM-41, Siral 10, Siral 20, and Siral 40) are mesoporous materials (20 Å < Dp < 500 Å). The MCM-41 presented a narrow distribution of porous diameter with an average dimension of 30 Å. This result agrees with the literature that reports 20–60 Å for MCM-41 pore diameter (Thangaraj and Solomon 2019). The silica-aluminas (Siral 10, 20, and 40) showed a wide distribution of pore diameter in the range of 30 and 200 Å with a predominance of pores between 50 and 100 Å and can allow the immobilization of significant amounts of the enzyme inside the pores (Hanefeld et al. 2009; Garcia-galan et al. 2011). Figure 1 shows the pore distribution of Siral 20 and Siral 40.
Kalantari et al. (2017) synthesized octadecyl alkyl-modified mesoporous-silica nanoparticles (C18-MSNs) for lipase immobilization. They observed that lipase immobilization was influenced by the support’s particle size and pore structure. The better results were observed when the support (C18-MSN-0.5) had a similar pore diameter slightly larger than the dimensions of lipase. The optimized pore size reduced the lipase leakage and retained the enzyme performance during the reuse.
All the studied supports have some acidic character, which could cause enzymatic deactivation. It is known that pH can significantly influence the enzyme’s microenvironment and protein structure and directly affect enzyme activity. Therefore, the pH of the solution was measured after adding the solid to phosphate buffer (5 mmol L−1 and pH 7.0) in an experiment without lipase, and the results are shown in Table 2. The final solution pH has dropped significantly, mainly for HZSM-5 zeolite. It is an important study and can help understand the phenomena that occur later.
Study of the immobilization process
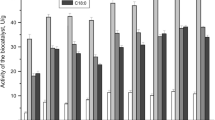
The zeolites HZSM-5 with SAR of 25 and 280, Si-MCM-41, and silica-aluminas with different Al2O3:SiO2 ratios (90:10, 80:20, and 60:40, that is, Siral 10, 20, and 40, respectively) were tested as a support for the lipase immobilization, and results are shown in Fig. 2.
The supports that provided the best CALB immobilization efficiency were Siral 40 (91.4%), HZSM-5 (SAR 280) (90.6%), and MCM-41 (89.4%). Siral 40 was chosen for CALB immobilization due to its high specific area and mesoporous volume. Although MCM-41 has similar characteristics, it is a low-density material that floated when in contact with the reaction media, making the experimental procedure difficult under the studied conditions. It can be observed that the supports that obtained the highest immobilization efficiencies are more hydrophobic, as they have high silica: alumina ratio. It has been widely reported that the CALB lipase is intensely activated in hydrophobic materials (Kim et al. 2006; Talbert and Goddard 2012) due to the large hydrophobic surface existing around its active site (Uppenberg et al. 1994).
The supports that allowed the highest RML immobilization efficiencies were Siral 20 (97.6%), HZSM-5 (SAR 25) (77.1%), and HZSM-5 (SAR 280) (62.7%). Due to its greater immobilization efficiency, Siral 20 was chosen as the immobilization support for this preparation. Lipases from different sources can show a different affinity for a given support. However, generally, the most hydrophobic support may immobilize more lipases than a less hydrophobic one (Rodrigues et al. 2019).
Siral 40 has a higher SiO2/Al2O3 ratio, a greater number of silanol groups (≡SiOH) on its surface, and ismore hydrophobic than Siral 20. Therefore, it was chosen to evaluate the buffer concentration and pH effect on immobilization since Siral 40 also has the greatest specific area and mesoporous volume from the set of three promising biocatalysts and the higher immobilization efficiency for CALB.
Effect of buffer concentration and pH on the immobilization process
Lipases can be adsorbed onto hydrophobic supports using phosphate buffer with different concentrations (5–25 mmol L−1). This adsorption involves the hydrophobic regions around the active site, stabilizing the enzyme in its open conformation (Fernandez-Lafuente et al. 1998). Generally, hydrophobic adsorptions are enhanced by increasing ionic strength, but the effect shows differently for lipase immobilization in hydrophobic supports. Higher ionic strength hampers the open conformation of lipases, avoiding the interfacial activation when interacting with the support (Manoel et al. 2015b; Rodrigues et al. 2019).
Some studies use sodium phosphate buffer with a concentration of 5 mmol L−1 at pH 7.0 for CALB and RML immobilization (Manoel et al. 2015a; Bassi et al. 2016; Barsé et al. 2019; Cipolatti et al. 2020). However, considering the pH change, as shown in Table 2, a more concentrated buffer solution (100 mmol L−1) was investigated to stabilize the pH medium. The higher the buffer concentration, the higher the capacity to resist pH changes. Thus, the immobilization of CALB and RML onto Siral 40 was performed using 2 concentrations (5 and 100 mmol L−1) of phosphate buffer at pH 7.0. The results are shown in Fig. 3. The pH of the solution, using the 100 mmol L−1 phosphate buffer, was 7 for enzymatic immobilization and in the control assay (performed only with the support without enzyme).
It is possible to observe from Fig. 3 that the increase in buffer concentration has significantly decreased the immobilization efficiency. The increase in buffer concentration results in an increase in the ionic strength of the solution, which could also cause the breakdown of ionic interactions between the enzyme molecule and the support (Bornscheuer 2002). Secundo et al. (2011) also studied the immobilization of α-chymotrypsin in Si-Al supports. They suggested that the ionic strength of higher concentrations of potassium phosphate buffers could cause a loss of the protein amount adsorbed on the support.
The concentration of phosphate buffer solution was then fixed at 5 mmol L−1 to avoid the decrease in immobilization efficiencies of both lipases. A higher buffer pH value (11.0) was evaluated since Siral 40 is a slightly acidic material. Figure 4 presents the immobilization efficiency using a phosphate buffer concentration of 5 mmol L−1 and two pH values (7.0 and 11.0). The 5 mmol L−1 phosphate buffer solution at pH 11.0 maintained the final pH at 8.0 for the tested support. The immobilization efficiency for both lipases was similar for different pH values, although the study of Yu et al. (Yu et al. 2013b) indicates that the adsorption media pH affects the immobilization efficiency.
The pH optimum for the enzyme preparations CALB and RML is in the range of 5–9 and 7–10, respectively. Therefore, the following experiments were conducted with 5 mmol L−1 buffer solution at pH 7.0.
Protein concentration effect on the immobilization process
The immobilization of lipases on silica-aluminas supports was studied using different lipase concentrations. CALB was immobilized on Siral 40, while RML was immobilized on Siral 20, and the results are shown in Fig. 5 and Table 3. The increase in the free-protein concentration increased the protein adsorbed mass onto the support until it reached a protein maximum value of 10.64 and 20.97 mgprotein gsupport−1 for CALB and RML, respectively.
Immobilizations were carried out at room temperature (25 ± 1 °C) for 120 min, using 10 mL of enzymatic solution (phosphate buffer 5 mmol L−1 pH 7.0) and 1 g of support.
Both experimental data fitted the Langmuir adsorption model with a high determination coefficient (Table 3), confirming the validity of this model to describe enzyme adsorption onto silica-aluminas. Langmuir’s adsorption model predicts adsorption in a monolayer of adsorbate molecules on a surface with a limited number of adsorption sites (Alves et al. 2017). The higher equilibrium constant obtained for RML adsorption could relate to electrostatic interactions involving alumina sites or surface silanol groups with the enzyme molecule (Macario et al. 2007). CALB adsorption onto Siral 40 probably occurs due to hydrophobic interactions since CALB has a highly hydrophobic nature and Siral 40 contains higher silica content, being more hydrophobic (Vescovi et al. 2016).
The effect of immobilized derivatives protein content in the esterification reaction (ethyl oleate synthesis) was studied, and the results are shown in Fig. 6. The CALB-Siral 40 esterification activity increased in the range of 0.8 and 3.3 mgprotein mL−1 of lipase solution. However, activity decreased for immobilized derivatives prepared with protein concentrations higher than 3.3 mgprotein mL−1. It can indicate the formation of lipase clusters at high protein concentrations studied, as reported by Al-Duri and Yong (Al-Duri and Yong 2000). The support saturation enables many protein–protein interactions, leading to conformational changes and decreasing the esterification activity (Brito et al. 2017). The RML-Siral 20 esterification activity increased with the initial protein concentration for all the values studied. Siral 20 has a higher protein adsorption capacity (Qm) than Siral 40 (Fig. 5).
The esterification activity values (CALB-Siral 40 and RML-Siral 20) were higher than those obtained for free lipase (results not shown), showing that the immobilization improved the enzyme performance on the esterification reaction.
Effect of temperature on ethyl oleate synthesis
The temperature effect on the ethyl oleate synthesis using the free or the immobilized enzyme was investigated, and the results are shown in Fig. 7. The protein amount was equal to 0.6, 0.4, 1.2, and 0.8 mg mL−1 for the reaction catalyzed by CALB, RML, CALB-Siral 40, and RML-Siral 20, respectively. The temperature optima were 30 °C for RML and RML-Siral 20. A sharp conversion decrease was observed at temperatures higher than 40 °C. Although the maximum temperature did not change, higher conversions were observed for reactions using the immobilized enzyme (RML-Siral 20).
Similarly, higher conversions were observed in reactions catalyzed with CALB-Siral 40 compared to CALB. The optimal temperature changed from 40 to 60 °C using CALB-Siral 40. Ma et al. (Ma et al. 2016) also observed that CALB immobilized onto epoxy-modified silica has greater activity than CALB in the temperature range of 0 to 60 °C. Therefore, immobilization improved the thermal stability of the biocatalyst. This higher stability may be related to a stronger interaction between the enzyme and the support, which promotes a reduction in the mobility of tertiary lipase structure, protecting it from denaturing effects caused by temperature and contact with the reaction medium. Thus, according to the results presented in Fig. 7, CALB-Siral 40 is more thermostable than CALB, which is a biocatalyst desirable feature in biotransformation processes.
Effect of enzyme concentration on the ethyl oleate synthesis
The effect of enzyme concentration on ethyl oleate synthesis is shown in Fig. 8. The X-axis was displayed as protein mass (mg) so that similar enzyme quantities in both forms (free and immobilized) could be compared. The maximum conversion was observed for a free lipase concentration of 30% v/v, corresponding to 1.8 and 1.2 mgprotein mL−1 of initial protein concentration for CALB and RML, respectively. It seems that enzymatic saturation occurred in an enzymatic concentration of 40% v/v (2.4 and 1.6 mgprotein mL−1 for CALB and RML, respectively).
CALB-Siral 40 showed slightly lower conversion values than CALB (Fig. 8A). RML-20 provided conversion values similar to those obtained with RML for initial enzymatic concentrations equal to or higher than 0.4 mgprotein mL−1 (Fig. 8B). It is important to highlight that both immobilized enzymes maintained the catalytic properties of lipases in nonaqueous media, showing that silica-aluminas oxides are promising supports for lipase immobilization and its application in organic synthesis. Foresti and Ferreira (2005) observed only an oleic acid conversion of 10% using CALB immobilized onto polypropylene.
Immobilized biocatalyst reuse
The advantage of using immobilized enzyme preparations is that they can be reused in processes, reducing costs. The reuse of CALB-Siral 40 and RML-Siral 20 was evaluated in the ethyl oleate synthesis. The immobilized biocatalyst was reused for 3 batch cycles, and after each batch, the immobilized derivative was filtered, washed with ethanol, and maintained in a desiccator until the subsequent batch. Ethanol was chosen as a washing solvent because it is one of the reactants, and considering the previous results obtained by our research group (Aguieiras et al. 2016). The use of hydrophobic solvents as a washing solvent was also avoided because they can reduce the strength of the adsorption of the enzyme on the hydrophobic support, favoring enzyme desorption. The results are shown in Fig. 9.
Both immobilized derivatives were reused for three batch cycles. However, the conversion values decreased more pronounced from the first to the second cycle than from the second to the third cycle. This behavior could be related to lipase desorption or denaturation. Lipase immobilized on hydrophobic supports has the active center more exposed to the medium. They could be more rapidly inhibited by the reactants and be especially sensitive to the negative effects of phosphate anions from the buffer solution (Rodrigues et al. 2019). José et al. (José et al. 2011) observed a secondary structural change in commercial immobilized lipase B from Candida antarctica (Novozym®435) after contact with ethanol. The polar solvents might distort the enzyme molecule by removing the water in the micro-layer surrounding the biocatalyst (Bezbradica et al. 2007; Aguieiras et al. 2013; Stergiou et al. 2013).
Immobilization on hydrophobic supports is very strong, but the lipases may be desorbed according to the reaction conditions. The lipases present in the pores of the support would be less subject to leaching (Rodrigues et al. 2019).
After the first cycle, infrared spectroscopy was used to evaluate whether the enzyme desorbed or chemical substances (reagents and products) were adsorbed on the solid support. FTIR spectrum is a useful tool to identify certain functional groups or chemical bonds on the solid surface and the occurrence of changes on the material’s surface (Li and Bai 2005). Figures 10 and 11 show the FTIR spectra for the support, the free-liquid enzyme, the immobilized enzyme, the immobilized enzyme after being used on oleic acid esterification and washed with ethanol, and the reactants (ethanol and oleic acid), and the reaction medium after the reaction.
Both free lipase spectra (CALB and RML) presented a broad band around 3600–2900 cm−1 that can be assigned to the stretching vibration of OH groups, which correspond to the asymmetric and symmetric stretching vibration of water molecules. Moreover, both spectra present a band around 1700–1600 cm−1 that is related to the amide I absorption of the polypeptide backbone and a band around 1400–1200 cm−1, which is assigned to CN stretching (NH bending) (Van De Weert et al. 2001; Barth 2007; Kong and Yu 2007).
The band around 1700–1600 cm−1 is present in both immobilized biocatalysts spectra. Still, it is hardly seen on CALB on Siral 40, which indicates a change in hydration of the native protein compared to the adsorbed one since the immobilization process is followed by a decrease of the band region related to water for CALB. Foresti et al. (2010) observed that lipase conformation could change slightly during adsorption on the immobilization process, mostly related to changes in the hydration state between native and immobilized forms.
Some bands (2950–2800 cm−1 and 1705 cm−1), related to the reactants (oleic acid and ethanol, respectively), were observed in the spectra of the immobilized enzymes after the reaction, which may reveal that the biocatalyst washing procedure was not enough to remove all the reactants from the immobilized enzyme surface. Reactants on the biocatalyst surface probably caused the conversion decrease when the biocatalyst was reused. Intensity decreases of some bands related to CALB and RML can also be related to enzyme leaching.
Conclusions
Six different materials were evaluated as support for enzyme immobilization, and silica-aluminas oxides were found to provide a great immobilization efficiency, maintaining the stability of the resulting biocatalyst. Lower pH value and concentration improved the immobilization efficiency. Then, lipase adsorption was studied using the best supports for each enzyme (Siral 40 for CALB and Siral 20 for RML) and an adjusted immobilization media. The Langmuir isotherm model described the adsorption of commercial liquid-free lipases (CALB and RML) satisfactorily onto silica-aluminas. Even though increased RML concentration on Siral 20 has improved esterification activity, this behavior was not observed for CALB on Siral 40. The esterification activity of CALB on Siral 40 presented a maximum value at experiment conditions of 3.3 mgprotein mL−1, which probably indicates the formation of lipase clusters with the increase in enzyme concentration. The optimal temperature observed for ethyl oleate synthesis was equal to 30 and 60 °C for RML-Siral 20 and CALB-Siral 40, respectively.
Both immobilized enzymes could maintain the catalytic properties of lipases in nonaqueous media. Moreover, the reuse of these biocatalysts was evaluated on ethyl oleate synthesis. Immobilized CALB and RML could be reused for three batch cycles. The pronounced decrease in oleic acid conversion was probably caused by insufficient reactants removal from the biocatalyst surface after each batch cycle and possible enzyme leaching, as observed by the FTIR-ATR spectra. The treatment of the enzyme immobilized with glutaraldehyde or polyethyleneimine is a strategy that should be studied to prevent the enzyme release. The obtention of new biocatalysts in this work and their use in esterification reactions shows these biocatalysts’ potential to obtain new industrial interest esters.
Data availability
Not applicable.
References
Aarthy M, Saravanan P, Gowthaman MK et al (2014) Enzymatic transesterification for production of biodiesel using yeast lipases: an overview. Chem Eng Res Des 92:1591–1601. https://doi.org/10.1016/j.cherd.2014.04.008
Aguieiras ECG, Souza SL, Langone MA (2013) Estudo do comportamento da lipase comercial lipozyme RM IM em reações de esterificação para obtenção de biodiesel. Quim Nova 36:646–650
Aguieiras ECG, Ribeiro DS, Couteiro PP et al (2016) Investigation of the reuse of immobilized lipases in biodiesel synthesis: influence of different solvents in lipase activity. Appl Biochem Biotechnol 179:485–496. https://doi.org/10.1007/s12010-016-2008-9
Al-Duri B, Yong YP (2000) Lipase immobilisation: an equilibrium study of lipases immobilised on hydrophobic and hydrophilic/hydrophobic supports. Biochem Eng J 4:207–215. https://doi.org/10.1016/S1369-703X(99)00050-9
Alves MD, Aracri FM, Cren ÉC, Mendes AA (2017) Isotherm, kinetic, mechanism and thermodynamic studies of adsorption of a microbial lipase on a mesoporous and hydrophobic resin. Chem Eng J 311:1–12. https://doi.org/10.1016/j.cej.2016.11.069
Amini Z, Ilham Z, Ong HC et al (2017) State of the art and prospective of lipase-catalyzed transesterification reaction for biodiesel production. Energy Convers Manag 141:339–353. https://doi.org/10.1016/j.enconman.2016.09.049
Ansorge-Schumacher MB, Thum O (2013) Immobilised lipases in the cosmetics industry. Chem Soc Rev 42:6475–6490. https://doi.org/10.1039/c3cs35484a
Antonopoulou I, Varriale S, Topakas E et al (2016) Enzymatic synthesis of bioactive compounds with high potential for cosmeceutical application. Appl Microbiol Biotechnol 100:6519–6543. https://doi.org/10.1007/s00253-016-7647-9
Baerlocher C, McCusker LB (2007) New advances in zeolite structure analysis. Stud Surf Sci Catal 170:657–665. https://doi.org/10.1016/S0167-2991(07)80905-0
Bajaj A, Lohan P, Jha PN, Mehrotra R (2010) Biodiesel production through lipase catalyzed transesterification: an overview. J Mol Catal B Enzym 62:9–14
Barsé LQ, Graebin NG, Cipolatti EP et al (2019) Production and optimization of isopropyl palmitate via biocatalytic route using home-made enzymatic catalysts. J Chem Technol Biotechnol 94:389–397. https://doi.org/10.1002/jctb.5782
Barth A (2007) Infrared spectroscopy of proteins. Biochim Biophys Acta Bioenerg 1767:1073–1101. https://doi.org/10.1016/j.bbabio.2007.06.004
Bassi JJ, Todero LM, Lage FAP et al (2016) Interfacial activation of lipases on hydrophobic support and application in the synthesis of a lubricant ester. Int J Biol Macromol 92:900–909. https://doi.org/10.1016/j.ijbiomac.2016.07.097
Bezbradica D, Mijin D, Šiler-Marinković S, Knežević Z (2007) The effect of substrate polarity on the lipase-catalyzed synthesis of aroma esters in solvent-free systems. J Mol Catal B Enzym 45:97–101. https://doi.org/10.1016/j.molcatb.2006.12.003
Bornscheuer UT (2002) Microbial carboxyl esterases: classi¢cation, properties and application in biocatalysis. FEMS Microbiol Rev 26:73–81
Boudrant J, Woodley JM, Fernandez-Lafuente R (2020) Parameters necessary to define an immobilized enzyme preparation. Process Biochem 90:66–80. https://doi.org/10.1016/j.procbio.2019.11.026
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brady D, Jordaan J (2009) Advances in enzyme immobilisation. Biotechnol Lett 31:1639–1650
Brautaset T, Ellingsen TE (2011) Comprehensive biotechnology. Elsevier
Brito MJP, Veloso CM, Bonomo RCF et al (2017) Activated carbons preparation from yellow mombin fruit stones for lipase immobilization. Fuel Process Technol 156:421–428. https://doi.org/10.1016/j.fuproc.2016.10.003
Campisano ISP, de Queiros EE, de Oliveira VC et al (2021) Solvent-free lipase-catalyzed synthesis of linear and thermally stable polyesters obtained from diacids and diols. Brazilian J Chem Eng 38:549–562. https://doi.org/10.1007/s43153-021-00137-y
Chandra P, Enespa Singh R, Arora PK (2020) Microbial lipases and their industrial applications: a comprehensive review. BioMed Central 19:1–42
Chen NY (1976) Hydrophobic properties of zeolites. J Phys Chem 80:60–64. https://doi.org/10.1021/j100542a013
Christopher LP, Kumar H, Zambare VP (2014) Enzymatic biodiesel: challenges and opportunities. Appl Energy 119:497–520
Cipolatti EP, Silva MJA, Klein M et al (2014) Current status and trends in enzymatic nanoimmobilization. J Mol Catal B Enzym 99:56–67. https://doi.org/10.1016/j.molcatb.2013.10.019
Cipolatti EP, Moreno-pérez S, Tereza L et al (2015) Synthesis and modification of polyurethane for immobilization of Thermomyces lanuginosus ( TLL ) lipase for ethanolysis of fish oil in solvent free system. J Mol Catal B Enzym 122:163–169. https://doi.org/10.1016/j.molcatb.2015.09.006
Cipolatti EP, Valério A, Henriques RO et al (2020) Production of new nanobiocatalysts via immobilization of lipase B from C. antarctica on polyurethane nanosupports for application on food and pharmaceutical industries. Int J Biol Macromol 165:2957–2963. https://doi.org/10.1016/j.ijbiomac.2020.10.179
Ćorović M, Milivojević A, Simović M et al (2020) Enzymatically derived oil-based L-ascorbyl esters: synthesis, antioxidant properties and controlled release from cosmetic formulations. Sustain Chem Pharm. https://doi.org/10.1016/j.scp.2020.100231
Costantini A, Califano V (2021) Lipase immobilization in mesoporous silica nanoparticles for biofuel production. Catalysts. https://doi.org/10.3390/catal11050629
Cunha AG, Besteti MD, Manoel EA et al (2014) Preparation of core-shell polymer supports to immobilize lipase B from Candida antarctica: effect of the support nature on catalytic properties. J Mol Catal B Enzym 100:59–67. https://doi.org/10.1016/j.molcatb.2013.11.020
da Dutra L, Pinto MCC, Cipolatti EP et al (2022) How the biodiesel from immobilized enzymes production is going on: an advanced bibliometric evaluation of global research. Renew Sustain Energy Rev 153:111765. https://doi.org/10.1016/j.rser.2021.111765
Date AA, Nagarsenker MS (2008) Parenteral microemulsions: an overview. Int J Pharm 355:19–30. https://doi.org/10.1016/J.IJPHARM.2008.01.004
Dumitriu E, Secundo F, Patarin J, Fechete I (2003) Preparation and properties of lipase immobilized on MCM-36 support. J Mol Catal B Enzym 22:119–133. https://doi.org/10.1016/S1381-1177(03)00015-8
Everton SS, Sousa I, da Silva DL et al (2022) The role of Brazil in the advancement of enzymatic biodiesel production. Brazil J Chem Eng. https://doi.org/10.1007/s43153-022-00229-3
Fasim A, More VS, More SS (2021) Large-scale production of enzymes for biotechnology uses. Curr Opin Biotechnol 69:68–76. https://doi.org/10.1016/j.copbio.2020.12.002
Fernandez-Lafuente R, Armisén P, Sabuquillo P et al (1998) Immobilization of lipases by selective adsorption on hydrophobic supports. Chem Phys Lipids 93:185–197. https://doi.org/10.1016/S0009-3084(98)00042-5
Foresti ML, Ferreira ML (2005) Solvent-free ethyl oleate synthesis mediated by lipase from Candida antarctica B adsorbed on polypropylene powder. Catal Today 107–108:23–30. https://doi.org/10.1016/J.CATTOD.2005.07.053
Foresti ML, Valle G, Bonetto R et al (2010) FTIR, SEM and fractal dimension characterization of lipase B from Candida antarctica immobilized onto titania at selected conditions. Appl Surf Sci 256:1624–1635. https://doi.org/10.1016/J.APSUSC.2009.09.083
Garcia-galan C, Berenguer-Murcia Á, Fernandez-Lafuente R, Rodrigues RC (2011) Potential of different enzyme immobilization strategies to improve enzyme performance. Adv Synth Catal 353:2885–2904. https://doi.org/10.1002/adsc.201100534
Gog A, Roman M, Toşa M et al (2012) Biodiesel production using enzymatic transesterification - current state and perspectives. Renew Energy 39:10–16
Gustafsson H, Johansson EM, Barrabino A et al (2012) Immobilization of lipase from Mucor miehei and Rhizopus oryzae into mesoporous silica–the effect of varied particle size and morphology. Colloids Surf B Biointerfaces 100:22–30. https://doi.org/10.1016/j.colsurfb.2012.04.042
Hanefeld U, Gardossi L, Magner E (2009) Understanding enzyme immobilisation. Chem Soc Rev 38:453–468. https://doi.org/10.1039/b711564b
Hensen EJM, Poduval DG, Ligthart DAJM et al (2010) Quantification of strong brønsted acid sites in aluminosilicates. J Phys Chem C 114:8363–8374. https://doi.org/10.1021/jp9106348
Hua YW, Fang M, Tong D, shen, et al (2013) Immobilization of Candida rugosa lipase on hexagonal mesoporous silicas and selective esterification in nonaqueous medium. Biochem Eng J 70:97–105. https://doi.org/10.1016/j.bej.2012.10.005
Jesionowski T, Zdarta J, Krajewska B (2014) Enzyme immobilization by adsorption: a review. Adsorption 20:801–821. https://doi.org/10.1007/s10450-014-9623-y
José C, Bonetto RD, Gambaro LA et al (2011) Investigation of the causes of deactivation–degradation of the commercial biocatalyst Novozym® 435 in ethanol and ethanol–aqueous media. J Mol Catal B Enzym 71:95–107. https://doi.org/10.1016/J.MOLCATB.2011.04.004
Kalantari M, Yu M, Yang Y et al (2017) Tailoring mesoporous-silica nanoparticles for robust immobilization of lipase and biocatalysis. Nano Res 10:605–617. https://doi.org/10.1007/s12274-016-1320-6
Kim H, Yoon SH, Choi HN et al (2006) Amperometric glucose biosensor based on sol-gel-derived zirconia / nafion composite film as encapsulation matrix. Bull Korean Chem Soc 27:65–70
Kong J, Yu S (2007) Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin (Shanghai) 39:549–559. https://doi.org/10.1111/j.1745-7270.2007.00320.x
Krajewska B (2004) Application of chitin- and chitosan-based materials for enzyme immobilizations: a review. Enzyme Microb Technol 35:126–139. https://doi.org/10.1016/j.enzmictec.2003.12.013
Kumar A, Verma V, Dubey VK et al (2023) Industrial applications of fungal lipases: a review. Front Microbiol. https://doi.org/10.3389/fmicb.2023.1142536
Li N, Bai R (2005) Copper adsorption on chitosan–cellulose hydrogel beads: behaviors and mechanisms. Sep Purif Technol 42:237–247. https://doi.org/10.1016/J.SEPPUR.2004.08.002
Ma HZ, Yu XW, Song C et al (2016) Immobilization of Candida Antarctica lipase B on epoxy modified silica by sol-gel process. J Mol Catal B Enzym 127:76–81. https://doi.org/10.1016/J.MOLCATB.2016.02.014
Macario A, Giordano G, Setti L et al (2007) Study of lipase immobilization on zeolitic support and transesterification reaction in a solvent free-system. Biocatal Biotransformation 25:328–335. https://doi.org/10.1080/10242420701444256
Macario A, Katovic A, Giordano G, et al (2005) Immobilization of Lipase on microporous and mesoporous materials: Studies of the support surfaces. In: Studies in Surface Science and Catalysis. Elsevier Inc., pp 381–394
Manoel EA, Dos Santos JCS, Freire DMG et al (2015a) Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme Microb Technol 71:53–57. https://doi.org/10.1016/j.enzmictec.2015.02.001
Manoel EA, José CS, Freire DMG et al (2015b) Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme Microb Technol 71:53–57. https://doi.org/10.1016/j.enzmictec.2015.02.001
Manoel EA, Pinto M, dos Santos JCS et al (2016) Design of a core–shell support to improve lipase features by immobilization. RSC Adv 6:62814–62824. https://doi.org/10.1039/C6RA13350A
Mateo C, Palomo JM, Fernandez-Lorente G et al (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol 40:1451–1463. https://doi.org/10.1016/j.enzmictec.2007.01.018
Mitchell S, Pérez-Ramírez J (2011) Mesoporous zeolites as enzyme carriers: synthesis, characterization, and application in biocatalysis. Catal Today 168:28–37. https://doi.org/10.1016/j.cattod.2010.10.058
Mokhtar NF, Raja Noor Zaliha RNZR, Muhd Noor ND et al (2020) The immobilization of lipases on porous support by adsorption and hydrophobic interaction method. Catalysts 10:1–17. https://doi.org/10.3390/catal10070744
Nakamoto H, Takahashi H (1982) Hydrophobic natures of zeolite ZSM-5. Zeolites 2:67–68. https://doi.org/10.1016/S0144-2449(82)80002-X
Pedro KCNR, Ferreira IEP, Henriques CA, Langone MAP (2019) Enzymatic fatty acid ethyl esters synthesis using acid soybean oil and liquid lipase formulation. Chem Eng Commun 207:43–55. https://doi.org/10.1080/00986445.2019.1572001
Pinto MCC, Everton SS, Cirilo LCM et al (2020) Effect of hydrophobicity degree of polymer particles on lipase immobilization and on biocatalyst performance. Biocatal Biotransformation 0:1–11. https://doi.org/10.1080/10242422.2020.1739026
Rashmi BS, Gayathri D (2014) Partial purification, characterization of Lactobacillus sp. G5 lipase and their probiotic potential. Int Food Res J 21:1737–1743
Reis P, Holmberg K, Watzke H et al (2009) Lipases at interfaces: a review. Adv Colloid Interface Sci 147–148:237–250
Rodrigues RC, Ortiz C, Berenguer-Murcia Á et al (2013) Modifying enzyme activity and selectivity by immobilization. Chem Soc Rev 42:6290–6307. https://doi.org/10.1039/c2cs35231a
Rodrigues RC, Virgen-Ortíz JJ, dos Santos JCS et al (2019) Immobilization of lipases on hydrophobic supports: immobilization mechanism, advantages, problems, and solutions. Biotechnol Adv 37:746–770. https://doi.org/10.1016/J.BIOTECHADV.2019.04.003
Sá AGA, de Meneses AC, de Araújo PHH, de Oliveira D (2017) A review on enzymatic synthesis of aromatic esters used as flavor ingredients for food, cosmetics and pharmaceuticals industries. Trends Food Sci Technol 69:95–105. https://doi.org/10.1016/j.tifs.2017.09.004
Secundo F, Roda G, Vittorini M et al (2011) Effect of chemical composition of SBA-15 on the adsorption and catalytic activity of α-chymotrypsin. J Mater Chem 21:15619–15628. https://doi.org/10.1039/c1jm11475a
Serralha FN, Lopes JM, Lemos F et al (1998) Zeolites as supports for an enzymatic alcoholysis reaction. J Mol Catal B Enzym 4:303–311. https://doi.org/10.1016/S1381-1177(98)00069-1
Sheldon RA, van Pelt S (2013) Enzyme immobilisation in biocatalysis: why, what and how. Chem Soc Rev 42:6223–35. https://doi.org/10.1039/c3cs60075k
Stergiou PY, Foukis A, Filippou M et al (2013) Advances in lipase-catalyzed esterification reactions. Biotechnol Adv 31:1846–1859. https://doi.org/10.1016/j.biotechadv.2013.08.006
Talbert JN, Goddard JM (2012) Enzymes on material surfaces. Colloids Surfaces B Biointerfaces 93:8–19. https://doi.org/10.1016/j.colsurfb.2012.01.003
Tan T, Lu J, Nie K et al (2010) Biodiesel production with immobilized lipase : a review. Biotechnol Adv. https://doi.org/10.1016/j.biotechadv.2010.05.012
Thangaraj B, Solomon PR (2019) Immobilization of lipases - a Review. Part II: carrier materials. Chem Bioeng Rev 6:167–194. https://doi.org/10.1002/cben.201900017
Tsutsumi K, Kawai T, Yanagihara T (1994) Adsorption characteristics of hydrophobic zeolites. Stud Surf Sci Catal 83:217–224. https://doi.org/10.1016/S0167-2991(08)63260-7
Uppenberg J, Hansen MT, Patkar S, Jones TA (1994) The sequence, crystal structure determination and refinement of two crystal forms of lipase B from Candida antarctica. Structure 2:293–308. https://doi.org/10.1016/S0969-2126(00)00031-9
Van De Weert M, Haris PI, Hennink WE, Crommelin DJA (2001) Fourier transform infrared spectrometric analysis of protein conformation: effect of sampling method and stress factors. Anal Biochem 297:160–169. https://doi.org/10.1006/ABIO.2001.5337
Vescovi V, Kopp W, Guisán JM et al (2016) Improved catalytic properties of Candida antarctica lipase B multi-attached on tailor-made hydrophobic silica containing octyl and multifunctional amino- glutaraldehyde spacer arms. Process Biochem 51:2055–2066. https://doi.org/10.1016/J.PROCBIO.2016.09.016
Wang C, feng, Zhou G wei, Li YJ, et al (2012) Biocatalytic esterification of caprylic acid with caprylic alcohol by immobilized lipase on amino-functionalized mesoporous silica. Colloids Surfaces A Physicochem Eng Asp 406:75–83. https://doi.org/10.1016/j.colsurfa.2012.04.053
Wenchao W, Fashe L, Ying L (2020) Effect of biodiesel ester structure optimization on low temperature performance and oxidation stability. J Mater Res Technol 9:2727–2736. https://doi.org/10.1016/J.JMRT.2020.01.005
Yu Z, Jameel H, Chang H et al (2013b) Quantification of bound and free enzymes during enzymatic hydrolysis and their reactivities on cellulose and lignocellulose. Bioresour Technol 147:369–377. https://doi.org/10.1016/j.biortech.2013.08.010
Zhou Z, Hartmann M (2013) Progress in enzyme immobilization in ordered mesoporous materials and related applications. Chem Soc Rev 42:3894–3912. https://doi.org/10.1039/c3cs60059a
Acknowledgements
The authors thank UFRJ, UERJ, and IFRJ for supporting, and thank Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro- FAPERJ (E-26/211.889/2021, E-26/200.144/2023, E-26/201.366/2021) for funding the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical standards
Tests on humans or animals were not carried out in the research. All authors are aware of and approve the submission of the work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pedro, K.C.N.R., da Silva, J.V.V., Cipolatti, E.P. et al. Adsorption of lipases on porous silica-based materials for esterification in a solvent-free system. 3 Biotech 13, 380 (2023). https://doi.org/10.1007/s13205-023-03801-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03801-x